Abstract
Objective
Recent evidence suggests involvement of coagulation factor XIa (FXIa) in thrombotic event development. This study was conducted to explore possible synergies between tissue factor (TF) and exogenous FXIa (E-FXIa) in thrombin generation.
Approach and Results
In thrombin generation assays, for increasing concentrations of E-FXIa with low, but not high TF concentrations, peak thrombin significantly increased while lag time and time to peak significantly decreased. Similar dependencies of lag times and rates of thrombin generation were found in mathematical model simulations. In both in vitro and in silico experiments that included E-FXIa, thrombin bursts were seen for TF levels much lower than those required without E-FXIa. For in silico thrombin bursts initiated by the synergistic action of TF and E-FXIa, the mechanisms leading to the burst differed substantially from those for bursts initiated by high TF alone. For the synergistic case, sustained activation of platelet-bound FIX by E-FXIa, along with feedback-enhanced activation of platelet-bound FVIIIa and FXa, were needed to elicit a thrombin burst. Further, the initiation of thrombin bursts by high TF levels relied on different platelet FIX/FIXa binding sites than those for bursts initiated with low TF levels with E-FXIa.
Conclusions
Low concentrations of TF and exogenous FXIa, each too low to elicit a burst in thrombin production alone, act synergistically when in combination to cause substantial thrombin production. The observation about FIX/FIXa binding sites may have therapeutic implications.
Keywords: coagulation/thrombosis, thrombin, platelet, modeling
Subject codes: Mechanisms, Pathophysiology, Physiology, Platelets, Thrombosis
Introduction
Thrombin generation occurs through a series of overlapping initiation, amplification, and propagation stages that involve platelets and TF-bearing cells such as fibroblasts and monocytes [1, 2, 3]. Based on in vitro experiments and in vivo observations, it is believed that in vivo thrombin generation in response to a vessel injury begins with the production of small amounts of FIXa and FXa by the TF:FVIIa complex on a TF-bearing surface (subendothelium) at the site of injury. The FXa may activate its cofactor FV on the subendothelium, form prothrombinase and generate small amounts of thrombin. If collagen in the subendothelium is also exposed, platelets that adhere may become directly activated by the collagen and provide an additional source of FV and FVa [4]. The amplification stage includes the small amounts of thrombin activating more platelets, platelet-associated coagulation cofactors (also activated by small amounts of FXa), and FXI. Together, these lead to FIXa, FVIIIa, and FVa becoming present on the activated platelets’ surfaces. Finally, in the propagation stage, tenase complexes (FVIIIa:FIXa) form on platelet surfaces and begin to rapidly produce FXa. The locally-made FXa binds to the platelet-bound FVa to make large quantities of prothrombinase (FXa:FVa) and an explosive burst of thrombin results.
Along with these biochemical reactions, biophysical mechanisms are important for thrombin generation in vivo. In particular, in addition to their procoagulant role in providing specific binding sites for the coagulation proteins [5, 6, 7, 8], platelets play an anticoagulant role when they adhere to and ‘pave over’ the TF-bearing cells, physically blocking the TF:FVIIa activity [3, 9, 10]. Our group’s mathematical model predicted that thrombin generation exhibits a threshold dependence on exposed TF levels [10]. Experiments directly followed that confirmed the threshold behavior in vitro with flow, but not without [11, 12]. Thus, explosive thrombin generation under flow may occur if there is sufficient TF exposure but this is affected by the rate and extent of platelet coverage. The mathematical models further showed that the occurrence of a thrombin burst depends on the formation of a sufficiently high density of tenase (FVIIIa:FIXa) on activated platelets before activity of TF:FVIIa is blocked because adherent platelets cover the subendothelium [13].
Since FIXa is one of the two components within the tenase complex, it is key to propagation of thrombin generation. FIX is activated by TF:FVIIa in the initiation stage but also by FXIa in the propagation stage. Although FIXa is not characterized as having an important role during initiation [2], its role is essential (and distinct from FXa [14]) in the propagation phase. This is because it binds to the platelet surfaces early, priming them for tenase formation once there is sufficient available FVIIIa. While trace amounts of FIXa with no TF have been shown to induce a thrombin burst in static coagulation experiments [15], our previous simulations of intravascular coagulation under flow indicate that for low levels of exposed TF, the tiny amounts of FIXa produced by TF:FVIIa during initiation are insufficient to induce a thrombin burst. However, if there were another way to activate FIX during initiation, it might be possible to generate large amounts of thrombin even for low levels of exposed TF.
With this in mind, we consider FXIa. There is growing evidence that either FXI or FXIa is involved in development of thrombotic events (TEs). For example, TEs following treatments with immune globulin intravenous (IGIV) are known to be associated with FXIa contamination. Adverse thrombotic events associated with IGIV were first reported in 1986 [16] and in 2003, black-box warnings for IGIV were requested by the FDA. Studies showed that some off- the-shelf IGIV products were able to generate thrombin and that these same products were contaminated with FXIa [17]. More recent studies found FXIa in batches of IGIV shown to be thrombotic [17, 18] and others, although not based on thrombotic batches, also found an association of FXIa with IGIV products [19, 20, 21, 22]. Another line of evidence is the thrombogenicity of FXI concentrates used to treat FXI deficient patients. The addition of AT or C1 inhibitor stabilized thrombogenic FXI concentrates and led to much lower elevation of coagulation markers in humans [23, 24, 25]. Coagulation factors FIX, FX, and prothrombin were activated in baboons given FXIa [26]. Finally, a number of studies have measured either circulating FXIa or FXIa-like activity in blood from patients with TEs [27, 28, 29, 30, 31, 32, 33, 34].
Circulating FXI and FXIa play different roles in thrombin generation and the clotting response. Zymogen FXI is activated (slowly) by thrombin during the amplification stage before it can take on a procoagulant role and activate FIX. Hence, the effects of thrombin-activated FXIa may occur after there has already been a significant thrombin response as reported in [35, 36]. Previous simulations with our model support this possibility in that they showed that the threshold level of TF exposure was insensitive to the presence of thrombin-activated FXIa [37]. On the other hand, circulating FXIa would presumably activate FIX in the initiation stage and thus might have a powerful impact on thrombin generation, even for low levels of exposed TF. In this study, we use a mathematical model of coagulation under flow together with an in vitro thrombin generation assay to explore how varying levels of TF and exogenous FXIa (E-FXIa) affect thrombin generation. Using the in silico model, we elucidate a biochemical mechanism that explains the difference in lag times with high TF alone versus the combination of low TF and low E-FXIa.
Methods & Materials
The simulations performed for this paper were conducted using the mathematical model described in [37]. Schematics of the reactions and of the physical setting in which the reactions are assumed to occur are given in Fig. M-I in the online-only Methods and Materials Supplement. A complete listing of the reactions included in the model and of the parameter values used in the simulations are given is Tables M-I – M-VII in that Supplement. See [37] for a listing of the model’s differential equations, and [10, 13, 37] for further discussions of the model’s derivation and behavior. Details about the thrombin generation assays are available in the Methods and Materials Supplement.
Results
Computer studies show threshold behavior and synergy
In silico experiments involving platelet deposition and coagulation were conducted in a segment of a blood vessel. These simulations involved all of the reactions depicted in Fig. M-IA in the Materials and Methods Supplement. The reactions were assumed to occur in a reaction zone (see Fig. M-IB) which included the site of a small (10–20 μm long) vessel wall injury, represented by a collagen-exposing and TF-bearing lipid surface; liquid flowing blood carrying zymogens, unactivated platelets, and exogenous FXIa; and accumulated activated platelets presenting binding sites for the coagulation proteins. We assume that each species is well-mixed within the small reaction zone and represent it by its average concentration in the zone. The model consists of a system of ordinary differential equations that tracks separately the concentrations of each type of protein, e.g., FXa, in the plasma and bound to the subendothelial or platelet surfaces. The inputs for individual simulations include zymogen concentrations, platelet count, and the flow shear rate. The outputs include the time course of the concentrations of all of the coagulation proteins, unactivated platelets, and accumulated wall-bound platelets. Below we discuss behaviors of key coagulation proteins. We note that, during a simulation, platelets accumulate in the reaction zone to concentrations of up to 100 times their bulk concentration (250,000/μl) in the blood.
