Abstract
Renal ischemia-reperfusion (RIR) injury is a common occurrence after major surgery and shock, leading to acute kidney injury (AKI). Osteopontin (OPN) is a secreted glycoprotein that acts as a proinflammatory cytokine and activator of T lymphocytes. We hypothesized that blockade of OPN reduces the severity of inflammation and injury in RIR. Renal ischemia was induced in adult C57BL/6 mice via bilateral clamping of renal pedicles for 35 min, followed by reperfusion for 24 h. Anti-OPN antibody (Ab), non-immunized isotype IgG, or normal saline was injected intravenously at time of reperfusion. Blood and kidneys were collected for analysis. At 24 h after RIR, OPN mRNA and protein levels were significantly increased in renal tissue compared to sham mice. In serum, elevated levels of BUN and creatinine were reduced in anti-OPN Ab-treated mice compared to vehicle. Anti-OPN Ab-treated mice also had decreased mRNA levels of injury markers neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) compared to the vehicle. The histologic architecture and apoptosis of renal tissue were improved in the anti-OPN Ab-treated mice. In renal tissue, inflammatory cytokines IL-6 and TNF-α protein levels were reduced in the Ab-treated mice. NK cell infiltration was decreased after anti-OPN Ab treatment, as was neutrophil infiltration, shown by reduced chemokine expression and Gr1 renal immunohistochemical staining. These findings demonstrate a beneficial role of OPN blockade in RIR associated with NK cell-mediated downregulation of inflammatory cytokines and chemokines. Administration of anti-OPN Ab may therefore serve as an immunomodulatory adjunct in the treatment of RIR-induced AKI.
Keywords: Acute kidney injury, inflammation, apoptosis, neutrophils, NK cells
INTRODUCTION
Renal ischemia-reperfusion (RIR) often occurs after major surgery such as kidney transplantation, or after hypovolemic or septic shock. RIR may lead to acute kidney injury (AKI), and is associated with high morbidity and mortality. Critically ill patients with AKI have an estimated mortality rate reported between 30–70%, higher when associated with stage 2 or 3 AKI (1–5). These patients are also twice as likely to be discharged to a short- or long-term care facility instead of home, which significantly adds to healthcare costs (1, 3). After the initial insult, many patients progress to chronic kidney disease and eventually, end-stage renal disease. Since there is no definitive treatment for AKI, management options solely focus on prevention and supportive care. Nevertheless, ongoing research continues to advance our understanding of the pathophysiology of ischemic AKI, bringing about novel approaches to treatment.
In ischemia, deprivation of oxygen and nutrient delivery to cells leads to accumulation of waste products of metabolism in the tissue, leading to cell death (1, 6). In the kidneys, anoxic cell injury develops in the renal tubular epithelial cells (TECs) (1). Innate immune cells such as natural killer (NK) cells, neutrophils, and macrophages become activated and migrate to the tissue, which causes further cellular injury of the TECs through the release of cytokines and reactive oxygen species (7, 8). Of the innate immune cells, NK cells are cytotoxic lymphocytes which play a critical role in RIR injury (9–11). These cells contain perforin and granzymes, and upon release in proximity to target cells, perforin forms pores in the cell membrane through which granzymes can enter, causing apoptosis or osmotic cell lysis (12). NK cells are also known to provide immunity against pathogens and tumors via their ability to secrete cytokines (7). These cells constitutively express receptors for cytokines and chemokines allowing for a rapid response of pro-inflammatory molecules that enhance the accumulation of polymorphonuclear granulocytes to cause tissue damage (7).
Several molecules/factors have been identified as regulators of NK cell activation and infiltration during RIR. Osteopontin (OPN) is a secreted glycoprotein that was originally identified as a bone matrix molecule, but later it was found to be ubiquitously expressed in a broad range of tissues, with multiple functions including cell migration, adhesion, and lymphocyte activation (13). After renal damage, OPN expression is significantly up-regulated in all tubular segments and glomeruli (13). The use of OPN knockout (KO) mice demonstrated the role of OPN in promoting NK cell-induced renal damage during RIR, and that under in vitro conditions, OPN can lead to NK cell migration and activation causing TEC death (10). On the other hand, OPN KO mice have also been demonstrated to have increased injury after RIR, suggesting a renoprotective role of OPN (14). This discrepancy may be due to differences in the genetic backgrounds of these KO mice strains. Additionally, the use of KO mice does not reflect normal physiology. To address the inconsistency between these two studies in OPN’s role during RIR, and ascertain whether OPN is a suitable target for therapeutic intervention, we evaluated the effect of blocking OPN activity on RIR injury in the wild-type (WT) animal with normal OPN expression.
In this study, using a mouse model of RIR, we showed that both mRNA and protein levels of OPN were significantly upregulated in renal tissue after ischemic injury. Renal injury, inflammation, and apoptosis were attenuated when anti-OPN antibody (Ab) was injected in RIR mice compared to vehicle-treated animals. This improved prognosis was related to reduced infiltration of NK cells in the kidney during RIR. These data support our hypothesis that neutralization of OPN can reduce the severity of renal injury by reducing NK cell infiltration in RIR.
MATERIALS AND METHODS
Animal model of RIR
Adult male C57BL/6 mice (ages 8–10 weeks, 20–25 g; Charles River Laboratories; Wilmington, MA) were induced with 2.5% inhalational isoflurane, and then prepped with 10% povidone-iodine wash on their abdomen. A midline incision was made, and bowel was displaced to reveal bilateral renal hila. Microvascular clips were applied to each renal pedicle for 35 min; after removal, the abdomen was closed with a running 6-0 nylon suture, and a 500-μl bolus of normal saline was given subcutaneously. Reperfusion was allowed for 24 h; animals were then harvested for blood and renal tissue. Sham animals underwent laparotomy without renal ischemia. All experiments were performed in accordance with the guidelines for the use of experimental animals by the National Institutes of Health (Bethesda, MD) and were approved by the Institutional Animal Care and Use Committee (IACUC) of the Feinstein Institute for Medical Research.
