Abstract
Background
As changes in thyroid stimulating hormone, thyroid hormones, and vital signs following administration of a single dose of liothyronine have typically only been documented for 24 hours, we documented these parameters over 96 hours.
Methods
Blood samples were obtained for 4 days after administration of 50-mcg liothyronine. Concentrations of total and free triiodothyronine, free and total thyroxine, and thyroid stimulating hormone were measured. Vital signs were documented.
Results
Triiodothyronine concentrations peaked at 2.5 hours following liothyronine administration. Heart rate increased by 5 hours after liothyronine administration, subsequently reaching a value higher than baseline (p value 0.009). Suppression of thyroid stimulating hormone concentrations began at 2 hours. The nadir thyroid stimulating hormone value at 12 hours was significantly different from baseline (p <0.001), and remained lower than baseline for 2–3 days.
Conclusions
A single dose of liothyronine has both short term and longer term effects. There is clearly a different lag time between the serum concentrations of triiodothyronine and its effects on the heart and pituitary respectively. The increase in serum triiodothyronine concentration occurred with hours and was then followed by an increase in heart rate. The increased heart rate was transient and was followed by a reduction in thyroid stimulating hormone concentration. The suppression of thyroid stimulating hormone was delayed, but was more sustained. Thus, sustained thyroid stimulating hormone reduction beyond 24 hours was achieved by a single dose of liothyronine that produced only brief increases in serum triiodothyronine levels and transient increases in heart rate.
Key terms: T3, triiodothyronine, kinetics, TSH, heart rate, temperature
INTRODUCTION
The kinetics of triiodothyronine (T3) following oral doses of various T3-containing preparations during the first 24 hours after its administration have been described in both hypothyroid and euthyroid individuals.
With respect to hypothyroid patients, Saberi and Utiger (1) described serum T3 concentrations in patients given daily doses of T3 of either 25 mcg or 50 mcg. Peak T3 levels of 220–230 ng/dL were reached about 4 hours after the 25-mcg dose and had fallen to baseline by 12 hours. The 50-mcg dose produced peak T3 levels of 350–375 ng/dL, also at about 4 hours, and these had fallen to baseline by 20 hours. Thyroid stimulating hormone (TSH) concentrations, which were slightly elevated to about 11–14 mIU/L at baseline, did not change during T3 therapy in either group. A 1983 study described T3 levels increasing from 86–236 ng/dL to 209–458 ng/dL 3 hours after thyroid extract administration in hypothyroid patients (2). TSH concentrations did not change. Thus, neither of these studies found a change in TSH after T3 administration. In contrast, in a study of combination therapy that included 10 mcg T3, free T3 (FT3) concentrations rose to 4.09 pg/mL (normal range for assay 1.82–4.6 pg/mL) 4 hours after the dose was administered and this was accompanied by a TSH value that was lower than baseline for about 12 hours (3). FT3 concentrations remained above baseline for about 12 hours also.
With respect to T3 administration in euthyroid patients, T3 kinetics are similar regardless of whether T3 was provided in the form of a synthetic preparation or a thyroid extract (4). Six grains of desiccated thyroid resulted in peak T3 levels of 640 ng/dL 2 hours after the dose was administered. Five grains of thyroglobulin produced a peak T3 level of 570 ng/dL at 2–4 hours. Seventy-five mcg of synthetic T3 resulted in a peak concentration of T3 of 550 ng/dL 4 hours after dosage administration. The area under the curve (AUC) corrected for the T3 content of the administered tablet did not differ between the 3 preparations and was in the range of 3,500–4,500 ng.hr/dL over a 26 hour period (4). Another study employing an oral dose of 75 mcg of T3 also showed a time to achieve maximum concentration (Tmax) of between 2–3 hours (5). Some data regarding T3 kinetics is available from bioequivalence studies performed with synthetic T3 preparations. For example, the test and reference T3 product had maximum concentrations (Cmax) of T3 of 422–477 ng/dL at 2–2.5 hours after administration of 50 mcg of T3 in one such test (6). The AUC0-24 hr was 2,022–2266 ng.hr/dL. A similar Tmax of 2.35 hours was also reported by Coastal Pharmaceuticals in their study of 100 mcg liothyronine (ANDA 90–097) (7).
Studies reported by Nicoloff and colleagues in 1972 calculated a half-life of T3 that varied with thyroid status (8). The mean half-life was 0.63 days in 7 hyperthyroid patients, 1.0 day in 8 euthyroid individuals, and 1.38 days in 9 hypothyroid patients.
We documented the effect of a single dose of T3 on serum T3, FT3, TSH, heart rate, and body weight over a 4-day period in euthyroid individuals.
METHODS
Overview
This was a phase 1 study to determine the safety and pharmacokinetics of a 50-mcg dose of a liothyronine preparation, Thyromax® (BCT303). This preparation is a liothyronine tablet made with microcrystalline cellulose and magnesium stearate and it was hoped to have a sustained profile of T3 release. The trial was registered at ClinicalTrials.gov as clinical trial NCT01581463. As a sustained release profile of serum T3 concentrations was not observed, in addition to documenting T3 and FT3, the data regarding TSH, heart rate, and body weight were also analyzed over the 4-day period, and the effect of gender and race was also studied.
