Abstract
Fanconi anemia (FA) is an autosomal recessive disease marked by congenital defects, bone marrow failure, and high incidence of leukemia and solid tumors. Eight genes have been cloned, with the accompanying protein products participating in at least two complexes, which appear to be functionally dependent upon one another. Previous studies have described chromatin localization of the FA core complex, except at mitosis, which is associated with phosphorylation of the FANCG protein (F. Qiao, A. Moss, and G. M. Kupfer, J. Biol. Chem. 276:23391-23396, 2001). The phosphorylation of FANCG at serine 7 by using mass spectrometry was previously mapped. The purpose of this study was to map the phosphorylation sites of FANCG at mitosis and to assess their functional importance. Reasoning that a potential kinase might be cdc2, which was previously reported to bind to FANCC, we showed that cdc2 chiefly phosphorylated a 14-kDa fragment of the C-terminal half of FANCG. Mass spectrometry analysis demonstrated that this fragment contains amino acids 374 to 504. Kinase motif analysis demonstrated that three amino acids in this fragment were leading candidates for phosphorylation. By using PCR-directed in vitro mutagenesis we mutated S383, S387, and T487 to alanine. Mutation of S383 and S387 abolished the phosphorylation of FANCG at mitosis. These results were confirmed by use of phosphospecific antibodies directed against phosphoserine 383 and phosphoserine 387. Furthermore, the ability to correct FA-G mutant cells of human or hamster (where S383 and S387 are conserved) origin was also impaired by these mutations, demonstrating the functional importance of these amino acids. S387A mutant abolished FANCG fusion protein phosphorylation by cdc2. The FA pathway, of which FANCG is a part, is highly regulated by a series of phosphorylation steps that are important to its overall function.
Fanconi anemia (FA) is an autosomal recessive disease of cancer susceptibility marked by congenital defects, bone marrow failure, and high incidence of leukemia and solid tumors (3, 5, 14, 15). Eleven complementation groups have been defined (22, 23, 31), with eight genes having been cloned (4, 7, 8, 10, 20, 33, 34, 46, 50, 52). However, the encoded protein products resemble no known proteins and have few identifiable functional motifs.
The one biological characteristic of FA is that cells in culture as well as the patients themselves exhibit hypersensitivity to DNA cross-linking agents. Indeed, such sensitivity results in chromosomal breakage, a phenotype utilized in a clinical test for FA. The reaction to cross-linking may also be manifest by the exhibition of G2 delay, which has been shown by some to be an S-phase defect in FA cells (12, 24, 28). Nonetheless, no biochemical mechanism has been elucidated to explain these findings.
Protein-protein interactions have shown that the FA proteins are interrelated and participate in at least two complexes (29, 36, 55). The first, termed the FA core complex, is nuclear and is comprised of FANCA, FANCC, FANCE, FANCF, FANCG, and FANCL (6, 9, 18, 34, 51). The second is made up of FANCD2 and FANCE. FANCD2 coimmunoprecipitates BRCA1 and is monoubiquitinated in an FA core complex, DNA damage, and S-phase-dependent fashion (19). In addition, BRCA2 has been shown to be the FANCD1 protein (21).
Only a few protein modifications have been reported for FA proteins, but they appear to be functionally important. FANCA, FANCD2, and FANCG have all been reported to be phosphorylated (16, 48, 55). For example, FANCA is phosphorylated only in wild-type, corrected, or mutant FA-D2 cells (1, 16, 40, 55). Also, it was recently found that FANCG is phosphorylated at serine 7 (40a). Knockout of this site results in impaired ability to correct FA-G cells.
Some evidence for activation of FA proteins has emerged in recent reports. The FANCD2 protein has been shown to be monoubiquitinated in response to DNA damage and during S phase (19). Others have shown links to S phase and to DNA repair complexes (1, 39, 42, 47). In addition, Pang et al. have demonstrated that STAT1 undergoes FANCC-dependent phosphorylation in response to γ-interferon (37). Recent work has revealed that at least a subset of the FA proteins resides in the nucleus bound to chromatin, where increased protein binding occurs in response to DNA damage. In addition, it was shown that during the cell cycle the FA proteins detach from chromatin during mitosis and FANCG becomes phosphorylated, all the while remaining part of the complex (40).
It was previously shown that the G2-M kinase cdc2 binds to FANCC (27) and that it is part of the FA core complex, as detected by mass spectrometry (49). In this paper we have defined the sites in FANCG that are phosphorylated at mitosis. These events are tightly related to the cell cycle and regulate localization of the entire FA complex. By using mass spectrometry, subsequent mutagenesis of candidate amino acids, and phosphospecific antibodies we show that FANCG is phosphorylated at serines 383 and 387. In particular, S387 is phosphorylated by cdc2, which is consistent with previous findings that cdc2 is part of the FA core complex.
MATERIALS AND METHODS
Production of Flag-FANCG mutants.
pMMP-Flag-FANCG was made as previously described (40). Primers homologous to FANCG nucleotides were used for PCR by using a Stratagene quick-change kit. Each set of primers contained a 1-nucleotide difference resulting in the change of amino acid 7. The resulting pMMP constructs were transfected into 293GPG producer lines, and viral supernatants were collected daily between 3 and 7 days after transfection. The retroviral supernatants were used for subsequent retroviral transduction.
Cell culture.
Cells were grown at 37°C in a 5% CO2 incubator. HeLa cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). FA-G mutant cells (326SV) were grown in DMEM plus 15% FBS. 293GPG producer cells were grown as described previously (40). HeLa cells were also transduced with pMMP-Flag-FANCA, and the resulting cells were selected with puromycin. EUFA143 FA-G mutant lymphoblasts were grown in RPMI 1640 plus 15% FBS. The Chinese hamster ovary (CHO) FANCG/XRCC9 mutant cell line NM3 was maintained in DMEM with 10% FBS, as described previously (54).
Immunoprecipitation.
For whole-cell lysate, pelleted cells were extracted in 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, protease inhibitors (1 μg of leupeptin/ml, 1 μg of aprotinin/ml, 1 μg of pepstatin/ml, 50 μg of phenylmethylsulfonyl fluoride/ml), and phosphatase inhibitors (1 mM sodium orthovanadate, 1 mM sodium pyrophosphate). The extract was tested for protein concentration by the Bradford assay. Two milligrams of protein in 1 ml of the respective buffer containing protease and phosphatase inhibitors was incubated with 50 μl of anti-Flag affinity gel (Sigma) for 1 h at 4°C with rotation. The beads were then washed three times in Tris-buffered saline (TBS; 50 mM Tris-HCl [pH 8.0], 150 mM NaCl) containing 0.1% Triton X-100 and protease and phosphatase inhibitors and was dried. Fifty microliters of loading buffer was added (29). For the immunoprecipitations in which cdc2 was detected, Tween 20 was used as the detergent.
