Abstract
Purpose of review
This review will provide the practitioner with an understanding of the spectrum of non-transplant surgical options for managing patients with short bowel syndrome.
Recent findings
Intestinal lengthening procedures are promising therapy to allow for autonomy from parenteral nutrition. The recently described serial transverse enteroplasty is an effective procedure that is easier to perform and has similar outcomes to the more standard longitudinal lengthening procedure described by Bianchi.
Summary
There are several surgical options for management of the short bowel syndrome and include construction of intestinal valves or reversed intestinal segments, interposition of segments of colon, or intestinal lengthening procedures. The choice of technique is dictated by the patients underlying pathophysiology and includes such factors as intestinal transit time, length of remnant bowel, presence of intact colon, and degree of small bowel dilation. Non-transplant surgical interventions are important adjuncts to the elimination of parenteral nutrition dependence and need for intestinal transplantation.
Keywords: Short bowel syndrome, intestinal adaptation, intestinal lengthening, intestinal valve, colon interposition, pediatric, intestinal failure, surgery
Introduction
In children, massive small bowel resection (SBR) results in short bowel syndrome (SBS), a serious condition in which the ability of the intestine to absorb and digest nutrition is insufficient. SBS, and the requirement for total parenteral nutrition (TPN) can lead to a wide array of serious and life-threatening consequences including diarrhea, dehydration, vitamin deficiencies, stunted growth, small bowel bacterial overgrowth, TPN-related cholestatic liver failure, venous thrombosis, and recurrent sepsis. After massive SBR, the main therapeutic goal is to obtain nutritional independence from TPN and its associated complications. Children who develop liver failure or recurrent catheter-related sepsis may eventually be considered for small bowel transplantation, but despite advances this therapy five-year patient survival is roughly 64%.1 Early in the post-operative period, an adaptive response typically occurs that increases both length and absorptive capacity of the remaining bowel. Often, this process alone is sufficient to provide intestinal autonomy. When adaptation is insufficient to achieve enteric autonomy, however, several surgical options may obviate the need for intestinal transplantation.
Timing is an essential consideration when considering a surgical intervention for intestinal rehabilitation. Early surgery may be an unnecessary, as bowel adaptation and lengthening may prevent the need for long term TPN. Late surgery exposes the patient to additional TPN-related costs and complications. Some series suggest a waiting period of at least one year as a minimum interval of time to allow for a robust adaptation response.2 A recent series by Wood et al, however, has suggested that patients undergoing operation at an early age (less than 365 days) received fewer central lines and weaned earlier from TPN than older (>365 days) patients undergoing operation.3 The approach to surgical timing needs to be individualized- if the patient is making progress towards enteral feeding tolerance with uncomplicated provision of TPN, operative intervention should be postponed. Earlier surgery must be considered, however, in the context of significant cholestasis or inability to advance enteral feeding.
Pre-operative planning
Intestinal failure in SBS patients is multifactorial and arises from a combination of pathophysiologic abnormalities, including rapid intestinal transit, decreased mucosal surface area, ineffective peristalsis, and reduced intestinal length. Several surgical approaches, which can be categorized based on the abnormality they primarily address, have been developed to address these abnormalities. A targeted and thorough assessment of a patient’s intestinal length, caliber, motility, and transit is required to determine the indications for each procedure and create an operative plan
As a first step, plain abdominal radiographs and a contrast upper gastrointestinal series with small bowel follow-through can provide a great deal of information regarding the patient’s intestinal anatomy and function. Areas of partial obstruction and efficiency of transit should be sought. Simple interventions to relieve areas of partial obstruction such as lysis of adhesions or resection of a focal stricture may be all that is necessary. Surgery designed to slow intestinal transit would obviously not be indicated for a patient with underlying delayed transit. Although not very precise, the upper GI series can provide an idea of small intestinal length and caliber. In patients with a proximal stoma, a contrast enema can estimate the amount of distal bowel available for subsequent reanastomosis and can identify areas of stricture.
The major goal of surgery is maximization of absorptive and digestive capacity, so when feasible, patients with diverting stomas should have intestinal continuity restored. One advantage of a stoma; however, is that accurate daily stool volumes can be recorded. A threshold of 40ml/kg/day of stool output generally should limit further advancement of enteral feeding.
