Abstract
Background
The hypercatabolic response in severely burned pediatric patients is associated with increased production of catecholamines and corticosteroids, decreased formation of testosterone, and reduced strength alongside growth arrest for up to 2 years post injury. We have previously shown that, in the pediatric burned population, the administration of the testosterone analog oxandrolone improves lean body mass accretion and bone mineral content and that the administration of the β1, β2 adrenoreceptor antagonist propranolol decreases cardiac work and resting energy expenditure while increasing peripheral lean mass. Here, we determined whether the combined administration of oxandrolone and propranolol has added benefit.
Methods
In this prospective, randomized study of 612 burned children (52 ± 1% of total body surface area burned, ages 0.5–14 years [males]; ages 0.5–12 years [females]), we compared controls to the individual administration of these drugs, and the combined administration of oxandrolone and propranolol at the same doses, for 1 year post burn. Data were recorded at discharge, 6 months, and 1 and 2 years post injury.
Results
Combined use of oxandrolone and propranolol shortened the period of growth arrest by 84 days (p=0.0125 vs. control) and increased growth rate by 1.7 cm/y (p=0.0024 vs. control).
Conclusion
Combined administration of oxandrolone and propranolol attenuates burn-induced growth arrest in pediatric burn patients. This study is registered at clinicaltrials.gov: NCT00675714 and NCT00239668.
| Growth Arrest and Growth Rate By Treatment Group | ||
|---|---|---|
| Treatment | Length of Growth Arrest (Days) |
Growth Rate (cm/y) |
| Control | 280 ± 19 | 5.9 ± 0.2 |
| Oxandrolone | 217 ± 17 | 5.8 ± 0.3 |
| Propranolol | 242 ± 13 | 6.3 ± 0.2 |
| Oxandrolone + Propranolol | 196 ± 15* | 7.6 ± 0.5* |
Data presented as mean ± standard error.
p<0.05 vs. control.
Keywords: Oxandrolone, propranolol, burns, growth arrest, rate of stature increase, pediatric
MINI ABSTRACT
The combined use of the testosterone analog oxandrolone and the β-adrenoreceptor antagonist propranolol shortened the period of growth arrest and increased the rate of growth compared to control and compared to the administration of oxandrolone and propranolol individually in pediatric burn subjects during growth spurt years.
INTRODUCTION
Severe burn injuries induce a significant hypermetabolic state characterized by a severe loss of lean body mass, muscle wasting, and growth delays.1–3 Bone loss also begins quickly and is sustained following burn injuries in children, increasing the risks of post-burn fractures and reducing bone mass and growth velocity.4, 5 We have previously reported that the daily administration of the non-aromatizable androgen oxandrolone (Ox), an orally-active synthetic non-virilizing testosterone derivative, for 1 year post burn significantly reduces hypermetabolism and significantly increases bone mineral content, lean body mass, and strength in pediatric burned patients when assessed 1 year post burn.6
Propranolol (Prop) is a non-selective β-adrenoreceptor antagonist that mitigates the catecholamine response associated with burns. Significant reductions in the percent of predicted heart rate and percent of predicted resting energy expenditure, prevention of bone loss, and improvement of lean body mass accretion result when Prop is administered at a dose to decrease heart rate by 15% for 1 year in pediatric burn patients.7 We have also shown that burned children receiving Prop have improved protein synthesis, muscle protein synthesis efficiency, and muscle protein net balance after treatment for 2 weeks compared to untreated burned children.8 Prop treatment attenuates the hyperdynamic, hypermetabolic, hypercatabolic, and osteopenic responses following severe burn injury in pediatric patients.7
The specific objectives of our study were to evaluate whether the combined administration of Ox and Prop attenuates growth arrest and improves the rate of growth, as measured in centimeters grown per year, compared to the administration of Ox or Prop alone. We hypothesized that the combined administration of Ox and Prop in burned pediatric subjects would have improved growth outcomes compared to untreated subjects or to subjects treated with Ox or Prop alone. Our study compared four groups of pediatric burn patients who were admitted to Shriners Hospital for Children®—Galveston from 1997 to 2015. Specifically, males between the ages of 0.5 and 14 years and females between the ages of 0.5 to 12 years were assessed. The upper limits of the age ranges were eliminates the variable onset of post-pubescent growth delay.