SDS-PAGE and immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was conducted, followed by gel transfer in 25 mM Tris and 200 mM glycine onto nylon-supported nitrocellulose. Filters were blocked for 1 h in 5% bovine serum albumin in TBS and then were incubated overnight at room temperature in TBS plus Tween 20 (TBS-T) containing primary antibody. Filters were then washed in TBS-T, incubated with horseradish peroxidase-linked secondary antibody (Amersham), washed again, and visualized by chemiluminescence (29).
Immunoblotting was performed with FANCG and FANCA antisera, as previously described (40). Rabbit polyclonal phosphospecific antibodies made against synthetic peptides were obtained from Upstate USA, Incorporated. Phosphospecific crude serum was successively passed over columns (Pierce) containing unphosphorylated and phosphorylated peptides, respectively. The antibody attached to the phosphorylated peptide column was eluted with glycine (0.1 M, pH 3.5).
Fusion protein expression and thrombin cleavage.
Glutathione S-transferase (GST)-FANCG(N) terminal and GST-FANCG(C) proteins were expressed as previously described (41a). Thrombin was added to 1 μg of fusion protein in TBS (Tris 50 mM, 150 mM NaCl, pH 8.0), and the resulting mixture was incubated overnight at room temperature. After SDS-PAGE the gel was stained with Coomassie and a 14-kDa fragment from FANCG(C) was excised.
Mass spectrometry analysis.
Trypsin in-gel digestion and extraction of peptides from the 14-kDa FANCG(C) band was performed as described by Shevchenko (44). Peptides were separated on microcapillary columns by reverse-phase nanoflow high-performance liquid chromatography (HPLC) (Applied Biosystems or Agilent HPLC) with a gradient consisting of 0.1 M acetic acid and 70% acetonitrile with 0.1 M acetic acid on either an Applied Biosystems or Agilent HPLC as previously described (43). Online analysis of the chromatographically separated tryptic peptides was performed by microelectrospray ionization interfaced to a ThermoFinnigan LCQ ion trap mass spectrometer. Data-dependent analysis was performed in which one full mass spectrometry scan from m/z of 300 to 2,000 was performed followed by five mass spectrometry/mass spectrometry scans of the most abundant ions present in the full mass scan. All mass spectrometry/mass spectrometry scans were performed with an isolation window of 3 Da (precursor m/z ± 1.5 Da) and using 35% collision energy.
ProteinFarm in-house software was used as an interface between the raw mass spectrometric information and a database searching program, SEQUEST (13). After deletion of poor quality spectra and conversion to .dta file format, SEQUEST was used to search against a FANCG database. The corresponding spectra of peptides with a cross-correlation score above 2 were manually confirmed for protein validation.
In vitro kinase assays.
In vitro kinase reactions were performed by incubation of a solution containing 1 μg of GST fusion proteins and 10 μCi of [γ-32P]ATP and also containing either 1 μl of extract or 10 U of recombinant cdc2 (New England Biolabs) in 25 μl of buffer (50 mM Tris [pH 7.5], 10 mM MgCl2, 1 mM dithiothreitol, 10 μM cold ATP) (25, 53). The reaction was stopped by addition of equal volumes of loading buffer, and the entire content was run on SDS-PAGE. The gel was transferred to membrane (Biotrace) and was subsequently exposed to film for autoradiography and immunoblotting, respectively.
For peptide-based assays FANCG peptides were synthesized with a series of lysines at the N terminus and encompassing FANCG amino acids 378 to 392, with singly mutated peptides at S383A and S387A and doubly mutated peptide S383A/S387A. The reaction mixture consisted of buffer, 10 μCi of [γ-32P]ATP, 10 μM cold ATP, and 10 U of recombinant cdc2 (New England Biolabs). The assay was incubated for specified times at 30°C. The reaction mixture was then spotted onto P81 paper (Whatman), which was then washed in 0.75% phosphoric acid and acetone. The paper was then analyzed by scintillation counter.
Cytotoxicity assays.
For human fibroblast lines, cells were plated at density to achieve 10 to 100 colonies per plate in a 6-well dish. Mitomycin C (MMC) was added in the indicated doses. Midway through a 7-day incubation, the cells were washed and MMC was re-added. After being washed the cells were stained with crystal violet followed by extraction in SDS-methanol. Absorbance was measured at an optical density of 595 nm (OD595). For the hamster lines, the cells were similarly plated but the colonies were counted with the microscope.
For lymphoblasts, 2 ml of cells was seeded into flasks at a density of 2 × 105 cells/ml. MMC was added at the indicated doses (0 to 300 nM) with duplicate or triplicate flasks being prepared for each dose. Following 24 h of incubation at 37°C, a further 2 ml of growth medium was added, along with sufficient MMC to maintain the indicated (starting) dose. This process was repeated for a further 2 days (i.e., the volume of medium in the flask was doubled each 24 h while maintaining the MMC concentration). After four days, 4 ml of the resulting 16-ml cultures was transferred to new flasks. Twelve milliliters of fresh medium containing MMC was added, and the cells were incubated for a further 3 to 4 days. Resulting cell densities in each individual culture were determined by hemocytometer counts (a minimum of four counts for each culture) and were normalized to the cell numbers obtained in untreated cultures. MMC sensitivity in CHO cell lines was determined by clonal survival assays as described previously (54).
Cell cycle analysis.
A total of 500,000 EUFA143 cells with various constructs of FANCG were treated with 1 μM MMC. After being washed in phosphate-buffered saline, the cells were resuspended in a solution containing 0.1% citrate, 0.03% NP-40, 100 μg of propidium iodide/ml, and 10 μg of RNase/ml and were analyzed by fluorescence-activated cell sorting (FACS) (45).
RESULTS
FANCG exhibits higher mobility forms at mitosis.
The phosphorylation of serine 7 of FANCG has previously been mapped (F. Qiao, submitted). We wondered if this phosphorylation could account for the higher mobility forms at mitosis that were reported previously (5). In order to test this idea, we treated HeLa plus vector, HeLa plus FANCG, and HeLa plus FANCG (S7A) cells with 1 μM nocodazole overnight to arrest the cells in mitosis. One-hundred percent synchrony at mitosis was verified by FACS. Asynchronous HeLa plus Flag-FANCG cells were used as a control. FANCG was directly immunoprecipitated with anti-Flag affinity gels (Fig. 1). Subsequent immunoblotting revealed that higher mobility forms of FANCG were detectable in both HeLa plus wild-type FANCG and HeLa plus FANCG(S7A) cells (lanes 3 and 4). Previous work showed that these high-mobility isoforms of FANCG were phosphatase sensitive, which is indicative of phosphorylation (40). These data suggest that phosphorylation of serine 7 is not responsible for the higher mobility isoforms seen at mitosis, although it is possible that serine 7 might still be phosphorylated at mitosis.
FIG. 1.