Intestinal Valves
Partially obstructing intestinal valves have traditionally employed for patients with short intestinal length and normal caliber, but who are also hampered by rapid transit. The simplest techniques include the placement of sutures or an external Teflon collar around the circumference of the bowel.4 Another method involves everting a bowel segment to create a small intussusceptum, slowing transit. This procedure is technically variable, particularly with regard to knowing the optimum length of bowel to evert and the direction of the intussusception (proximal versus distal bowel). Too long of an intussusceptum can easily cause a bowel obstruction.
The clinical experience with intestinal valves is sparse and limited to small series or case reports5. In animal models, a partially obstructing valve induces intestinal dilatation and mucosal adaptation6. Noting this observation, Georgeson et al. began performing staged intestinal lengthening procedures after constructing intussuscepting valves in which the partially obstructed bowel became dilated. In their series, several patients improved from lengthening of the previously non-dilated bowel.7
Reversed Intestinal Segments
Whereas the intestinal valves cause anatomic obstruction, interposition of a reversed, antiperistaltic segment of bowel creates a “physiologic” valve. Reversed segments of intestine have been employed mostly in patients with postvagotomy diarrhea or dumping syndrome.8 The exact length of bowel to be reversed is critical: too short a segment may ineffective in slowing peristalsis, while too long a segment may create functional obstruction. Outcomes obtained in experimental and clinical series are somewhat disparate, which may be attributable to various lengths of reversed bowel used. While 10 cm seems to be the most common length utilized in adult patients, beneficial effects have been described in infants when as little as 3 cm has been reversed.9 Technically, the reversed segment should be placed as distally as possible maximize the length of proximal bowel that can benefit from the slowed transit and enhanced mucosal absorption. Reversed segments may have limited applicability in patients with extreme SBS, as construction of the reversed segment may compromise an already tenuous absorptive mucosal mass. In children, the reversed segment can potentially continue to grow and cause bowel obstruction. In addition, the reversed segment may not function normally and thus effectively shorten the intestinal remnant, particularly if obstruction or ischemia occur.
The largest series of reversed intestinal segments was recently reported and comprised of 38 adult patients with the SBS.10 With a median length of follow-up of 57.7 months, 45% of patients had weaned from parenteral nutrition and 5-year survival was 84%. A confounding variable in several reports of reversed segments is that the procedure is done concurrently with ostomy closure, making it difficult to determine which factor was most important (adding more bowel in continuity versus the reversed segment).2, 6 Although outcomes are mixed,11 there may be a role for this procedure as well as for intestinal valves when the bowel caliber is normal and transit is rapid.
Colon Interposition
A surgical option less-widely used consists of interposing of a segment of colon between two limbs of small bowel in patients with rapid intestinal transit. Although the exact mechanism is not well understood, colonic interposition has been studied experimentally and applied clinically in the management of SBS. The interposed colon segment is generally placed proximally and its slow, segmental peristaltic contractions probably slow the rate at which nutrients are delivered to the distal bowel. This interposed colon can also absorb nutrients, water, and electrolytes. There appears to be adaptive changes within the transposed colon segment involving the mucosa (which contains Paneth cells normally exclusive to small bowel)12 and the longitudinal smooth muscle layer.13
Isoperistaltic colon interposition in the management of the SBS was first reported in beagle puppies following 90% small bowel resection.14 When compared with animals undergoing resection alone, the dogs with the interposition had prolonged intestinal transit, better weight gain, less stool output fecal fat. Colon interposition has been clinically beneficial in both children and adults. In the largest series of children with SBS, isoperistaltic colon interposition allowed six of nine patients to wean from parenteral nutrition completely.15 The other three patients died from complications secondary to parenteral nutrition. No reported morbidity or mortality was associated with the interposition procedure.
The small number of patients reported precludes determination of the ideal length of colon to utilize, but reports range from 8 to 24 cm. This appears to be useful for slowing intestinal transit, with success in about 50%, while not compromising the viability of the small bowel. This procedure may be considered in patients with an intact colon when the small bowel has not become dilated.