Administration of neutralizing OPN antibody
At time of reperfusion, mice were injected with either one of the following: (1) mouse affinity purified polyclonal Ab, anti-OPN Ab (R&D Systems; Minneapolis, MN; Catalog No.: AF808), (2) normal (non-immunized) goat immunoglobulin G (IgG) (R&D Systems; Catalog No.: AB-108-C), or (3) PBS (vehicle). Antibodies were given at a dose of 1.5 mg/kg in 100-μl volume of PBS. Injections were made into the tail vein at the base of the tail using a 29G × 1/2″ U-100 insulin syringe (Terumo Medical Corporation; Elkton, MD). The skin was prepped with 70% isopropyl alcohol prior to injection, and pressure was held over the site afterward to prevent bleeding and promote hemostasis.
Analysis of serum renal function markers
Blood samples were centrifuged at 2,000g for 15 min to collect serum and then either analyzed for injury parameters immediately, or stored at −80°C. Blood urea nitrogen (BUN) and creatinine were measured by using commercial assay kits according to the manufacturer’s instructions (Pointe Scientific; Lincoln Park, MI).
Quantitative real-time PCR analysis
Total RNA was extracted from renal tissue and homogenized using a sonic dismembrator in TRIzol (Invitrogen; Carlsbad, CA). RNA was then reverse-transcribed into cDNA using murine leukemia virus reverse transcriptase (Applied Biosystems; Foster City, CA). The PCR reaction was performed in 20 μl of final volume containing 1.25 pmol of forward and reverse primer, ~350 ng of cDNA, and 10 μl SYBR Green PCR Master Mix (Applied Biosystems). The thermal profile used by the Applied Biosystems 7300 real-time PCR machine was: 50°C for 2 min, 95°C for 10 min, 45 cycles of 95°C for 15 seconds, and 60°C for 1 min. Mouse β-actin was used for normalization. Relative expression of mRNA was represented as fold change in comparison to the sham group. The primer sequences were designed by using the Primer 3 software (15), which include: OPN (NM_001204201) Forward: TCTGATGAGACCGTCACTGC, Reverse: AGGTCCTCATCTGTGGCATCOPN; NGAL (NM_005564.4): Forward: CTCAGAACTTGATCCCTGCC, Reverse: TCCTTGAGGCCCAGAGACTT; KIM-1 (NM_134248.2): TGCTGCTACTGCTCCTTGTG, Reverse: GGGCCACTGGTACTCATTCT; KC (NM_008176): Forward: GCTGGGATTCACCTCAAGAA, Reverse: ACAGGTGCCATCAGAGCAGT; β-actin (NM_007393): Forward: CGTGAAAAGATGACCCAGATCA, Reverse: TGGTACGACCAGAGGCATACAG.
Measurement of proinflammatory cytokines and OPN
Proinflammatory cytokines, interleukin 6 (IL-6) and tumor necrosis factor (TNF)-α in renal tissue were quantified by using the mouse enzyme-linked immunosorbent assay (ELISA) kits (BD Biosciences, Franklin Lakes, NJ). OPN in renal tissue was measured by using the mouse ELISA kit (R&D Systems).
Histological evaluation of renal injury and immunohistochemistry
Renal tissue was fixed in 10% formalin and embedded in paraffin. Tissue was sectioned into 5 μm slices and stained with hematoxylin and eosin (H&E). Using light microscopy, the level of injury was assessed in a blinded fashion in the following categories: tubular cell injury, tubular cell detachment, loss of brush border, tubular simplification, and cast formation. Histologic changes were assessed in a blinded fashion according to a modified scoring system based on the extent of tissue injury (16). Briefly, a score of 0 represents 0% injury, 1 (<10%), 2 (10–25%), 3 (25–50%), 4 (50–75%), and 5 (>75%), for a maximal score of 25. Scores were averaged for each sample over 10 randomly selected fields.
For Gr-1 immunohistochemistry, paraffin-embedded renal tissues were dewaxed in xylene and rehydrated in serial ethanol washes. The slides were heated in 0.92% citric acid buffer (Vector Laboratories; Burlingame, CA) at 95°C for 30 min. After cooling, the slides were incubated with 2% H2O2/60% methanol and blocked in normal rabbit serum/Tris-buffered saline. The anti-Gr-1 Ab (Abcam; Cambridge, MA) was applied and incubated overnight at 4°C. Vectastain ABC reagent and a DAB kit (Vector Laboratories) were used to detect the immunohistochemical reaction. Slides were counterstained with hematoxylin and examined under a phase contrast Eclipse Ti-S light microscope (Nikon; Melville, NY). NIS-Elements BR imaging software (Nikon) was used to quantify brown stained Gr-1+ cells.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
The presence of apoptotic cells was measured by in situ labeling of DNA fragmentation using a TUNEL assay. An in situ cell death detection kit (Roche Diagnostics; Indianapolis, IN) was used. Slides were counterstained with 4′, 6-diamidino-2′-phenylindole dihydrochloride (DAPI). Analysis was performed using an Eclipse Ti-S light microscope (Nikon), and the NIS-Elements BR imaging software (Nikon) was used to quantify fluorescent cells.
