Abstract
OBJECTIVE
To estimate whether race or ethnic and socioeconomic strata are independently associated with advanced-stage ovarian cancer–specific survival after adjusting for adherence to National Comprehensive Cancer Network treatment guidelines.
METHODS
The design was a retrospective population-based cohort study of patients with stage IIIC–IV epithelial ovarian cancer identified from the Surveillance, Epidemiology, and End Results–Medicare database (1992–2009). Quartile of census tract median household income was used as the measure of socioeconomic status (quartiles 1–4). A multivariable logistic regression model was used to identify characteristics predictive of adherence to National Comprehensive Cancer Network guidelines for surgery and chemotherapy. Cox proportional hazards models and propensity score matching were used for survival analyses.
RESULTS
A total of 10,296 patients were identified, and 30.2% received National Comprehensive Cancer Network guideline–adherent care. Among demographic variables, black race (adjusted odds ratio [OR] 1.53, 95% confidence interval [CI] 1.22–1.92) and low socioeconomic status (quartile 1, adjusted OR 1.32, 95% CI 1.14–1.52) were independently associated with nonguideline care. Stratified multivariate survival analysis using the propensity score-matched sample (n55,124) revealed that deviation from treatment guidelines was associated with a comparable risk of disease-related death across race-ethnicity: whites (adjusted hazard ratio [HR] 1.59, 95% CI 1.48–1.71), blacks (adjusted HR 1.66, 95% CI 1.19–2.30), Asian or Pacific Islanders (adjusted HR 1.52, 95% CI 0.99–1.92), and Hispanics (adjusted HR1.91, 95% CI 0.98–3.72). Across socioeconomic status, deviation from treatment guidelines was also associated with a comparable risk of ovarian cancer mortality for quartile 1 (adjusted HR 1.69, 95% CI 1.47–1.95), quartile 2 (adjusted HR 1.63, 95% CI1.42–1.87), quartile 3 (adjusted HR 1.51, 95% CI1.32–1.73), and quartile 4 (adjusted HR 1.57, 95% CI 1.38–1.79).
CONCLUSION
Adherence to treatment guidelines for advanced-stage ovarian cancer is associated with equivalent survival benefit across racial or ethnic and socioeconomic strata. Ensuring equal access to standard treatment is a viable strategic approach to reduce survival disparities.
LEVEL OF EVIDENCE: II
In the United States, there are 22,000 new cases of ovarian cancer diagnosed and more than 14,000 disease-related deaths annually, accounting for more deaths than all other gynecologic cancers combined.1 Adherence to National Comprehensive Cancer Network treatment guidelines for ovarian cancer correlates with improved survival with a proportionally greater benefit for women with advanced-stage disease.2, 3 Sociodemographic disparities in ovarian cancer survival are thought to be largely the result of unequal access to care and administration of nonstandard treatment regimens, primarily as a consequence of lower socioeconomic status and lack of private health insurance among minority populations.4 Supporting data suggest that when patients receive comparable treatment, racial disparities in ovarian cancer survival are largely mitigated.5–7 In contrast, other studies have shown worse survival for racial minorities and the socioeconomically disadvantaged after controlling for treatment received, suggesting that there may be either intrinsic or modifiable factors affecting the effectiveness of standard treatment among vulnerable populations.2, 8–10
From a health care policy perspective, correcting sociodemographic disparities in ovarian cancer survival hinges on ensuring equal access to contemporary, state-of-the-art treatment. However, the cornerstone of this strategic approach, that equal ovarian cancer treatment is accompanied by equivalent survival benefit, has not been definitively established. Therefore, the current study aimed to test the hypothesis that adherence to National Comprehensive Cancer Network treatment guidelines for advanced-stage epithelial ovarian cancer is associated with equivalent disease specific-survival benefit across racial or ethnic and socioeconomic strata in the Medicare population using propensity score matching.11
MATERIALS AND METHODS
The study design was a retrospective population-based cohort study of patients with International Federation of Gynecology and Obstetrics and American Joint Commission on Cancer stages IIIC and IV epithelial ovarian cancer using the linked Surveillance, Epidemiology, and End Results (SEER)– Medicare database. The study received exempt status by the institutional review board of the University of California, Irvine (HS#2012–9076). The SEER program of the National Cancer Institute contains approximately 97% of all incident cancer cases from tumor registries that covered 14% of the U.S. population in 1995 and 28% of the population in 2010.12–14 Among patients aged older than 65 years in the SEER database, 93% were identified in the Medicare enrollment file and their records successfully matched in the linkage process performed by the National Cancer Institute and Center for Medicare and Medicaid Services.13, 14 Medicare claims included all inpatient hospitalizations, outpatient, physician or supplier data, durable medical equipment, hospice, and home health care. All claims were longitudinal from the time of Medicare eligibility until death. The current analysis included SEER cases from 1992 through 2009 and corresponding Medicare claims from 1991 through 2010.
A total of 38,792 patients were identified with invasive epithelial ovarian cancer (SEER primary site code C569) as their only tumor or first primary tumor. Patients were sequentially removed with: missing tumor histology (n = 287), nonepithelial tumors (n = 117), diagnosis at autopsy or death certificate only (n = 820), age younger than 66 years (n = 10,468), missing tumor stage (n = 3,341), stage I–IIIB disease (n = 8,731), missing month of diagnosis (n = 36), and enrollment in a health maintenance organization or discontinuous enrollment in Medicare parts A and B (n = 4,696). The remaining 10,296 patients comprised the study population (see the Appendix, available online at http://links.lww.com/AOG/A614).