Tapering Enteroplasty
Patients with short bowel syndrome often undergo progressive dilation of the remnant bowel. This can be segmental, but often affects a large portion of the bowel. Dilated segments have ineffective peristalsis due to failure of bowel wall apposition during contraction. The resulting low contraction pressures cause a to-and-fro motion of the enteric contents, setting the stage for stasis, functional bowel obstruction, and bacterial overgrowth. Optimally, such a segment of bowel can be resected if the affected length is short and sufficient healthy intestine remains. Unfortunately, the bowel of SBS patients is short in length and predominantly dilated, a situation that precludes resection.
Tapering enteroplasty reduces the bowel caliber while preserving intestinal length. This is accomplished by excising an antimesenteric portion of the bowel, leaving the mesenteric tube of bowel intact. The tapered intestine caliber is significantly smaller and therefore peristalsis becomes more effective and improves adaptive capacity of the bowel.16
In a large series of 160 adult and pediatric patients with SBS,17 11 children ranging in age from 6 months to 9 years had dilated intestinal segments and remnant lengths longer than 30 cm. All had associated bacterial overgrowth and malabsorption. Enteroplasty was performed on the duodenum (n=3), jejunum (n=3), or ileum (n=5). Nine of the 11 patients were weaned completely from parenteral nutrition. Two patients experienced recurrent malabsorption and underwent a subsequent lengthening surgery. Compared to a lengthening procedure, tapering is associated with lower operative morbidity and is preferred when small intestinal length and degree of dilatation are sufficient.18
An alternative to resection of the antimesenteric bowel is plication, wherein the bowel is folded and sutured into the lumen. This theoretically preserves the mucosa for better absorption while at the same time decreasing the overall bowel caliber.19 Unfortunately, the suture lines tend to eventually break down, and bowel dilation and functional obstruction recur.
Intestinal Lengthening Procedures
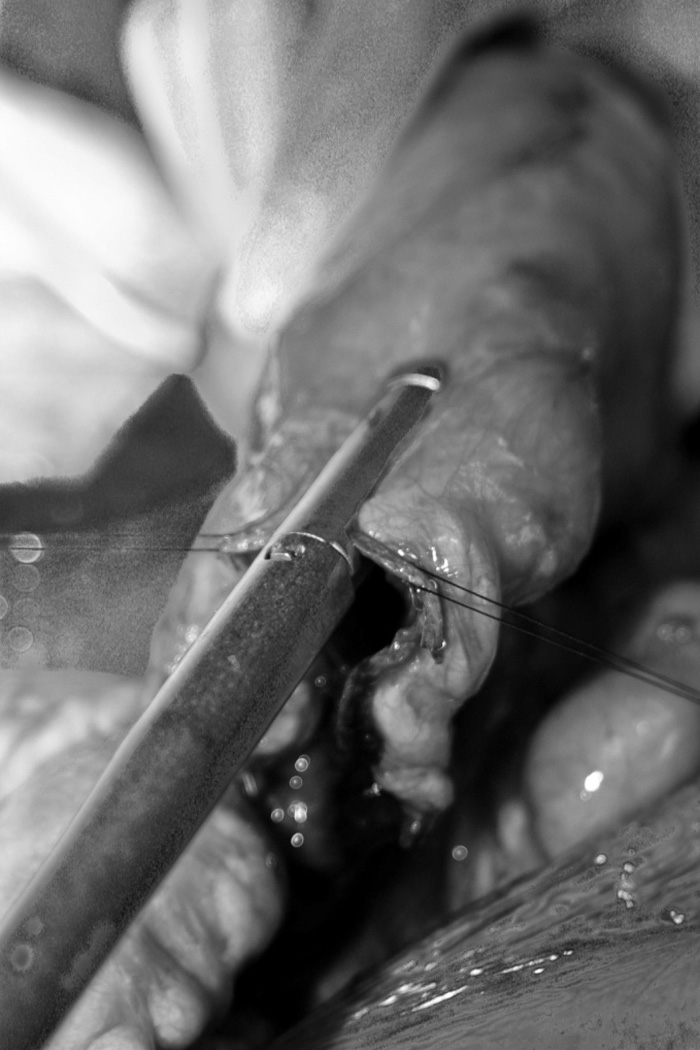
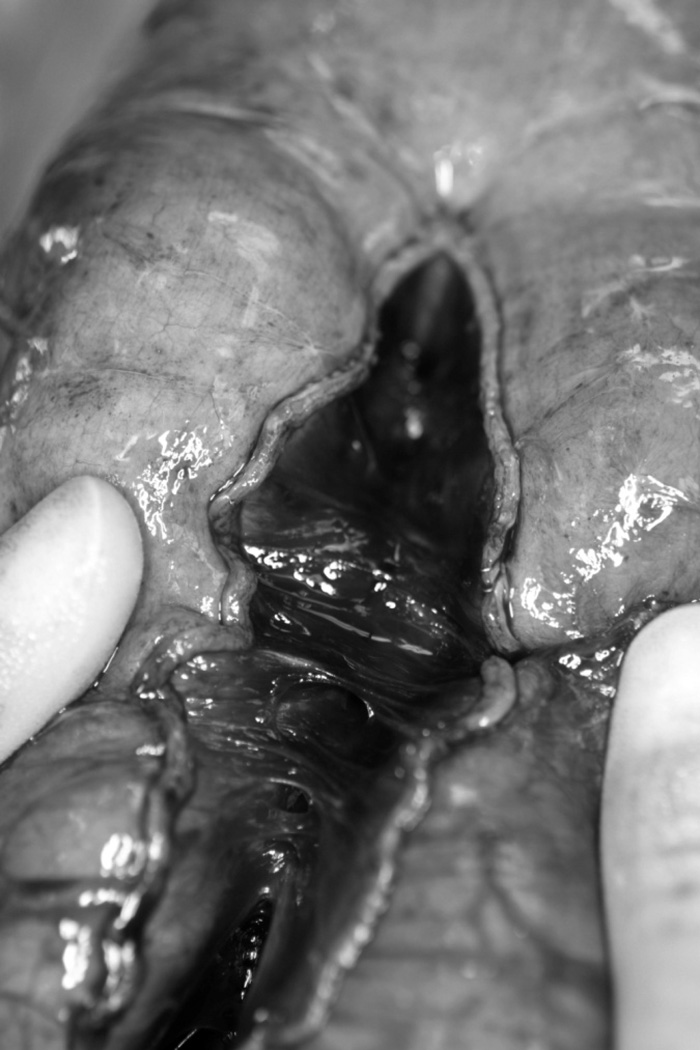