Participation Recruitment and Study Procedures
Healthy participants without thyroid disease were recruited for the study. Initial screening involved a screening questionnaire that was administered over the telephone. Volunteers taking steroids, or any medications known to affect thyroid hormone metabolism, thyroid hormone absorption, or thyroxine-binding globulin were also excluded. Women who were pregnant, lactating, or taking oral contraceptives were ineligible. Subjects who appeared to be eligible for the study were scheduled for a visit to the Georgetown Clinical Research Unit (GCRU). They were then consented for the study and signed a written informed consent form. They were determined to be in general good health based on the results of medical history, physical examination, 12-lead electrocardiogram (ECG), and a screening TSH. Women underwent a screening pregnancy test. Participants were eligible if clinical laboratory testing showed that they had a normal serum TSH (0.4 – 4.5 mIU/L).
Eligible participants were studied for five days on the GCRU following ingestion of a single dose of liothyronine (Thyromax®). On the first day of the study the participants refrained from taking their usual vitamins, and supplements and reported to the GCRU in a fasting state, after not having eaten since 10:00 pm the previous day. Vital signs (heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), respiratory rate (RR), and weight) were obtained. An ECG was recorded. An intravenous catheter was inserted into a forearm vein and was left in place for 12 hours for subsequent blood sampling.
Baseline thyroid function tests (TSH, total T4, free T4, total T3, free T3) were obtained at 7:30 AM, 7:45 AM and 8:00 AM, with 8:00 AM being designated as time 0. Ten mL of blood were withdrawn for this and all subsequent blood tests. One tablet of Thyromax® containing 50 mcg of T3 was administered orally at approximately 8:00 AM with a glass of water. The Thyromax® (BCT303) was provided by Dr. Keith Latham from IPE, Inc. Thyroid function tests (T3, free T3, free T4, T4, and TSH) were obtained at 15 minute intervals after taking the T3 for 4 samples, then at 30 minutes intervals for 6 additional samples, at hourly intervals for 2 samples, and then at 6 hours, 8 hours, 12 hours, 24 hours, 48 hours, 72 hours, and 96 hours following T3 ingestion. A set of vital signs was obtained with most blood samplings. An electrocardiogram was recorded 1 hour, 4 hours, and 12 hours after T3 administration. Light meals were provided at approximately 12.00 noon and 6.00 PM on the first day of the study. Once the 12 one-hourly blood samples were obtained at approximately 8.00 PM, a set of vital signs were obtained and a final electrocardiogram (ECG) was recorded. The participant was asked to return to the GCRU in a fasting state at 8.00 AM the following 4 days for additional sets of thyroid function tests at 24 hours, 48 hours, 72 hours, and 96 hours after T3 administration. A set of vital signs was obtained with each of these additional four blood samples. Each set of vital signs was obtained after a minimum of 5 minutes rest in a seated position.
Laboratory testing
The 10 ml blood samples were collected in a plain red top tube and allowed to clot. The resulting serum was sent to the main Georgetown laboratory for measuring thyroid hormone levels. All samples for one patient were assayed in a single batch. The reference range for the thyroid assays were TSH 0.4–4.5 mIU/L (immunometric assay), free thyroxine (FT4) 0.8–1.8 ng/dL, thyroxine (T4) 4.7–13.3 mcg/dL, FT3 2.18–3.98 pg/mL, and total T3 76–181 ng/dL (all immunoassays).
Data analysis
The maximum observed concentration (Cmax) and the time at which this occurred (Tmax) were calculated. The area under the plasma concentration–time curves from zero to the last sampling time (i.e., 96 hrs) and 24 hrs [AUC(0,t)] was calculated according to the trapezoidal rule using the actual times of measurements. Baseline-corrected AUC(0,t) values were calculated using the following equation: AUC(0,t)=AUC(0,t), uncorrected-predose level×t. Mean with standard deviation or median with interquartile range were calculated according to gender and race. Wilcoxon rank-sum test was used to compare the difference. Pearson and Spearman correlation coefficients were calculated to examine the correlation of physiologic parameters (height, weight, BMI, and age) with Cmax, Tmax, and AUC. Wilcoxon rank-sum test was also used to compare baseline clinical and laboratory parameters with the peak or nadir response following T3 administration. A mixed linear model with time-varying covariates was used to assess the association of T3 and FT3 concentrations with TSH concentrations, FT4 concentrations, T4 concentrations, weight, temperature, HR, SBP, DBP and RR. Two-tailed P values <0.05 were considered statistically significant. Data were analyzed using SAS version 9.3 (SAS institute, Cary, NC).
RESULTS
Forty-three volunteers inquired about the study. Thirteen individuals did not wish to participate. Fifteen women were ineligible because they were taking oral contraceptives. Fifteen participants underwent screening for the study. Three volunteers were ineligible based on their screening. Twelve participants were eligible (4 females and 8 males, ages 19–41) and all completed the entire study. Their mean age was 29.3 ± 8.6 years (range: 18–43). Of the 12 subjects, 33.3% were women and 41.7% were African American. None of these 12 volunteers were taking any medications at the time of the study. The sequence of events for the study with respect to blood sampling and T3 administration is shown in the left hand column of table 1. Participants completed the study between 6/11/12 and 9/3/12.
Table 1.