The first main outcome was adherence to treatment guidelines for ovarian cancer and was based on National Comprehensive Cancer Network recommendations for surgery and chemotherapy (1996– 2008).15–20 For stage IIIC–IV disease, a minimum of oophorectomy (±hysterectomy) and omentectomy was considered adherent surgery and administration of multiagent chemotherapy that included a platinum agent was considered appropriate care. For patients diagnosed after 1996, the chemotherapy requirement was expanded to include administration of a taxane in addition to platinum.15 Either initial surgery or chemotherapy was considered adherent care. Because the SEER–Medicare database does not provide information on progression-free survival, no exception in regard to guideline adherence was made for patients treated with initial chemotherapy and not undergoing surgery as a result of disease progression. Using International Classification of Disease, 9th Revision, Clinical Modification procedure and diagnosis codes in claims data (Box 1), the type of surgery was identified from hospital claims files, whereas the administration of chemotherapy and type of agents were identified from inpatient, outpatient, and physician or supplier claims files. A dichotomous variable, adherence or nonadherence, was created for adherence of the overall treatment plan (both surgery and chemotherapy) to recommended National Comprehensive Cancer Network treatment guidelines. The second main outcome was ovarian cancer–specific mortality, defined as the time between diagnosis and death from ovarian cancer or the date of last follow-up.
Box 1. Procedure and Diagnosis Codes.
Billing codes for ovarian cancer surgery
-
ICD-9 procedure codes (inpatient hospital claims data)
54.4: Omentectomy, excision, destruction peritoneal tissue
65.2: Wedge resection or partial excision of ovary
65.3: Unilateral oophorectomy
65.4: Bilateral oophorectomy
65.6: Bilateral salpingo-oophorectomy
68.3–9: Hysterectomy
70.32: Excision or destruction cul de sac lesion
Billing codes for chemotherapy
-
DRG (inpatient hospital claims data)
410
-
ICD-9 procedure code (inpatient, outpatient, and physician or supplier claims data)
99.25
-
ICD-9 diagnosis code (inpatient, outpatient, and physician or supplier claims data)
V58.1, V66.2, V67.2, E9331, E9307
-
HCPCS codes (outpatient and physician claims data)
Q0083-Q0085, G0355-G0356, G0359-G0362, J8530, J8560, J8565, J8600, J8700,
J9000–J9999, 964–965
Billing codes for chemotherapy agents
-
Platinum agents—HCPCS codes (outpatient and physician claims data)
J9060, J9062, J9045
-
Taxane agents—HCPCS codes (outpatient and physician claims data)
J9264, J9265, J9170, J9171
ICD-9, International Classification of Diseases, 9th Revision; DRG, diagnosis-related group; HCPCS, Healthcare Common Procedure Coding System.
Analytical covariates included patient demographic variables and disease-related characteristics. Patient characteristics were age at diagnosis (66–69, 70–74, 75–79, 80–84, 85 years or older) and race or ethnicity (white, black, Asian or Pacific Islander, Hispanic, or other or unknown). Race or ethnicity was coded by SEER registrars with the following priorities in available data: patient self-declared identification, documentation in the medical record, or death certificate. Quartile of the median household income in each patient’s census tract was used as the measure of socioeconomic status (lowest = quartile 1, low-middle = quartile 2, high-middle = quartile 3, highest = quartile 4). Patient comorbidity was measured by Deyo adaptation of the Charlson Comorbidity Index. The Charlson-Deyo comorbidity score was calculated by using all International Classification of Diseases, 9th Revision diagnosis codes, procedure codes, and Healthcare Common Procedure Coding System procedure codes included in the inpatient, outpatient, and physician claims in the 12-month period before ovarian cancer diagnosis.21,22 To prevent overestimation of the comorbidity score when using physician or outpatient claims, a patient’s diagnoses had to appear on at least two different claims that were more than 30 days apart.23 Tumor characteristic included International Federation of Gynecology and Obstetrics and American Joint Committee on Cancer tumor stage, tumor differentiation or grade, tumor histology, and tumor size.
Frequency distributions of patients’ demographic and clinical characteristic were analyzed with χ2 test or Fisher’s exact test for categorical variables in bivariate analyses. Multivariate logistic regression analysis was performed to identify adjusted odds ratios (ORs) for demographic and clinical–pathologic characteristics predictive of non–guideline-adherent treatment. Survival curves of 5-year disease-specific survival were generated using the Kaplan-Meier estimate of survival probability and analyzed using the log rank test. The estimated hazard function was plotted against follow-up time. After verifying the proportionality assumption, a Cox proportional hazards model was fitted to evaluate the effects of demographic and clinical–pathologic variables on survival. Possible interaction terms of main effects were also tested by comparing a reduced model with the full model.
Propensity score matching was used to evaluate the effect of adherence to National Comprehensive Cancer Network treatment guidelines on ovarian cancer–specific mortality while adjusting for characteristics affecting the likelihood of guideline adherence. A multivariate logistic model for predictors of nonadherent care was fitted using baseline demographic and clinical characteristics including age, race or ethnicity, quartile of socioeconomic status, Charlson-Deyo comorbidity score, stage of disease, tumor histology, degree of differentiation, and tumor size. From the logistic model, the propensity score was calculated as the predicted probability that each patient would receive non–guideline-adherent care. Using a matching algorithm with a caliper of 0.001, a propensity score–matched sample of guideline-adherent and -nonadherent cases was created (one to one) with similar distribution of the characteristics using the patient’s propensity score from the logistic model. A Cox proportional hazards model for ovarian cancer–specific mortality was fitted using the propensity score–matched cohort. Stratified analyses were performed to further examine the effect of treatment guideline adherence on survival according to racial or ethnic classification and socioeconomic status quartile strata in both the propensity score–matched sample and the entire study population cohort. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were generated. All P values are two-sided. Statistical analysis was performed using SAS 9.2.