MATERIALS AND METHODS
Subject Demographics and Injury Characteristics
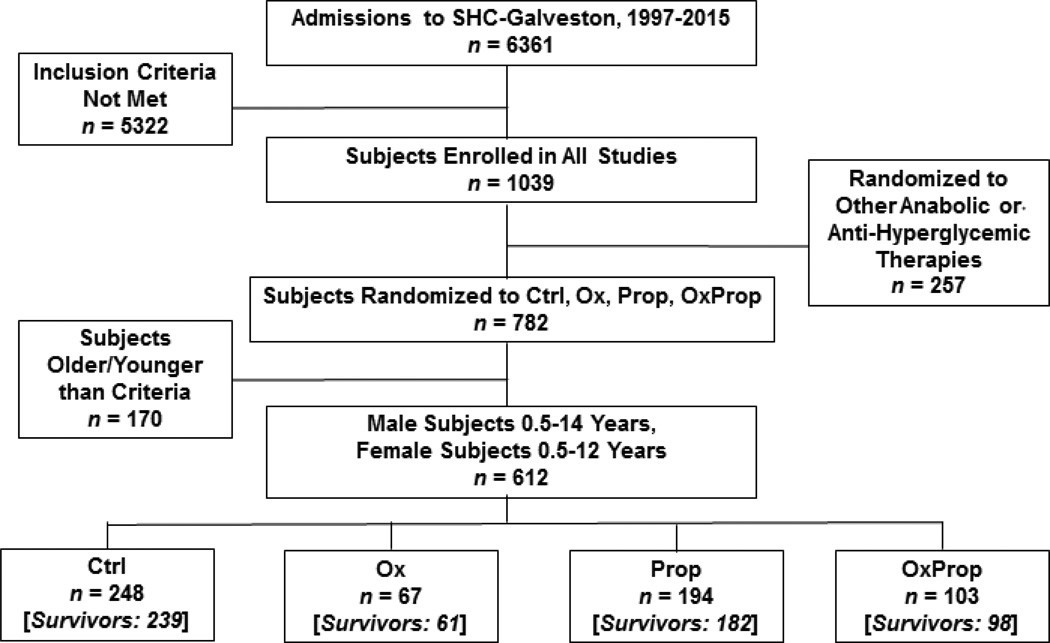
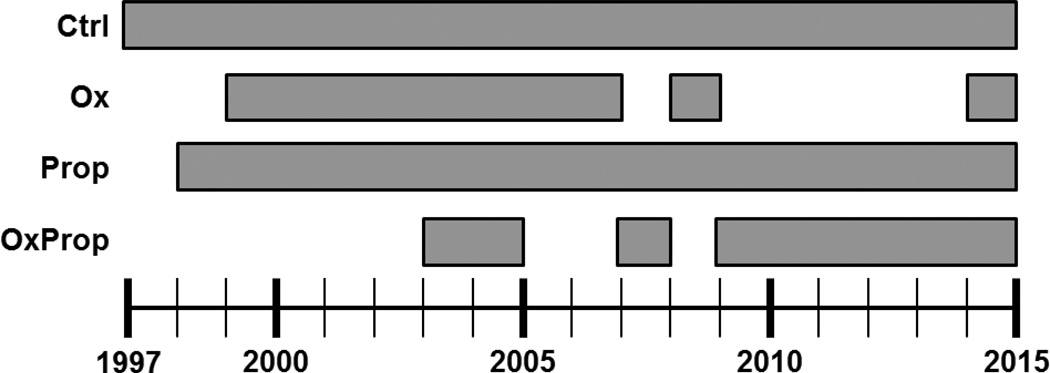
A total of 6,361 patients were admitted to Shriners Hospital for Children®—Galveston between November 1997 and October 2015. Of the 6,361 patients, 1,039 consented to participate in research protocols after meeting the following inclusion criteria: (1) 0.5 to 18 years of age and (2) ≥30% of the total body surface area (TBSA) burned. Six hundred twelve subjects of the 1,039 subjects were included in this prospective, intent-to-treat study. All were males between 0.5 or 14 years or females between 0.5 and 12 years. Subjects were prospectively assigned to one of four treatment groups using a randomization schedule generated with random allocation sequence software. Subjects received one of the following treatments during their acute stay and for 1 year post discharge: (a) control treatment (Ctrl; n=248), (b) Ox (BTG Pharmaceuticals, West Conshohocken, PA; 0.1 mg/kg every 12 hours for 1 year minimum; n=67), (c) Prop (Roxane Laboratories, Columbus, OH; 4.0 ± 0.2 mg/kg/day for 1 year minimum, dose titrated to decrease heart rate by 15%; n=194), (d) combined administration of Ox and Prop [OxProp; n=103] (Figure 1). If bradycardia occurred, a single dose of propranolol was held and administration recommenced after 16 hours with one half of the original dose. The dose was then escalated back to the original dose over the following 24 hours. Ctrl subjects were enrolled continuously from 1997 to 2015, and Ctrl subjects outnumbered subjects in the Ox, Prop, and OxProp groups because of the balanced design of the randomization schedule for subjects in all studies at Shriners Hospital for Children®—Galveston (Figure 2). Subjects were enrolled in OxProp beginning in 2003, and breaks in OxProp enrollment were taken from 2005 to 2007 and from 2008 to 2009 to study Ox and Prop subjects. Study drugs were started within 4 ± 0.5 days post admission. Subjects were blinded to their treatment group, and the appropriate dose of propranolol was determined by a physician blinded to the study treatments. Patients were excluded due to decision not to treat due to severity of injury, and/or anoxic brain injury. Patient age, sex, ethnicity, percent of TBSA burned, and percent of TBSA with third-degree burns were recorded at the time of admission. Age-appropriate diagrams were used to determine burn size.9 All subjects received the standard of care for wound treatment and nutrition as described previously.10
Figure 1.
Flow Diagram. Out of 6,361 acute pediatric admissions to Shriners Hospitals for Children®—Galveston between 1997 and 2015, 612 patients were included in our study. Inclusion criteria were based on age (0.5–12 years for male subjects, 0.5–14 years for female subjects at the time of the admission) and burn size (≥30% total body surface area burned).
Figure 2.
Years of enrollment for each group.