Measurement of caspase-3 activity
Renal tissue was homogenized in lysis buffer (10 mM HEPES, pH 7.4; 5 mM MgCl2; 1 mM DTT; 1% Triton X-100; 2 mM EGTA; 2 mM EDTA) containing protease inhibitor (Roche Diagnostics; Indianapolis, IN). Protein concentrations were determined by Bio-Rad protein assay reagent (Bio-Rad Laboratories; Hercules, CA). Caspase-3 substrate peptide (DEVD-AMC) was added to an assay buffer (20 mM HEPES, pH 7.4; 5 mM DTT, 2 mM EDTA, 0.1% CHAPS). After a 60–90 min incubation at 37°C in the dark, a fluorometric reader was used to measure excitation (365–380 nm) and emission (430–460 nm). The results were divided by the protein concentration and expressed as fold change.
Assessment of NK cells with flow cytometry
NK cells were purified from mouse kidneys at 24 h after RIR or sham laparotomy. Cells were isolated via pancreatin (Sigma-Aldrich; St. Louis, MO) and collagenase digestion (Worthington; Lakewood, NJ) as described previously (17). Briefly, kidney digests were incubated at 37°C for 10 min, and then passed through a 70-μm strainer. Cells were stained with fluorescent antibodies APC-CD3, PE-CD49b, and PE-isotype control (Biolegend, San Diego, CA) in flow cytometry buffer (PBS, 2% fetal bovine serum, 0.1% sodium azide). For each flow cytometric experiment for the detection of cell surface antigens by using multicolor fluorescent antibodies, we used unstained and single color compensation controls to get rid of any possible spillover of individual fluorescence. Data was acquired using a BD LSRFortessa flow cytometer (BD Biosciences; San Jose, CA), and analyzed using FlowJo 7.6.5 software (FlowJo LLC; Ashland, OR).
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM) and compared via one way analysis of variance (ANOVA) and Student-Newman-Keuls (SNK) test for multiple group comparisons. Significance was considered if p < 0.05 between the experimental groups.
RESULTS
RIR increases OPN expression in the kidney
We first determined whether OPN expression was increased in the kidney after RIR. The expression of OPN at its mRNA level was increased 13-fold in the RIR kidneys compared to the sham-operated animals (Fig. 1A). Similarly, the protein levels of OPN were also found to be increased 3-fold in the RIR kidneys compared to the sham mice (Fig. 1B).
Figure 1. OPN expression increases in the kidney after RIR.
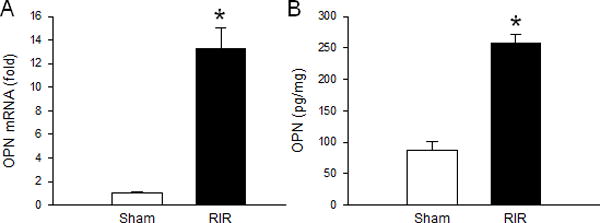
Renal tissue from sham, vehicle, IgG control, and anti-OPN Ab treated mice were collected at 24 h after RIR. OPN (A) mRNA and (B) protein levels were determined by qRT-PCR and ELISA, respectively. Data are expressed as mean ± SEM (n=6–10 mice/group) and compared by one-way ANOVA by SNK method. *p<0.05 vs. sham. RIR, renal ischemia-reperfusion; OPN, osteopontin; ELISA, enzyme-linked immunosorbent assay.
Neutralization of OPN improves renal function after RIR
We examined renal function by assaying for serum levels of BUN and creatinine. BUN and creatinine were both significantly increased in the vehicle after RIR compared to the sham mice. Mice treated with non-immunized IgG control Ab showed no significant difference compared to the vehicle-treated mice. In the anti-OPN Ab treated mice, although BUN and creatinine levels were still significantly higher than the sham-operated animals, we noticed a significant decrease of BUN and creatinine levels by 25% and 22%, respectively compared to the vehicle-treated mice (Fig. 2A and B).
Figure 2. Neutralization of OPN improves renal function.
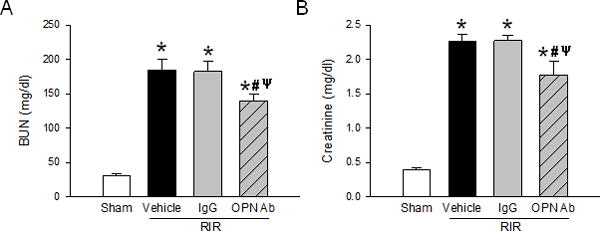
Serum samples from sham, vehicle, IgG control, and anti-OPN Ab groups were collected to measure (A) BUN and (B) creatinine. Data are expressed as mean ± SEM (n=7–9 mice/group) and compared by one-way ANOVA by SNK method. *p<0.05 vs. sham; #p<0.05 vs. vehicle; ψp<0.05 vs. IgG. BUN, blood urea nitrogen; OPN, osteopontin.
Neutralization of OPN reduces levels of renal injury markers after RIR
To determine renal injury, we assayed for additional markers which have been highlighted in recently published studies (18, 19). First, we looked at neutrophil gelatinase-associated lipocalin (NGAL) which serves as an early biomarker for AKI (18). It is part of the lipocalin superfamily of secreted proteins markedly upregulated in stimulated epithelial cells (18). The mRNA level of NGAL was reduced by 41% in the anti-OPN Ab treated mice compared to the vehicle mice (Fig. 3A). We then looked at the expression of kidney injury molecule-1 (KIM-1) which is a transmembrane protein expressed at a low level in normal kidney but increased dramatically in the post-ischemic kidney (19). The mRNA level of KIM-1 was significantly reduced by 49% in the kidneys of anti-OPN Ab-treated mice compared to the vehicle mice (Fig. 3B).