In 1980, Bianchi provided an early description of a surgery designed to actually increase the length of the intestine.20 Alternately referred to as longitudinal intestinal lengthening and tailoring (LILT), the Bianchi procedure appears to be most successful for patients who have undergone a period of normal bowel adaptation following resection.21 LILT utilizes the anatomy of the bowel’s mesenteric blood supply, which bifurcates just proximal to the edge of the bowel wall to supply one half of the circumference. The bowel can be safely divided along its longitudinal axis into two tubes with independent blood supply that are half the circumference of the original bowel (Figure 1A). These “tubes” can be created either with a gastrointestinal anastomosis (GIA) stapler or hand-sewn. The two newly constructed tubes of bowel are then connected in an isoperistaltic fashion, resulting in a segment of bowel with double the length and half the circumference of the original (Figure 1B). Thus, LILT both increases the contact time of luminal nutrient with the absorptive mucosa (increased length) and improves peristalsis (smaller caliber). A variation of this technique involves entering one side of the bowel limb at the initiation of the lengthening and exiting the other side once the lengthening has completed.22 This permits the use of only a single anastomosis and is a very useful technical modification.
Figure 1.
longitudinal division of bowel (A) and creation of two isoperistaltic channels (B)
Bianchi reported a series of 20 children who underwent this lengthening procedure over a 16 year period of time.23 Long-term survival was 45%, and intestinal length greater than 40 cm and no evidence of significant liver disease (determined by absence of clinical jaundice) at the time of reconstruction predicted survival. An analysis of children referred for intestinal transplantation after failed lengthening procedures at the University of Pittsburgh revealed similar concerns for the application of this procedure in neonatal patients, those with extremely short intestinal length (< 50cm), or with the presence of jaundice.24 Finally, this procedure is certainly not without long-term morbidity. As documented by Waag et al, who reported on 18 survivors of the Bianchi procedure, morbidities included hyperphagia, hyponatremia and hypochloremia, metabolic acidosis, D-lactic acidosis, cholelithiasis and urolithiasis, gastro-esophageal reflux, and symptoms caused by secondary dilatation of the lengthened bowel loops.25 It is therefore essential that patients are followed closely after this procedure.