This study was approved by the Institutional Review Board of the University of Texas Medical Branch (Galveston, TX). Informed written consent was obtained from the subject’s legal guardian before enrollment in the study. Written assent was also obtained from the subject if he/she was 7 years old or older. Subjects were assessed at discharge, 6 months, 1 and 2 years post burn, and subjects who withdrew from the study were included in the data analysis until the time of withdrawal. All study staff, including study physicians, attended protocol initiation meetings at the outset of the study to review enrollment criteria and all study-related procedures. They met weekly thereafter to review progress of all subjects and ensure continued protocol adherence. Subject data entered into CRFs and the study database was subject to regular quality-control checks. This study is part of a larger clinical trial (clinicaltrials.gov: No. NCT00675714) evaluating outcomes following administration of several therapeutic agents in burns.
Growth Arrest and Growth Rate
Endpoints for growth, which were assessed using height and body weight measurements, were collected from each subject at admission, throughout their acute stay, at discharge, 6 months post burn, and annually at all follow-up appointments. The duration of growth arrest was defined as the days from the date of burn to the date at which each subject was determined to have passed their admission height by at least 0.25 cm and where the height increase was persistent and progressive. This end date of growth arrest was collectively determined by the examination of growth data by three physicians who were blinded to drug cohort assignment. Growth rate was modeled using linear regression of the heights at the end date of growth arrest through subsequent follow-up visits males reached the age of 14 years and females reached the age of 12 years. The latter was determined clinically by using sex, age, and height-for-age percentiles; only data from males ages 2–14 years and females ages 2–12 years was included in the growth rate analysis.11
Statistical Analysis
Differences among groups in days between burn and end of growth arrest were assessed by permutation t-tests. This approach was chosen due to heterogeneous skewness of distributions between the groups; the data from the Ctrl and Prop-treated groups were strongly right-skewed, while the Ox and OxProp groups showed little evidence of skewness. Hommel-adjusted p values compensated for multiple comparisons among groups.12 Growth slope (cm/y) was modeled by multiple linear regression with relation to age at burn, percent of TBSA burned, and treatment group. Differences among treatments and times were assessed by Tukey-adjusted contrasts. All statistical testing assumed a 95% level of confidence (p<0.05), and all analyses were performed using R statistical software.13
RESULTS
The demographic data from our burned patient population randomized into the Ctrl, Ox, Prop, and OxProp groups are presented in Table 1. Ctrl subjects (n=248) did not receive study drug. Subjects who were randomized into the Ox group (n=67) received 0.1 mg/kg Ox every 12 hours for 1 year, while Prop subjects (n=194) received 4.0 ± 0.2 mg/kg/day Prop for 1 year, and subjects randomized to OxProp (n=103) received both 0.1 mg/kg Ox every 12 hours for 1 year and 4.0 ± 0.2 mg/kg/day Prop for 1 year. There were no significant differences among the four groups in age (p=0.47), sex (p=0.20), ethnicity (p=0.28), percent of TBSA burned (p=0.47), percent of TBSA with third-degree burns (p=0.69), mortality (p=0.31), length of stay (p=0.48), body mass index (p=0.51), or weight (p=0.23). The range of admission times in all groups is shown in Figure 2.
Table 1.
Patient Demographics and Characteristics
| Ctrl (n=248) | Ox(n=67) | Prop(n=194) | OxProp (n=103) | p-Values | |
|---|---|---|---|---|---|
| Age (Years) | 6 ± 0.2 | 6 ± 0.5 | 5 ± 0.3 | 5 ± 0.4 | 0.47 |
| Sex (Male, %) | 146 (59) | 43 (64) | 128 (66) | 72 (70) | 0.20 |
| Hispanic (%) | 224 (90) | 60 (90) | 166 (86) | 91 (88) | 0.28 |
| TBSA Burned (%) | 52 ± 1 | 54 ± 2 | 52 ± 1 | 51 ± 1 | 0.47 |
| TBSA w/3rd Degree Burns (%) | 38 ± 1 | 39 ± 3 | 39 ± 2 | 36 ± 2 | 0.69 |
| Mortality (%) | 9 (4) | 6 (9) | 12 (6) | 5 (5) | 0.31 |
| Length of Stay (Days) | 26 ± 1 | 28 ± 3 | 29 ± 2 | 28 ± 2 | 0.48 |
Data presented as mean ± standard error.
There was a bimodal temporal pattern from burn to time of admission in our subjects. Table 2 summarizes the burn to admission times of the immediate (burn to admission: <7.5 days) and delayed (burn to admission: >7.5 days) subjects. Both groups were included in our study because the primary outcome, which is the number of days of growth arrest between the Ctrl and OxProp groups, was significant with or without the inclusion of delayed admission subjects (all subjects: p=0.0125, Table 3).
Table 2.
Immediate and Delayed Admission
| Ctrl (n=248) | Ox (n=67) | Prop (n=194) | OxProp (n=103) | p-Values | |
|---|---|---|---|---|---|
| Immediate Admit*, n | 207 | 46 | 169 | 79 | |
| Burn to Admit (Days) | 3 ± 0.1 | 3 ± 0.2 | 2 ± 0.1 | 2 ± 0.2 | 0.01 |
| Median | 2 | 3 | 2 | 2 | |
| Delayed Admit‡, n | 41 | 21 | 25 | 24 | |
| Burn to Admit (Days) | 20 ± 2 | 25 ± 8 | 21 ± 5 | 27 ± 5 | 0.61 |
| Median | 14 | 15 | 12 | 19 | |
Data presented as mean ± standard error.
<7 days between burn and admission.
>7 days between burn and admission.
Table 3.