Figure 3. Neutralization of OPN reduces novel renal injury markers.
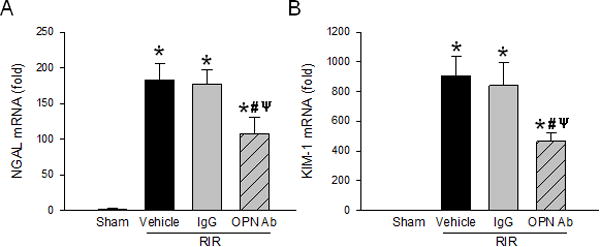
Renal tissue from sham, vehicle, IgG control, and anti-OPN Ab treated mice were collected to measure (A) NGAL and (B) KIM-1 mRNA by qRT-PCR. Their expression levels were normalized to β-actin, and sham was designated as 1 for comparison. Data are expressed as mean ± SEM (n=6–8 mice/group) and compared by one-way ANOVA by SNK method. *p<0.05 vs. sham; #p<0.05 vs. vehicle; ψp<0.05 vs. IgG. OPN, osteopontin; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1 kidney injury molecule-1.
Neutralization of OPN protects renal tissue damge after RIR
To gauge whether inhibition of OPN activity by Ab led to lessened renal injury, we evaluated tissue histology with a hematoxylin & eosin (H&E) stain. At 24 h after RIR, there was a noticeable difference in the amount of tubular cell injury and cast formation in the anti-OPN Ab treated animals compared to the vehicle- and IgG-treated control mice (Fig. 4A). Using semiquantitative histological evaluation, an injury score was calculated based on criteria described in Materials and Methods. A 37% improvement was noticed in the anti-OPN Ab treated mice compared to the vehicle group (Fig. 4B). Beside this, brief examination of our kidney tissue sections following RIR we have observed some qualitative differences in the levels of injury at glomeruli and podocytes, in which treatment with anti-OPN Ab showed reduced injury (data not shown).
Figure 4. Neutralization of OPN reduces renal histologic injury.
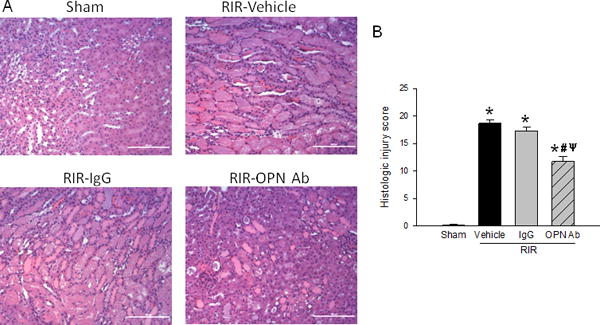
Sections of kidney tissue from sham, vehicle, IgG control, and anti-OPN Ab treated mice were collected after RIR and stained with H&E. (A) Representative sections are shown at 200× magnification. (B) Histologic injury grading score as described in Materials and Methods. The score represents the percentage of morphologic alterations as follows: 1, <10%; 2, 10–25%; 3, 26–50%; 4, 51%–75%; 5+, >75%. Data are expressed as mean ± SEM (n=10 microscopic fields/group) and compared by one-way ANOVA by SNK method. *p<0.05 vs. sham; #p<0.05 vs. vehicle; ψp<0.05 vs. IgG. H&E, hematoxylin and eosin; OPN, osteopontin.
Neutralization of OPN inhibits renal apoptosis after RIR
Cellular apoptosis is a hallmark feature of tissue injury during RIR condition. A TUNEL assay was performed on renal tissue sections, and it showed a significant increase in TUNEL-positive staining in the vehicle and IgG control group compared to the sham animals (Fig. 5A). In contrast, the RIR mice treated with anti-OPN Ab had a 79% decrease in TUNEL-positive staining compared to the vehicle mice (Fig. 5A and B). Caspase-3 serves as a crucial mediator of cellular apoptosis to cause tissue damage during inflammation (20). We performed a caspase-3 enzymatic activity assay, which showed significant up-regulation in the renal tissues during RIR induction; however, its activity was significantly decreased in the anti-OPN Ab-treated group by 59% compared to the vehicle animals (Fig. 5C).
Figure 5. Neutralization of OPN reduces renal apoptosis.
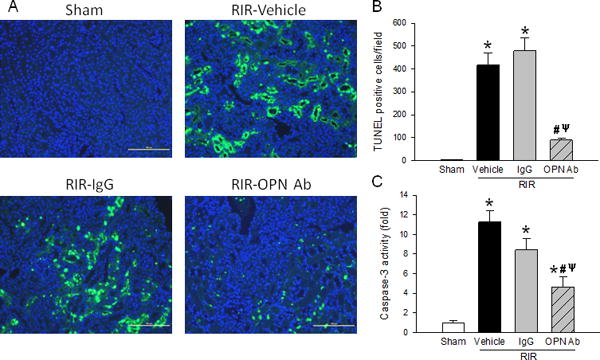
Sections of kidney tissue from sham, vehicle, IgG control, and anti-OPN Ab treated mice were collected after 24 h after RIR. (A) Representative photomicrographs show TUNEL-positive (green) and DAPI (blue) nuclear counterstaining. (B) TUNEL-positive cells per 200× field. (C) Caspase-3 enzyme activity assay was performed with kidney tissue. Data are expressed as mean ± SEM (TUNEL assay, n=20 microscopic fields/group; Caspase assay n=6–9 mice/group) and compared by one-way ANOVA by SNK method. *p<0.05 vs. sham; #p<0.05 vs. vehicle; ψp<0.05 vs. IgG. OPN, osteopontin; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; DAPI, 4′, 6-diamidino-2′-phenylindole dihydrochloride.