FANCG is phosphorylated at mitosis. Whole-cell extracts from HeLa cells plus pMMP-vector, pMMP-FANCG, or pMMP-FANCG(S7A) cells asynchronous or synchronized into mitosis via nocodazole were prepared and run on SDS-PAGE. Immunoprecipitation (IP) was conducted with anti-Flag affinity gel. Immunoblotting with anti-FANCG antiserum showed the appearance of two more bands of higher mobility in mitotic cells (lanes 3 and 4) than for FANCG seen in asynchronous cells (lane 3). Mutation of FANCG at serine 7, a phosphorylation site that has been previously described (41a), does not account for mitotic phosphorylation (lane 4). Lane 1, vector-only control; lane 2, asynchronous plus wild-type FANCG; lane 3, mitotic plus wild-type FANCG; lane 4, mitotic plus S7A.
cdc2 phosphorylates a 14-kDa piece of the carboxyl terminus of FANCG.
It was shown previously by coprecipitation experiments with FANCC and work in which mass spectrometry detected cdc2 in purified samples that cdc2 is part of the FA core complex (27, 49). Because cdc2 acts chiefly at G2-M, we performed in vitro kinase reactions using recombinant cdc2 and FANCG fusion proteins covering the full length of FANCG in order to determine (i) if cdc2 could phosphorylate FANCG and (ii) which portion of the FANCG protein became phosphorylated. We cleaved GST by thrombin digestion and then incubated the mixture with recombinant cdc2 and [γ-32P]ATP. The reaction was then run over SDS-PAGE. The proteins were transferred to membrane. Immunoblotting with anti-FANCG(C) and autoradiography showed that not only was the carboxyl terminus phosphorylated but also that the signal was chiefly restricted to a 14-kDa thrombin cleavage product (Fig. 2). The FANCG(C) mixture was also Coomassie stained to show the full-length FANCG(C) and the thrombin cleavage products.
FIG. 2.
cdc2 phosphorylates a 14-kDa piece of the carboxyl terminus of FANCG. GST fusion proteins containing the amino half (N) and carboxyl half (C), respectively, of FANCG were thrombin cleaved. The resulting mixture was then subjected to in vitro kinase reaction, using beads containing cdc2 immunoprecipitated from mitotic HeLa cell extract. In vitro kinase reaction mixtures were subjected to SDS-PAGE. After transfer, the same filter was subjected both to autoradiography and to immunoblotting using anti-FANCG(C) antiserum. The immunoblot showed full-length FANCG(C) as well as a 14-kDa piece of FANCG(C). GST was also detected, because the original antibody was raised against a GST fusion protein. Autoradiography showed that not only full-length FANCG(C) but also a 14-kDa piece of FANCG(C) became phosphorylated by cdc2. A portion of the thrombin cleavage reaction mixture was run on SDS-PAGE and was Coomassie stained. The 14-kDa piece of FANCG(C) was excised and subjected to mass spectrometric analysis. The resulting spectrum of peptides corresponded to FANCG amino acids 374 to 504. ns, nonspecific.
In order to determine the approximate span that the 14-kDa piece of FANCG represented, we ran a preparative SDS-PAGE gel after thrombin cleavage of GST-FANCG(C), stained the gel with Coomassie, and excised the 14-kDa band. Subsequent mass spectrometric analysis revealed that this fragment of FANCG spanned at least amino acids 374 to 504.
No obvious cdc2 consensus sequence for phosphorylation exists in any of the FANCG protein. In order to determine if any amino acids appeared likely to be phosphorylated, we analyzed FANCG (amino acids 374 to 504) by using a kinase predictor program (www.kinase.com). This analysis revealed that three amino acids were the most likely candidates for phosphorylation: S383, S387, and T487.
S383 and S387 are phosphorylated at mitosis.
Based on the analysis of the most likely amino acids to be phosphorylated in FANCG (374 to 504), we used PCR-directed mutagenesis to mutate S383, S387, and T487 to alanine. Each mutant FANCG was then introduced into HeLa cells, which were then forced into mitosis by using nocodazole. Subsequent immunoblotting revealed that mutating S383A (lane 4) and S387A (lane 5) each eliminated one of the mitotic isoforms of FANCG (Fig. 3A). Indeed, the double S383A/S387A (lane 6) resulted in the removal of both isoforms, suggesting that S383 and S387 may be the phosphoamino acids of FANCG during mitosis. The T487A (lane 7) mutant did not affect the expression of the mitotic isoforms. None of the FANCG mutants affected the ability to bind to FANCA, suggesting that the FA complex remains intact, although decreased affinity for or diminished stability of FANCA may have resulted in the case of FANCG(S387A) (17).
FIG. 3.
S383 and S387 are phosphorylated at mitosis. pMMP-Flag-FANCG was mutated to alanine at S383, S387, or both by PCR-directed mutagenesis. The resulting constructs were then transduced into HeLa cells. Extracts from these cells synchronized to mitosis were prepared and immunoprecipitated (IP) with anti-Flag affinity gel. (A) Wild-type (WT) FANCG displayed two isoforms above the basal FANCG. Extra bands above the basal FANCG protein corresponding to phosphoproteins are marked by arrowheads. FANCG immunoblotting revealed that S383A and S387A mutations each eliminated one of the phospho-FANCG isoforms (lanes 4 and 5), while the double mutant eliminated both (lane 6). The T487A mutant did not alter the phospho-FANCG isoforms (lane 7). In all cases, the mutants coprecipitated FANCA. Lane 1, vector-only control; lane 2, wild-type FANCG, asynchronous; lane 3, wild-type mitotic; lane 4, S383A mitotic; lane 5, S387A mitotic; lane 6, S383A/S387A mitotic; lane 7, T487A mitotic. (B) Anti-phosphospecific antibodies to S383 and S387 were used to immunoblot a filter similar to that described for panel A. Anti-phosphoserine 383 specifically detected phosphorylated FANCG in all cell lines except FANCG(S383A) and FANCG (S383A/S387A). Similarly, anti-phosphoserine 387 antibody specifically detected phosphorylated FANCG in all cell lines except FANCG(S387A) and FANCG(S383A/S387A). Lane 1, preimmune control; lane 2, S383A; lane 3, S387A; lane 4, S383A/S387A; lane 5, T487A; lane 6, wild-type FANCG. (C) Asynchronous and mitotic HeLa whole-cell lysates were immunoprecipitated with FANCA antibody. Immunoblotting with phosphospecific antibodies revealed phosphoserine 383 and 387 on FANCG present only during mitosis. Lanes 1 and 5, mutant FA-G control; lanes 2 and 6, preimmune control; lanes 3 and 7, asynchronous; lanes 4 and 8, nocodazole-arrested mitotic cells. Asy, asynchronous; mit, mitotic.