Study sequence and vital signs
| Time (hours) | Blood sampling | Other PO intake | Heart rate Mean (SD) | SBP in mmHg Mean (SD) | DBP in mmHg Mean (SD) | Temperature in Celsius Mean (SD) | ECG | Weight in kg Mean (SD) |
|---|---|---|---|---|---|---|---|---|
| -0.50 | X | 58 (9) | 116 (13) | 65 (5) | 36.4 (0.4) | normal | ||
| -0.25 | X | 59 (8) | 115 (13) | 66 (10) | 35.9 (0.5) | |||
| 0* | X | 60 (7) | 117 (9) | 67 (8) | 36.2 (0.4) | 84.3 (18) | ||
| 0.25 | X | 58 (8) | 117 (10) | 68 (9) | 36.1 (0.3) | |||
| 0.50 | X | 59 (7) | 116 (12) | 67 (9) | 36.1 (0.5) | |||
| 0.75 | X | 61 (8) | 115 (11) | 67 (9) | 36.2 (0.5) | |||
| 1.00 | X | 59 (7) | 116 (11) | 66 (10) | 36.2 (0.4) | normal | ||
| 1.50 | X | 61 (9) | 115 (14) | 64 (11) | 36.1 (0.4) | |||
| 2.00 | X | 59 (7) | 115 (10) | 66 (9) | 36.3 (0.3) | |||
| 3.00 | X | 61 (8) | 120 (12) | 65 (8) | 36.5 (0.3) | |||
| 4.00 | X | Lunch | 72 (9) | 119 (14) | 64 (7) | 36.6 (0.4) | normal | |
| 5.00 | X | 73 (9) | 120 (10) | 64 (7) | 36.5 (0.4) | |||
| 6.00 | X | 72 (11) | 123 (12) | 66 (8) | 36.6 (0.4) | |||
| 8.00 | X | 71 (11) | 121 (14) | 65 97) | 36.6 (0.4) | |||
| 10.00 | Dinner | 76 (9) | 118 914) | 68 (9) | 36.5 (0.5) | |||
| 12.00 | X | 71 (11) | 121 929) | 71 (11) | 36.4 (0.4) | normal | ||
| 24.00 | X | 75 11) | 119 (10) | 68 (9) | 36.5 (0.4) | 83.7 (18.1) | ||
| 48.00 | X | 65 (8) | 120 (16) | 70 (8) | 36.6 (0.5) | 83.0 (17.5) | ||
| 72.00 | X | 66 (8) | 116 (10) | 65 (9) | 36.4 (0.4) | 83.6 (18.4) | ||
| 96.00 | X | 69 (9) | 115 (14) | 66 (10) | 35.9 (0.3) | 83.9 (18.2) |
T3 administration at time 0
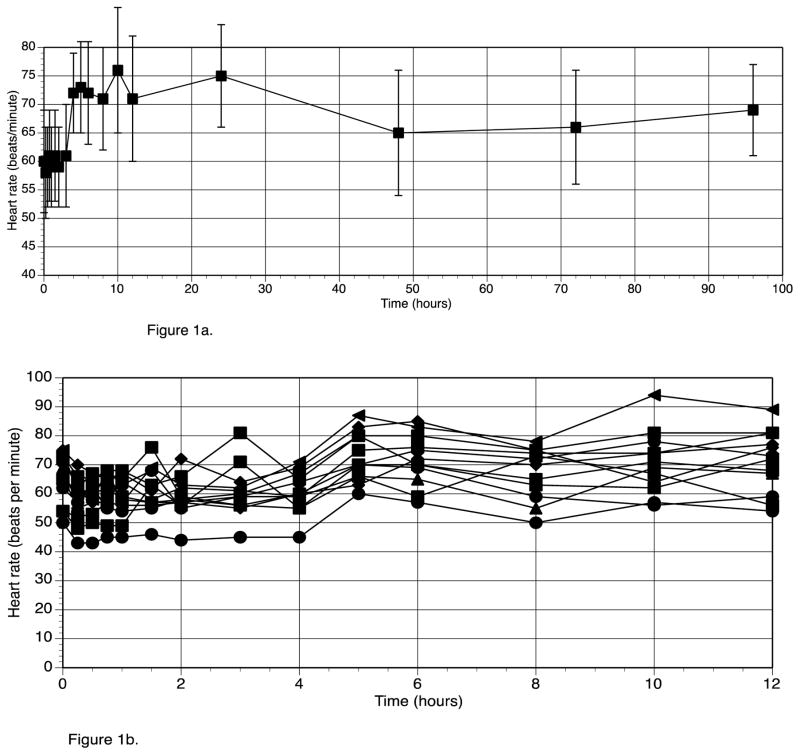
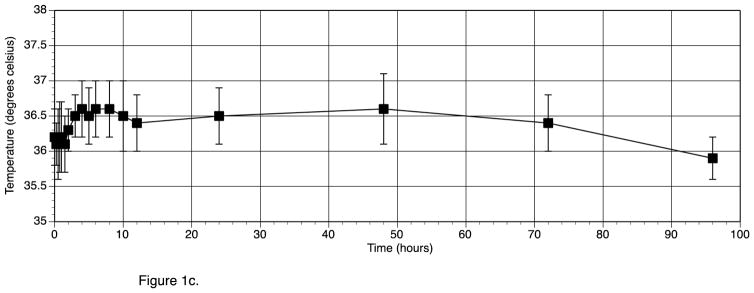
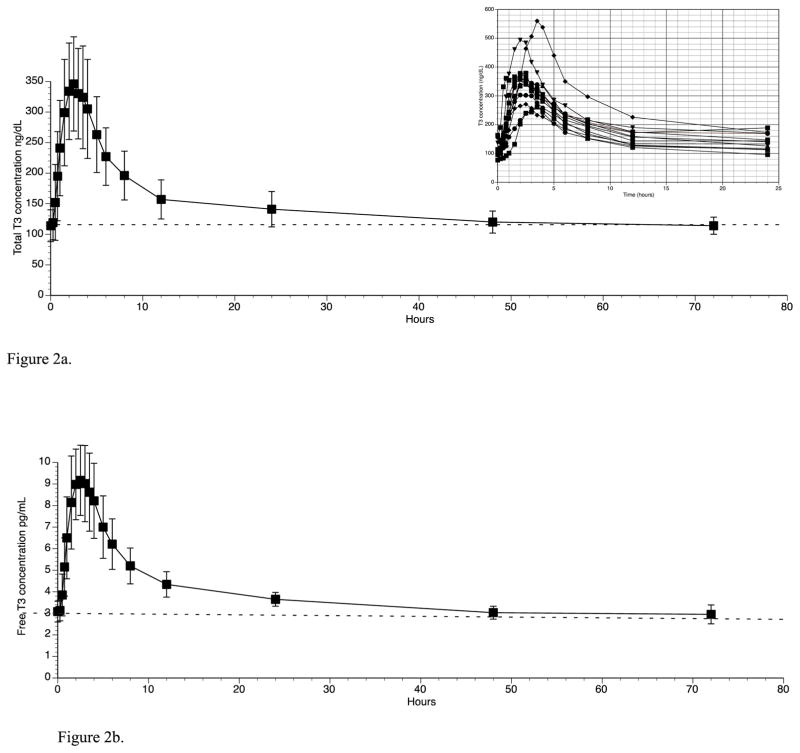