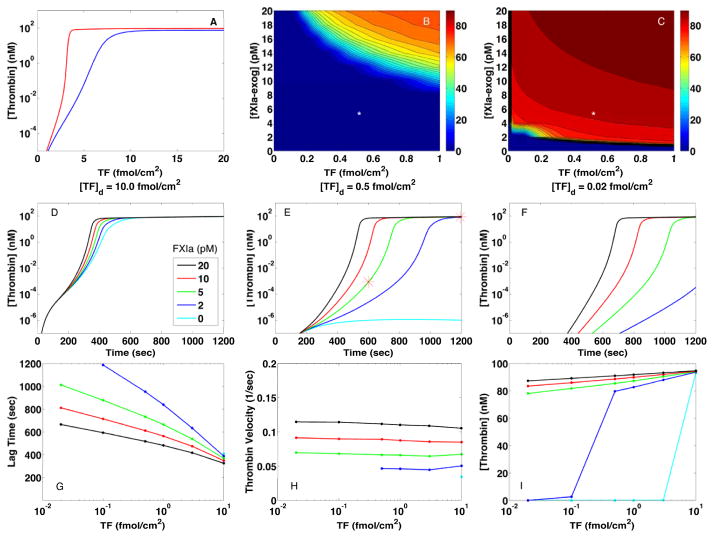
Fig. 1A–C displays plasma thrombin concentrations at 10 and 20 min from simulations in which we specified a density (amount/area) of TF, [TF]d, initially exposed by injury and a concentration of exogenous FXIa, [E-FXIa], in the bulk plasma. Fig. 1A shows that, without E-FXIa, thrombin has a threshold dependence on [TF]d. The threshold at 10 min (blue curve) is at approximately 7–9 fmol/cm2, while at 20 min (red curve) a thrombin burst occurs for all [TF]d > 3.2 fmol/cm2. For [TF]d < 1 fmol/cm2, little thrombin is produced, consistent with results in our earlier studies [10, 13]. In the presence of E-FXIa, threshold behavior persists, but shifts to much lower densities of TF as shown in Fig. 1B–C. The heat maps in these figures show the concentration of thrombin after 10 min (B) and 20 min (C) of clotting activity for various [TF]d and [E-FXIa] values. Substantial thrombin is produced by 10 min when [TF]d > 0.14 fmol/cm2 and [E-FXIa]> 14 pM and by 20 min comparable amounts are produced for a much wider range of both TF and E-FXIa ([TF]d > 0.02 fmol/cm2 and [E-FXIa]> 4 pM). Thus, even small concentrations of E-FXIa sharply increase the system’s response to exposed TF; there is a strong synergy between the two. After a burst, thrombin plateaus at a concentration of 70–90 nM for shear rate 100/sec (Fig. 1C). For lower shear rates, synergy was also seen (Fig. S-I) but for slightly higher [TF]d and [E-FXIa] and yielded higher thrombin plateaus. The in silico simulations normally include endogenous FXI and, during a simulation, thrombin activates some of this FXI to produce endogenous FXIa. For simulations without endogenous FXI, the threshold for a thrombin burst was only slightly different (not shown). This indicates that endogenously-produced FXIa does not contribute to synergistic behavior in a meaningful way. The reason is that endogenous FXI activation occurs slowly and only after thrombin has formed. The thrombin concentration plateau was somewhat higher without endogenous FXI because no thrombin was sequestered in complex with FXI in that case.
Figure 1.
Effects of TF and FXIa on intravascular thrombin generation in in silico experiments. (A) Plasma thrombin concentration (nM) at 10 min and 20 min for [TF]d between 0 and 20 fmol/cm2 and no exogenous fXIa. Plasma thrombin concentration (nM) at 10 min (B) and 20 min (C) for [TF]d between 0 and 1 fmol/cm2 and [E-FXIa] between 0 and 20 pM. Each point on a curve in (A) or the heat-maps in (B–C) was obtained from a single simulation with specified values of [TF]d and [E-FXIa], e.g., the points indicated by white stars in (B) and (C) came from the red starred points in the time course curve in (E). Panels (D–F) show thrombin generation curves (plasma thrombin concentration vs time) for three representative TF-expressing vessel wall injuries (TF density: 10 (D), 0.5 (E), and 0.02 (F) fmol/cm2) and for five FXIa concentrations as indicated. Panels (G–I) show thrombin generation parameters derived from the in silico experiments shown in (D–F). (G) Thrombin lag time (time for [Thrombin] to first reach 1 nM), (H) Relative maximum velocity (1/sec) of [Thrombin] increase, and (I) [Thrombin] after 20 min. Platelet count 250,000/μL, shear rate 100/sec.
Dynamics of intravascular thrombin production
Fig. 1D-F shows plasma thrombin concentrations from three sets of simulations; in each set, [TF]d was held fixed and [E-FXIa] was varied. For [TF]d = 10 fmol/cm2, a value which is above threshold without E-FXIa (Fig. 1A), E-FXIa dose-dependently decreased the lag time before a thrombin burst occurred but had little effect on the plateau thrombin concentration (≈ 93 nM). (Note that the constant influx of prothrombin with the flow leads to a thrombin plateau, not the peak seen in static experiments as in Fig. 2.). For all levels of [TF]d, increasing the [E-FXIa] shortened the lag time in a dose-dependent manner with higher sensitivity to [E-FXIa] and longer lag times occurring at lower [TF]d. Fig. 1H shows that the thrombin velocity, i.e., the maximum relative rate of thrombin concentration increase , was largely independent of [TF]d for each [E-FXIa], but that it increased with [E-FXIa] (see Supplement Section 1.2, especially Fig. S-II, for further discussion). Thus, in these simulations, [TF]d was important in determining whether a thrombin burst occurred and what the lag was before the burst, but it only weakly influenced how fast thrombin increased during the burst.
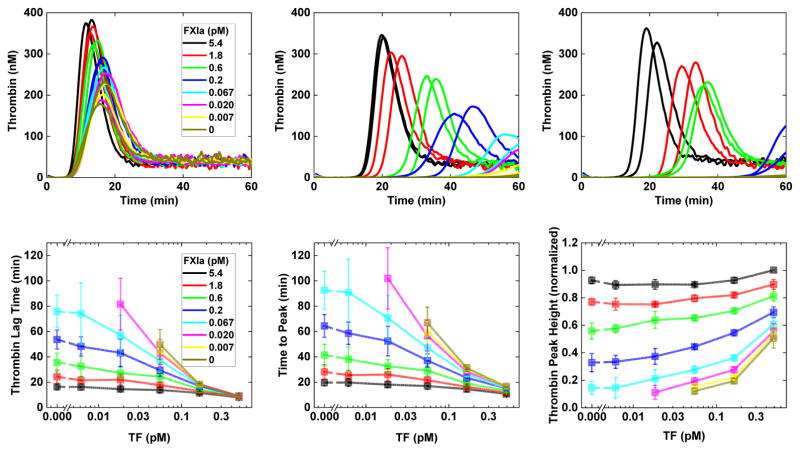
Figure 2.
Effects of exogenously added TF and FXIa on thrombin generation in CTI-treated normal pooled human plasma supplemented with human platelets to a final concentration of about 250,000/μl. Top: Time course of fluid-phase thrombin concentration for [TF]= 0.5 (A), 0.06 (B), 0.02 (C) pM and for [E-FXIa] values as indicated. Bottom: thrombin generation parameters (D) Thrombin lag time, (E) Time to peak thrombin concentration, and (F) Peak thrombin. Panels (A–C) show results from representative experiments, panels (D–F) show averaged results from four experiments using platelets from individual donors. In panel F, results within each exper- imental run are normalized against the highest stimulus condition: 280 nM, 390 nM, 429 nM, and 592 nM. Peak height normalization accounts for variability in the amount of thrombin generated by the platelets of different donors and allows evaluation of trends.
For bursts elicited by high [TF]d without E-FXIa, the story is different. In that situation, the thrombin velocity became progressively larger as [TF]d increased from 5 to 25 fmol/cm2, and the lag time decreased (Fig. S-III). Fig. 1I shows that for initiation by a combination of TF and E-FXIa, the thrombin plateau was only mildly sensitive to [TF]d and [E-FXIa]. These results indicate that the dynamics induced by high [TF]d alone are different than those triggered by a combination of low [TF]d and low [E-FXIa]. Below we compare the time courses for these two situations in more detail.