In order to detect in vivo phosphorylation of FANCG at mitosis, we obtained phosphospecific antibodies produced against phosphoserine 383 and phosphoserine 387 FANCG peptides (Upstate USA, Inc.). We immunoprecipitated Flag-FANCG from mitotic HeLa lysates containing all the mutated versions of FANCG. Immunoblotting with the anti-phosphoserine 383 antibody specifically detected an isoform of FANCG in wild-type FANCG (Fig. 3B, lane 6) and in FANCG(S387A) (lane 3) but not in the mutant S383A (lane 2) or S383A/S387A (lane 4). Conversely, the anti-phosphoserine 387 antibody detected only isoforms containing phosphorylated serine 387. In order to demonstrate phosphorylation of endogenous FANCG, we immunoprecipitated FANCA from either asynchronous or mitotic HeLa whole-cell lysates. Again, immunoblotting with the anti-phosphoserine antibodies specifically detected FANCG isoforms at mitosis (Fig. 3C, lanes 4 and 8).
S383 and S387 are functionally important in FANCG.
To show that S383 and S387 not only were phosphorylated but also were functionally important, we introduced Flag-FANCG(S383A), Flag-FANCG(S387A), and Flag-FANCG(S383A/S387A) constructs into FA-G mutant EUFA143 cells. Cell survival assays measuring sensitivity to MMC revealed that knockout of S383 and/or S387 resulted in the inability to fully correct FA-G mutant cells (Fig. 4A). Equal expression of mutant FANCG species was demonstrated by immunoblotting of FANCG in EUFA143 cell lysates (Fig. 4B). Ku80 immunoblotting showed equal loading. Thus, not only are S383 and S387 phosphorylated, but they are also functionally important to the FA pathway.
FIG. 4.
S383 and S387 are functionally important in FANCG. (A) The mutant FANCG constructs used for experiments depicted in Fig. 3 were transduced into EUFA143 FA-G mutant cells. MMC cytotoxicity assays revealed that only wild-type FANCG (wtFANCG) fully corrected the mutant cells. (B) Whole-cell lysates of EUFA143 cells containing FANCG constructs were immunoblotted with FANCG antibody in order to demonstrate expression of FANCG. Lane 1, wild-type FANCG; lane 2, S383A/S387A; lane 3, S387A; lane 4, S383A; lane 5, vector-only control. Ku80 immunoblotting was performed on the same filter as a loading control. (C) The mutant FANCG constructs were transduced into 326SV FA-G mutant cells. MMC cytotoxicity assays revealed that only wild-type FANCG fully corrected the mutant cells. (D) Whole-cell lysates of 326SV cells containing FANCG constructs were immunoblotted with FANCG antibody in order to demonstrate equal expression of FANCG. Lane 1, vector-only control; lane 2, wild-type FANCG; lane 3, S383A/S387A; lane 4, S387A; lane 5, S383A. β-Tubulin immunoblotting was performed on the same filter as a loading control. (E) A total of 500,000 EUFA143 cells containing indicated FANCG constructs were treated with 1 μM MMC and were subsequently analyzed by FACS. All FA-G mutant constructs demonstrated marked increases in G2 accumulation similar to that of the mutant cell line compared to that of the cell line corrected with wild-type FANCG. The data presented are representative of three independent experiments.
We also tested these constructs by transducing them into another FA-G mutant cell line, 326SV. In these cells, only wild-type FANCG fully corrected the MMC hypersensitivity (Fig. 4C). The effect of the mutations in this cell line is more complete, perhaps because of the different cell type (fibroblast). Equal expression of FANCG was shown by immunoblotting of 326SV whole-cell lysates (Fig. 4D). Immunoblotting of β-tubulin demonstrated equal loading.
Another phenotype associated with FA cells is increased G2 accumulation after MMC treatment (12, 24, 28). To assess the effect of these mitotic phosphorylation mutations on cell cycle, we subjected the EUFA143 cells to FACS analysis after MMC treatment. Corrected cells displayed markedly lower G2 accumulation than all the mutants (Fig. 4E). These data are consistent with the cytotoxicity results that S383 and S387 are functionally important phosphoamino acids.
Mitotic mutants fail to correct CHO cells.
FANCG was first cloned as XRCC9 by using the radiation-sensitive mutant CHO cell line UV40, in which the FANCG homolog XRCC9 is mutant (10, 32). To assess if S383 and S387 have homologous amino acids, we performed alignments against murine and hamster versions of FANCG. This analysis revealed that both S383 and S387 are conserved in both murine and hamster fancg in highly homologous stretches of sequence (Fig. 5A). In contrast, T487 is not conserved.
FIG. 5.
Homologous hamster amino acids of FANCG are also functionally important. (A) FANCG amino acid sequences from human, hamster, and mouse were aligned. S383 and S387 have homologous amino acids in hamster and mouse, while T487 did not. (B) The mutant and wild-type FANCG (wtFANCG) constructs were transduced into mutant FA-G CHO cell lines. Cytotoxicity assays revealed that only wild-type FANCG was able to fully correct the mutant CHO cells. (C) Whole-cell lysates of CHO cells containing FANCG constructs were immunoblotted with FANCG antibody in order to demonstrate equal expression of FANCG. Ku80 immunoblotting was performed on the same filter as a loading control. Lane 1, CHO plus FANCG (wild type); lane 2, S383/S387A; lane 3, S387A; lane 4, S383A; lane 5, vector-only control.
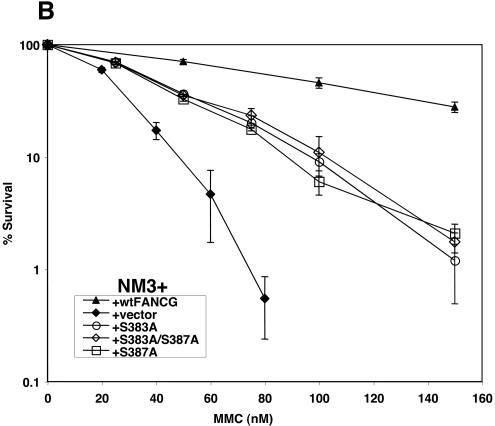
In a submitted work showing that FANCG is phosphorylated at S7, the authors could correct CHO cells mutant for FANCG/XRCC9 with wild-type human FANCG but not FANCG(S7A) (41a). To test the effect of mutations at S383 and S387, we transduced FANCG(S383A), FANCG(S387A), and FANCG(S383A/S387A) into the mutant CHO cell line NM3 and assessed cellular sensitivity to MMC. NM3 has a frameshift mutation in exon 3 of FANCG (30). As expected, human wild-type FANCG corrected the CHO cells (Fig. 5B; D37 = 120 nM versus D37 = 28 nM [D37 is dose of 37% survival]). However, S383A (D37 = 50 nM)-, S387A (D37 = 46)-, and S383/S387A (D37 = 48)-mutated FANCG species were unable to fully correct. Immunoblotting with FANCG and Ku80 antibody demonstrated equal expression of the human FANCG forms in CHO cells (Fig. 5C). These data support the fact that serines 383 and 387 are important for FA function in a way that is conserved evolutionarily between hamsters and humans.
cdc2 phosphorylates FANCG at S387.