Table 1 (right hand columns) also shows the vital signs for the participants during the study No individual participant had a baseline HR of greater than 75 beats/min. Following T3 administration the highest individual HR recorded was 94 beats/min, and no significant ECG abnormalities developed. Mean HR increased by a maximum of 18 beats/min within the first 12 hours after T3 administration (see figure 1a). The peak increase in HR at 10 hours was significantly different from baseline HR. (p value 0.009). The heart rate of the individual participants for the first 12 hours after T3 administration is shown in figure 1b. Oral temperature increased from a mean of 36.2 C to a maximum of 36.6 C, and then dropped back to 35.9 C (see figure 1c). The increase in temperature was not significant (p value 0.56). SBP, DBP, RR, and weight did not change following T3 administration (see table 1). The only clinical parameter that was significantly related to T3 over 24 hours was HR (p value <0.0001).
Figure 1.
Figure 1a. Heart Rate before and after T3 administration (T3 given at time 0 hours)
Figure 1b. Heart Rate for each individual participant during the first 12 hrs after T3 given at time 0 hrs
Figure 1c. Temperature before and after T3 administration (T3 given at time 0 hours)
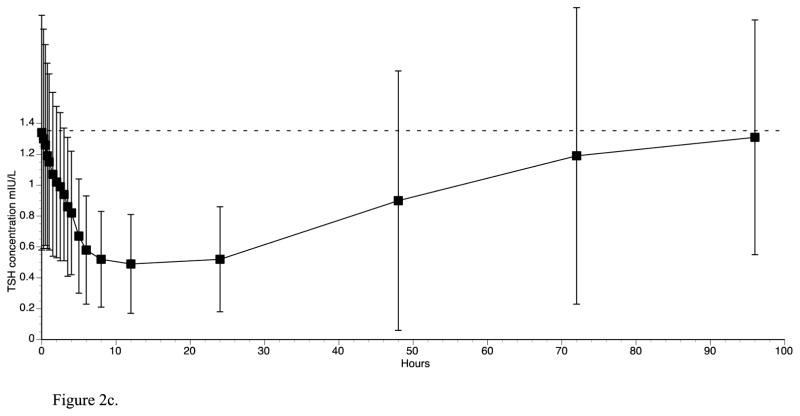
The concentrations of the various thyroid parameters prior to and after T3 administration are shown in table 2. T3 concentrations began to increase 45 minutes after T3 administration and peaked at 2.5 hours (table 2 and 3, figure 2a). FT3 concentrations similarly reached their maximum concentration at 2.5 hours (table 2 and figure 2b). Both T3 and FT3 had essentially returned to baseline by 48 hours. TSH concentrations showed an inverse pattern to T3 and FT3, but the nadir was reached at 12 hours and TSH values remained below their baseline concentrations for approximately 72 hours (table 2 and figure 2c). The nadir TSH value at 12 hours was significantly different from baseline (p <0.001). FT4 and total T4 concentrations did not change substantially with T3 administration (see table 2). The T3 values were significantly negatively correlated with TSH values over 24 hours (p value = 0.0044).
Table 2.