Neutralization of OPN decreases proinflammatory cytokines in the kidney after RIR
Inflammation is another important pathway to induce injury after RIR. We measured levels of proinflammatory cytokines IL-6 and TNF-α in renal tissue. Using ELISA, the level of IL-6 was found to be increased significantly in the vehicle and IgG control mice, but decreased significantly by 30% when RIR mice were treated with anti-OPN Ab compared to the vehicle mice (Fig. 6A). TNF-α was also found to be increased significantly in the vehicle and IgG control mice, but dcreased by 51% in the anti-OPN Ab-treated group compared to the vehicle mice (Fig. 6B). Thus, renal inflammation is significantly decreased when OPN is effectively targeted by its neutralizing Ab.
Figure 6. Neutralization of OPN reduces kidney inflammation.
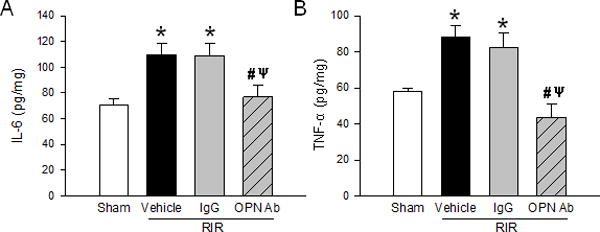
Renal tissue from sham, vehicle, IgG control, and anti-OPN Ab treated mice were collected to measure levels of cytokines (A) IL-6 and (B) TNF-α by ELISA. Data are expressed as mean ± SEM (n=6–9 mice/group) and compared by one-way ANOVA by SNK method. *p<0.05 vs. sham; #p<0.05 vs. vehicle; ψp<0.05 vs. IgG. OPN, osteopontin; IL, interleukin; TNF, tumor necrosis factor; ELISA, enzyme-linked immunosorbent assay.
Neutralization of OPN lowers NK cell infiltration in the kidney after RIR
NK cells are an important contributor to renal injury after RIR (9). Using fluorescently labeled antibodies for NK cell-specific markers, flow cytometry was used to identify the NK cell population from a whole kidney cell suspension. CD3−CD49b+ NK cells were selected through a series of gates; first, the leukocytes in the total kidney cell population were gated, then CD3− cells, and finally, CD49b+ cells (Fig. 7A). The NK cell population was found to be increased in the vehicle mice 7-fold over sham mice. On the other hand, the frequency of NK cells in the kidneys of RIR mice treated with anti-OPN Ab was significantly decreased by 31% compared to the vehicle mice (Fig. 7B).
Figure 7. Neutralization of OPN reduces NK cell infiltration.
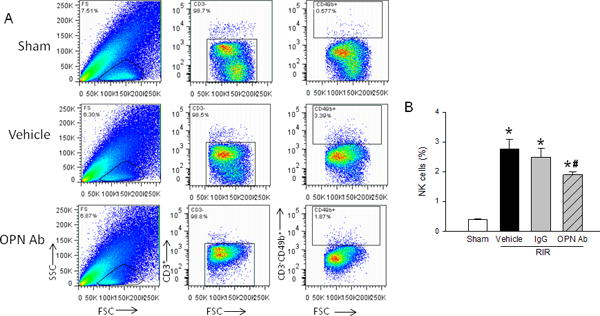
Renal tissue from sham, vehicle, IgG control, and anti-OPN Ab treated mice were collected 24 h after RIR. Cells were isolated and stained for CD3−CD49b+ NK cells. (A) Representative flow images with gating strategies shown. (B) NK cell frequencies expressed as a percentage of CD3− leukocytes. Data are expressed as mean ± SEM (n=8–10 animals/group) and compared by one-way ANOVA by SNK method. *p<0.05 vs. sham; #p<0.05 vs. vehicle; ψp<0.05 vs. IgG. NK, natural killer; OPN, osteopontin.
Neutralization of OPN reduces neutrophil infiltration in the kidney after RIR
NK cells have been shown to promote neutrophil infiltration into tissues in inflammation (21). To evaluate for neutrophils, immunohistochemistry was performed for Gr-1, a neutrophil specific Ab, on renal tissue sections. Gr-1 was found to be increased in the vehicle and IgG control mice kidneys, but decreased by 85% in the anti-OPN Ab treated mice (Fig. 8A and B). NK cells are also known to express pro-inflammatory chemokines during inflammation (12). To look for neutrophil recruitment, we performed qPCR for chemokine (C-X-C motif) ligand-1 (CXCL-1) also known as keratinocyte chemoattractant (KC). mRNA levels of the chemokine KC increased 13- and 15-fold in the vehicle and IgG control mice, respectively; however, the RIR mice treated with anti-OPN Ab decreased KC level by 39% compared to vehicle treated animals (Fig. 8C).
Figure 8. Neutralization of OPN reduces neutrophil infiltration.