Previous work has demonstrated that FANCC and cdc2 bind and that cdc2 can be detected in the FA core complex by mass spectrometry (8, 27). The data depicted in Fig. 2 show that cdc2 can phosphorylate a 14-kDa thrombin cleavage product of FANCG. To determine if any of the mitotic phosphoamino acids could be phosphorylated by cdc2, we mutated GST-FANCG(C) to alanine at S383, S387, or T487 by PCR-mediated mutagenesis. After thrombin cleavage, we performed an in vitro kinase reaction using recombinant cdc2. While cdc2 could phosphorylate GST-FANCG(S383A) (Fig. 6A, lane 3), albeit at lower intensity, it failed to phosphorylate GST-FANCG(S387A) (lane 4) at all, indicating that S387 was a phosphorylation site for cdc2. Use of recombinant polo-like kinase was able to phosphorylate either mutant form of FANCG, suggesting that it was not a likely kinase for FANCG at mitosis (data not shown). Fifteen amino acid peptides (amino acids 378 to 392) spanning the S383 and S387 sites of FANCG were synthesized that were variably mutated at serines 383 and 387. Using recombinant cdc2 in an in vitro kinase reaction, the wild-type peptide was phosphorylated more than 40-fold (Fig. 6B) by 60 min. Mutation at serine 383 resulted in a greater than 50% diminution of phosphorylation. Interestingly, both mutation at 387 and a doubly mutated 383/0.387 resulted in a completely phosphorylation-dead peptide.
FIG. 6.
cdc2 phosphorylates FANCG at S387. (A) FANCG(C) fusion protein was mutated by PCR-directed mutagenesis. The resulting proteins were then thrombin cleaved and subjected to in vitro kinase reaction with recombinant cdc2. Autoradiography revealed that GST-FANCG(S387A) and GST-FANCG(S387A/S387A) were unable to be phosphorylated. Lane 1, GST only; lane 2, wild-type FANCG(C); lane 3, S383A; lane 4, S387A; lane 5, S383A/S387A; lane 6, T487. (B) Wild-type and mutant peptides covering FANCG amino acids 378 to 392 were incubated in an in vitro kinase reaction mixture. A portion of each reaction mixture was spotted onto P81 paper at the indicated times and was subjected to scintillation counting. S387A and S383A/387A peptides completely eliminated cdc2 kinase activity.
cdc2 binds to FA core complex during mitosis.
Based on the above data and published data suggesting cdc2 interaction with FANCC (27), we investigated the cell cycle dependence of this interaction. We synchronized HeLa plus Flag-FANCA cells to the G1-S border by double thymidine blockade and released them into thymidine-free medium. Cells were collected at 0, 3, 6, and 9 h after release. Synchrony was verified by FACS analysis. The DNA histograms corresponding to each point are shown in Fig. 7. In addition, a portion of cells was forced into mitosis by overnight treatment in 1 μM nocodazole. Flag immunoprecipitation followed by immunoblotting revealed the coprecipitation of cdc2 peaking at 9 h after release (Fig. 7, lane 6). This is consistent with a role for cdc2 in regulating the nuclear egress of the FA core complex at mitosis.
FIG. 7.
cdc2 binds to FA core complex maximally at late G2. HeLa cells plus Flag-FANCA were synchronized to the G1-S border and then were released. Cells were collected at 0, 3, 6, and 9 h postrelease along with mitotic arrested cells. Lysates were prepared, and immunoprecipitations (IP) were performed with anti-Flag affinity gel. Immunoblotting revealed that cdc2 maximally was coprecipitated in late G2, 9 h after release. DNA histograms display synchronized cells at the indicated time points. Lane 1, vector-only control (Vec); lane 2, asynchronous cells (asy); lane 3, G1-S-arrested cells; lane 4, 3 h postrelease from G1-S; lane 5, 6 h; lane 6, 9 h; and lane 7, mitotic cells (mit).
FANCG mitotic mutants are aberrantly localized in mitosis.
In order to demonstrate the effect of mitotic phosphorylation mutants on subcellular localization, we transfected cyano fluorescent protein (CFP)-FANCG constructs into HeLa cells. These cells were arrested in mitosis with nocodazole, collected after being shaken from the plate, and cytospun onto slides. Fluorescent microscopy revealed that wild-type FANCG localized to fine perinuclear foci (Fig. 8A). However, FANCG(S383A) localized to large globules still evident in the periphery of the 4,6-diamidino-2-phenylindole-stained distorted nucleus. FANCG(S387A) was dispersed throughout the nucleus, and the doubly mutated FANCG contained features of both. We prepared chromatin extracts from mitosis-arrested HeLa cells with the FANCG-mutated forms and subjected them to SDS-PAGE. Immunoblotting with FANCG antibody revealed that mutated FANCG proteins were retained on chromatin along with FANCA (Fig. 8B). Ku80 immunoblot demonstrated equal loading. These data are consistent with the fact that wild-type FANCG protein is functional and serines 383 and 387 are functionally critical for normal FA pathway activity, including proper detachment from mitotic chromatin.
FIG. 8.
FANCG mitotic mutants are aberrantly localized in mitosis. (A) An ECFP-FANCG construct was mutated by PCR-directed mutagenesis and was transfected into HeLa cells. Cells were then arrested in mitosis by using nocodazole. Cells were shaken from plates, collected, and cytospun onto slides. Fluorescent microscopy revealed that wild-type (wt) FANCG protein was perinuclear and in fine foci. The other mitotic mutants were in larger aggregates (S383A and double mutant) and/or dispersed in the nucleus (S387A and double mutant). (B) Chromatin extracts were prepared from mitotic HeLa cells containing the FANCG constructs shown in panel A. Lane 1, S383A; lane 2, S387A; lane 3, S383A/S387A; lane 4, wild-type FANCG; lane 5, vector only. Immunoblotting with FANCG antibody revealed that mutant FANCG was retained in chromatin. A Ku80 immunoblot on the same filter demonstrated equal loading. DAPI, 4,6-diamidino-2-phenylindole.
DISCUSSION
We have determined that the FA core complex appears to operate at the level of chromatin and that the complex undertakes a regulated exit from chromatin at mitosis when the chromosomes condense (40). The basis of this regulation is phosphorylation of the FANCG protein. In this study we have identified the two amino acids that are phosphorylated, and we have determined that cdc2 appears to phosphorylate at least one of these amino acids. More importantly, these residues are functionally significant to the FA pathway, as mutation results in impairment of the ability of FANCG to correct FA-G mutant cells in both human and hamster cells.