Duration of Growth Arrest & Growth Rate
| Group | Growth Arrest Duration (days) |
Growth Rate (cm/y) |
|---|---|---|
| Ctrl | 280 ± 19 | 5.9 ± 0.2 |
| Ox | 217 ± 17 | 5.8 ± 0.3 |
| Prop | 242 ± 13 | 6.3 ± 0.2 |
| OxProp | 196 ± 15* | 7.6 ± 0.5§†‡ |
Data presented as mean ± standard error.
p=0.0125 vs. Ctrl.
p=0.0024vs. Ctrl;
p=0.0145 vs. Ox;
p=0.0145 vs. Prop.
Duration of Growth Arrest & Growth Rate
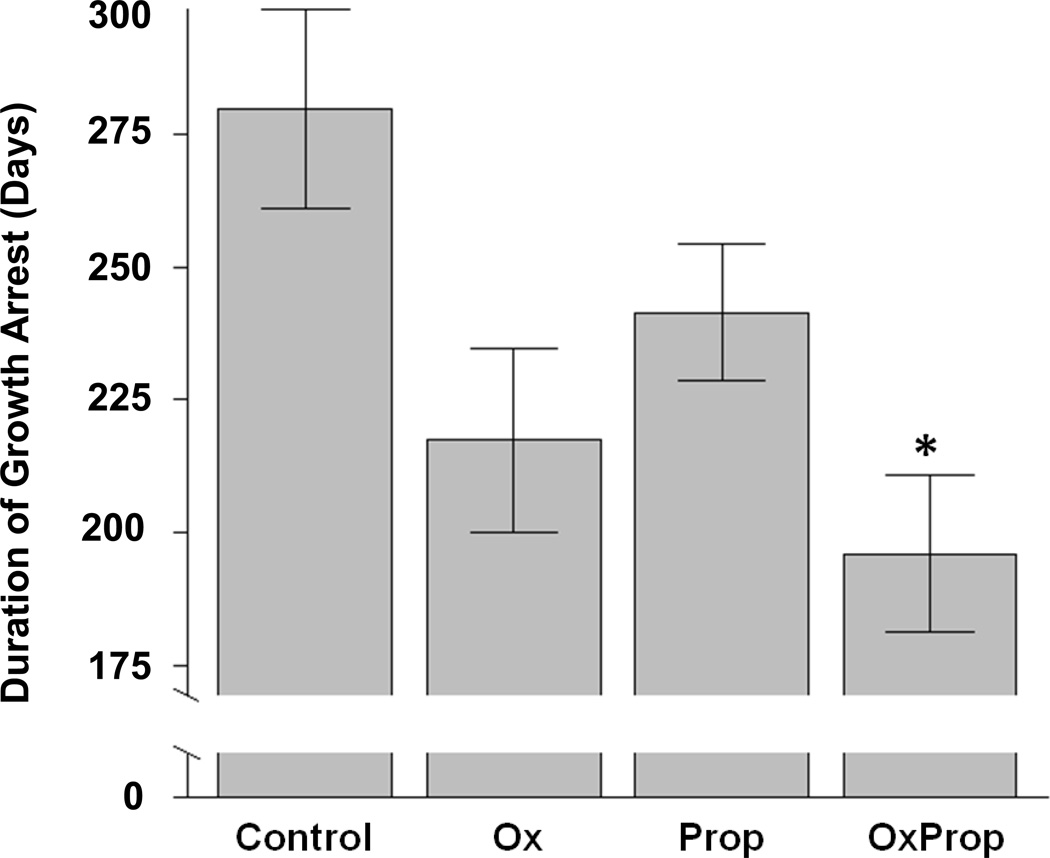
Table 3 shows means and p values for Ctrl (median; 221 days of growth arrest), Ox (median, 239 days), Prop (median, 206 days), and OxProp (median, 180 days; Figure 3). The Ctrl group averaged 84 more days of growth arrest than the OxProp group (p=0.0125).
Figure 3.
Difference in duration of growth arrest among groups. Data are shown as mean ± standard error. *p=0.0125 vs. Ctrl.