A large series of patients (n=43) undergoing longitudinal lengthening was reported by Sudan et al in which 55% of patients weaned completely from parenteral nutrition.26 In another series of 53 patients, 79% successfully weaned from parenteral nutrition with overall survival of 77%.27 Both reports found significance in bowel length, liver function, and ability to wean completely from parenteral nutrition as prognostic features for survival.
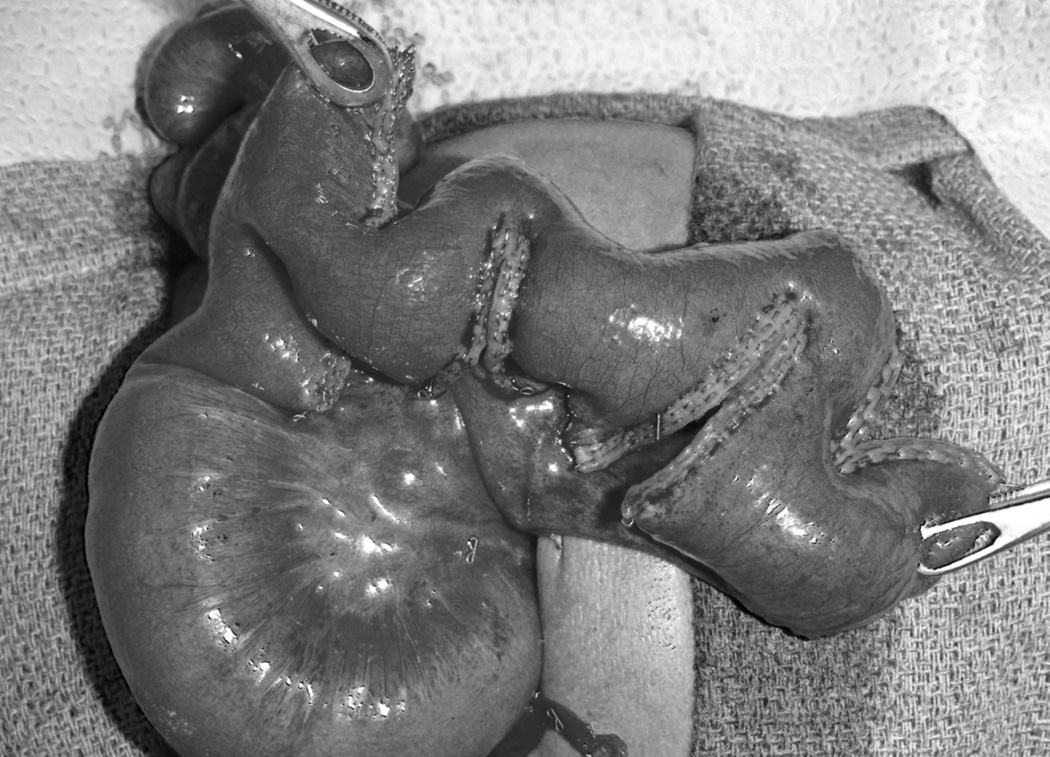
The most recently described operation aimed at intestinal lengthening is the serial transverse enteroplasty (STEP) procedure.28 As the name suggests, the dilated small bowel is lengthened by serial transverse applications of a stapler from opposite directions, creating a zig zag channel (Figure 2). STEP can provide near doubling of bowel length. In a recent report of the International Serial Transverse Enteroplasty Data Registry, overall mortality was 11%, and 47% of patients attained full enteral nutritional support.29 As with LILT, predictors of death or progression to intestinal transplant after STEP were higher direct bilirubin and shorter bowel length. In a study of 5 year outcomes of 12 patients following the STEP procedure in children, revealed 2 that underwent intestinal transplantation and 2 patients who died.30 Of the remaining 8 patients, 7 weaned successfully from TPN. Another report from Seattle Children’s Hospital performed nutritional analysis on 15 children undergoing STEP procedure. Twelve of the patients in this series experienced improved enteral tolerance, and 9 achieved enteral autonomy. Interestingly, 5 of the 6 patients remaining TPN-dependent had an initial diagnosis of gastroschisis, and no gastroschisis patient achieved enteral autonomy. Acknowledging the small numbers in this series off of which to base conclusions, the authors suggest that gastroschisis might present specific abnormalities with respect to intestinal motility that interfere with some of the benefits of the STEP procedure.31
Figure 2.