RESULTS
A total of 10,296 patients were identified for study inclusion. The median follow-up time was 57.0 months (95% CI 54.0–60.0 months). Patients’ age at diagnosis ranged from 66 to 107 years with 56.9% of patients aged 75 years or older (Table 1). Most patients were white (88.2%) and presented with stage IV disease (62.4%). Forty-one percent of patients had a Charl-son-Deyo score 1 or greater. Overall, 30.2% of patients received guideline-adherent care. Proper surgery was performed in 44.5% of patients, and 48.7% of patients received the recommended chemotherapy. Collectively, half of all patients received no treatment (22.3%), only surgery (11.1%), or only chemotherapy (16.4%). Only 18.9% of blacks received guideline-adherent care compared with 31.2% of whites, 32.1% of Asian or Pacific Islanders, and 24.6% of Hispanics (P < .001). Adherence to treatment guidelines increased with socioeconomic status, ranging from 25.0% for quartile 1 to 36.1% for quartile 4 (P <.001).
Table 1.
Study Population Characteristics and Logistic Regression Analysis of Predictors of Nonadherence to National Comprehensive Cancer Network Treatment Guidelines (Dependent Variable) for Advanced-Stage Ovarian Cancer
| Characteristic | Overall | Adherent | Nonadherent | aOR | 95% CI |
|---|---|---|---|---|---|
| Total | 10,296 (100.0) | 3,109 (30.2) | 7,187 (69.8) | ||
| Age at diagnosis (y) | |||||
| 66–69 | 1,950 (18.9) | 857 (43.9) | 1,093 (56.1) | 1.00 | |
| 70–74 | 2,482 (24.1) | 992 (39.9) | 1,492 (60.1) | 1.07 | 0.93–1.22 |
| 75–79 | 2,553 (24.8) | 821 (32.2) | 1,732 (67.8) | 1.43 | 1.25–1.64 |
| 80–84 | 1,882 (18.3) | 359 (19.1) | 1,523 (80.9) | 2.38 | 2.03–2.80 |
| 85 or older | 1,427 (13.9) | 80 (5.6) | 1,347 (94.4) | 7.52 | 5.82–9.72 |
| Race–ethnicity | |||||
| White | 9,083 (88.2) | 2,831 (31.2) | 6,252 (68.8) | 1.00 | |
| Black | 679 (6.6) | 128 (18.9) | 551 (81.1) | 1.53 | 1.22–1.92 |
| Asian or Pacific Islander | 215 (2.1) | 69 (32.1) | 146 (67.9) | 1.11 | 0.79–1.55 |
| Hispanic | 118 (1.2) | 29 (24.6) | 89 (75.4) | 1.31 | 0.81–2.11 |
| Other or unknown | 201 (2.0) | 52 (25.9) | 149 (74.1) | 1.57 | 1.09–2.26 |
| Socioeconomic status | |||||
| Quartile 1 (lowest) | 2,566 (25.0) | 641 (25.0) | 1,925 (75.0) | 1.32 | 1.14–1.52 |
| Quartile 2 (low-middle) | 2,566 (25.0) | 735 (28.6) | 1,831 (71.4) | 1.22 | 1.06–1.39 |
| Quartile 3 (high-middle) | 2,567 (25.0) | 797 (31.0) | 1,770 (69.0) | 1.10 | 0.96–1.25 |
| Quartile 4 (highest) | 2,566 (25.0) | 927 (36.1) | 1,639 (63.9) | 1.00 | |
| Charlson-Deyo score | |||||
| 0 | 6,068 (58.9) | 2,105 (34.7) | 3,963 (65.3) | 1.00 | |
| 1 | 2,367 (23.0) | 663 (28.0) | 1,704 (72.0) | 1.14 | 1.01–1.29 |
| 2 or greater | 1,861 (18.1) | 341 (18.3) | 1,520 (81.7) | 1.60 | 1.38–1.85 |
| FIGO–AJCC stage | |||||
| IIIC | 3,871 (37.8) | 1,867 (48.2) | 2,004 (51.8) | 1.00 | |
| IV | 6,425 (62.4) | 1,242 (19.3) | 5,183 (80.7) | 2.31 | 2.09–2.56 |
| Tumor histology | |||||
| Serous | 4,562 (44.3) | 2,129 (46.7) | 2,433 (53.3) | 1.00 | |
| Mucinous | 255 (2.5) | 50 (19.6) | 205 (80.4) | 2.31 | 1.64–3.26 |
| Endometrioid | 364 (3.5) | 142 (39.0) | 222 (61.0) | 1.73 | 1.36–2.19 |
| Clear cell | 123 (1.2) | 54 (43.9) | 69 (56.1) | 0.92 | 0.62–1.38 |
| Adenocarcinoma, NOS | 2,336 (22.7) | 313 (13.4) | 2,023 (86.6) | 2.62 | 2.26–3.04 |
| Other | 2,656 (25.8) | 421 (15.9) | 2,235 (86.5) | 2.54 | 2.23–2.90 |
| Tumor differentiation | |||||
| Grade 1–2 | 1,152 (11.2) | 443 (38.5) | 709 (61.5) | 1.00 | |
| Grade 3 | 3,759 (36.5) | 1,599 (42.5) | 2,160 (57.5) | 0.83 | 0.72–0.97 |
| Grade 4 | 949 (9.2) | 467 (49.2) | 482 (50.8) | 0.69 | 0.57–0.84 |
| Grade unknown | 4,436 (43.1) | 600 (13.5) | 3,836 (86.5) | 1.79 | 1.52–2.12 |
| Tumor size (cm) | |||||
| Less than 5 | 1,069 (10.4) | 507 (47.4) | 562 (52.6) | 1.00 | |
| 5–10 | 1,257 (12.2) | 532 (42.3) | 725 (57.7) | 1.17 | 0.97–1.41 |
| Greater than 10 | 1,307 (12.7) | 529 (40.5) | 778 (59.5) | 1.20 | 1.00–1.45 |
| Unknown | 6,663 (64.7) | 1,541 (23.1) | 5,122 (76.9) | 1.73 | 1.48–2.01 |
aOR, adjusted odds ratio; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; AJCC, American Joint Committee on Cancer; NOS, not otherwise specified.