In Fig. 2 we show results from in vitro thrombin generation experiments as described in the Methods section. These experiments were performed without flow, with CTI-treated normal pooled human plasma, supplemented with human platelets to a final platelet concentration of about 250,000/μl, and added TF-bearing vesicles. The top panels show the time course of the thrombin concentration for various TF and E-FXIa concentrations. For [TF]= 5 pM (Fig. 2A), no E-FXIa was needed to cause a burst of thrombin production. For larger [E-FXIa], the lag time decreased slightly and the peak height increased, both in a dose-dependent way. For [TF]= 0.06 or 0.02 pM (Fig. 2B–C), a thrombin burst occurred only for sufficiently high [E-FXIa]. For the higher doses of E-FXIa used, the peak heights were similar to those seen for higher [TF], but the lag times were progressively longer with smaller [TF]. For lower levels of [E-FXIa] that still produced a burst, the resulting lag times were longer with corresponding peak heights reduced. Fig. 2D-E show that the lag time and time to the peak both decreased with increasing [TF], for each [E-FXIa]; these changes in timing were more significant at lower [E-FXIa]. The peak heights always increased (Fig. 2F) when [TF] or [E-FXIa] increased. For higher [E-FXIa], the peak heights were only weakly dependent on [TF], but for lower [E-FXIa], they varied substantially with [TF]. For combinations of very low [TF] and very low [E-FXIa], no burst was seen. The dependence of lag times on [TF] and [E-FXIa] in the in vitro experiments is similar to that seen in the model simulations.
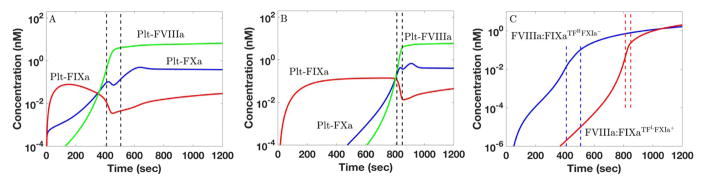
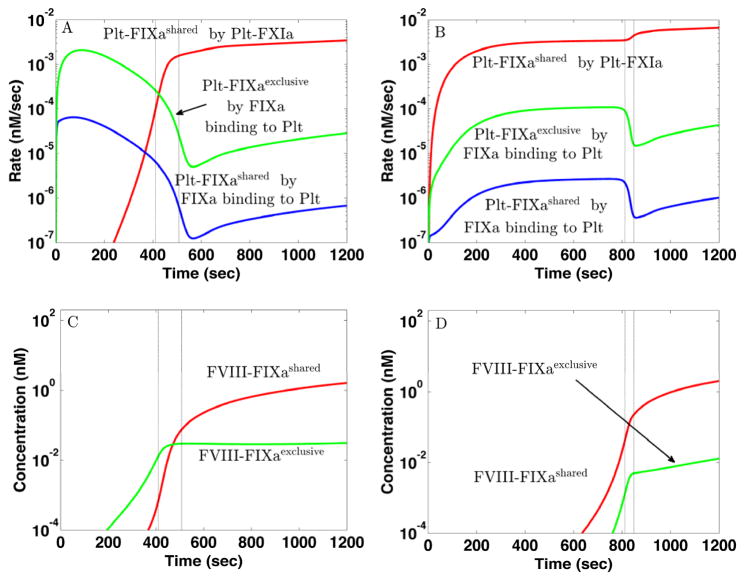
In both the mathematical simulations and in vitro experiments we see that small doses of TF and E-FXIa, each far from sufficient to elicit a burst in thrombin production alone, act synergistically when in combination to cause substantial thrombin production. The thrombin concentration after 20 min in the in silico simulations for [TF]d = 10 fmol/cm2 and no E-FXIa is very close to that seen with [TF]d = 0.02 fmol/cm2 with [E-FXIa]= 10 pM. Similarly, in the in vitro experiments, [TF]= 0.5 pM alone (Fig. 2F, black curve) leads to a peak height similar to that seen with the much lower [TF]= 0.006 pM with [E-FXIa] = 0.6 pM (Fig. 2F, dark blue curve). In the following, we focus on two of the simulations, the one with [TF]d = 10 fmol/cm2 and no E-FXIa which we denote TFHFXIa− and the one with [TF]d = 0.02 fmol/cm2 and [E-FXIa]= 10 pM which we denote TFLFXIa+. It is noteworthy that as shown in Fig. 3, the platelet-bound FVIIIa, FIXa, FXa, and tenase concentrations at 20 min are almost the same in the two simulations despite the different stimuli that trigger coagulation. Yet, comparing the time course of the thrombin concentration for the TFHFXIa− case to that for the TFLFXIa+ one, we see strong differences that are similar in both simulations and experiments. In particular, the thrombin burst in the low TF, low E-FXIa situation occurs substantially later than in the high TF one.
Figure 3.
FVIIIa:FIXa formation and FXa production on platelets in in silico simulations. For (A) TFHFXIa− ([TF]d = 10 fmol/cm and [E-FXIa]= 0) and (B) TFLFXIa+ ([TF]d = 0.02 fmol/cm2and [E-FXIa]= 10 pM), time course of platelet-bound FIXa (r), FVIIIa (g), and FXa (b). (C) Time course of FVIIIa:FIXa formation for TFHFXIa− (b) and TFLFXIa+ (r). The onset of the thrombin burst is indicated by the dashed lines which indicate when the plasma thrombin concentration reached 1 nM and 30 nM in the respective simulations. Platelet count 250,000/μL. Shear rate 100/sec.
Mechanisms underlying different time courses
In the in silico simulations, a thrombin burst occurred for both TFHFXIa− and TFLFXIa+, but in the latter case the burst happened much later. Similar behavior was seen in the in vitro experiments. In this section, we elucidate the biochemical and biophysical mechanisms behind the different response times in the simulations. In doing so, we make use of the fact that the simulations provide concentrations of all of the coagulation species shown in Fig. MI-A as well as the rates at which the individual reactions shown there occur. Understanding the reason for these differences in the simulations may provide hints to what underlies the analogous differences in the experiments.
The critical event in determining whether and when a thrombin burst occurs is the formation of sufficient quantities of the platelet-bound FVIIIa:FIXa complex [13]. As shown in Fig. 3A–B (red curves), there are significant amounts of platelet-bound FIXa (Plt-FIXa) available very early for both TFHFXIa− and TFLFXIa+, so what is needed for FVIIIa:FIXa formation is a sufficient concentration of Plt-FVIIIa. In the simulations, we assume that Plt- FVIIIa may form by activation of Plt-FVIII by either platelet-bound FXa or thrombin, or by binding of thrombin-activated FVIIIa from the plasma [38, 39]. In Fig. 3A we see that for TFHFXIa−, the Plt-FVIIIa concentration rises sharply between 200–400 sec (green curve), following an earlier rise in Plt-FXa (blue curve). A sharp rise in the concentration of FVIIIa:FIXa occurs shortly thereafter (Fig. 3C, blue curve), and is closely followed by sharp rises in the prothrombinase and thrombin concentrations (not shown). For TFLFXIa+, the sharp rises in Plt-FXa and then Plt-FVIIIa occur much later (Fig. 3B), and consequently, formation of FVIIIa:FIXa (Fig. 3C, red curve), prothrombinase (not shown) and the thrombin burst (not shown) are substantially delayed. There is more to the story than just a delay; in the simulations, the dynamics of FVIIIa:FIXa formation for TFHFXIa− are significantly different from those for TFLFXIa+.
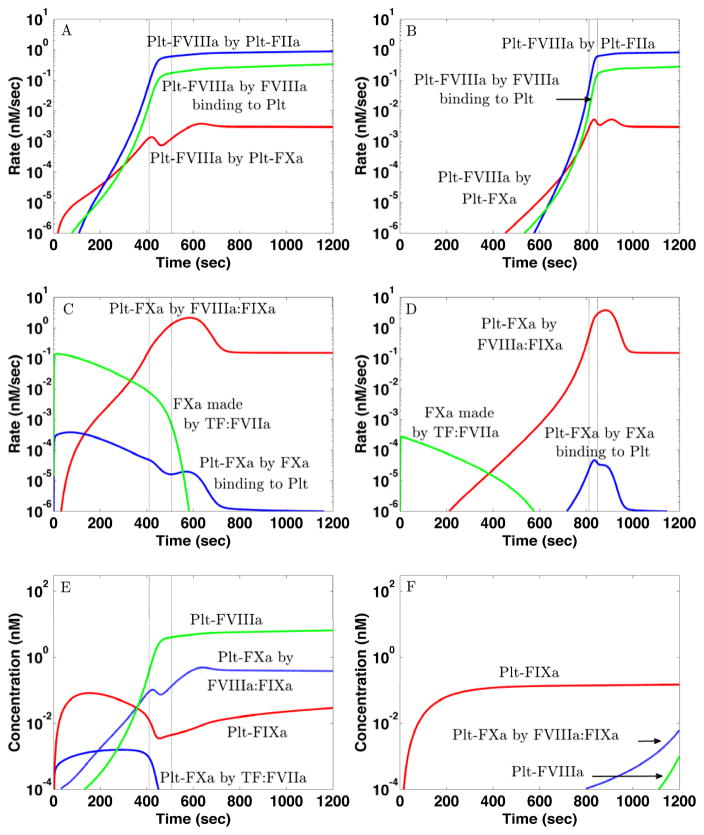
Fig. 4A-B show the rates of production of Plt-FVIIIa by different mechanisms. For both TFHFXIa− and TFLFXIa+, FXa-mediated activation of FVIII on platelet surfaces occurs earliest and is the primary means of producing Plt-FVIIIa for about 200 sec. Later, thrombin-mediated activation of FVIII on the platelet surface be- comes the dominant way Plt-FVIIIa is produced. Activation of plasma FVIII by thrombin followed by its binding to the platelet surface always contributes less. Although the lag time to the rise of Plt-FVIIIa for TFLFXIa+ is much longer than for TFHFXIa−, the rates of activation of Plt-FVIIIa by Plt-FXa starting after the respective lag times are comparable in the two cases.