Important examples exist in the literature, as the human SWI/SNF complex is phosphorylated at mitosis concomitant with its egress from the nucleus (35, 41). In addition, the BLM protein is also phosphorylated at mitosis and retains the ability to become dephosphorylated in response to DNA damage during G2-M (11). We will be exploring this same possibility for the FA core complex as well, given the excessive G2-M phenotype noted in FA after DNA damage. Phosphorylated FANCG and regulated exit of the FA core complex may be necessary for proper transit into mitosis. These complexes appear to be preserved and not simply degraded; they stand ready for reuse as cells reenter S phase (J. Mi, submitted for publication). Experiments which track individual complexes in pulse-chase fashion will be necessary to show just how long-lived FA complexes are.
The FA pathway appears to be tied to the cell cycle in some way. First, mutant FA cells accumulate in the G2 phase of the cell excessively after MMC treatment (12, 28). Second, we have shown that cdc2 and FANCC interact and that cdc2 can be detected in the FA core complex by mass spectrometry (27, 49). Third, recent data imply that the FA complex may function at an S-phase checkpoint (1, 2, 19, 38, 39, 42, 47). Thus, while the checkpoint machinery, which causes arrest in FA mutant cells, might be intact, the ability to coordinate a downstream repair function could be impaired.
The notion that S383 and S387 are important amino acids in FANCG is further borne out by the fact that these are conserved amino acids in the mouse and hamster. FANCG was first cloned from a CHO line hypersensitive to ionizing radiation and MMC (10, 32). Thus, the mouse and hamster would be attractive organisms for the performance of transgenic experiments for the expression of these FANCG mutants. Phosphorylation of FANCG is not the first such posttranslational modification to be described. FANCA phosphorylation was shown to be functionally important as well, as it is lacking in all but the FA-D complementation group cell lines (1, 26, 55). No obvious stimulation has been described for this event. Researchers have reported on FANCG phosphorylation (16, 40). We also have mapped a site at FANCG serine 7, but the stimulus for this is unknown. By contrast, FANCG phosphorylation is associated with mitosis. It remains to be seen whether the FA core complex plays a mechanistic role in G2-M or simply that phosphorylation of FANCG signals the end of DNA replication and, thus, the end of FA core complex function in that cell cycle. Our new phosphospecific antibodies should be useful in this regard as well as in immunofluorescence studies of mitotic cells.
Acknowledgments
G.M.K. is supported by K02 and R01 funding from the National Heart, Lung, and Blood Institute. N.J.J. is supported by the North West Cancer Research Fund (NWCRF-CR624). D.F.H. is supported by NIH GM37537.
We thank Sahana Ananth for technical assistance.
REFERENCES
- 1.Adachi, D., T. Oda, H. Yagasaki, K. Nakasato, T. Taniguchi, A. D. D'Andrea, S. Asano, and T. Yamashita. 2002. Heterogeneous activation of the Fanconi anemia pathway by patient-derived FANCA mutants. Hum. Mol. Genet. 11:3125-3134. [DOI] [PubMed] [Google Scholar]
- 2.Akkari, Y. M., R. L. Bateman, C. A. Reifsteck, S. B. Olson, and M. Grompe. 2000. DNA replication is required to elicit cellular responses to psoralen-induced DNA interstrand cross-links. Mol. Cell. Biol. 20:8283-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alter, B. P., and N. S. Young. 1993. The bone marrow failure syndromes, p. 216-316. In D. G. Nathan and F. A. Oski (ed.), Hematology of infancy and childhood, vol. 1. W.B. Saunders, Philadelphia, Pa. [Google Scholar]
- 4.Apostolou, S., S. A. Whitmore, J. Crawford, et al. 1996. Positional cloning of the Fanconi anaemia group A gene. Nat. Genet. 14:324-328. [DOI] [PubMed] [Google Scholar]
- 5.Auerbach, A., M. Buchwald, and H. Joenje. 1997. Fanconi anemia, p. 317-332. In B. Vogelstein and K. Kinzler (ed.), Genetics of cancer. McGraw-Hill, New York, N.Y.
- 6.Christianson, T. A., and G. C. Bagby. 2000. FANCA protein binds FANCG proteins in an intracellular complex. Blood 95:725-726. [PubMed] [Google Scholar]
- 7.de Winter, J. P., F. Leveille, C. G. van Berkel, M. A. Rooimans, L. van Der Weel, J. Steltenpool, I. Demuth, N. V. Morgan, N. Alon, L. Bosnoyan-Collins, J. Lightfoot, P. A. Leegwater, Q. Waisfisz, K. Komatsu, F. Arwert, J. C. Pronk, C. G. Mathew, M. Digweed, M. Buchwald, and H. Joenje. 2000. Isolation of a cDNA representing the Fanconi anemia complementation group E gene. Am. J. Hum. Genet. 67:1306-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Winter, J. P., M. A. Rooimans, L. van Der Weel, C. G. van Berkel, N. Alon, L. Bosnoyan-Collins, J. de Groot, Y. Zhi, Q. Waisfisz, J. C. Pronk, F. Arwert, C. G. Mathew, R. J. Scheper, M. E. Hoatlin, M. Buchwald, and H. Joenje. 2000. The Fanconi anaemia gene FANCF encodes a novel protein with homology to ROM. Nat. Genet. 24:15-16. [DOI] [PubMed] [Google Scholar]
- 9.de Winter, J. P., L. van Der Weel, J. de Groot, S. Stone, Q. Waisfisz, F. Arwert, R. J. Scheper, F. A. Kruyt, M. E. Hoatlin, and H. Joenje. 2000. The Fanconi anemia protein FANCF forms a nuclear complex with FANCA, FANCC and FANCG. Hum. Mol. Genet. 9:2665-2674. [DOI] [PubMed] [Google Scholar]
- 10.de Winter, J. P., Q. Waisfisz, M. A. Rooimans, C. G. van Berkel, L. Bosnoyan-Collins, N. Alon, M. Carreau, O. Bender, I. Demuth, D. Schindler, J. C. Pronk, F. Arwert, H. Hoehn, M. Digweed, M. Buchwald, and H. Joenje. 1998. The Fanconi anaemia group G gene FANCG is identical with XRCC9. Nat. Genet. 20:281-283. [DOI] [PubMed] [Google Scholar]