Data are n (%) unless otherwise specified.
All variables were included in the final model and adjusted for the effects of other variables. Bold indicates statistical significance.
The multivariate logistic regression model for non-adherence to National Comprehensive Cancer Network treatment guidelines revealed statistically significant and independent increased risk associated with increasing age, Charlson-Deyo score, and stage of disease (Table 1). Compared with whites, black race was associated with a statistically significant and independent 53% increase in the risk of non–guideline-adherent care. Asian or Pacific Islander race and Hispanic ethnicity were associated with an increased risk of nonadherent care, although these differences did not reach statistical significance. There was a significant and independent inverse linear relationship between socioeconomic status and the likelihood non–guideline-adherent care with the highest risk associated with quartile 1 (adjusted OR 1.32, 95% CI 1.14–1.52).
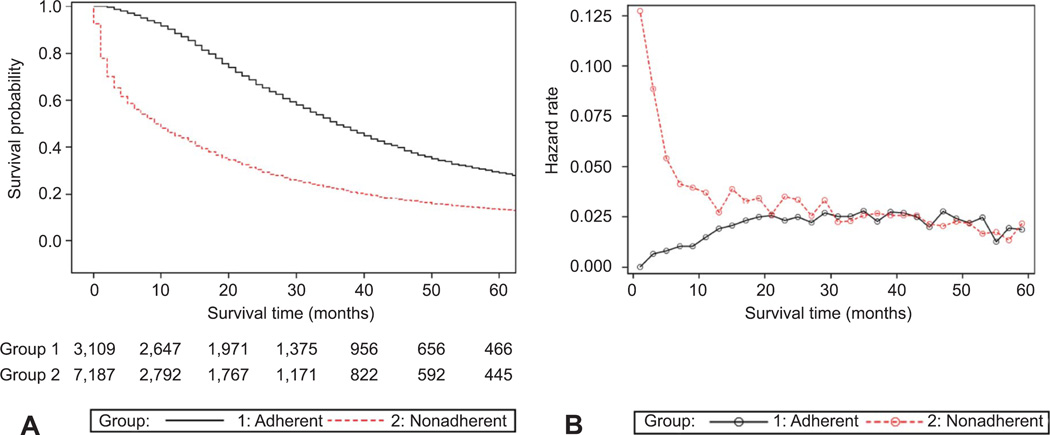
The median ovarian cancer–specific survival time for all patients was 18.0 months. For patients receiving guideline-adherent care, the median survival time was 36.0 months (95% CI 35.0–38.0 months; 5-year 26.9%, standard error 0.9) compared with 9.0 months (95% CI 9.0–10.0 months; 5-year 13.9%, standard error 0.5) for non–guideline-adherent care (P < .0001) (Fig. 1A). The estimated hazard function plotted according to treatment guideline adherence and nonadherence reflects the probability of ovarian cancer–related mortality during each 2-month observation time interval according to whether a patient survived the preceding interval (Fig. 1B). The hazard function plot shows an increased probability of death during the first 18 months of observation for patients in the nonadherent treatment group. Similar results were obtained for subsets of younger patients (66–69 years) and older patients (85 years or older) (data not shown).
Fig. 1.
A. Five-year ovarian cancer–specific survival stratified according to adherence (group 1 adherent, n = 3,109) or non-adherence (group 2 nonadherent, n = 7,187) to National Comprehensive Cancer Network treatment guidelines for advanced-stage ovarian cancer. For patients receiving guideline adherent care, the median survival time (36.0 months, 95% confidence interval 35.0–38.0 months) was significantly longer compared with non–guideline-adherent care (9.0 months, 95% confidence interval 9.0–10.0 months) (log-rank P < .001). B. Estimated hazard function (probability of ovarian cancer–related mortality) plotted against follow-up time (2-month intervals) stratified according to adherence (group 1 adherent, n = 3,109) or nonadherence (group 2 nonadherent, n = 7,187) to National Comprehensive Cancer Network treatment guidelines for advanced-stage ovarian cancer showing an increased probability of death during the first 18 months of observation for patients in the nonadherent treatment group.
The multivariate proportional hazards model revealed that adherence to National Comprehensive Cancer Network treatment guidelines (HR 1.00) was associated with a statistically significant and independent improvement in ovarian cancer–specific survival compared with nonadherent care (adjusted HR 1.69, 95% CI 1.60–1.79) (Table 2). Increasing age, higher Charlson-Deyo score, mucinous tumor histology, and stage IV disease were statistically significantly associated with worse survival. After adjusting for the effects of other variables, race or ethnicity was not a significant predictor of ovarian cancer–specific survival; however, lower socioeconomic status (quartile 1 through quartile 3) was associated with a consistent, independent, and statistically significant negative effect on survival relative to patients in the highest socioeconomic status category (quartile 4).
Table 2.