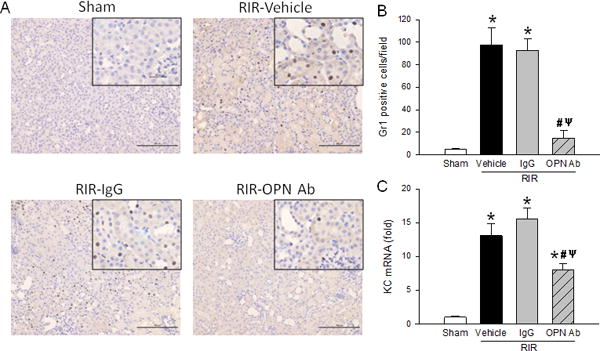
Sections of kidney tissue from sham, vehicle, IgG control, and anti-OPN Ab treated mice were collected 24 h after RIR. (A) Representative immunohistochemical images of the kidney sections are shown at 200× with 400× insets. Brown dots indicate Gr1 positive staining, while nuclei are counterstained in blue. (B) Gr1 positive cells per 200× field are quantified. (C) Renal tissue from sham, vehicle, IgG control, and anti-OPN Ab treated mice were collected to measure KC mRNA by qPCR. Their expression levels were normalized to β-actin, and sham was designated as 1 for comparison. Data are expressed as mean ± SEM (IHC n=20 fields/group; qRT-PCR n=7–10 mice/group) and compared by one-way ANOVA by SNK method. *p<0.05 vs. sham; #p<0.05 vs. vehicle; ψp<0.05 vs. IgG. IHC, immunohistochemistry; OPN, osteopontin; KC, keratinocyte chemoattractant.
DISCUSSION
AKI induced by RIR injury is a costly and burdensome event that complicates illness for many patients. Here, we performed a study utilizing anti-OPN Ab to mitigate RIR-mediated AKI. We have found that inhibition of OPN by its neutralizing Ab was able to improve renal function in a mouse model of RIR. Mouse kidneys exhibited protection from injury, as observed by histological analysis, and reduced apoptosis, as seen by TUNEL and caspase-3 assays. Blockade of OPN also led to reduced renal tissue inflammation. Flow cytometry for NK cells in the kidney showed a decreased population in mice with anti-OPN Ab treatment. Furthermore, decreased KC chemokine expression and decreased Gr-1 neutrophil infiltration on immunohistochemistry support the hypothesis that inhibition of NK cell infiltration was the mechanism by which OPN blockade was found to be beneficial in RIR.
Blockade of OPN led to preservation of renal function and a reduction in renal injury. This was demonstrated by decreases in BUN, creatinine, NGAL, and KIM-1 in the anti-OPN Ab treated mice, which correlated to histological findings. BUN and creatinine are the most widely assessed diagnostic and prognostic markers of renal diseases, but new injury markers such as NGAL and KIM-1 have become promising next-generation biomarkers in clinical nephrology (18, 19). NGAL was first purified from human neutrophils because of its association with gelatinase, and it is highly upregulated in the early post-ischemic mouse kidney (22). KIM-1 is a transmembrane glycoprotein that promotes the obstruction of the tubule lumen that characterizes AKI (23). In clinical studies, both NGAL and KIM-1 are biomarkers that have shown an ability to predict AKI days before an elevation in serum creatinine (24). In our study, targeting OPN led to significant reductions in both NGAL and KIM-1, emphasizing the value of OPN blockade in reducing RIR injury.
Apoptosis is a major pathophysiological cause of tissue injury during RIR. Our TUNEL assay revealed significantly less TUNEL-positive cells in the kidneys treated with anti-OPN Ab, as well as less caspase-3 enzymatic activity compared to the vehicle and IgG control kidneys. A promising area of RIR research specifically involves anti-apoptotic treatment. A strategy using short hairpin RNA (shRNA) that targets and silences Fas and caspase-8 has shown to be protective in RIR (25). In a more recent study, apoptosis inhibitor of macrophage (AIM) protein has been shown to promote recovery from AKI (26). To another extent, protecting the integrity of mitochondria, a main organelle participated in apoptosis induction, by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) and carnitine has also been shown to improve renal function after RIR (27). Consistent with these studies, attenuating apoptosis by anti-OPN Ab treatment also leads to improved outcomes after RIR.
Acute inflammation caused by RIR is considered to be another important pathway for injury. We found upregulation of OPN expression after RIR in the kidney, as well as in the circulation (data not shown). Like other pro-inflammatory cytokines and chemokines, induction of OPN expression during acute and chronic inflammatory diseases has been reported (28, 29). OPN acts as a chemotactic agent that promotes migration of inflammatory cells to the site of injury, and acts as an adhesive protein to retain cells (29, 30). In this study, neutralization with anti-OPN Ab significantly downregulated expression of pro-inflammatory cytokines IL-6 and TNF-α in the kidney compared to the vehicle. In the serum, levels of TNF-α and IL-1β were also shown to be decreased with anti-OPN Ab treatment in RIR animals (data not shown). Similarly, Hirano et al. utilized anti-OPN Ab in a murine model of sepsis to downregulate inflammation leading to beneficial outcomes (28). By targeting OPN with a neutralizing Ab, we were able to reduce excessive cytokine production, and reduce TEC injury, leading to overall improvement after RIR.
NK cells have previously been shown to be important for RIR injury (9–11). NK cells produce toxic molecules such as perforin and granzymes that induce cell death (12). We found that NK cell infiltration was reduced after treatment with anti-OPN Ab in the kidney after RIR. Zhang et al. first described interactions between TECs and NK cells which lead to TEC apoptotic death (9). Furthermore, using OPN KO mice, they found that the KO mice had reduced NK cell infiltration and less tissue damage during RIR compared to WT mice (10). We also showed that neutrophil infiltration was attenuated in mice given anti-OPN Ab, correlating with reduced chemokine KC expression. Previously, Kim et al. showed that signaling through co-stimulatory ligand CD137L in TECs is essential for NK cell-mediated neutrophil recruitment (21). Reverse signaling through the CD137L led to production of chemokines CXCL1/KC and CXCL2 (21). Aside from NK cell-mediated neutrophil infiltration, a direct role of OPN for inducing neutrophil infiltration cannot be ruled out based on a previous report showing that OPN served as a chemoattractant for neutrophil migration in vitro (31). Nevertheless, NK cells are an important part of the pathogenesis of OPN-mediated injury via recruitment of neutrophils after RIR.