Serial transverse enteroplasty procedure (STEP)
One of the notable risks of either a Bianchi intestinal lengthening or the STEP procedure is recurrent bowel dilation. In this setting, repeat STEP can be performed,32 and there is even a description of a successful post-STEP LILT33 Recurrent dilation, regardless of the initial lengthening procedure appears to be associated with a worse prognosis.34
Conclusion
Given the rarity of lengthening procedures, there is no good evidence suggesting superiority of either LILT or STEP procedures. A recent review by Frongia et al35 extracted data from 39 retrospective case series on 363 and 109 patients undergoing LILT and STEP procedures, respectively. They found the procedures to have similar historical impact on improvement of enteral nutrition and reversal of parenteral nutrition-related complications, but a slightly lower rate of mortality and progression to transplantation for the STEP procedure. Certainly a larger study would need to be conducted to prove the superiority of one procedure over the other. Both options, however, appear to be safe and successful in preventing the most severe sequelae of short bowel syndrome, parenteral nutrition, and the need for intestinal transplantation.
Summary points.
Timing of surgical intervention in short bowel syndrome patients is crucial in preventing unnecessary procedures and maximizing the likelihood of enteral independence
Lengthening procedures commonly used in patients with dilated bowel are safe and moderately successful in achieving enteral independence in carefully selected patients
Both longitudinal and serial transverse lengthening procedures have shown good results and no evidence supports the superiority of one procedure over the other.
Footnotes
Conflicts of interest: The authors have no financial or other conflicts of interest to disclose.
References
- 1.Desai CS, Khan KM, Gruessner AC, Fishbein TM, Gruessner RW. Intestinal retransplantation: analysis of Organ Procurement and Transplantation Network database. Transplantation. 2012;93(1):120–125. doi: 10.1097/TP.0b013e31823aa54d. [DOI] [PubMed] [Google Scholar]
- 2.Chaet MS, Farrell MK, Ziegler MM, Warner BW. Intensive nutritional support and remedial surgical intervention for extreme short bowel syndrome. J Pediatr Gastroenterol Nutr. 1994;19(3):295–298. doi: 10.1097/00005176-199410000-00006. [DOI] [PubMed] [Google Scholar]
- 3. Wood SJ, Khalil B, Fusaro F, Folaranmi SE, Sparks SA, Morabito A. Early structured surgical management plan for neonates with short bowel syndrome may improve outcomes. World J Surg. 2013;37(7):1714–1717. doi: 10.1007/s00268-013-2011-z. Case series suggesting that in select patients, early surgery for short bowel syndrome is associated with fewer central line complications and earlier weaning from parenteral nutrition
- 4.Stahlgren LH, Roy RH, Umana G. A Mechanical Impediment to Intestinal Flow; Physiological Effects on Intestinal Absorption. JAMA. 1964;187:41–44. doi: 10.1001/jama.1964.03060140047011. [DOI] [PubMed] [Google Scholar]
- 5.Thompson JS, Sudan DA, Gilroy R. Predicting outcome of procedures to slow intestinal transit. Transpl Proc. 2006;38(6):1838–1839. doi: 10.1016/j.transproceed.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Collins J, 3rd, Vicente Y, Georgeson K, Kelly D. Partial intestinal obstruction induces substantial mucosal proliferation in the pig. J Pediatr Surg. 1996;31(3):415–419. doi: 10.1016/s0022-3468(96)90750-2. [DOI] [PubMed] [Google Scholar]