Thyroid parameters prior to and after administration of 50 mcg T3
| Time after 50 mcg T3 (hours) | T3 ng/dL | FT3 pg/mL | TSH mIU/L | T4 mcg/dL | FT4 ng/dL | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| -0.50 | 120 | 25 | 3.12 | 0.52 | 1.58 | 0.89 | 6.5 | 1.7 | 0.99 | 0.14 |
| -0.25 | 118 | 24 | 3.04 | 0.48 | 1.42 | 0.77 | 8.1 | 1.7 | 1.00 | 0.13 |
| 0.00 | 114 | 26 | 3.08 | 0.48 | 1.34 | 0.76 | 7.6 | 1.7 | 0.97 | 0.14 |
| 0.25 | 119 | 28 | 3.13 | 0.48 | 1.30 | 0.71 | 7.5 | 1.9 | 0.98 | 0.15 |
| 0.50 | 152 | 62 | 3.85 | 0.97 | 1.26 | 0.65 | 7.7 | 1.9 | 0.99 | 0.14 |
| 0.75 | 195 | 73 | 5.15 | 1.35 | 1.19 | 0.60 | 8.0 | 2.3 | 0.99 | 0.14 |
| 1.00 | 241 | 78 | 6.50 | 1.90 | 1.15 | 0.57 | 8.3 | 2.0 | 1.00 | 0.15 |
| 1.50 | 299 | 87 | 8.14 | 2.16 | 1.07 | 0.53 | 7.7 | 2.0 | 0.99 | 0.14 |
| 2.00 | 334 | 79 | 8.98 | 1.64 | 1.02 | 0.49 | 7.6 | 1.7 | 0.99 | 0.15 |
| 2.50 | 346 | 77 | 9.17 | 1.63 | 0.99 | 0.48 | 7.3 | 1.8 | 0.97 | 0.15 |
| 3.00 | 330 | 74 | 9.02 | 1.77 | 0.94 | 0.43 | 7.2 | 1.7 | 0.97 | 0.15 |
| 3.50 | 324 | 84 | 8.62 | 1.81 | 0.86 | 0.45 | 7.1 | 2.2 | 0.97 | 0.15 |
| 4.00 | 305 | 81 | 8.22 | 1.74 | 0.82 | 0.40 | 7.5 | 2.1 | 0.98 | 0.16 |
| 5.00 | 263 | 62 | 7.00 | 1.45 | 0.67 | 0.37 | 7.5 | 2.3 | 0.96 | 0.16 |
| 6.00 | 227 | 47 | 6.21 | 1.17 | 0.58 | 0.35 | 8.0 | 2.1 | 0.93 | 0.12 |
| 8.00 | 196 | 40 | 5.20 | 0.83 | 0.52 | 0.31 | 7.7 | 1.9 | 0.94 | 0.12 |
| 12.00 | 157 | 32 | 4.34 | 0.59 | 0.49 | 0.32 | 7.2 | 1.9 | 0.90 | 0.12 |
| 24.00 | 141 | 29 | 3.65 | 0.32 | 0.52 | 0.34 | 6.8 | 1.9 | 0.92 | 0.13 |
| 48.00 | 120 | 18 | 3.03 | 0.30 | 0.90 | 0.84 | 7.3 | 1.4 | 0.87 | 0.11 |
| 72.00 | 114 | 14 | 2.95 | 0.44 | 1.19 | 0.96 | 7.4 | 1.3 | 0.89 | 0.10 |
| 96.00 | 113 | 21 | 3.07 | 0.50 | 1.31 | 0.76 | 7.2 | 1.2 | 0.89 | 0.10 |
Table 3.
Pharmacokinetic parameters following administration of 50 mcg T3
| PK Parameters for Total T3 | BCT303 | ||
|---|---|---|---|
| Baseline T3 (ng/dL) | 120 ng/dL | ||
| Cmax (ng/dL) | 346 ng/dL | ||
| Tmax hrs | 2.5 hours | ||
| AUC 0–24 (ng.hr/dL) | 4,740 ng.hr/dL | ||
| AUC 0–24, corrected for baseline (ng.hr/dL) | 1,860 ng.hr/dL | ||
| Half-life | 22.04 hours | ||
| Pharmacokinetic parameter | Females n=4 | Males n=8 | P values* |
| Baseline T3 (ng/dL) | 126.4 ± 29.7 | 112.9 ± 21.8 | 0.67 |
| T3 Cmax (ng/dL) | 404 ± 60 | 342 ± 98 | 0.05 |
| T3 Tmax (hrs) | 2.00 (1.75 – 2.25) | 2.50 (2.25 – 3.50) | 0.07 |
| T3 AUC0-96 (ng.hr/dL) | 13938 ± 2029 | 12929 ± 2117 | 0.55 |
| T3 AUC0-24 (ng.hr/dL) | 4926 ± 605 | 4370 ±970 | 0.20 |
| T3 AUC0-24 corrected for baseline (ng.hr/dL) | 1892 ± 568 | 1660 ± 815 | 0.35 |
| Caucasian n=7 | African Americans n=5 | ||
| Baseline T3 (ng/dL) | 112 ± 24 | 124 ± 25 | 0.74 |
| T3 Cmax (ng/dL) | 353 ± 105 | 376 ± 70 | 0.74 |
| T3 Tmax (hrs) | 2.50 (2.50 – 3.50) | 2.00 (2.00 – 2.00) | 0.02 |
| T3 AUC0-96 (ng.hr/dL) | 12761 ± 2278 | 13970 ± 1662 | 0.32 |
| T3 AUC0-24 (ng.hr/dL) | 4433 ± 1047 | 4725 ± 648 | 0.4168 |
| T3 AUC0-24 corrected for baseline (ng.hr/dL) | 1735 ± 931 | 1740 ± 378 | 0.6261 |
Data are means ± STD or median (1st quartile, 3rd quartile)
Wilcoxon rank-sum test
Figure 2.