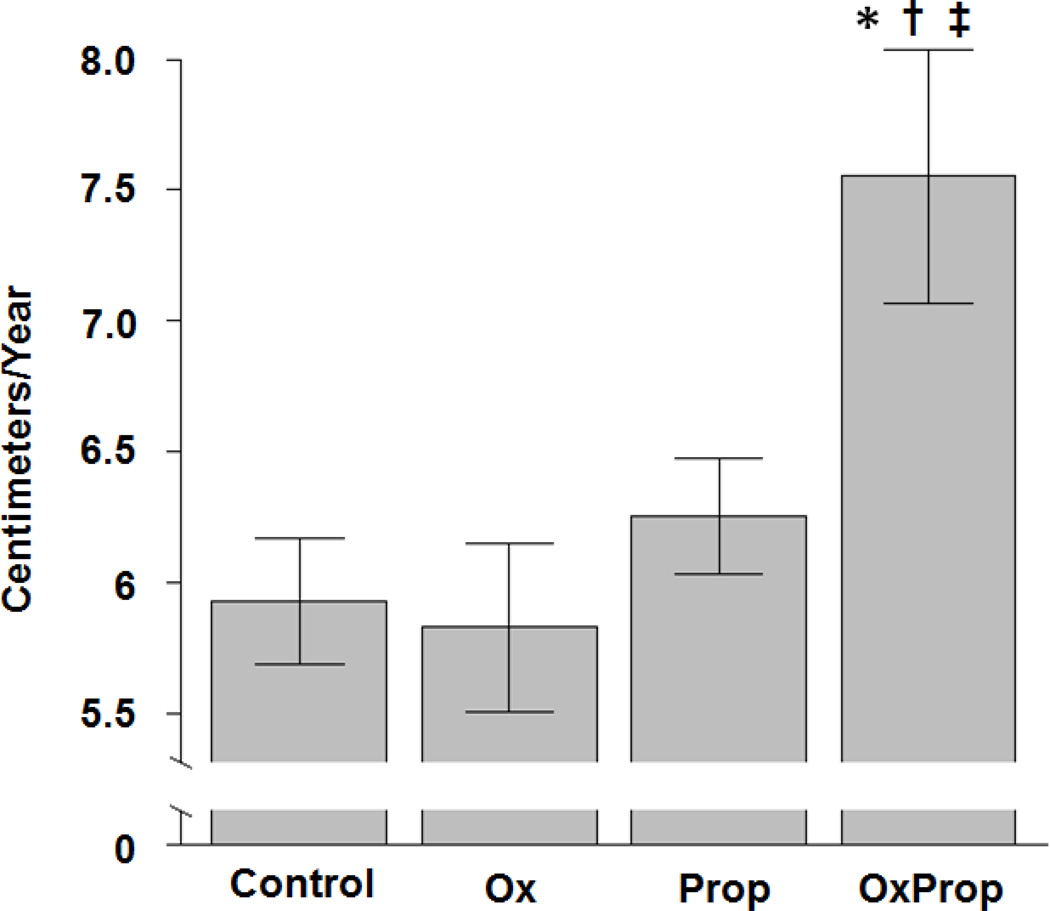
The subjects treated with OxProp grew 1.7 cm/y more than Ctrl-treated subjects (median, 6.0 cm/y; p=0.0024), with an adjusted 95% CI spanning 0.4 to 2.6 (Table 3, Figure 4). OxProp (median, 6.9 cm/y) averaged 1.8 cm/y greater growth than Ox (median, 5.6 cm/y; p=0.0145), with an adjusted 95% CI spanning 0.2 to 3.0. OxProp also averaged 1.3 cm/y greater growth than Prop (median, 6.4 cm/y; p=0.0145), with an adjusted CI spanning 0.2 to 2.4.
Figure 4.
Difference in growth slope among groups. Data are shown as mean ± standard error. *p=0.0024 vs. Ctrl; †p=0.0145 vs. Ox;‡ p=0.0145 vs. Prop.
There was no evidence of a significant effect due to sex (p=0.40 for days of growth arrest, p=0.09 for growth rate). Age had significant effects on time to growth arrest and on growth rate and thus was included as a covariate in our statistical models. The year of burn was a significant covariate; however, the increases in growth rate with OxProp treatment remained significantly different compared to the Ctrl group (p=0.0337) and compared to the Prop group (p=0.0178). Also, growth arrest was significantly different between the Ox and Ctrl groups (p=0.0009) and the Prop and Ox groups (p=0.0062).
Safety Profile
Subjects were closely monitored for adverse events during their acute stay and at every follow-up visit post-discharge; all adverse events were reviewed by the Data Safety Monitoring Board. Clitoral hood edema developed in three female subjects randomized to the Ox group, and the edema resolved in 3 months.
DISCUSSION
The trauma from burn injury results in adaptive responses by the body including the systemic inflammatory and stress hormone responses. The consequences of these sequelae include muscle wasting and bone loss in conjunction with stunted growth. Our present study showed that the combined administration of Ox and Prop significantly improved growth arrest by an average of 84 days compared to the Ctrl treatment. Also, we showed that OxProp significantly increased growth rate compared to the Ctrl treatment and compared to Ox or Prop alone. In this analysis, we included males between ages 0.5 to 14 years and females between ages 0.5 to 12 years at the time of burn. This age range was used to examine the pediatric growth period. During these age spans, unburned males grow at an average rate 6.5 cm/y and unburned females grow at an average rate of 6.7 cm/y.14, 15 Height velocity reaches a plateau at 14 years for males and 12 years for females in the non-burned population.11
In our previous studies, Ox (0.1 mg/kg every 12 hours) administered for 1 year post burn to pediatric burned subjects increased muscle mass at 6 months post burn and improved bone mineral content and density at 1 year post burn.6 The reasons for the delay in the effects of Ox on the bone and muscle are unknown. Oxidative stress increases after burn, and scavengers of reactive oxygen and nitrogen species are significantly depleted in burned patients.16 We postulate that the elevated inflammatory cascade and additional oxidative stress associated with burns may interfere with bone strength and muscle integrity and may delay the effects of Ox.
A wide array of inflammatory cytokines are consistently elevated after burn injury, including IL-1β, IL-6, and TNF-α.17 We have previously shown that Prop decreases inflammatory cytokines such as TNF-α and IL-1β in pediatric burn subjects during their acute hospitalization.18 Also, our studies showed that combined administration of Prop with recombinant human growth hormone in 15 pediatric burned subjects significantly decreased serum C-reactive protein, cortisone, IL-6, and IL-8 as well as significantly increased serum insulin-like growth factor-I, insulin-like growth factor binding protein-3, and growth hormone compared to control treatment.19 TNF-α and IL-1β have been shown to decrease osteoclast formation and bone resorption in murine models, and TNF-α, IL-1β, and IL-6 increase bone loss in mice.20, 21 The anti-inflammatory properties of Prop have been well-documented; its use has been associated with decreased edema in mesenchymal stromal cells with traumatic brain injury22 as well as decreased cardiac inflammation and oxidative stress markers such as glutathione and isoprostanes.23 Prop attenuates inflammatory cell infiltration, expression of the cytokines TNF-α and IL-8,24 and airway inflammation25 in a rat model of cigarette smoke exposure. The combined administration of Ox and Prop may stimulate protein synthesis and anabolism owing to Ox and decrease the inflammatory cascade owing to Prop, resulting in an additive effect that increases growth of pediatric burn patients.