- 7.Georgeson K, Halpin D, Figueroa R, Vincente Y, Hardin W., Jr Sequential intestinal lengthening procedures for refractory short bowel syndrome. J Pediatr Surg. 1994;29(2):316–320. doi: 10.1016/0022-3468(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 8.Cuschieri A. Surgical management of severe intractable postvagotomy diarrhoea. The Br J Surg. 1986;73(12):981–984. doi: 10.1002/bjs.1800731212. [DOI] [PubMed] [Google Scholar]
- 9.Warden MJ, Wesley JR. Small bowel reversal procedure for treatment of the "short gut" baby. J Pediatr Surg. 1978;13(3):321–323. doi: 10.1016/s0022-3468(78)80407-2. [DOI] [PubMed] [Google Scholar]
- Beyer-Berjot L, Joly F, Maggiori L, et al. Segmental reversal of the small bowel can end permanent parenteral nutrition dependency: an experience of 38 adults with short bowel syndrome. Ann Surg. 2012;256(5):739–744. doi: 10.1097/SLA.0b013e31827387f5. Largest case series describing efficacy of reversed intestinal segments in adults with short bowel syndrome
- 11.Miranda R, Steffes B, O'Leary JP, Woodward ER. Surgical treatment of the postgastrectomy dumping syndrome. Am J Surg. 1980;139(1):40–43. doi: 10.1016/0002-9610(80)90227-5. [DOI] [PubMed] [Google Scholar]
- 12.Kono K, Sekikawa T, Iizuka H, et al. Interposed colon between remnants of the small intestine exhibits small bowel features in a patient with short bowel syndrome. Dig Surg. 2001;18(3):237–241. doi: 10.1159/000050143. [DOI] [PubMed] [Google Scholar]
- 13.King DR, Anvari M, Jamieson GG, King JM. Does the colon adopt small bowel features in a small bowel environment? Austr NZ J Surgery. 1996;66(8):543–546. doi: 10.1111/j.1445-2197.1996.tb00806.x. [DOI] [PubMed] [Google Scholar]
- 14.Hutcher NE, Mendez-Picon G, Salzberg AM. Prejejunal transposition of colon to prevent the development of short bowel syndrome in puppies with 90 per cent small intestine resection. J Pediatr Surg. 1973;8(5):771–777. doi: 10.1016/0022-3468(73)90420-x. [DOI] [PubMed] [Google Scholar]
- 15.Glick PL, de Lorimier AA, Adzick NS, Harrison MR. Colon interposition: an adjuvant operation for short-gut syndrome. J Pediatr Surg. 1984;19(6):719–725. doi: 10.1016/s0022-3468(84)80358-9. [DOI] [PubMed] [Google Scholar]
- 16.Almond SL, Haveliwala Z, Khalil B, Morabito A. Autologous intestinal reconstructive surgery to reduce bowel dilatation improves intestinal adaptation in children with short bowel syndrome. J Pediatr Gastroenterol Nutri. 2013;56(6):631–634. doi: 10.1097/MPG.0b013e318287de8d. [DOI] [PubMed] [Google Scholar]
- 17.Thompson JS, Langnas AN, Pinch LW, Kaufman S, Quigley EM, Vanderhoof JA. Surgical approach to short-bowel syndrome. Experience in a population of 160 patients. Ann Surg. 1995;222(4):600–605. doi: 10.1097/00000658-199522240-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber TR, Vane DW, Grosfeld JL. Tapering enteroplasty in infants with bowel atresia and short gut. Arch Surg. 1982;117(5):684–688. doi: 10.1001/archsurg.1982.01380290130023. [DOI] [PubMed] [Google Scholar]
- 19.de Lorimier AA, Harrison MR. Intestinal plication in the treatment of atresia. J Pediatr Surg. 1983;18(6):734–737. doi: 10.1016/s0022-3468(83)80014-1. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi A. Intestinal loop lengthening--a technique for increasing small intestinal length. J Pediatr Surg. 1980;15(2):145–151. doi: 10.1016/s0022-3468(80)80005-4. [DOI] [PubMed] [Google Scholar]
- 21.Bianchi A. Autologous gastrointestinal reconstruction. Sem Pediatric Surgery. 1995;4(1):54–59. [PubMed] [Google Scholar]