Figure 2a. Triiodothyronine concentrations for 3 days following oral administration of a single 50 mcg dose of liothyronine (insert shows triiodothyronine concentrations of individual patients over the first 24hrs)
Figure 2b. Free triiodothyronine concentrations for 3 days following oral administration of 50 mcg liothyronine
Figure 2c. TSH concentrations for 4 days following oral administration of a single 50 mcg dose of liothyronine
The pharmacokinetic parameters for total T3 are shown in table 3. The mean Cmax was 346 ng/dL and the mean time to maximum concentration (Tmax) was 2.5 hours. The mean AUC 0–24 hrs, corrected for baseline, was 1,860 ng.hr/dL. The half-life of the T3 preparation was calculated as 22 hours when the decline in T3 concentration between 2.5 and 94 hours was employed. The Cmax, Tmax, and AUC for T3 were unaffected by weight, height, BMI, and age when either Pearson or Spearman correlation coefficients were calculated (data not shown). The pharmacokinetics parameters for T3 were unaffected by sex (see table 3), with the exception of a slightly higher Cmax in women (404 vs. 342 ng/dL, p value 0.05). As shown in table 3, the Tmax was slightly delayed in Caucasians compared with African Americans (2.5 vs. 2.0 hours, p value 0.027).
The number of study participants was too small to examine gender difference by race, or racial differences by gender for any of the parameters mentioned above.
DISCUSSION
The Cmax of 346 ng/dL and Tmax of 2.5 hours for liothyronine documented in our study were both fairly similar to those reported by Watson (Cmax 422 ng/dL; T max 2.5 hours). Our Tmax was also similar to the value of 2.35 hours reported by Coastal Pharmaceuticals in their study of 100 mcg liothyronine (ANDA 90–097) (7).
Since blood is an intermediate compartment for distribution of T3 from the gut, the initial decline of blood levels of T3 following achievement of Cmax is partly due to further distribution of T3 to target tissues and hydrophobic sites, such as adipose tissue. The best explanation for the shape and duration of T3 blood levels over time as shown in figure 2a could be represented by multiple release rates from the gut into the blood. Otherwise, a linear loss of T3 from the blood following Cmax, and further distribution to other compartments, would be observed. A similar T3 profile to ours was predicted by DiStefano and colleagues based on oral administration of a single dose of T3 and use of a “two-compartment gut model” (9). This model was based on a study of T3 administration in euthyroid individuals (5).
Calculation of T3 half-life in the blood may also be complicated by distribution from the blood into other compartments and re-distribution back into the blood. Thus, the apparent half-life of T3 in the blood depends on the choice of time points for the calculation. For example, an average half-life of 22 hours can be calculated, but a maximum half-life of 71 hours can be calculated from the decline of T3 in the blood between 48 and 72 hours. As stated earlier, this product was thus shown not to have an extended serum half-life compared with the similar 24-hour half-life of currently available T3 preparations.
Most studies, such as bioequivalence studies, do not typically report thyroid-related laboratory values beyond 24 hours following T3 administration. During the course of our study we continued to collect data for the 24–96 hour period and were able demonstrate that despite the return of T3 concentrations to baseline by 48 hours, the response of TSH lagged behind the normalization of T3 values. The nadir of TSH values coincided with the earliest return of T3 to baseline. TSH values then gradually returned to normal between 48 and 96 hours. Interestingly, the TSH values of 3 volunteers rebounded to above their baseline values. However, we could not identify any characteristic of the thyroid hormones profiles that predicted this overshoot in the TSH values. A limitation of our study is that we did not have control participants who did not receive T3 to serve as an illustration of the normal circadian rhythm of TSH. However, the fall in serum TSH from 8am to 8pm is approximately 0.5 mIU/L in most studies (10, 11), a change that is of lesser magnitude than the change we observed following T3 administration.
FT4 did not fall in response to either the increase in T3 or the decrease in TSH in this single-dose study, in contrast to the reduction in FT4 concentrations seen in patients taking T3 preparations on a long-term basis. Interestingly, there was a drop in FT4 in three patients, suggesting some differences in response to T3. Although there were no notable changes in SBP, DBP, respiratory rate, oral temperature, ECG recordings or body weight in any of the patients, this single dose of T3 did appear to result in an increase in heart rate that was sustained for about 24 hours.
Interestingly, and as a separate issue, oral body temperature ranged from a minimal of 35.9°C (96.6°F) to a maximum of 36.6°C (97.9°F) and never reached 37.0°C (98.6°F). However, admittedly, only two temperature measurements were taken in the evening hours (6pm and 8pm) when body temperature is generally highest. A mean oral temperature of 36.8°C (98.2°F) has previously been described in 148 healthy volunteers (12). The issue of body temperature has been hotly debated with respect to Wilson’s Low Temperature Syndrome in which inability to maintain a body temperature of 98.6°F is considered one of the features of this syndrome (13).