Burn injury induces production of epinephrine26, 27 and glucocorticoids,28 both of which have anti-chondrogenic effects,29 and these increases last up to 3 years post burn. High concentrations of glucocorticoids are associated with shorter height in children,30 shorter femur growth and reduced bone density in young rabbits,31, 32 and reduced size of the growth plates in the tibiae of rats.33 Exposure to 30 nM of testosterone improves chondrogenesis in human intervertebral disc cells by increasing the expression of aggrecan, collagen type I, and type II collagen in particular.34 Chondrogenesis and myogenesis are induced in the larynx of South African clawed frogs by testosterone, which stimulates satellite cell division, further increasing muscle mass.35 Additionally, Takarada and colleagues showed that epinephrine inhibits the gene transactivation necessary for chondrogenic differentiation. Furthermore, Prop prevented this response to epinephrine in ATDC5 cells and primary cultured mouse costal chondrocytes.29 The administration of Prop significantly increased the expression of collagen I and X mRNA in fractured murine femurs in similar experiments.29 Since testosterone has chondrogenic properties and Ox is a testosterone analog, we speculate that Ox and Prop both have chondrogenic properties that may synergistically stimulate linear growth in pediatric burn patients.
Ox may cause perturbation of the immune response, which could potentially be attenuated by co-administering Ox with Prop. The use of Ox has been associated with the impairment of host defense by exacerbating systemic inflammatory response syndrome. In contrast, the influx of inflammatory neutrophils and monocytes associated with burn has been significantly mitigated in burned models treated with Prop,36, 37 and the addition of Prop to Ox may decrease the incidence of immunosuppression and improve host antibacterial defenses.
Limitations of our study include (1) the temporal differences between burn and admission among the immediate and delayed groups, and (2) the year of admission between all four groups. We have previously obtained substantial evidence of the beneficial effects of Ox and Prop alone in short-term studies38–44 and then began enrolling subjects in respective long-term clinical trials beginning in 1997 (Figure 2). Ctrl subjects were enrolled continuously from 1997 to 2015 to correct for the evolving standard of care at our burn unit. Once we determined the efficacy and clinical outcomes of Ox and Prop individually,8, 45, 46 subjects were enrolled in OxProp beginning in 2003. Breaks in OxProp enrollment were taken from 2005 to 2007 and 2008 to 2009 to confirm that the effects of Ox and Prop individually were maintained, regardless of the year of admission. There is no evidence of any differences among the groups in regards to age, percent of TBSA burned, percent of TBSA with third-degree burns, sex, race, length of stay, or mortality (Table 1), and the clinical care from 1997 to 2015 has been consistently guided by one attending Chief-of-Staff/Surgeon to ensure continuity of care. The differences among groups in time between burn and admission do not appear to underlie the observed effects since there is no significant difference in growth arrest between the Ctrl and OxProp groups regardless of admission time from burn.
Within 6 weeks of severe burn injury, lumbar spine bone density is reduced by 7% from admission bone mass with no improvement seen during a 2-year follow-up.5, 47 There is also a 3% loss of total body bone mineral content, which does appear to recover between 18 to 24 months post burn.47 With no apparent improvement in bone mass, severely burned children are at risk for reduced peak bone mass and stunted growth. Our findings show that the combined use of Ox and Prop improve growth arrest and growth rate. The use of both therapies together in pediatric burn patients should be strongly considered in the clinical setting.
Acknowledgments
Source of Funding. This work was supported by NIDILRR (90DP0043-02-01 [DNH]), National Institutes of Health (P50GM060338, R01GM056687, and T32GM008256 [DNH]; R01HD049471 [OES]; and R01GM112936 [CCF]), Shriners Hospitals for Children (84080, 79135, 80100, and 71008 [DNH]; 71009 [OES]; and 84291 [CCF]), the Department of Surgery at UTMB (2014-667 [LES]), and the Remembering the 15 Research Education Endowment Fund. This study was also conducted with the support of UTMB’s Institute for Translational Sciences, supported in part by a Clinical and Translational Science Award (UL1TR001439) from the National Center for Advancing Translational Sciences (NIH).
The authors would like to thank the medical and research staff of Shriners Hospitals for Children®—Galveston for their valuable assistance, as well as the Data Safety Monitoring Board. We would also like to thank Drs. Jong Lee, Carlos Jimenez, Ludwik Branski, and William Norbury for their clinical contributions and their work in maintaining the protocols, Dr. Kristofer Jennings for his assistance in recommending permutation t-tests to cope with heterogeneous distributions in time to growth arrest, Dr. Kasie Cole for proofreading the manuscript, Mr. Michael Ben Silva and Ms. Kaitlin Watson for their help in collecting samples, Ms. Maricela Pantoja for her help with analyses, and Ms. Lisa Molina and Pamela Stevens for their assistance in retrieving the data.
Footnotes
Conflicts of Interest. No conflicts of interest declared.