Figure 4.
Differences in platelet-bound FVIIIa and FXa formation in in silico experiments for (Left) TFHFXIa− ([TF]d = 10 fmol/cm2and [E-FXIa]= 0) and (Right) TFLFXIa+ ([TF]d = 0.02 fmol/cm2 and [E-FXIa]= 10 pM). (A–B) Rates of formation of platelet-bound FVIIIa by activation of FVIII on platelet by FXa (r), by activation of FVIII on platelet by thrombin (b) and by the binding of fluid-phase FVIIIa to platelet surfaces (g). (C–D) Rates of production of FXa by TF:FVIIa (g) and by FVIIIa:FIXa (r) and rates of binding of fluid-phase FXa to the platelet surfaces (b). (E–F) To test the importance of FXa produced on the platelet for platelet-bound FVIII activation and FVIIIa:FIXa formation, we blocked the ability to activate FVIII only for FXa produced on the platelets. The time courses shown in (E) for TFHFXIa− are little different from those shown in Fig. 3A, while those in (F) for TFLFXIa+ are very much reduced compared to those in Fig. 3B. The onset of the thrombin burst is indicated by the dashed lines which show when the plasma thrombin concentration reached 1 nM and 30 nM in the respective simulations. Platelet count 250,000/μL, shear rate 100/sec.
Fig. 4C–D show how Plt-FXa is created in these simulations. For TFHFXIa−, TF:FVIIa produces substantial FXa and, although less than 1% of it binds to platelet surfaces because most is washed away by the flow, this is how most Plt-FXa is produced for the first 200 sec (Fig. 4C). This Plt-FXa activates sufficient FVIII on the platelets (Fig. 4E) so that a thrombin burst results by 400 sec (Fig. 1D, cyan curve). After the burst has started, FVIIIa:FIXa produces much more Plt-FXa. For TFLFXIa+, TF:FVIIa activates FXa about 1000-fold more slowly than for TFHFXIa− and thus, much less plasma FXa binds to platelets and the Plt-FXa concentration only becomes significant around 600 sec (Fig. 4B) due to FVIIIa:FIXa (Fig. 4B,D red curves). This prompts the question: For TFLFXIa+, where does the Plt-FVIIIa come from to allow FVIIIa:FIXa to produce this additional platelet-bound FXa? The answer is that Plt-FVIIIa and Plt-FXa increase together by a bootstrap feedback process; a little Plt-FVIIIa allows some FVIIIa:FIXa formation which activates additional Plt-FXa that in turn activates more Plt-FVIIIa and allows further FVIIIa:FIXa formation. This story is suggested by the rate curves in Fig. 4B,D, and is confirmed by the simulation experiments shown in Fig. 4E–F.
For simulations shown in Fig. 4E–F, we slightly modified the model to allow us to track separately the FXa made by TF:FVIIa and the FXa made by FVIIIa:FIXa. Further, we blocked the ability of FVIIIa:FIXa-activated Plt-FXa to activate Plt-FVIII, while allowing all of its other functions to occur as usual. Plt-FXa formed by direct binding of TF:VIIa-activated FXa retained its ability to activate Plt-FVIII. Fig. 4E shows the concentrations of Plt-FXa containing FXa activated by TF:FVIIa (solid blue curve) or by FVIIIa:FIXa (dashed blue curve), for TFHFXIa−. Comparing the green and dashed blue curves in Fig. 4E to the green and blue curves in Fig. 3A, we can see that these model modifications did not change the time courses of Plt-FVIIIa and Plt-Xa concentrations, or the subsequent formation of prothrombinase and thrombin (not shown). For an analogous experiment for TFLFXIa+ in which only TF:VIIa-activated FXa was allowed to activate FVIII on platelet surfaces, Plt-FVIIIa and Plt-FXa production were delayed and attenuated (Fig. 4F). Comparing these results with those in Fig. 3B, we see that without the bootstrap feedback process, the Plt-FXa and Plt-FVIIIa concentrations are at least two orders of magnitude lower. As a consequence, this simulation did not produce a thrombin burst, even after 20 min, showing that this feedback loop is essential for the production of thrombin in the TFLFXIa+ situation.
Role of platelet FIX/FIXa binding sites
For the simulations described above, each activated platelet was assumed to have equal numbers of two types of binding sites to which FIXa can bind [40, 41, 42, 43]. One is a shared binding site for which FIXa and FIX compete. We call these “shared” receptors and denote them . The other is one to which FIXa, but not FIX, can bind, and we call these “exclusive” receptors and denote them . We refer to FIXa bound to the and sites as Plt-FIXashared and Plt-FIXaexclusive, respectively. Each of these can bind with Plt-FVIIIa to form the respective tenase complexes FVIIIa:FIXashared and FVIIIa:FIXaexclusive which we refer to collectively as the tenase complexes. The Plt-FIXa and FVIIIa:FIXa concentrations displayed in Figs. 3–4 are the total concentrations for FIXa bound to either type of binding sites. We emphasize that the synergistic response to TF and E-FXIa stimulation shown earlier does not depend on the assumption that platelets have two types of FIXa binding sites. A synergistic response with approximately the same thresholds also occurs if platelets have either distinct sites for FIX and FIXa, (Fig. S-IV, top), or only shared receptors (Fig. S-IV, bottom). We showed [10] that the primary effect of the exclusive binding sites is to allow TF-initiated thrombin bursts to occur at much lower TF levels than when only shared binding sites are present. The story is different for bursts initiated by low TF levels in conjunction with E-FXIa. When all 500 binding sites for FIX and FIXa on each activated platelet can bind FIX, there is a higher concentration of Plt-FIX to be activated by Plt-FXIa. Hence, synergistic initiation of thrombin bursts happens at even lower [TF]d when each activated platelet has 500 shared FIX/FIXa binding sites. Compare Fig. 1 with Fig. S-IV, bottom.
Fig. 3A–B shows that the total Plt-FIXa concentration rises early and rapidly for both the TFHFXIa− and TFLFXIa+ simulations. For TFHFXIa−, it is overwhelmingly the Plt-FIXaexclusive concentration that is increasing during this period, while for TFLFXIa+, it is primarily the Plt-FIXashared concentration that is rising (not shown). This can be inferred from Fig. 5A–B where we show the rates of formation of platelet-bound FIXa by different routes. The rate of change of the Plt-FIXa concentration is affected by the rates at which FIXa in the plasma binds to the platelet or binding sites, and by the rate at which Plt-FIX is activated by Plt-FXIa. For TFHFXIa−, the early increase in Plt-FIXa is almost entirely due to plasma FIXa binding to the platelets (Fig. 5A). During this time, there is little FXIa and so TF:FVIIa is by far the dominant source of FIXa activation.
Figure 5.