While OPN has been examined by other groups in the context of RIR injury (10), its findings in the literature have been conflicting. As mentioned previously, Zhang et al. found OPN KO mice to have improved renal function with less tissue damage after RIR compared to WT mice. Another study using OPN KO mice found worsened renal function associated with higher structural damage than WT mice after RIR (14). Since OPN has many important functions relating to bone formation/remodeling (13), its absence may lead to difficulties in normal bone formation, and to development of bone related disorders. In the kidney, OPN is also produced by luminal epithelia in nephrons under homeostatic conditions (13). It is secreted into tubule fluid, but its function is not exactly known (13). In this study, we developed a therapeutic approach utilizing anti-OPN Ab to treat RIR animals, while more closely reflecting normal physiology by keeping the basal homeostatic functions of OPN intact.
Preserving the function of kidney grafts is of crucial importance for effective transplantation. Current experimental strategies involving proteins that downregulate inflammation, oxidative stress and apoptosis are shown to improve graft survival and function in animal models of transplantation (32). Although there is no such literature demonstrating the role of OPN for kidney transplant preservation, a recent study has focused on the role of OPN in kidney allograft injury (33). Transplant rejection is a process in which a transplant recipient’s immune system attacks the transplanted organ or tissue by considering it as a foreign substance. Recent studies demonstrated that natural killer (NK) cells are cytotoxic to tubular epithelial cells and contribute to acute kidney ischemia-reperfusion injury (9, 10). OPN, which can activate NK cells to mediate tubular epithelial cell death, was shown to be highly expressed in kidney grafts (33). OPN null kidney grafts had reduced injury after transplantation into mice, indicating OPN to play deleterious role in kidney allograft injury (33). Since our current study demonstrated beneficial outcome after treatment with anti-OPN Ab in terms of attenuating renal tissue injury and inflammation by inhibiting NK cell infiltration in the kidney, further research utilizing anti-OPN Ab could provide additional information for protecting kidney allograft injury during transplantation.
In our study, we obtained a modest effect on the renal functional markers by treatment with anti-OPN Ab in mice with RIR-induced injuries. Here, we adopted a RIR model which focused only on the acute phase by collecting samples after 24 hours of reperfusion. However, we speculate that experiments done at later time periods would provide valuable information regarding the extent of anti-OPN Ab treatment effectiveness to profoundly downregulate disease parameters. Further studies to look at longer time points of RIR other than 24 hours would be of great interest. One of the pitfalls of our study is that we could not provide strong experimental links between anti-OPN treatment in RIR mice and modulation of NK cell infiltration. Nonetheless, based on current literature findings we infer to their cause and effect relationship as Zhang et al and Victorino et al showed that AKI pathology is directly linked to NK cells (9, 11), and that OPN is directly linked to NK cells (10).
In summary, we have demonstrated that neutralization of OPN can reduce renal dysfunction caused by excessive inflammation, cell injury, apoptosis, and neutrophil infiltration after RIR. These effects are likely due to decreased recruitment of NK cells to the kidney. While the role of OPN has been widely studied in the field of RIR, conclusions have diverged widely. Our aim to investigate OPN via antibody neutralization provides further evidence toward OPN as a deleterious agent in renal ischemia. Therefore, targeting OPN after RIR may be a promising therapeutic option for patients at high risk of renal ischemic injury.
Acknowledgments
The authors thank Drs. Mahendar Ochani and Fangming Zhang for their technical assistance.
Source of Funding: This study was supported by the National Institutes of Health (NIH) grants HL076179 (PW) and R35GM118337 (PW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of Interest: All authors report no financial conflicts of interest.
References
- 1.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121(11):4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(6):961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munshi R, Hsu C, Himmelfarb J. Advances in understanding ischemic acute kidney injury. BMC Med. 2011;9:11. doi: 10.1186/1741-7015-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian H, Sun T, Hao D, Wang T, Li Z, Han S, Qi Z, Dong Z, Lv C, Wang X. The optimal timing of continuous renal replacement therapy for patients with sepsis-induced acute kidney injury. Int Urol Nephrol. 2014;46(10):2009–2014. doi: 10.1007/s11255-014-0747-5. [DOI] [PubMed] [Google Scholar]
- 5.Lopes JA, Jorge S, Resina C, Santos C, Pereira A, Neves J, Antunes F, Prata MM. Acute kidney injury in patients with sepsis: a contemporary analysis. Int J Infect Dis. 2009;13(2):176–181. doi: 10.1016/j.ijid.2008.05.1231. [DOI] [PubMed] [Google Scholar]
- 6.Le Dorze M, Legrand M, Payen D, Ince C. The role of the microcirculation in acute kidney injury. Curr Opin Crit Care. 2009;15(6):503–508. doi: 10.1097/MCC.0b013e328332f6cf. [DOI] [PubMed] [Google Scholar]