Other limitations of our study, other than the lack of a control group, include the supraphysiologic dose of T3 that was administered, and the small, group of participants we studied who were heterogeneous with respect to sex and ethnicity. In addition our healthy volunteers were mostly young males, which contrasts to the typical population requiring treatment for hypothyroidism, which is mostly a middle aged female population. However, the strength of our study is the duration of our observations, which exceed the period of observation reported in most similar studies.
Serum TSH levels fell with 2 hours of T3 administration, possibly due to inhibition of its release. There was then a prolonged effect of T3 on serum TSH. This subsequent, prolonged effect of a single dose of T3, outlasting the actual period of the T3 elevation, is in keeping with the known action of T3 to trigger nuclear events, possibly inhibiting TSH synthesis. TSH values then began to recover. Our data are in agreement with a prior study in which TSH values began to escape from suppression from a single T3 dose at a T3 concentration of approximately 153 ng/dL with a gradual recovery over several days (14). However, although this prior study showed an interval of about 96 hours before TSH values returned to baseline with a 100-mcg dose of T3, they did not follow TSH values beyond 48 hours with the smaller 40-mcg dose of T3 that they studied. Another study also examining TSH suppression after a single dose of 75 mcg T3 showed that TSH suppression was present at 24 hours, but did not collect data at further time points (5). These data collectively provide insight into the dynamics and duration of pituitary suppression by T3, although they do not address the issue of pituitary versus hypothalamic influences (15).
Once daily administration of short-acting T3 preparations in hypothyroid individuals would not be predicted to be associated with steady T3 levels based on pharmacokinetic data (16), but based on our data could possibly be associated with steady lowering of TSH levels. Daily dosing of 50 mcg T3 was studied in athyreotic patients and resulted in persistent suppression of TSH levels over a 3-week period (17). However, blood sampling was only performed on a weekly basis, so peaks and troughs in TSH or T3 levels would not have been captured. Dividing T3 doses that averaged approximately 40 mcg daily into three times daily dosing regimens in hypothyroid patients, however, avoided any peaks or troughs in T3 or TSH concentrations within the selected periods of sampling (18).
The effects of short-acting T3 preparations on parameters such as vital signs and body weight in hypothyroid patients cannot be predicted from our data in euthyroid volunteers, although increased heart rate might be postulated. The increase in heart rate seen in these participants, however, argues against administration of this dose of T3 as a single dose. Studies using thrice daily administration of T3 in athyreotic patients suggest that there may be hepatic effects and also effects on body weight, without effects on heart rate (19). There is continued interest in developing a sustained release T3 preparation that maintains stable concentrations of T3, such that daily administration of T3 provides steady serum levels of T3. This interest is fueled by the fact that enzymatic conversion of T4 to T3 is necessary for thyroid hormone action, and the evidence in secondary analyses that a specific polymorphism in the type 2 deiodinase, possibly associated with unsatisfactory response to T4 monotherapy (20), affects approximately 15% of the population.
In summary, based on the present observations of sustained reduction of TSH concentrations beyond 24 hours by a single 50 mcg dose of liothyronine, it is possible that a once per day dosing regimen of liothyronine could result in stable lowering of TSH and also stable regulation of other T3 regulated gene products, even without achievement of steadily maintained serum levels of T3. This observation could be consistent with discordance between serum and tissue levels of T3.
Acknowledgments
Funded in part with Federal funds (Grant # UL1TR000101 previously UL1RR031975) from the National Center for Research Resources, NIH, through the Clinical and Translational Science Awards Program (CTSA), a trademark of DHHS, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise. Statistical analyses were provided by the Design, Biostatistics, and Population Studies component of the Georgetown-Howard Universities Center for Clinical and Translational Science.
The authors could not have performed this study without the invaluable expert assistance of Amber Surian who served as study coordinator. The authors also wish to sincerely thank the members of the data safety and monitoring board: Dr. Meeta Sharma, Dr. Andrea Singer, Dr. Jane Otado, and Alice Aukaegbu, FNP for their dedicated service and generous donation of their time. Finally we wish to thank Mark Lund for serving as the research assistant for the study.
Abbreviations
- AUC
area under the curve
- BMI
body mass index
- Cmax
maximum concentration
- DBP
diastolic blood pressure
- ECG
electrocardiogram
- FT4
free thyroxine
- FT3
free triiodothyronine HR, heart rate
- GCRU
Georgetown Clinical Research Unit
- RR
respiratory rate
- SBP
systolic blood pressure
- T3
triiodothyronine
- T4
thyroxine
- Tmax
time to maximum concentration
- TSH
thyroid stimulating hormone
Footnotes
Conflict of interest: JJ and KDB are sponsors of the IND for the T3 preparation used in this study (BCT303), but have no financial interests in the product. JJ has no other conflicts of interest relevant to this article. HW has no conflicts of interest. KDB has or has recently had research grants from Pfizer, Eisei, Amgen, and Astra Zeneca. He serves on the Food and Drug Administration Endocrine Advisory Committee as an ad hoc member. KRL is the inventor and IPE is the manufacturer of BCT303. KRL is the sponsor of this study.