Production of platelet-bound FIXa and timing of FVIIIa:FIXa formation in in silico experiments for (Left) TFHFXIa− ([TF]d = 10 fmol/cm2and [E-FXIa]= 0) and (Right) TFLFXIa+ ([TF]d = 0.02 fmol/cm2 and [E-FXIa]= 10 pM). The model includes 250 ‘shared’ binding sites per activated platelet for which FIX and FIXa compete and 250 ‘exclusive’ binding sites per activated platelet to which only FIXa can bind. (A) For TFHFXIa−, the formation of platelet-bound FIXa for the first ≈ 400 sec occurs almost exclusively by fluid-phase FIXa binding to the exclusive sites. FXIa-mediated activation of platelet-bound FIX (red curve) contributes negligibly early in the simulation, but becomes dominant at later times. (B) For TFLFXIa+, platelet-bound FIXa formation is dominated throughout the simulation by the activation of platelet-bound FIX (bound to the shared binding sites) by platelet-bound FXIa (red curve). Little platelet-bound FIXa is formed by plasma FIXa binding to either type of platelet binding site (green and blue curves). (C) For TFHFXIa−, platelet-bound FVIIIa becomes available early (see Fig. 3A and 4A) because of activation by platelet-bound FXa originally produced by TF:FVIIa, and so [FVIIIa:FIXa] also rises early and involves FIXa bound to the exclusive binding sites (green curve). Compared to these results, in (D) we see that for TFLFXIa+, platelet-bound FVIIIa becomes available later (see Fig. 3C and 4B), because of the bootstrap feedback process, and so [FVIIIa:FIXa] rises much later, and involves FIXa bound to the shared binding sites (red curve). The onset of the thrombin burst is indicated by the dashed lines which show when the plasma thrombin concentration reached 1 nM and 30 nM in the respective simulations. Platelet count 250,000/μL, shear rate 100/sec.
Since plasma FIXa competes with the more plentiful plasma FIX for binding sites, the rate at which plasma FIXa binds these sites (blue curve) is much lower than that at which it binds the ones (green curve). Only after about 400 sec when TF:FVIIa activation of FIX has slowed because platelets pave over the subendothelium and thrombin has activated FXI, does the FXIa-mediated production of Plt-FIXa become dominant (red curve), resulting in more FIXa bound to sites than to ones. Fig. 5B shows that for TFLFXIa+, Plt-FIXa formation is always dominated by activation of Plt-FIX by Plt-FXIa (red curve), which is present from the beginning because of E-FXIa in the plasma. The sites play a much smaller role in this case (green curve) because there are only small amounts of FIXa activated by TF:VIIa or plasma FXIa, and most of this plasma FIXa is washed away by flow. For TFHFXIa−, FVIIIa:FIXa formation primarily involves Plt-FIXaexclusive for the first 400 sec (Fig. 5C), and it is these FVIIIa:FIXa molecules that determine whether a thrombin burst occurs. For TFLFXIa+, high concentrations of FVIIIa:FIXa are formed much later and mostly involve Plt-FIXashared (Fig. 5D).
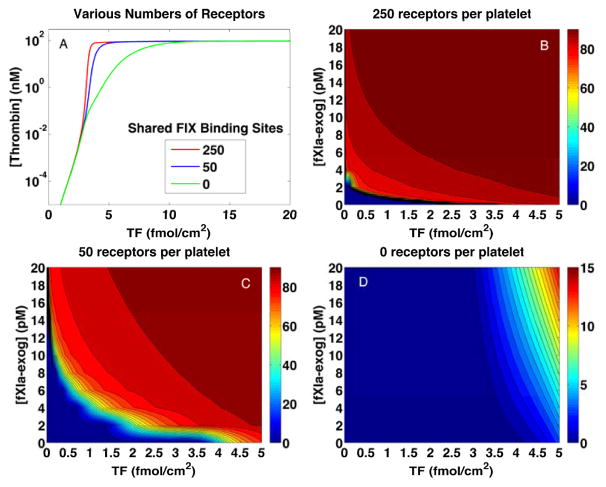
The simulation results in Fig. 5 revealed major differences in the relative importance of the exclusive FIXa platelet binding sites and the shared FIX/FIXa ones between TFHFXIa− and TFLFXIa+. These observations motivated an additional set of simulations (Fig. 6A) in which all or some of the shared sites were removed. The thrombin bursts elicited by high [TF]d were affected only marginally when 80% of the sites were removed (compare the red and blue curves). Even when all sites were removed (green curve), a thrombin burst occurred for almost the entire range of [TF]d values for which it occurred when these sites were available. In contrast, the effect of removing the sites was profound for low [TF]d. Fig. 6B shows the plasma thrombin concentration at 20 min for a range of [TF]d and [E-FXIa] values with the usual number of sites (these conditions are the same as for Fig. 1 but include a wider range of [TF]d values). Fig. 6C–D shows the thrombin concentration when 80% or 100% of the sites were removed. For the 80% case, there is a much greater range of [TF]d and [E-FXIa] values for which a thrombin burst does not occur. For example, [E-FXIa]=10 pM, [TF]d =0.02 fmol/cm2 led to a thrombin plateau concentration of ≈ 62 nM in the usual case, while [TF]d greater than 1.4 fmol/cm2 was required to elicit a comparable thrombin response in the 80% removed case. In the complete absence of the shared FIX/FIXa sites, combinations of relatively high [TF]d and [E-FXIa] were required for even a moderate thrombin response. Thus, preventing FIX/FIXa from binding to sites, substantially reduces the synergistic response to TF and E-FXIa, but has little effect on thrombin production elicited by high [TF]d values.
Figure 6.
Regulation of thrombin generation by FIX/FIXa binding site concentrations. (A) Plasma thrombin concentration at 20 min vs [TF]d with no exogenous FXIa with 250 (red), 50 (blue), 0 (green) binding sites per platelet. (B–D) Plasma thrombin concentration at 20 min for various [TF]d and [E-FXIa] with 250 (B), 50 (C), 0 (D) binding sites per platelet. Note different scale for panel (D). Platelet count 250,000/μL. Shear rate 100/sec.
Discussion
Our mathematical model of platelet deposition and coagulation under flow in response to vessel injury has previously predicted that occurrence of a thrombin burst depends on the outcome of a race between platelet coverage of subendothelial TF and establishment of a sufficiently high density of the tenase (FIXa:FVIIIa) complex on those platelets [13]. In those earlier studies, done without E-FXIa, TF:FVIIa produced FIXa and FXa only as long as part of the subendothelium remained exposed. Further, it did so at a rate that was higher for a higher initial density of exposed TF and that progressively decreased as more and more of the subendothelium became covered by adherent platelets. Almost all of the FIXa and FXa made by TF:FVIIa was carried away by the flow but some of each became bound to the activated platelets. Plt-FXa was the dominant enzyme activating Plt-FVIII early in the coagulation response. For any positive concentration of Plt-FXa, the concentration of Plt-FVIIIa increased in time, but how rapidly it reached a particular value depended on how much TF:FVIIa-activated FXa had bound to the platelet surface. In contrast to Plt-FVIIIa, Plt-FIXa was produced at this stage of the coagulation response only by the binding of TF:FVIIa-activated FIXa to the platelets from the plasma. A thrombin burst occurred if the rise of Plt-FVIIIa was sufficiently rapid that it became plentiful while TF:FVIIa was still producing significant amounts of FIXa. In our simulations this occurred for TF density of 3–4 fmol/cm2 or more, the exact value depending on the near-wall speed of the blood flow (i.e., whether we simulated venous or arterial conditions). For a below-threshold density of exposed TF, it was the insufficiency of FIXa, not that of Plt-FVIIIa, that accounted for insufficient tenase formation and consequently weak thrombin production.
Both the experiments and simulations reported in this paper show that, with low concentrations of E-FXIa, a thrombin burst can occur for much lower TF levels than required without E-FXIa. That is, TF and FXIa act synergistically to elicit a thrombin burst. This is similar to the recent observation that rFXIIa and rFVIIa act synergistically [44].
Qualitative Correspondence Between in vitro and in silico Models
Because our model and in silico experiments pertain to an open system in which flow brings new zymogens to and removes enzymes from the site of the coagulation reactions, and our in vitro experiments involve a closed static system without replenishment of zymogens, close quantitative agreement between their results is not expected. We found, however, wide areas of qualitative correspondence between the behaviors seen in the simulations and experiments. Most prominent, of course, was the existence of the synergy between TF and E-FXIa in triggering a thrombin burst. Other corresponding behaviors (see Figs. 1–2) include:
For sufficiently high TF levels, no E-FXIa was needed to elicit a thrombin burst.
As the TF level dropped, the E-FXIa level needed to trigger a thrombin burst increased.
Thrombin bursts initiated by high TF levels occurred with a much shorter lag time than those initiated by low TF and E-FXIa levels.
For TF and E-FXIa levels that triggered a burst, increasing the TF or the E-FXIa level shortened the lag time.
The lag time and the time to peak or plateau was quite sensitive to [E-FXIa] for low TF levels and much less sensitive for high TF levels
The lag time and the time to peak or plateau showed substantial or little variation with the TF level for low [E-FXIa] and high [E-FXIa], respectively.
For sufficiently high [E-FXIa], the maximum rate at which thrombin increased in the simulations and the peak thrombin height attained in the experiments were both sensitive to variations in [E-FXIa], but not to variations in the TF level.