Predictors of Ovarian Cancer–Specific Survival (Dependent Variable) Analyzed Using Cox Proportional Hazards Model (N=10,296)*
| Characteristic | aHR | 95% CI |
|---|---|---|
| Age at diagnosis (y) | ||
| 66–69 | 1.00 | |
| 70–74 | 1.08 | 1.00–1.16 |
| 75–79 | 1.24 | 1.15–1.33 |
| 80–84 | 1.53 | 1.42–1.66 |
| 85 or older | 2.02 | 1.85–2.20 |
| Race–ethnicity | ||
| White | 1.00 | |
| Black | 0.95 | 0.86–1.05 |
| Asian or Pacific Islander | 0.94 | 0.80–1.12 |
| Hispanic | 1.02 | 0.81–1.28 |
| Other or unknown | 1.24 | 1.05–1.46 |
| Socioeconomic status quartile | ||
| 1 (lowest quartile) | 1.25 | 1.17–1.34 |
| 2 (low-middle quartile) | 1.14 | 1.06–1.21 |
| 3 (high-middle quartile) | 1.09 | 1.02–1.17 |
| 4 (highest quartile) | 1.00 | |
| Charlson-Deyo score | ||
| 0 | 1.00 | |
| 1 | 1.14 | 1.07–1.20 |
| 2 or greater | 1.28 | 1.20–1.37 |
| FIGO–AJCC stage | ||
| IIIC | 1.00 | |
| IV | 1.25 | 1.19–1.32 |
| Tumor histology | ||
| Serous | 1.00 | |
| Mucinous | 1.92 | 1.65–2.22 |
| Endometrioid | 0.80 | 0.70–0.92 |
| Clear cell | 1.06 | 0.85–1.33 |
| Adenocarcinoma, NOS | 1.27 | 1.19–1.35 |
| Other | 1.33 | 1.25–1.42 |
| Tumor differentiation | ||
| Grade 1–2 | 1.00 | |
| Grade 3 | 1.01 | 0.92–1.09 |
| Grade 4 | 0.98 | 0.88–1.09 |
| Grade unknown | 1.24 | 1.14–1.34 |
| Tumor size (cm) | ||
| Less than 5 | 1.00 | |
| 5–10 | 0.97 | 0.88–1.08 |
| Greater than 10 | 0.95 | 0.86–1.06 |
| Unknown | 1.13 | 1.04–1.22 |
| Adherence to NCCN guidelines | ||
| Adherent | 1.00 | |
| Nonadherent | 1.69 | 1.60–1.79 |
aHR, adjusted hazard ratio; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; AJCC, American Joint Committee on Cancer; NOS, not otherwise specified; NCCN, National Comprehensive Cancer Network.
Bold indicates statistical significance.
All variables were included in the final model and adjusted for the effects of other variables.
The propensity score–matched sample for treatment guideline adherence and nonadherence included 5,124 patients who were well matched according to demographic and disease-related characteristics (Table 3). The median survival for all patients in the propensity score sample was 22.0 months. In the propensity score sample, adherence to treatment guidelines (HR 1.00) was associated with a statistically significant and independent improvement in ovarian cancer–specific survival compared with nonadherent care (adjusted HR 1.60, 95% CI 1.49–1.71). Stratified survival analyses according to race or ethnicity and socioeconomic status were performed by generating separate multivariate proportional hazards models using both the propensity score–matched sample and the entire study population for comparison (Tables 4 and 5). Stratification of the propensity score–matched sample according to race or ethnicity revealed that, compared with guideline-adherent care (HR 1.00), deviation from treatment guidelines was associated with a comparable risk of disease-related death for whites (adjusted HR 1.59, 95% CI 1.48– 1.71), blacks (adjusted HR 1.66, 95% CI 1.19–2.30), Asian or Pacific Islanders (adjusted HR 1.52, 95% CI 0.99–1.92), and Hispanics (adjusted HR 1.91, 95% CI 0.98–3.72). Stratification of the propensity score– matched sample across quartiles of socioeconomic status also showed that deviation from treatment guidelines was associated with a comparable risk of ovarian cancer mortality for quartile 1 (adjusted HR 1.69, 95% CI 1.47–1.95), quartile 2 (adjusted HR 1.63, 95% CI 1.42–1.87), quartile 3 (adjusted HR 1.51, 95% CI 1.32–1.73), and quartile 4 (adjusted HR 1.57, 95% CI 1.38–1.79). In the stratification analyses for the entire study population, the directionality of the effect of deviation from National Comprehensive Cancer Network treatment guidelines was consistently preserved across race or ethnicity and socioeconomic status; however, the magnitude of the survival effect in median survival times was proportionally greater compared with those for the propensity sample estimates (Tables 6 and 7).
Table 3.
Comparison of Propensity Score–Matched Sample of Patients With Advanced-Stage Epithelial Ovarian Cancer Overall and Stratified According to Adherence and Nonadherence to National Comprehensive Cancer Network Treatment Guidelines
| Characteristic | Overall | Adherent | Nonadherent | χ2 Test P |
|---|---|---|---|---|
| Total | 5,124 (100.0) | 2,562 (50.0) | 2,562 (50.0) | |
| Age at diagnosis (y) | ||||
| 66–69 | 1,263 (24.6) | 629 (49.8) | 634 (50.2) | |
| 70–74 | 1,597 (31.2) | 794 (49.7) | 803 (50.3) | |
| 75–79 | 1,450 (28.3) | 733 (50.6) | 717 (49.4) | .85 |
| 80–84 | 6,66 (13.0) | 338 (50.8) | 328 (49.2) | |
| 85 or older | 148 (2.9) | 69 (45.9) | 80 (54.1) | |
| Race or ethnicity | ||||
| White | 4,630 (90.4) | 2,307 (49.8) | 2,323 (50.2) | |
| Black | 238 (4.6) | 123 (51.7) | 115 (48.3) | |
| Asian or Pacific Islander | 97 (1.9) | 44 (45.4) | 53 (54.6) | .54 |
| Hispanic | 58 (1.1) | 31 (53.4) | 27 (46.6) | |
| Other or unknown | 101 (2.0) | 57 (56.4) | 44 (43.6) | |