- 11.Dutertre, S., R. Sekhri, L. A. Tintignac, R. Onclercq-Delic, B. Chatton, C. Jaulin, and M. Amor-Gueret. 2002. Dephosphorylation and subcellular compartment change of the mitotic Bloom's syndrome DNA helicase in response to ionizing radiation. J. Biol. Chem. 277:6280-6286. [DOI] [PubMed] [Google Scholar]
- 12.Dutrillaux, B., A. Aurias, A. M. Dutrillaux, D. Buriot, and M. Prieur. 1982. The cell cycle of lymphocytes in Fanconi anemia. Hum. Genet. 62:327-332. [DOI] [PubMed] [Google Scholar]
- 13.Eng, J. K., A. L. McCormack, and J. R. Yates. 1994. An approach to correlate tandem mass-spectral data of peptides with amino-acid-sequences in a protein database. J. Am. Soc. Mass Spectr. 5:976-989. [DOI] [PubMed] [Google Scholar]
- 14.Fanconi, G. 1967. Familial constitutional panmyelocytopathy, Fanconi's anemia (FA). I. Clinical aspects. Semin. Hematol. 4:233-240. [PubMed] [Google Scholar]
- 15.Fanconi, G. 1927. Familiar infantile perniziosaartige Anamie (pernizioses Blutbild und Konstitution). Z. Kinderheil. 117:257-280. [Google Scholar]
- 16.Futaki, M., S. Watanabe, S. Kajigaya, and J. M. Liu. 2001. Fanconi anemia protein, FANCG, is a phosphoprotein and is upregulated with FANCA after TNF-alpha treatment. Biochem. Biophys. Res. Commun. 281:347-351. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Higuera, I., Y. Kuang, J. Denham, and A. D. D'Andrea. 2000. The Fanconi anemia proteins FANCA and FANCG stabilize each other and promote the nuclear accumulation of the Fanconi anemia complex. Blood 96:3224-3230. [PubMed] [Google Scholar]
- 18.Garcia-Higuera, I., Y. Kuang, D. Naf, J. Wasik, and A. D. D'Andrea. 1999. Fanconi anemia proteins FANCA, FANCC, and FANCG/XRCC9 interact in a functional nuclear complex. Mol. Cell. Biol. 19:4866-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Higuera, I., T. Taniguchi, S. Ganesan, M. S. Meyn, C. Timmers, J. Hejna, M. Grompe, and A. D. D'Andrea. 2001. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol. Cell 7:249-262. [DOI] [PubMed] [Google Scholar]
- 20.Hejna, J. A., C. D. Timmers, C. Reifsteck, D. A. Bruun, L. W. Lucas, P. M. Jakobs, S. Toth-Fejel, N. Unsworth, S. L. Clemens, D. K. Garcia, S. L. Naylor, M. J. Thayer, S. B. Olson, M. Grompe, and R. E. Moses. 2000. Localization of the Fanconi anemia complementation group D gene to a 200-kb region on chromosome 3p25.3. Am. J. Hum. Genet. 66:1540-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howlett, N. G., T. Taniguchi, S. Olson, B. Cox, Q. Waisfisz, C. De Die-Smulders, N. Persky, M. Grompe, H. Joenje, G. Pals, H. Ikeda, E. A. Fox, and A. D. D'Andrea. 2002. Biallelic inactivation of BRCA2 in Fanconi anemia. Science 297:606-609. [DOI] [PubMed] [Google Scholar]
- 22.Joenje, H., M. Levitus, Q. Waisfisz, A. D'Andrea, I. Garcia-Higuera, T. Pearson, C. G. van Berkel, M. A. Rooimans, N. Morgan, C. G. Mathew, and F. Arwert. 2000. Complementation analysis in Fanconi anemia: assignment of the reference FA-H patient to group A. Am. J. Hum. Genet. 67:759-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joenje, H., A. Oostra, M. Wijker, F. d. Summa, C. V. Berkel, M. Rooimans, W. Ebell, M. V. Wel, J. Pronk, M. Buchwald, and F. Arwert. 1997. Evidence for at least eight Fanconi anemia genes. Am. J. Hum. Genet. 61:940-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiser, T. N., A. Lojewski, C. Dougherty, L. Juergens, E. Sahar, and S. A. Latt. 1982. Flow cytometric characterization of the response of Fanconi's anemia cells to mitomycin C treatment. Cytometry 2:291-297. [DOI] [PubMed] [Google Scholar]
- 25.Kumagai, A., and W. G. Dunphy. 1996. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science 273:1377-1380. [DOI] [PubMed] [Google Scholar]
- 26.Kupfer, G., D. Naf, I. Garcia-Higuera, J. Wasik, A. Cheng, T. Yamashita, A. Tipping, N. Morgan, C. G. Mathew, and A. D. D'Andrea. 1999. A patient-derived mutant form of the Fanconi anemia protein, FANCA, is defective in nuclear accumulation. Exp. Hematol. 27:587-593. [DOI] [PubMed] [Google Scholar]
- 27.Kupfer, G., T. Yamashita, S. Asano, A. Suliman, and A. D. D'Andrea. 1997. The Fanconi anemia protein, FAC, binds to the cyclin-dependent kinase, cdc2. Blood 90:1047-1054. [PubMed] [Google Scholar]
- 28.Kupfer, G. M., and A. D. D'Andrea. 1996. The effect of the Fanconi anemia polypeptide, FAC, upon p53 induction and G2 checkpoint regulation. Blood 88:1019-1025. [PubMed] [Google Scholar]
- 29.Kupfer, G. M., D. Naf, A. Suliman, M. Pulsipher, and A. D. D'Andrea. 1997. The Fanconi anaemia proteins, FAA and FAC, interact to form a nuclear complex. Nat. Genet. 17:487-490. [DOI] [PubMed] [Google Scholar]
- 30.Lamerdin, J. E., N. A. Yamada, J. W. George, B. Souza, A. T. Christian, N. J. Jones, and L. H. Thompson. 2004. Characterization of the hamster FancG/Xrcc9 gene and mutations in CHO UV40 and NM3. Mutagenesis 19:237-244. [DOI] [PubMed] [Google Scholar]
- 31.Levitus, M., M. A. Rooimans, J. Steltenpool, N. F. Cool, A. B. Oostra, C. G. Mathew, M. E. Hoatlin, Q. Waisfisz, F. Arwert, J. P. De Winter, and H. Joenje. 2003. Heterogeneity in Fanconi anemia: evidence for two new genetic subtypes. Blood 103:2498-2503. [DOI] [PubMed] [Google Scholar]
- 32.Liu, N., J. E. Lamerdin, J. D. Tucker, Z. Q. Zhou, C. A. Walter, J. S. Albala, D. B. Busch, and L. H. Thompson. 1997. The human XRCC9 gene corrects chromosomal instability and mutagen sensitivities in CHO UV40 cells. Proc. Natl. Acad. Sci. USA 94:9232-9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo Ten Foe, J. R., M. A. Rooimans, L. Bosnoyan-Collins, et al. 1996. Expression cloning of a cDNA for the major Fanconi anemia gene, FAA. Nat. Genet. 14:320-323. [DOI] [PubMed] [Google Scholar]