To understand why this last correspondence is meaningful, note that for a system like that used for our in vitro experiments in which a fixed quantity of prothrombin is converted to thrombin while at the same time thrombin is inactivated by antithrombin, the peak thrombin concentration that is attained directly reflects the maximum rate of activation of prothrombin to thrombin. This wide-ranging qualitative similarity in the nature of the thrombin bursts, their timing, and their various sensitivities to changes in the TF and exogenous FXIa levels, gives confidence in the model’s value for exploring the mechanisms behind these behaviors.
Distinct Mechanisms to Tenase Formation for Different Initial Stimulus
To probe these mechanisms, we focused on two sets of initiating stimuli, TFHFXIa− with [TF]d =10 fmol/cm2 and no E-FXIa and TFLFXIa+ with [TF]d =0.02 fmol/cm2 and [E-FXIa]= 10 pM. Both sets of stimuli led to a burst in thrombin production, but the thrombin dynamics are quite different for these two cases. For TFHFXIa−, the plasma thrombin concentration first reaches 1 nM at 410 sec, the maximum relative rate ( ) at which the thrombin concentration increases following that time is 0.035/sec, and the thrombin concentration at 20 min is approximately 93 nM. For TFLFXIa+, the lag time before the concentration of thrombin in the plasma reaches 1 nM is longer at 812 sec, the maximum relative rate at which the thrombin concentration increases, 0.092/sec, is much higher, and the concentration at 20 min, 94 nM, is almost the same. These differences can be traced to the different dynamics by which the FVIIIa:FIXa complexes form and by the three-fold difference in the concentrations of these complexes, 0.014 nM for TFHFXIa− and 0.045 nM for TFLFXIa+, at the time the plasma thrombin concentration reaches 1 nM in the respective cases. The formation of both Plt-FVIIIa and Plt-FIXa is necessary in order that the FVIIIa:FIXa complex form, and the dynamics of each of these is different for the two types of stimuli.
Positive Feedback Aids in Formation of Platelet-Bound FVIIIa with Low TF
The in silico model incorporates our assumptions that FVIII can be activated in plasma by thrombin and on activated platelet surfaces by thrombin and FXa. There is solid evidence that FVIII binds to activated platelets and that these reactions occur [38, 39]. But the fact that FVIII circulates in the plasma, bound with vWF, along with evidence that FVIII bound to vWF is protected from activation by FXa [45, 46] has led some to conclude that FXa-mediated activation of Plt-FVIII occurs to a minimal extent in vivo. Others note that the affinities of FVIII for vWF and for anionic phospholipids are similar [47, 48] and that FVIII bound to vWF distributes between vWF and the phospholipids when both are present [47, 49]. Since the larger vWF multimers to which most FVIII is bound [47] readily bind to activated platelets, FVIII would come into close proximity to platelet surfaces, and thus be in position to bind to the platelet surface, be activated by Plt-FXa, and participate in the feedback loop involving FVIIIa:FIXa [39, 45, 50].
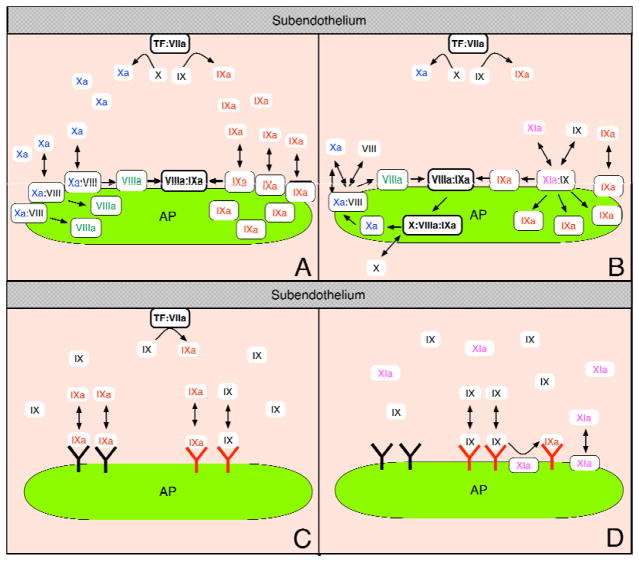
The simulations show that the important paths to formation of Plt-FVIIIa involve activation of FVIII already bound to the platelet surface. Two paths involve activation of Plt-FVIII by Plt-FXa because we distinguish between activation by Plt-FXa that originated with FXa produced by subendothelial TF:FVIIa and activation by Plt-FXa formed by the platelet tenase complexes. The third path involves activation of Plt-FVIII by platelet-bound thrombin. Thrombin also activates FVIII in the plasma and some of the resulting FVIIIa binds to platelets, but this route was always less productive than one of the three already mentioned. For the TFHFXIa− case, Plt-FXa formed from TF:FVIIa-activated FXa was the dominant activator of Plt-VIII for the first ≈ 200 sec and the rate of Plt-VIII activation by FXa increased during this period in tandem with the increasing concentration of Plt-FXa (Fig. 4A). After this initial period, platelet-bound thrombin became the dominant producer of Plt-FVIIIa. Blocking either of these pathways to producing Plt-VIIIa prevented a thrombin burst (results not shown). In contrast, activation of Plt-VIII by Plt-FXa that was produced by platelet tenase played a small role in this case, as demonstrated when this reaction was blocked (Fig. 4E). For TFLFXIa+, Plt-FVIIIa buildup proceeded more slowly than for TFHFXIa−. It was initiated by FXa produced by TF:FVIIa, but this FXa contributed little beyond starting the process. Instead, most Plt-FXa-activated FVIIIa involved FXa that was itself activated on the platelet surface by the tenase complexes FVIIIa:FIXashared and FVIIIa:FIXaexclusive. Since these complexes were scarce until sufficient Plt-FVIIIa had been produced, this was a bootstrap process (illustrated in Fig. 7) in which feedback activation of FVIIIa by platelet-tenase-activated FXa led to more platelet tenase production. If this feedback was blocked in the TFLFXIa+ case (Fig. 4F), then platelet tenase and prothrombinase formation, and thrombin production were substantially delayed. If the feedback functioned normally, but thrombin activation of FVIII was blocked, thrombin production was also reduced substantially (not shown).
Figure 7.
Schematic illustrating the different routes to tenase formation for (A) high densities of exposed TF without E-FXIa and for (B) low densities of exposed TF together with a low concentration of E-FXIa. Panel (A) shows sufficient FXa binding to the platelet surface to activate Plt-FVIII and thus allow tenase formation. Panel (B) shows the feedback loop in which small amounts of plasma FXa bind to the platelet surface and use bootstrapping to create more Plt-FXa. Note that all reactions shown take place on the platelet surface only. Schematic illustrating the initial formation of Plt-FIXa for (C) high densities of exposed TF without E-FXIa and for (D) low densities of exposed TF together with a low concentration of E-FXIa. Panel (C) shows that with high density of TF, TF-activated FIXa initially binds both the FIX/FIXa shared and FIXa exclusive binding sites, but predominately the FIXa exclusive ones. In contrast, panel (D) shows that the FIXa exclusive sites are, initially, mostly unbound and that FIX is activated on the platelet surface by Plt-FXIa, while it is bound to the FIX/FXIa shared sites.
Shared FIX/FIXa Binding Sites Important for Synergy
In the model we assume that activated platelets display a population of binding sites shared by FIX and FIXa, and another population of binding sites exclusive to FIXa. Only the shared sites are important for synergy between TF and E-FXIa, but looking at the roles of both populations in the TFHFXIa− and TFLFXIa+ cases is instructive. Before doing so, we note that support for the existence of two populations of FIXa receptors comes from a series of studies [40, 41, 42, 43], performed by the Walsh lab using a variety of equilibrium and kinetic approaches, in which they characterized the numbers and binding parameters of the receptors, and identified the portion of the FIX/FIXa Gla domain required for binding to the shared sites and the part of FIXa’s EGF2 domain required for its binding to the exclusive sites.
Even in the TFHFXIa− case, the plasma concentration of FIXa was much lower than that of the zymogen FIX. Because of this, binding of FIXa to platelets from the plasma was overwhelmingly to the exclusive FIXa binding sites rather than to the shared FIX/FIXa ones (Fig. 5A). The presence of FIXa bound to the shared sites only become significant later in the process, after the initiation of the thrombin burst, when thrombin-activated Plt-FXIa activated Plt-FIX that was already bound to the shared sites. In contrast, for the TFLFXIa+ case, plasma E-FXIa was available from the beginning, it bound rapidly to platelets as they became activated because of their interactions with subendothelial collagen, and the resulting Plt-FXIa activated Plt-FIX that was bound to the shared FIX/FIXa binding sites (Fig. 5B). In this case, because the TF level was low, there was little production of FIXa by TF:FVIIa, and so little binding of FIXa from the plasma to the exclusive binding FIXa binding sites. Hence, the formation of Plt-FIXa in the two situations occurred by totally different routes and, importantly, involved two different types of platelet binding sites (see Fig. 7).