REFERENCES
- 1.Branski LK, Herndon DN, Barrow RE, et al. Randomized controlled trial to determine the efficacy of long-term growth hormone treatment in severely burned children. Ann Surg. 2009;250(4):514–523. doi: 10.1097/SLA.0b013e3181b8f9ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128(2):312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 3.Alloju SM, Herndon DN, McEntire SJ, et al. Assessment of muscle function in severely burned children. Burns. 2008;34(4):452–459. doi: 10.1016/j.burns.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Klein GL, Herndon DN, Goodman WG, et al. Histomorphometric and biochemical characterization of bone following acute severe burns in children. Bone. 1995;17(5):455–460. doi: 10.1016/8756-3282(95)00279-1. [DOI] [PubMed] [Google Scholar]
- 5.Klein GL, Herndon DN, Langman CB, et al. Long-term reduction in bone mass after severe burn injury in children. J Pediatr. 1995;126(2):252–256. doi: 10.1016/s0022-3476(95)70553-8. [DOI] [PubMed] [Google Scholar]
- 6.Porro LJ, Herndon DN, Rodriguez NA, et al. Five-year outcomes after oxandrolone administration in severely burned children: a randomized clinical trial of safety and efficacy. J Am Coll Surg. 2012;214(4):489–502. doi: 10.1016/j.jamcollsurg.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herndon DN, Rodriguez NA, Diaz EC, et al. Long-term propranolol use in severely burned pediatric patients: a randomized controlled study. Ann Surg. 2012;256(3):402–411. doi: 10.1097/SLA.0b013e318265427e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345(17):1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 9.Mlcak R, Buffalo M. Pre-hospital management, transport, and emergency care. In: Herndon DN, editor. Total Burn Care. Vol. 3. Philadelphia: Saunders; 2007. pp. 81–92. [Google Scholar]
- 10.Finnerty CC, Ali A, McLean J, et al. Impact of stress-induced diabetes on outcomes in severely burned children. J Am Coll Surg. 2014;218(4):783–795. doi: 10.1016/j.jamcollsurg.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention NCfHS. CDC Growth Charts: United States 2000. [Accessed March 12, 2016]; Available at: http://www.cdc.gov/growthcharts/
- 12.Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika. 1988;75:383–386. [Google Scholar]
- 13.Team RC. R: A language and environment for statistical computing. Vienna: Foundation for Statistical Computing; 2012. [Google Scholar]
- 14.Rogol AD, Clark PA, Roemmich JN. Growth and pubertal development in children and adolescents: effects of diet and physical activity. Am J Clin Nutr. 2000;72(2 Suppl):521S–528S. doi: 10.1093/ajcn/72.2.521S. [DOI] [PubMed] [Google Scholar]
- 15.Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107(3):317–329. doi: 10.1016/s0022-3476(85)80501-1. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen TT, Cox CS, Traber DL, et al. Free radical activity and loss of plasma antioxidants, vitamin E, sulfhydryl groups in patients with burns: the 1993 Moyer Award. J Burn Care Rehabil. 1993;14:602–609. doi: 10.1097/00004630-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Finnerty CC, Herndon DN, Przkora R, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26(1):13–19. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 18.Jeschke MG, Norbury WB, Finnerty CC, et al. Propranolol does not increase inflammation, sepsis, or infectious episodes in severely burned children. J Trauma. 2007;62(3):676–681. doi: 10.1097/TA.0b013e318031afd3. [DOI] [PubMed] [Google Scholar]
- 19.Jeschke MG, Finnerty CC, Kulp GA, et al. Combination of recombinant human growth hormone and propranolol decreases hypermetabolism and inflammation in severely burned children. Pediatr Crit Care Med. 2008;9(2):209–216. doi: 10.1097/PCC.0b013e318166d414. [DOI] [PubMed] [Google Scholar]
- 20.Kitazawa R, Kimble RB, Vannice JL, et al. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J Clin Invest. 1994;94(6):2397–2406. doi: 10.1172/JCI117606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimble RB, Bain S, Pacifici R. The functional block of TNF but not of IL-6 prevents bone loss in ovariectomized mice. J Bone Miner Res. 1997;12(6):935–941. doi: 10.1359/jbmr.1997.12.6.935. [DOI] [PubMed] [Google Scholar]
- 22.Kota DJ, Prabhakara KS, van Brummen AJ, et al. Propranolol and Mesenchymal Stromal Cells Combine to Treat Traumatic Brain Injury. Stem Cells Transl Med. 2015 doi: 10.5966/sctm.2015-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer JH, Spurney CF, Iantorno M, et al. d-Propranolol protects against oxidative stress and progressive cardiac dysfunction in iron overloaded rats. Can J Physiol Pharmacol. 2012;90(9):1257–1268. doi: 10.1139/y2012-091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, Xu M, Zhang Y, et al. Effects of long-term application of metoprolol and propranolol in a rat model of smoking. Clin Exp Pharmacol Physiol. 2014;41(9):708–715. doi: 10.1111/1440-1681.12261. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y, Zhang Y, Shen N, et al. Effects of one month treatment with propranolol and metoprolol on the relaxant and contractile function of isolated trachea from rats exposed to cigarette smoke for four months. Inhal Toxicol. 2014;26(5):271–277. doi: 10.3109/08958378.2014.885098. [DOI] [PubMed] [Google Scholar]