- 22.Chahine AA, Ricketts RR. A modification of the Bianchi intestinal lengthening procedure with a single anastomosis. J Pediatr Surg. 1998;33(8):1292–1293. doi: 10.1016/s0022-3468(98)90171-3. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi A. Experience with longitudinal intestinal lengthening and tailoring. Eur J Pediatr Surg. 1999;9(4):256–259. doi: 10.1055/s-2008-1072258. [DOI] [PubMed] [Google Scholar]
- 24.Bueno J, Guiterrez J, Mazariegos GV, et al. Analysis of patients with longitudinal intestinal lengthening procedure referred for intestinal transplantation. J Pediatr Surg. 2001;36(1):178–183. doi: 10.1053/jpsu.2001.20047. [DOI] [PubMed] [Google Scholar]
- 25.Waag KL, Hosie S, Wessel L. What do children look like after longitudinal intestinal lengthening. Eur J Pediatr Surg. 1999;9(4):260–262. doi: 10.1055/s-2008-1072259. [DOI] [PubMed] [Google Scholar]
- 26.Sudan D, Thompson J, Botha J, et al. Comparison of intestinal lengthening procedures for patients with short bowel syndrome. Ann Surg. 2007;246(4):593–601. doi: 10.1097/SLA.0b013e318155aa0c. [DOI] [PubMed] [Google Scholar]
- 27.Reinshagen K, Kabs C, Wirth H, et al. Long-term outcome in patients with short bowel syndrome after longitudinal intestinal lengthening and tailoring. J Pediatr Gastroenterol Nutr. 2008;47(5):573–578. doi: 10.1097/mpg.0b013e31816232e3. [DOI] [PubMed] [Google Scholar]
- 28.Kim HB, Fauza D, Garza J, Oh JT, Nurko S, Jaksic T. Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. J Pediatr Surg. 2003;38(3):425–429. doi: 10.1053/jpsu.2003.50073. [DOI] [PubMed] [Google Scholar]
- 29.Jones BA, Hull MA, Potanos KM, et al. Report of 111 consecutive patients enrolled in the International Serial Transverse Enteroplasty (STEP) Data Registry: a retrospective observational study. J Am Coll Surg. 2013;216(3):438–446. doi: 10.1016/j.jamcollsurg.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira C, de Silva N, Wales PW. Five-year outcomes after serial transverse enteroplasty in children with short bowel syndrome. J Pediatr Surg. 2012;47(5):931–937. doi: 10.1016/j.jpedsurg.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 31.Javid PJ, Sanchez SE, Horslen SP, Healey PJ. Intestinal lengthening and nutritional outcomes in children with short bowel syndrome. Am J Surg. 2013;205(5):576–580. doi: 10.1016/j.amjsurg.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Piper H, Modi BP, Kim HB, Fauza D, Glickman J, Jaksic T. The second STEP: the feasibility of repeat serial transverse enteroplasty. J Pediatr Surg. 2006;41(12):1951–1956. doi: 10.1016/j.jpedsurg.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Fusaro F, Hermans D, Wanty C, Veyckemans F, Pirenne J, Reding R. Post-serial transverse enteroplasty bowel redilatation treated by longitudinal intestinal lengthening and tailoring procedure. J Pediatr Surg. 2012;47(10):e19–e22. doi: 10.1016/j.jpedsurg.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 34.Miyasaka EA, Brown PI, Teitelbaum DH. Redilation of bowel after intestinal lengthening procedures--an indicator for poor outcome. J Pediatr Surg. 2011;46(1):145–149. doi: 10.1016/j.jpedsurg.2010.09.084. [DOI] [PubMed] [Google Scholar]
- 35.Frongia G, Kessler M, Weih S, Nickkholgh A, Mehrabi A, Holland-Cunz S. Comparison of LILT and STEP procedures in children with short bowel syndrome - A systematic review of the literature. J Pediatr Surg. 2013;48(8):1794–1805. doi: 10.1016/j.jpedsurg.2013.05.018. [DOI] [PubMed] [Google Scholar]