A potentially important prediction of the model is that inhibiting the binding of FIX to the platelet’s shared FIX/FIXa binding sites substantially reduces thrombin production induced by combinations of low density TF exposure and circulating plasma FXIa without substantially changing the rapid and robust production of thrombin in response to higher densities of exposed TF (see Fig. 6). This observation may have therapeutic utility in providing an avenue to reduce thrombosis without significantly affecting hemostasis in response to injury.
Supplementary Material
Highlights.
This study shows through in vitro experiments and mathematical simulations that low concentrations of TF and exogenous FXIa, each far from sufficient to elicit a burst in thrombin production on its own, act synergistically when in combination to cause substantial thrombin production. This may have bearing on thrombotic event occurrence associated with use of FXIa-contaminated intravenous immune globulin treatments.
Using the mathematical model, we elucidate a biochemical mechanism that explains a large difference in lag times associated with high TF alone versus the combination of low TF and low E-FXIa.
We suggest possible therapeutic interventions, via platelet-bound FIXa, that block thrombin production under the synergistic conditions, but leave it largely unchanged in the case of initiation by high TF levels alone.
Acknowledgments
Sources of Funding
This study was supported in part by a Postgraduate and Postbaccalaureate Research Fellowship Award to W.C.C. from the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration (FDA). W.C.C. and M.V.O. are employees of the FDA. This paper is an informal communication and represents the authors’ best judgment. These comments do not bind or obligate FDA. This work was also supported in part by NSF grants DMS-1160432 and DMS-1521748 and NHLBI grant 1R01HL126864 to A.L.F. and NHLBI grant 1R01HL120728 to A.L.F. and K.L..
Footnotes
Disclosures:
None
Contributor Information
Karin Leiderman, Applied Mathematics and Statistics, Colorado School of Mines Golden, CO, USA.
William C. Chang, Office of Blood Research and Review, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD, USA
Mikhail Ovanesov, Office of Blood Research and Review, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD, USA.
Aaron L. Fogelson, Departments of Mathematics and Bioengineering, University of Utah, Salt Lake City, UT, USA
References
- 1.Hoffman M, Monroe DM. A cell-based model of hemostasis. Thromb Haemost. 2001;85:958–65. [PubMed] [Google Scholar]
- 2.Hoffman M, Monroe DM. Coagulation 2006: A modern view of hemostasis. Hematol Oncol Clin N. 2007;21(1):1–11. doi: 10.1016/j.hoc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Monroe DM, Hoffman M. What does it take to make a perfect clot? Arterioscler Thromb Vasc Biol. 2006;26 doi: 10.1161/01.ATV.0000193624.28251.83. [DOI] [PubMed] [Google Scholar]
- 4.Monkovic DD, Tracy PB. Functional characterization of human platelet-released Factor V and its activation by Factor Xa and thrombin. J Biol Chem. 1990;265:17132–40. [PubMed] [Google Scholar]
- 5.Mann KG, Bovill EG, Krishnaswamy S. Surface-dependent reactions in the propagation phase of blood coagulation. Semin Hematol. 1992;29:213–226. doi: 10.1111/j.1749-6632.1991.tb43692.x. [DOI] [PubMed] [Google Scholar]
- 6.Nesheim ME, Pittman DD, Wang JH, Slonosky D, Giles AR, Kaufman RJ. The binding of s-labeled recombinant Factor VIII to activated and unactivated human platelets. J Biol Chem. 1988;263:16467. [PubMed] [Google Scholar]
- 7.Tracy PB, Nesheim ME, Mann KG. Platelet Factor Xa receptor. Methods Enzymol. 1992;215:329–360. doi: 10.1016/0076-6879(92)15075-n. [DOI] [PubMed] [Google Scholar]
- 8.Walsh PN. Platelet-coagulant protein interactions. In: Colman RW, Hirsh J, Marder VJ, Salzman EW, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. 3. J B Lippincott Company; Philadelphia, PA: 1994. pp. 629–651. [Google Scholar]
- 9.Hathcock JJ, Nemerson Y. Platelet deposition inhibits tissue factor activity: In vitro clots are impermeable to Factor Xa. Blood. 2004;104:123–127. doi: 10.1182/blood-2003-12-4352. [DOI] [PubMed] [Google Scholar]
- 10.Kuharsky AL, Fogelson AL. Surface-mediated control of blood coagulation: The role of binding site densities and platelet deposition. Biophys J. 2001;80:1050–1074. doi: 10.1016/S0006-3495(01)76085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee MS, Denney WS, Jing H, Diamond SL. Systems biology of coagulation initiation: Kinetics of thrombin generation in resting and activated human blood. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okorie U, Denney WS, Chatterjee MS, Neeves KB, Diamond SL. Determination of surface tissue factor thresholds that trigger coagulation at venous and arterial shear rates: Amplification of 100 fM circulating tissue factor by flow. Blood. 2008;111:3507–13. doi: 10.1182/blood-2007-08-106229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogelson AL, Tania N. Coagulation under flow: The influence of flow-mediated transport on the initiation and inhibition of coagulation. Pathophysiol Haemost Thromb. 2005;34:91–108. doi: 10.1159/000089930. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman M, Monroe DM, Oliver JA, Roberts HR. Factors IXa and Xa play distinct roles in tissue factor-dependent initiation of coagulation. Blood. 1995;86:1794–1801. [PubMed] [Google Scholar]
- 15.Butenas S, Orfeo T, Gissel MT, Brummel KE, Mann KG. The significance of circulating factor IXa in blood. J Biol Chem. 2004;279(22):22875–22882. doi: 10.1074/jbc.M400531200. [DOI] [PubMed] [Google Scholar]
- 16.Woodruff RK, Grigg AP, Firkin FC, Smith IL. Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet. 1986;328(8500):217–218. doi: 10.1016/s0140-6736(86)92511-0. [DOI] [PubMed] [Google Scholar]
- 17.Roemisch JR, Kaar W, Zoechling A, Kannicht C, Putz M, Kohla G, Schulz P, Pock K, Huber S, Fuchs B, Buchacher A, Drause D, Weinberger J, Rembpeters G. Identification of activated FXI as the major biochemical root cause in IVIG batches associated with thromboembolic events. analytical and experimental approaches resulting in corrective and preventive measures implemented into the Octagam® manufacturing process. WebmedCentral Immunotherapy. 2011;2:WMC002002. [Google Scholar]
- 18.Ovanesov M, Shibeko AM, Woodle SA, Anderson CM, Hogwood J, Barson H, Gray E, Scott D. Association of factor XIa with intravenous immune globulin products implicated in thrombotic adverse events: biochemical root cause investigation. J Thromb Haem. 2011;9:272–272. [Google Scholar]
- 19.Etscheid M, Breitner-Ruddock S, Gross S, Hunfeld A, Seitz R, Dodt J. Identification of kallikrein and FXIa as impurities in therapeutic immunoglobulins: Implications for the safety and control of intravenous blood products. Vox Sang. 2012;102(1):40–46. doi: 10.1111/j.1423-0410.2011.01502.x. [DOI] [PubMed] [Google Scholar]
- 20.Funk MB, Gross N, Gross S, Hunfeld A, Lohmann A, Guenay S, Hanschmann KM, Keller-Stanislawski B. Thromboembolic events associated with immunoglobulin treatment. Vox Sang. 2013;105(1):54–64. doi: 10.1111/vox.12025. [DOI] [PubMed] [Google Scholar]
- 21.Menis M, Sridhar G, Selvam N, Ovanesov MV, Divan HA, Liang Y, Scott D, Golding B, Forshee R, Ball R, Anderson SA, Izurieta HS. Hyperimmune globulins and same-day thrombotic adverse events as recorded in a large healthcare database during 2008–2011. Am J Hematol. 2013;88(12):1035–1040. doi: 10.1002/ajh.23559. [DOI] [PubMed] [Google Scholar]