| Socioeconomic status | ||||
| Quartile 1 (lowest) | 1,133 (22.1) | 578 (51.0) | 555 (49.0) | |
| Quartile 2 (low-middle) | 1,224 (23.9) | 610 (49.8) | 614 (50.2) | .87 |
| Quartile 3 (high-middle) | 1,327 (25.9) | 663 (50.0) | 664 (50.0) | |
| Quartile 4 (highest) | 1,440 (28.1) | 711 (49.4) | 729 (50.6) | |
| Charlson-Deyo score | ||||
| 0 | 3,399 (66.3) | 1,712 (50.4) | 1,687 (49.6) | |
| 1 | 1,096 (21.4) | 540 (49.3) | 556 (50.7) | .76 |
| 2 or greater | 629 (12.3) | 310 (49.3) | 319 (50.7) | |
| FIGO –AJCC stage | ||||
| IIIC | 2,761 (53.9) | 1,366 (49.5) | 1,395 (50.5) | .42 |
| IV | 2,363 (46.1) | 196 (50.6) | 1,167 (49.4) | |
| Tumor histology | ||||
| Serous | 3,281 (64.0) | 1,645 (50.1) | 1,636 (49.9) | |
| Mucinous | 93 (1.8) | 48 (51.6) | 45 (49.4) | |
| Endometrioid | 248 (4.8) | 124 (50.0) | 124 (50.0) | .99 |
| Clear cell | 84 (1.6) | 41 (48.8) | 43 (51.2) | |
| Adenocarcinoma, NOS | 603 (11.8) | 297 (49.3) | 306 (50.7) | |
| Other | 815 (15.9) | 407 (49.9) | 408 (50.1) | |
| Tumor differentiation | ||||
| Grade 1–2 | 742 (14.5) | 376 (50.7) | 358 (51.2) | |
| Grade 3 | 2,577 (50.3) | 1,306 (50.7) | 407 (51.4) | .55 |
| Grade 4 | 680 (13.3) | 338 (49.7) | 411 (49.4) | |
| Grade unknown | 1,125 (22.0) | 542 (48.2) | 1,386 (49.5) | |
| Tumor size (cm) | ||||
| Less than 5 | 699 (13.6) | 341 (48.8) | 358 (51.2) | |
| 5–10 | 792 (15.5) | 385 (48.6) | 407 (51.4) | .70 |
| Greater than 10 | 832 (16.2) | 421 (50.6) | 411 (49.4) | |
| Unknown | 2,801 (54.7) | 1,415 (50.5) | 1,386 (49.5) |
FIGO, International Federation of Gynecology and Obstetrics; AJCC, American Joint Committee on Cancer; NOS, not otherwise specified. Data are n (%) unless otherwise specified.
Table 4.
Stratified Cox Proportional Hazards Models of the Effect of Adherence and Nonadherence to National Comprehensive Cancer Network Treatment Guidelines on Ovarian Cancer–Specific Survival (Dependent Variable) According to Race–Ethnicity for the Propensity Score–Matched Sample (n=5,124) and for the Entire Study Population Cohort (N=10,296), Adjusted for Age at Diagnosis, Charlson-Deyo Comorbidity Score, Tumor Stage, Histology, Grade, and Size
| Guideline Adherence | White (n = 4,630) |
Black (n = 238) |
Asian or Pacific Islander (n = 97) |
Hispanic (n = 58) |
||||
|---|---|---|---|---|---|---|---|---|
| aHR | 95% CI | aHR | 95% CI | aHR | 95% CI | aHR | 95% CI | |
| Propensity score–matched sample | ||||||||
| Adherent | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Nonadherent | 1.59 | 1.48–1.71 | 1.66 | 1.19–2.30 | 1.52 | 0.99–1.92 | 1.91 | 0.98–3.72 |
| Study population cohort | ||||||||
| Adherent | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Nonadherent | 1.69 | 1.59–1.80 | 1.62 | 1.23–2.14 | 1.76 | 1.12–2.76 | 2.73 | 1.40–5.30 |
aHR, adjusted hazard ratio; CI, confidence interval. Bold indicates statistical significance.
Table 5.
Stratified Cox Proportional Hazards Models of the Effect of Adherence and Nonadherence to National Comprehensive Cancer Network Treatment Guidelines on Ovarian Cancer–Specific Survival (Dependent Variable) According to Socioeconomic Status for the Propensity Score– Matched Sample (n=5,124) and for the Entire Study Population Cohort (N=10,296), Adjusted for Age at Diagnosis, Charlson-Deyo Comorbidity Score, Tumor Stage, Histology, Grade, and Size
| Guideline Adherence | Quartile 1 (n = 1,133) |
Quartile 2 (n = 1,224) |
Quartile 3 (n = 1,327) |
Quartile 4 (n = 1,440) |
||||
|---|---|---|---|---|---|---|---|---|
| aHR | 95% CI | aHR | 95% CI | aHR | 95% CI | aHR | 95% CI | |
| Propensity score–matched sample | ||||||||
| Adherent | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Nonadherent | 1.69 | 1.47–1.95 | 1.63 | 1.42–1.87 | 1.51 | 1.32–1.73 | 1.57 | 1.38–1.79 |
| Study population cohort | ||||||||
| Adherent | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Nonadherent | 1.81 | 1.60–2.05 | 1.70 | 1.52–1.91 | 1.66 | 1.48–1.87 | 1.66 | 1.48–1.86 |
aHR, adjusted hazard ratio; CI, confidence interval.
Bold indicates statistical significance.
Table 6.
Median Disease-Specific Survival Time According to Race–Ethnicity for the Propensity Score– Matched Sample (n = 5,124) and for the Entire Study Population Cohort (N = 10,296)
| Guideline Adherence |
White |
Black |
Asian–Pacific Islander |
Hispanic |
||||
|---|---|---|---|---|---|---|---|---|
| Median Survival (mo) |
95% CI | Median Survival (mo) |
95% CI | Median Survival (mo) |
95% CI | Median Survival (mo) |
95% CI | |
| Propensity score– matched sample |
||||||||
| Adherent | 35.0 | 34.0–37.0 | 38.0 | 28.0–47.0 | 39.0 | 23.0–59.0 | 35.0 | 24.0–85.0 |
| Nonadherent | 20.0 | 18.0–22.0 | 18.0 | 8.0–29.0 | 31.0 | 11.0–42.0 | 14.0 | 5.0–70.0 |
| Study population cohort |
||||||||
| Adherent | 36.0 | 35.0–38.0 | 32.0 | 25.0–46.0 | 42.0 | 32.0–61.0 | 31.0 | 24.0–85.0 |
| Nonadherent | 10.0 | 9.0–10.0 | 8.0 | 1.0–10.0 | 8.0 | 6.0–17.0 | 9.0 | 5.0–14.0 |
CI, confidence interval.