- 26.Gauglitz GG, Herndon DN, Kulp GA, et al. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009;94(5):1656–1664. doi: 10.1210/jc.2008-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norbury WB, Herndon DN, Branski LK, et al. Urinary cortisol and catecholamine excretion after burn injury in children. J Clin Endocrinol Metab. 2008;93(4):1270–1275. doi: 10.1210/jc.2006-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perlman WR, Webster MJ, Kleinman JE, et al. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biol Psychiatry. 2004;56(11):844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Takarada T, Hojo H, Iemata M, et al. Interference by adrenaline with chondrogenic differentiation through suppression of gene transactivation mediated by Sox9 family members. Bone. 2009;45(3):568–578. doi: 10.1016/j.bone.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Lai HC, FitzSimmons SC, Allen DB, et al. Risk of persistent growth impairment after alternate-day prednisone treatment in children with cystic fibrosis. N Engl J Med. 2000;342(12):851–859. doi: 10.1056/NEJM200003233421204. [DOI] [PubMed] [Google Scholar]
- 31.Kugelberg M, Shafiei K, Ohlsson C, et al. Glucocorticoid eye drops inhibit growth in the newborn rabbit. Acta Paediatr. 2005;94(8):1096–1101. doi: 10.1111/j.1651-2227.2005.tb02051.x. [DOI] [PubMed] [Google Scholar]
- 32.Kugelberg M, Ohlsson C, Savendahl L. Reduced bone mineral density and radial bone growth in young rabbits treated with dexamethasone eye drops. Horm Res. 2005;63(4):165–170. doi: 10.1159/000084684. [DOI] [PubMed] [Google Scholar]
- 33.Chrysis D, Ritzen EM, Savendahl L. Growth retardation induced by dexamethasone is associated with increased apoptosis of the growth plate chondrocytes. J Endocrinol. 2003;176(3):331–337. doi: 10.1677/joe.0.1760331. [DOI] [PubMed] [Google Scholar]
- 34.Bertolo A, Baur M, Aebli N, et al. Physiological testosterone levels enhance chondrogenic extracellular matrix synthesis by male intervertebral disc cells in vitro, but not by mesenchymal stem cells. Spine J. 2014;14(3):455–468. doi: 10.1016/j.spinee.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Sassoon D, Segil N, Kelley D. Androgen-induced myogenesis and chondrogenesis in the larynx of Xenopus laevis. Dev Biol. 1986;113(1):135–140. doi: 10.1016/0012-1606(86)90115-6. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi M, Jeschke MG, Asai A, et al. Propranolol as a modulator of M2b monocytes in severely burned patients. J Leukoc Biol. 2011;89(5):797–803. doi: 10.1189/jlb.1010553. [DOI] [PubMed] [Google Scholar]
- 37.Romana-Souza B, Nascimento AP, Monte-Alto-Costa A. Low-dose propranolol improves cutaneous wound healing of burn-injured rats. Plast Reconstr Surg. 2008;122(6):1690–1699. doi: 10.1097/PRS.0b013e31818cbf67. [DOI] [PubMed] [Google Scholar]
- 38.Wolfe RR, Herndon DN, Jahoor F, et al. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987;317(7):403–408. doi: 10.1056/NEJM198708133170702. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe RR, Herndon DN, Peters EJ, et al. Regulation of lipolysis in severely burned children. Ann Surg. 1987;206(2):214–221. doi: 10.1097/00000658-198708000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herndon DN, Barrow RE, Rutan TC, et al. Effect of propranolol administration on hemodynamic and metabolic responses of burned pediatric patients. Ann Surg. 1988;208(4):484–492. doi: 10.1097/00000658-198810000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minifee PK, Barrow RE, Abston S, et al. Improved myocardial oxygen utilization following propranolol infusion in adolescents with postburn hypermetabolism. J Pediatr Surg. 1989;24(8):806–810. doi: 10.1016/s0022-3468(89)80541-x. [DOI] [PubMed] [Google Scholar]
- 42.Gore DC, Honeycutt D, Jahoor F, et al. Propranolol diminishes extremity blood flow in burned patients. Ann Surg. 1991;213(6):568–573. doi: 10.1097/00000658-199106000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herndon DN, Nguyen TT, Wolfe RR, et al. Lipolysis in burned patients is stimulated by the beta 2-receptor for catecholamines. Arch Surg. 1994;129(12):1301–1304. doi: 10.1001/archsurg.1994.01420360091012. [DOI] [PubMed] [Google Scholar]
- 44.Baron PW, Barrow RE, Pierre EJ, et al. Prolonged use of propranolol safely decreases cardiac work in burned children. J Burn Care Rehabil. 1997;18(3):223–227. doi: 10.1097/00004630-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Herndon DN, Dasu MR, Wolfe RR, et al. Gene expression profiles and protein balance in skeletal muscle of burned children after beta-adrenergic blockade. Am J Physiol Endocrinol Metab. 2003;285(4):E783–E789. doi: 10.1152/ajpendo.00508.2002. [DOI] [PubMed] [Google Scholar]
- 46.Morio B, Irtun O, Herndon DN, et al. Propranolol decreases splanchnic triacylglycerol storage in burn patients receiving a high-carbohydrate diet. Ann Surg. 2002;236(2):218–225. doi: 10.1097/00000658-200208000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Przkora R, Herndon DN, Sherrard DJ, et al. Pamidronate preserves bone mass for at least 2 years following acute administration for pediatric burn injury. Bone. 2007;41(2):297–302. doi: 10.1016/j.bone.2007.04.195. [DOI] [PMC free article] [PubMed] [Google Scholar]