- 22.Wolberg AS, Kon RH, Monroe DM, Hoffman M. Coagulation factor XI is a contaminant in intravenous immunoglobulin preparations. Amer J Hematol. 2000;65(1):30–34. doi: 10.1002/1096-8652(200009)65:1<30::aid-ajh5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Bolton-Maggs PH, Wensley RT, Kernoff PB, Kasper CK, Winkelman L, Lane RS, Smith JK. Production and therapeutic use of a factor XI concentrate from plasma. Thromb Haemost. 1992;67(3):314–319. [PubMed] [Google Scholar]
- 24.Burnouf-Radosevich M, Burnouf T. A therapeutic, highly purified factor XI concentrate from human plasma. Transfusion. 1992;32(9):861–867. doi: 10.1046/j.1537-2995.1992.32993110761.x. [DOI] [PubMed] [Google Scholar]
- 25.Richards EM, Makris MM, Cooper P, Preston FE. In vivo coagulation activation following infusion of highly purified factor XI concentrate. Br J Haematol. 1997;96(2):298–297. doi: 10.1046/j.1365-2141.1997.d01-2015.x. [DOI] [PubMed] [Google Scholar]
- 26.Ten Cate H, Biemond BJ, Levi M, Wuillemin WA, Bauer KA, Barzegar S, Buller HR, Hack CE, Ten Cate JW, Rosenberg RD. Factor XIa induced activation of the intrinsic cascade in vivo. Thromb Haemost. 1996;75(3):445–449. [PubMed] [Google Scholar]
- 27.Butenas S, Undas A, Gissel MT, Szuldrzynski K, Zmudka K, Mann KG. Factor XIa and tissue factor activity in patients with coronary artery disease. Thromb Haemost. 2008;99(1):142. doi: 10.1160/TH07-08-0499. [DOI] [PubMed] [Google Scholar]
- 28.Jankowski M, Undas A, Kaczmarek P, Butenas S. Activated factor XI and tissue factor in chronic obstructive pulmonary disease: Links with inflammation and thrombin generation. Thromb Res. 2011;127(3):242–246. doi: 10.1016/j.thromres.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luszczak J, Undas A, Gissel M, Olszowska M, Butenas S. Activated factor XI and tissue factor in aortic stenosis: Links with thrombin generation. Blood coagulation & fibrinolysis. 2011;22(6):473. doi: 10.1097/MBC.0b013e328346c2bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Undas A, Jankowski M, Kaczmarek P, Sladek K, Brummel-Ziedins K. Thrombin generation in chronic obstructive pulmonary disease: Dependence on plasma factor composition. Thromb Res. 2011;128(4):e24–e28. doi: 10.1016/j.thromres.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Undas A, Owczarek D, Gissel M, Salapa K, Mann KG, Butenas S. Activated factor XI and tissue factor in inflammatory bowel disease. Inflamm Bowel Dis. 2010;16(9):1447–1448. doi: 10.1002/ibd.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Undas A, Slowik A, Gissel M, Mann KG, Butenas S. Active tissue factor and activated factor XI in patients with acute ischemic cerebrovascular events. E J Clin Invest. 2012;42(2):123–129. doi: 10.1111/j.1365-2362.2011.02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zabczyk M, Butenas S, Palka I, Nessler J, Undas A. Active tissue factor and activated factor XI in circulating blood of patients with systolic heart failure due to ischemic cardiomyopathy. Pol Arch Med Wewn. 2010;120(9):334. [PMC free article] [PubMed] [Google Scholar]
- 34.Zabczyk M, Butenas S, Plicner D, Fijorek K, Sadowski J, Undas A. Factors associated with the presence of circulating active tissue factor and activated factor XI in stable angina patients. Blood Coagul Fibrin. 2012;23(3):189–194. doi: 10.1097/MBC.0b013e32834ee194. [DOI] [PubMed] [Google Scholar]
- 35.Panteleev MA, Ovanesov MV, Kireev DA, Shibeko AM, Sinauridze EI, Ananyeva NM, Butylin AA, Saenko EL, Ataullakhanov FI. Spatial propagation and localization of blood coagulation are regulated by intrinsic and protein C pathways, respectively. Biophys J. 2006;90(5):1489–1500. doi: 10.1529/biophysj.105.069062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von dem Borne PA, Cox LMP, Bouma BN. Factor XI enhances fibrin generation and inhibits fibrinolysis in a coagulation model initiated by surface-coated tissue factor. Blood Coagul Fibrin. 2006;17(4):251–257. doi: 10.1097/01.mbc.0000224843.33216.5f. [DOI] [PubMed] [Google Scholar]
- 37.Fogelson AL, Hussain YH, Leiderman K. Blood clot formation under flow: The importance of factor XI depends strongly on platelet count. Biophys J. 2012;102:10–18. doi: 10.1016/j.bpj.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furby FH, Berndt MC, Castaldi PA, Koutts J. Characterization of calcium-dependent binding of endogenous factor VIII/von Willebrand factor to surface activated platelets. Thrombosis Research. 1984;35:501–511. doi: 10.1016/0049-3848(84)90282-2. [DOI] [PubMed] [Google Scholar]
- 39.Neuenschwander P, Jesty J. A comparison of phospolipid and platelets in the activation of human Factor VIII by thrombin and Factor Xa, and in the activation of Factor X. Blood. 1988;72:1761–1770. [PubMed] [Google Scholar]
- 40.Ahmad SS, Rawala-Sheikh R, Walsh PN. Comparative interactions of Factor IX and Factor IXa with human platelets. J Biol Chem. 1989;264:3244–3251. [PubMed] [Google Scholar]
- 41.London FS, Marcinkiewicz M, Walsh PN. A subpopulation of platelets responds to thrombin- or SFLLRN-stimulation with binding sites for Factor IXa. J Biol Chem. 2004;279:19854–18859. doi: 10.1074/jbc.M310624200. [DOI] [PubMed] [Google Scholar]
- 42.Wilkinson FH, Ahmad SS, Walsh PN. The Factor IXa second epidermal growth factor (EGF2) domain mediates platelet binding and assembly of the Factor X activating complex. Journal of Biological Chemistry. 2002;277:5734–5741. doi: 10.1074/jbc.M107753200. [DOI] [PubMed] [Google Scholar]
- 43.Wong MY, Gurr JA, Walsh PN. The second epidermal growth factor-like domain of human Factor IX mediates Factor IXa binding to platelets and assembly of the Factor X activating complex. Biochemistry. 1999;38:8948–8960. doi: 10.1021/bi982835g. [DOI] [PubMed] [Google Scholar]
- 44.Li R, Panceri KA, Fogarty PF, Diamond SL. Recombinant factor VIIa enhances platelet deposition from flowing haemophilic blood but requires the contact pathway to promote fibrin deposition. Haemophilia. 2015;21:266–274. doi: 10.1111/hae.12558. [DOI] [PubMed] [Google Scholar]
- 45.Donath MSH, Lenting PJ, van Mourik JA, Mertens K. The role of cleavage of the light chain at positions Arg1669 or Arg1721 in subunit interaction and activation of human blood coagulation Factor VIII. J Biol Chem. 1995;270:3648–55. doi: 10.1074/jbc.270.8.3648. [DOI] [PubMed] [Google Scholar]
- 46.Koedam JA, Hamer RJ, Beeser-Visser NH, Bouma BN, Sixma JJ. The effect of von Willebrand factor on the activation of factor VIII by factor Xa. European Journal of Biochemistry. 1990;189:229–234. doi: 10.1111/j.1432-1033.1990.tb15481.x. [DOI] [PubMed] [Google Scholar]
- 47.Saenko EL, Shima M, Sarafanov AG. Role of activation of the coagulation FVIII in interaction with vWF, phospholipid, and functioning within the Factor Xase complex. Trends in Cardiovascular Medicine. 1999;9:185–192. doi: 10.1016/s1050-1738(00)00019-0. [DOI] [PubMed] [Google Scholar]
- 48.Spaargaren J, Giesen PLA, Janssen MP, Voorberg J, Willems GM, van Mourik JA. Binding of blood coagulation Factor VIII and its light chain to phosphatidylserine/phosphatidylcholine bilayers as measured by ellipsometry. Biochemistry Journal. 1995;310:539–545. doi: 10.1042/bj3100539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moake JL, Weinstein MJ, Troll JH, Chute LE, Colannino NM. Direct radioimmune detection in human plasma of the association between Factor VIII procoagulant protein and von Willebrand factor, and the interaction of vWF-bound procoagulant VIII with platelets. Blood. 1983;61:1163–1173. [PubMed] [Google Scholar]
- 50.Griffiths AE, Wintermute J, Newell-Caito JL, Fay PJ. Residues flanking scissle bonds in Factor VIII modulate rates of cleavage and proteolytic activation catalyzed by Factor Xa. Biochemistry. 2013;52:8060–8068. doi: 10.1021/bi4010123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.