- 7.Akcay A, Nguyen Q, Edelstein CL. Mediators of Inflammation in Acute Kidney Injury. Mediators of Inflammation. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emlet DR, Shaw AD, Kellum JA. Sepsis-Associated AKI: Epithelial Cell Dysfunction. Seminars in Nephrology. 2015;35(1):85–95. doi: 10.1016/j.semnephrol.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Zhang ZX, Wang S, Huang X, Min WP, Sun H, Liu W, Garcia B, Jevnikar AM. NK cells induce apoptosis in tubular epithelial cells and contribute to renal ischemia-reperfusion injury. J Immunol. 2008;181(11):7489–7498. doi: 10.4049/jimmunol.181.11.7489. [DOI] [PubMed] [Google Scholar]
- 10.Zhang ZX, Shek K, Wang S, Huang X, Lau A, Yin Z, Sun H, Liu W, Garcia B, Rittling S, Jevnikar AM. Osteopontin expressed in tubular epithelial cells regulates NK cell-mediated kidney ischemia reperfusion injury. J Immunol. 2010;185(2):967–973. doi: 10.4049/jimmunol.0903245. [DOI] [PubMed] [Google Scholar]
- 11.Victorino F, Sojka DK, Brodsky KS, McNamee EN, Masterson JC, Homann D, Yokoyama WM, Eltzschiq HK, Clambey ET. Tissue-Resident NK Cells Mediate Ischemic Kidney Injury and Are Not Depleted by Anti-Asialo-GM1 Antibody. J Immunol. 2015;195(10):4973–4985. doi: 10.4049/jimmunol.1500651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 13.Xie Y, Sakatsume M, Nishi S, Narita I, Arakawa M, Gejyo F. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int. 2001;60(5):1645–1657. doi: 10.1046/j.1523-1755.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 14.Noiri E, Dickman K, Miller F, Romanov G, Romanov VI, Shaw R, Chambers AF, Rittling SR, Denhardt DT, Goligorsky MS. Reduced tolerance to acute renal ischemia in mice with a targeted disruption of the osteopontin gene. Kidney Int. 1999;56(1):74–82. doi: 10.1046/j.1523-1755.1999.00526.x. [DOI] [PubMed] [Google Scholar]
- 15.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3–new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97(4):1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown AC, Blank U, Adams DC, Karolak MJ, Fetting JL, Hill BL, Oxburgh L. Isolation and culture of cells from the nephrogenic zone of the embryonic mouse kidney. J Vis Exp. 2011;(50):e2555. doi: 10.3791/2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 19.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 20.Aziz M, Jacob A, Wang P. Revisiting caspases in sepsis. Cell Death Dis. 2014;5:e1526. doi: 10.1038/cddis.2014.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HJ, Lee JS, Kim JD, Cha HJ, Kim A, Lee SK, Lee SC, Kwon BS, Mittler RS, Cho HR, Kwon B. Reverse signaling through the costimulatory ligand CD137L in epithelial cells is essential for natural killer cell-mediated acute tissue inflammation. Proc Natl Acad Sci U S A. 2012;109(1):E13–22. doi: 10.1073/pnas.1112256109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest Suppl. 2008;241:89–94. doi: 10.1080/00365510802150158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24(11):3265–3268. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 24.Sprenkle P, Russo P. Molecular markers for ischemia, do we have something better then creatinine and glomerular filtration rate? Arch Esp Urol. 2013;66(1):99–114. [PubMed] [Google Scholar]
- 25.Du C, Wang S, Diao H, Guan Q, Zhong R, Jevnikar AM. Increasing resistance of tubular epithelial cells to apoptosis by shRNA therapy ameliorates renal ischemia-reperfusion injury. Am J Transplant. 2006;6(10):2256–2267. doi: 10.1111/j.1600-6143.2006.01478.x. [DOI] [PubMed] [Google Scholar]
- 26.Arai S, Kitada K, Yamazaki T, Takai R, Zhang X, Tsugawa Y, Sugisawa R, Matsumoto A, Mori M, Yoshihara Y, Doi K, Maehara N, Kusunoki S, Takahata A, Noiri E, Suzuki Y, Yahagi N, Nishiyama A, Gunaratnam L, Takano T, Miyazaki T. Apoptosis inhibitor of macrophage protein enhances intraluminal debris clearance and ameliorates acute kidney injury in mice. Nat Med. 2016;22(2):183–193. doi: 10.1038/nm.4012. [DOI] [PubMed] [Google Scholar]
- 27.Idrovo JP, Yang WL, Matsuda A, Nicastro J, Coppa GF, Wang P. Post-treatment with the combination of 5-aminoimidazole-4-carboxyamide ribonucleoside and carnitine improves renal function after ischemia/reperfusion injury. Shock. 2012;37(1):39–46. doi: 10.1097/SHK.0b013e31823185d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirano Y, Aziz M, Yang WL, Ochani M, Wang P. Neutralization of Osteopontin Ameliorates Acute Lung Injury Induced by Intestinal Ischemia-Reperfusion. Shock. 2016 doi: 10.1097/SHK.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lund SA, Giachelli CM, Scatena M. The role of osteopontin in inflammatory processes. J Cell Commun Signal. 2009;3(3–4):311–322. doi: 10.1007/s12079-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber GF, Zawaideh S, Hikita S, Kumar VA, Cantor H, Ashkar S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J Leukoc Biol. 2002;72(4):752–761. [PubMed] [Google Scholar]
- 31.Hirano Y, Aziz M, Yang WL, Wang Z, Zhou M, Ochani M, Khader A, Wang P. Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury. Crit Care. 2015;19:53. doi: 10.1186/s13054-015-0782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bon D, Chatauret N, Giraud S, Thuillier R, Favreau F, Hauet T. New strategies to optimize kidney recovery and preservation in transplantation. Nat Rev Nephrol. 2012;8(6):339–347. doi: 10.1038/nrneph.2012.83. [DOI] [PubMed] [Google Scholar]
- 33.Zhang ZX, Huang X, Jiang J, Lau A, Yin Z, Liu W, Haig A, Jevnikar AM. Natural Killer Cells Mediate Long-term Kidney Allograft Injury. Transplantation. 2015;99(5):916–924. doi: 10.1097/TP.0000000000000665. [DOI] [PubMed] [Google Scholar]