Table 7.
Median Disease-Specific Survival Time According to Socioeconomic Status for the Propensity Score–Matched Sample (n=5,124) and for Entire Study Population Cohort (N = 10,296)
| Guideline Adherence |
Quartile 1 |
Quartile 2 |
Quartile 3 |
Quartile 4 |
||||
|---|---|---|---|---|---|---|---|---|
| Median Survival (mo) |
95% CI | Median Survival (mo) |
95% CI | Median Survival (mo) |
95% CI | Median Survival (mo) |
95% CI | |
| Propensity score– matched sample |
||||||||
| Adherent | 35.0 | 32.0–39.0 | 35.0 | 31.0–39.0 | 35.0 | 32.0–37.0 | 37.0 | 33.0–40.0 |
| Nonadherent | 15.0 | 14.0–19.0 | 19.0 | 16.0–21.0 | 22.0 | 18.0–23.0 | 23.0 | 19.0–25.0 |
| Study population cohort |
||||||||
| Adherent | 36.0 | 33.0–39.0 | 35.0 | 32.0–39.0 | 35.0 | 33.0–38.0 | 38.0 | 35.0–41.0 |
| Nonadherent | 6.0 | 6.0–8.0 | 9.0 | 8.0–10.0 | 10.0 | 9.0–11.0 | 14.0 | 12.0–15.0 |
CI, confidence interval.
DISCUSSION
The current data indicate that, after controlling for other variables, deviation from treatment guidelines for advanced ovarian cancer was associated with a 69% and 60% increase in the risk of disease-related death in the entire study population and propensity score–matched sample, respectively. The main the objective of this study, however, was to test the hypothesis that adherence to treatment guidelines for advanced-stage ovarian cancer is associated with equivalent disease-specific survival benefit across racial or ethnic and socioeconomic strata. Indeed, in the propensity score–matched sample, the magnitude of survival benefit associated with adherence to treatment guidelines was remarkably consistent across racial or ethnic and socioeconomic groups. The reproducibility of a nearly identical survival benefit associated with adherence to treatment guidelines stratified according to race or ethnicity and socioeconomic status in the Cox proportional hazards model for the entire study population suggests that differences in treatment are important contributing factors to unadjusted survival disparities for women with advanced-stage ovarian cancer.
Improving the health of all sociodemographic groups through the elimination of health disparities has become a national priority.24 Prior work investigating disparities in ovarian cancer has been limited by small numbers of minority populations, exclusion of nonwhite, nonblack patients, and a limited capacity to disarticulate the combined effects of race, socioeconomic status, and medical comorbidity.2, 3, 5–9, 25–28 Several studies have examined disparities in ovarian cancer in the Medicare population but did not use the stringent treatment criteria of National Comprehensive Cancer Network guidelines, have limited sociodemographic diversity, or both.10, 28, 29 The current project examined a large and sociodemographically diverse study population. By including only Medicare beneficiaries, the potential effect of the type of health insurance on treatment and survival was essentially negated, because the lack of adequate health insurance has previously been cited as an impediment to appropriate care.2, 30
There are several limitations that must be considered when interpreting these data. First, the retrospective, population-based cohort study design is subject to the potential for selection and reporting bias inherent to such methodology and may limit the assumption of causality between guideline-adherent care and improved survival. As an observational study, the possibility exists that unmeasured confounding characteristics could have affected the observed results. Second, the use of claims data to identify treatment and comorbidity may have resulted in some underestimation of treatment actually received and severity of illness.29 Although Medicare claims data have been shown to have high concordance with formal medical record review for surgery and chemotherapy, it may be less reliable for diagnostic codes of comorbid illness.31 Third, the current findings may not be generalizable to the broader population of patients with ovarian cancer, because a substantial proportion of eligible participants were excluded because of missing information, and approximately half of eligible SEER patients were excluded based on age. Whether the observed results are applicable to younger segments of the population, those with early-stage disease, and patients with other forms of health insurance coverage cannot be determined.
Despite these limitations, several important conclusions, relevant to both clinicians and health care policy administrators, can be drawn from the current data. First, adherence to National Comprehensive Cancer Network treatment guidelines for advanced-stage ovarian cancer is an independent predictor of improved disease-specific survival after controlling for medical comorbidities and should appropriately be regarded as the therapeutic standard of care. Second, and most importantly, adherence to treatment guidelines is associated with a comparable survival benefit across race or ethnicity and socioeconomic status. These findings suggest that efforts to eliminate sociodemographic-based disparities in ovarian cancer survival should be focused on ensuring equivalent access to expert care by health care providers most likely to deliver treatment consistent with standard recommended guidelines. Finally, fewer than one in three women with Medicare was treated according to recommended guidelines, which is undoubtedly attributable at least in part to the composition of the study population with regard to advanced age and the high frequency of medical comorbidities. The observation that black race and low socioeconomic status are independent risk factors for nonstandard care, even after adjusting for the presence of medical comorbidities, is troubling. Additional research is needed to: 1) design interventions to maximize access to appropriate care for all women with ovarian cancer, where race or ethnicity and socioeconomic status are important considerations; and 2) more precisely define which segments of the population are most likely to benefit from the standard therapeutic approach and those for whom an alternative treatment strategy would be more appropriate, a distinction in which race or ethnicity and socioeconomic status appear to be irrelevant.