- 34.Meetei, A. R., J. P. de Winter, A. L. Medhurst, M. Wallisch, Q. Waisfisz, H. J. van de Vrugt, A. B. Oostra, Z. Yan, C. Ling, C. E. Bishop, M. E. Hoatlin, H. Joenje, and W. Wang. 2003. A novel ubiquitin ligase is deficient in Fanconi anemia. Nat. Genet. 35:165-170. [DOI] [PubMed] [Google Scholar]
- 35.Muchardt, C., J. C. Reyes, B. Bourachot, E. Leguoy, and M. Yaniv. 1996. The hbrm and BRG-1 proteins, components of the human SNF/SWI complex, are phosphorylated and excluded from the condensed chromosomes during mitosis. EMBO J. 15:3394-3402. [PMC free article] [PubMed] [Google Scholar]
- 36.Naf, D., G. M. Kupfer, A. Suliman, K. Lambert, and A. D. D'Andrea. 1998. Functional activity of the Fanconi anemia protein FAA requires FAC binding and nuclear localization. Mol. Cell. Biol. 18:5952-5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pang, Q., S. Fagerlie, T. A. Christianson, W. Keeble, G. Faulkner, J. Diaz, R. K. Rathbun, and G. C. Bagby. 2000. The Fanconi anemia protein FANCC binds to and facilitates the activation of STAT1 by gamma interferon and hematopoietic growth factors. Mol. Cell. Biol. 20:4724-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pichierri, P., D. Averbeck, and F. Rosselli. 2002. DNA cross-link-dependent RAD50/MRE11/NBS1 subnuclear assembly requires the Fanconi anemia C protein. Hum. Mol. Genet. 11:2531-2546. [DOI] [PubMed] [Google Scholar]
- 39.Pichierri, P., and F. Rosselli. 2004. The DNA crosslink-induced S-phase checkpoint depends on ATR-CHK1 and ATR-NBS1-FANCD2 pathways. EMBO J. 23:1178-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiao, F., A. Moss, and G. M. Kupfer. 2001. Fanconi anemia proteins localize to chromatin and the nuclear matrix in a DNA damage- and cell cycle-regulated manner. J. Biol. Chem. 276:23391-23396. [DOI] [PubMed] [Google Scholar]
- 40a.Qiao, F., J. M., J. B. Wilson, G. Zhi, N. R. Bucheimer, N. J. Jones, and G. M. Kupfer. Phosphorylation of Fanconi anemia complementation group G protein, FANCG, at serine 7 is important for function of the FA pathway. J. Biol. Chem., in press. [DOI] [PubMed]
- 41.Reyes, J. C., C. Muchardt, and M. Yaniv. 1997. Components of the human SWI/SNF complex are enriched in active chromatin and are associated with the nuclear matrix. J. Cell. Biol. 137:263-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothfuss, A., and M. Grompe. 2004. Repair kinetics of genomic interstrand DNA cross-links: evidence for DNA double-strand break-dependent activation of the Fanconi anemia/BRCA pathway. Mol. Cell. Biol. 24:123-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroeder, M. J., J. Shabanowitz, J. C. Schwartz, D. F. Hunt, and J. J. Coon. A Neutral loss activation method for improved phosphopeptide sequence analysis by quadruple ion trap mass spectrometry. Analyt. Chem., in press. [DOI] [PubMed]
- 44.Shevchenko, A. 2001. Evaluation of the efficiency of in-gel digestion of proteins by peptide isotopic labeling and MALDI mass spectrometry. Anal. Biochem. 296:279-283. [DOI] [PubMed] [Google Scholar]
- 45.Stonesifer, K., J. Xiang, E. Wilkinson, N. Benson, and R. Braylan. 1987. Flow cytometric analysis and cytopathology of body cavity fluids. Acta Cytologica 31:125-130. [PubMed] [Google Scholar]
- 46.Strathdee, C. A., H. Gavish, W. R. Shannon, and M. Buchwald. 1992. Cloning of cDNAs for Fanconi's anaemia by functional complementation. Nature 356:763-767. [DOI] [PubMed] [Google Scholar]
- 47.Taniguchi, T., I. Garcia-Higuera, P. R. Andreassen, R. C. Gregory, M. Grompe, and A. D. D'Andrea. 2002. S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood 100:2414-2420. [DOI] [PubMed] [Google Scholar]
- 48.Taniguchi, T., I. Garcia-Higuera, B. Xu, P. R. Andreassen, R. C. Gregory, S. T. Kim, W. S. Lane, M. B. Kastan, and A. D. D'Andrea. 2002. Convergence of the Fanconi anemia and ataxia telangiectasia signaling pathways. Cell 109:459-472. [DOI] [PubMed] [Google Scholar]
- 49.Thomashevski, A., A. A. High, M. Drozd, J. Shabanowitz, D. F. Hunt, P. A. Grant, and G. M. Kupfer. 2004. The Fanconi anemia core complex forms 4 different sized complexes in different subcellular compartments. J. Biol. Chem. 279:26201-26209. [DOI] [PubMed] [Google Scholar]
- 50.Timmers, C., T. Taniguchi, J. Hejna, C. Reifsteck, L. Lucas, D. Bruun, M. Thayer, B. Cox, S. Olson, A. D. D'Andrea, R. Moses, and M. Grompe. 2001. Positional cloning of a novel Fanconi anemia gene, FANCD2. Mol. Cell. 7:241-248. [DOI] [PubMed] [Google Scholar]
- 51.Waisfisz, Q., J. P. de Winter, F. A. Kruyt, J. de Groot, L. van der Weel, L. M. Dijkmans, Y. Zhi, F. Arwert, R. J. Scheper, H. Youssoufian, M. E. Hoatlin, and H. Joenje. 1999. A physical complex of the Fanconi anemia proteins FANCG/XRCC9 and FANCA. Proc. Natl. Acad. Sci. USA 96:10320-10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitney, M., et al. 1995. Microcell mediated chromosome transfer maps the Fanconi anemia group D gene to chromosome 3p. Nat. Genet. 11:341-343. [DOI] [PubMed] [Google Scholar]
- 53.Wilhelm, H., S. Anderson, and E. Karsenti. 1997. Purification of recombinant cyclin B1/cdc2 kinase from Xenopus egg extracts. Methods Enzymol. 283:12-28. [DOI] [PubMed] [Google Scholar]
- 54.Wilson, J. B., M. A. Johnson, A. P. Stuckert, K. L. Trueman, S. May, P. E. Bryant, R. E. Meyn, A. D. D'Andrea, and N. J. Jones. 2001. The Chinese hamster FANCG/XRCC9 mutant NM3 fails to express the monoubiquitinated form of the FANCD2 protein, is hypersensitive to a range of DNA damaging agents and exhibits a normal level of spontaneous sister chromatid exchange. Carcinogenesis 22:1939-1946. [DOI] [PubMed] [Google Scholar]
- 55.Yamashita, T., G. Kupfer, D. Naf, A. Suliman, H. Joenje, S. Asano, and A. D'Andrea. 1998. The Fanconi anemia pathway requires FAA phosphorylation and FAA/FAC nuclear accumulation. Proc. Natl. Acad. Sci. USA 95:13085-13090. [DOI] [PMC free article] [PubMed] [Google Scholar]