References
- 1.Saberi M, Utiger RD. Serum thyroid hormone and thyrotropin concentrations during thyroxine and triiodothyronine therapy. J Clin Endocrinol Metab. 1974;39:923–927. doi: 10.1210/jcem-39-5-923. [DOI] [PubMed] [Google Scholar]
- 2.Lev-Ran A. Part-of-the-day hypertriiodothyroninemia caused by desiccated thyroid. JAMA. 1983;250:2790–2791. [PubMed] [Google Scholar]
- 3.Saravanan P, Siddique H, Simmons DJ, Greenwood R, Dayan CM. Twenty-four hour hormone profiles of TSH, Free T3 and free T4 in hypothyroid patients on combined T3/T4 therapy. Exp Clin Endocrinol Diabetes. 2007;115:261–267. doi: 10.1055/s-2007-973071. [DOI] [PubMed] [Google Scholar]
- 4.LeBoff MS, Kaplan MM, Silva JE, Larsen PR. Bioavailability of thyroid hormones from oral replacement preparations. Metabolism. 1982;31:900–905. doi: 10.1016/0026-0495(82)90179-2. [DOI] [PubMed] [Google Scholar]
- 5.Ueda S, Takamatsu J, Fukata S, Tanaka K, Shimizu N, Sakata S, Yamaji T, Kuma K, Ohsawa N. Differences in response of thyrotropin to 3,5,3′-triiodothyronine and 3,5,3′-triiodothyroacetic acid in patients with resistance to thyroid hormone. Thyroid. 1996;6:563–570. doi: 10.1089/thy.1996.6.563. [DOI] [PubMed] [Google Scholar]
- 6.Watson ANDA study (data provided in FDA review of original ANDA).
- 7.http://www.accessdata.fda.gov/drugsatfda_docs/.../090097Orig1s000.pdf.
- 8.Nicoloff JT, Low JC, Dussault JH, Fisher DA. Simultaneous measurement of thyroxine and triiodothyronine peripheral turnover kinetics in man. J Clin Invest. 1972;51:473–483. doi: 10.1172/JCI106835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenberg M, Samuels M, DiStefano JJ., 3rd Extensions, validation, and clinical applications of a feedback control system simulator of the hypothalamo-pituitary-thyroid axis. Thyroid. 2008;18:1071–1085. doi: 10.1089/thy.2007.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brabant G, Prank K, Ranft U, Bergmann P, Schuermeyer T, Hesch RD, von zur Muhlen A. Circadian and pulsatile TSH secretion under physiological and pathophysiological conditions. Horm Metab Res Suppl. 1990;23:12–17. [PubMed] [Google Scholar]
- 11.Azukizawa M, Pekary AE, Hershman JM, Parker DC. Plasma thyrotropin, thyroxine, and triiodothyronine relationships in man. J Clin Endocrinol Metab. 1976;43:533–542. doi: 10.1210/jcem-43-3-533. [DOI] [PubMed] [Google Scholar]
- 12.Mackowiak PA, Wasserman SS, Levine MM. A critical appraisal of 98.6 degrees F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA. 1992;268:1578–1580. [PubMed] [Google Scholar]
- 13.http://www.wilsonssyndrome.com/.
- 14.Spencer CA, LoPresti JS, Nicoloff JT, Dlott R, Schwarzbein D. Multiphasic thyrotropin responses to thyroid hormone administration in man. J Clin Endocrinol Metab. 1995;80:854–859. doi: 10.1210/jcem.80.3.7883842. [DOI] [PubMed] [Google Scholar]
- 15.Chiamolera MI, Wondisford FE. Minireview: Thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology. 2009;150:1091–1096. doi: 10.1210/en.2008-1795. [DOI] [PubMed] [Google Scholar]
- 16.Brunton L, Chabner B, Knollman B. Chapter 2: Pharmacokinetics, Steady State. McGraw-Hill Companies; Goodman & Gilman’s The Pharmacological Basis of Therapeutics, Online edition. [Google Scholar]
- 17.Leboeuf R, Perron P, Carpentier AC, Verreault J, Langlois MF. L-T3 preparation for whole-body scintigraphy: a randomized-controlled trial. Clin Endocrinol (Oxf) 2007;67:839–844. doi: 10.1111/j.1365-2265.2007.02972.x. [DOI] [PubMed] [Google Scholar]
- 18.Celi FS, Zemskova M, Linderman JD, Babar NI, Skarulis MC, Csako G, Wesley R, Costello R, Penzak SR, Pucino F. The pharmacodynamic equivalence of levothyroxine and liothyronine: a randomized, double blind, cross-over study in thyroidectomized patients. Clin Endocrinol (Oxf) 2010;72:709–715. doi: 10.1111/j.1365-2265.2009.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celi FS, Zemskova M, Linderman JD, Smith S, Drinkard B, Sachdev V, Skarulis MC, Kozlosky M, Csako G, Costello R, et al. Metabolic effects of liothyronine therapy in hypothyroidism: a randomized, double-blind, crossover trial of liothyronine versus levothyroxine. J Clin Endocrinol Metab. 2011;96:3466–3474. doi: 10.1210/jc.2011-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, Dayan CM. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009;94:1623–1629. doi: 10.1210/jc.2008-1301. [DOI] [PubMed] [Google Scholar]