Acknowledgments
Dr. Bristow was supported in part by the Queen of Hearts Foundation.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Bristow RE, Powell MA, Al-Hammadi N, Chen L, Miller JP, Roland PY, et al. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst. 2013;105:823–832. doi: 10.1093/jnci/djt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013;121:1226–1234. doi: 10.1097/AOG.0b013e3182922a17. [DOI] [PubMed] [Google Scholar]
- 4.Chornokur G, Amankwah EK, Schildkraut JM, Phelan CM. Global ovarian cancer health disparities. Gynecol Oncol. 2013;129:258–264. doi: 10.1016/j.ygyno.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bristow RE, Ueda S, Gerardi MA, Ajiboye OB, Ibeanu OA. Analysis of racial disparities in stage IIIC epithelial ovarian cancer care and outcomes in a tertiary gynecologic oncology referral center. Gynecol Oncol. 2011;122:319–323. doi: 10.1016/j.ygyno.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 6.Farley JH, Tian C, Rose GS, Brown CL, Birrer M, Maxwell GL. Race does not impact outcomes for advanced ovarian cancer patients treated with cisplatin/paclitaxel: an analysis of Gynecologic Oncology Group trials. Cancer. 2009;115:4210–4217. doi: 10.1002/cncr.24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terplan M, Temkin S, Tergas A, Lengyel E. Does equal treatment yield equal outcomes? The impact of race on survival in epithelial ovarian cancer. Gynecol Oncol. 2008;111:173–178. doi: 10.1016/j.ygyno.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnholtz-Sloan JS, Tainsky MA, Abrams J, Severson RK, Qureshi F, Jacques SM, et al. Ethnic differences in survival among women with ovarian carcinoma. Cancer. 2002;94:1886–1893. doi: 10.1002/cncr.10415. [DOI] [PubMed] [Google Scholar]
- 9.Bristow RE, Palis BE, Chi DS, Cliby WA. The National Cancer Database report on advanced-stage epithelial ovarian cancer: impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol Oncol. 2010;118:262–267. doi: 10.1016/j.ygyno.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 10.Fairfield KM, Lucas FL, Earle CC, Small L, Trimble EL, Warren JL. Regional variation in cancer-directed surgery and mortality among women with epithelial ovarian cancer in the Medicare population. Cancer. 2010;116:4840–4848. doi: 10.1002/cncr.25242. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 12.Overview of the SEER program. [Retrieved August 20, 2014]; Available at: http://seer.can-cer.gov/about/overview.html.
- 13.Brief description of the SEER-Medicare database. [Retrieved August 20, 2014]; Available at: http://appliedresearch.cancer.gov/seermedicare/overview/
- 14.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States Elderly population. Med Care. 2002;40(suppl):3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 15.Morgan RJ, Jr, Copeland L, Gershenson D, Locker G, McIntosh D, Ozols R, et al. Oncology. suppl. Vol. 10. Williston Park; 1996. NCCN Ovarian Cancer Practice Guidelines. The National Comprehensive Cancer Network; pp. 293–310. [PubMed] [Google Scholar]
- 16.Ozols R. Oncology. Vol. 11. Williston Park; 1997. Update of the NCCN ovarian cancer practice guidelines; pp. 95–105. [PubMed] [Google Scholar]
- 17.Morgan R, Alvarez RD, Armstrong DK, et al. NCCN practice guidelines for ovarian cancer. Version 2000. Fort Washington (PA: National Comprehensive Cancer Network; 2000. [Google Scholar]
- 18.Morgan R, Alvarez RD, Armstrong DK, et al. Ovarian cancer guidelines. Version 1, 2002. Fort Washington (PA: National Comprehensive Cancer Network; 2002. [Google Scholar]
- 19.Morgan R, Alvarez RD, Armstrong DK, et al. NCCN ovarian cancer practice guidelines—version 1, 2005. Fort Washington (PA: National Comprehensive Cancer Network; 2005. [Google Scholar]
- 20.Morgan R, Alvarez RD, Armstrong DK, et al. NCCN ovarian cancer practice guidelines—version 1, 2007. Fort Washington (PA: National Comprehensive Cancer Network; 2007. [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in healthcare. Washington, DC: National Academy Press; 2003. [Google Scholar]
- 25.Merrill RM, Anderson AE, Merrill JG. Racial/ethnic differences in the use of surgery for ovarian cancer in the United States. Adv Med Sci. 2010;55:93–98. doi: 10.2478/v10039-010-0021-8. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Dolecek TA, Davis FG. Racial differences in stage at diagnosis and survival from epithelial ovarian cancer: a fundamental cause of disease approach. Soc Sci Med. 2010;71:274–281. doi: 10.1016/j.socscimed.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parham G, Phillips JL, Hicks ML, Andrews N, Jones WB, Shingleton HM, et al. The National Cancer Data Base report on malignant epithelial ovarian carcinoma in African-American women. Cancer. 1997;80:816–826. [PubMed] [Google Scholar]
- 28.Howell EA, Egorova N, Hayes MP, Wisnivesky J, Franco R, Bickell N. Racial disparities in the treatment of advanced epithelial ovarian cancer. Obstet Gynecol. 2013;122:1025–1032. doi: 10.1097/AOG.0b013e3182a92011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thrall MM, Gray HJ, Symons RG, Weiss NS, Flum DR, Goff BA. Trends in treatment of advanced epithelial ovarian caner in the Medicare population. Gynecol Oncol. 2011;122:100–106. doi: 10.1016/j.ygyno.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harlan LC, Clegg LX, Trimble EL. Trends in surgery and chemotherapy for women diagnosed with ovarian cancer in the United States. J Clin Oncol. 2003;21:3488–3494. doi: 10.1200/JCO.2003.01.061. [DOI] [PubMed] [Google Scholar]
- 31.Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER-Medicare data for measuring cancer surgery. Med Care. 2002;40(suppl):43–48. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]