Abstract
Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV) was first identified in 2012, and it continues to threaten human health worldwide. No MERS vaccines are licensed for human use, reinforcing the urgency to develop safe and efficacious vaccines to prevent MERS. MERS-CoV spike protein forms a trimer, and its receptor-binding domain (RBD) serves as a vaccine target. Nevertheless, the protective efficacy of RBD in its native trimeric form has never been evaluated. In this study, a trimeric protein, RBD-Fd, was generated by fusing RBD with foldon trimerization motif. It bound strongly to the receptor of MERS-CoV, dipeptidyl peptidase 4 (DPP4), and elicited robust RBD-specific neutralizing antibodies in mice, maintaining long-term neutralizing activity against MERS-CoV infection. RBD-Fd potently protected hDPP4 transgenic mice from lethal MERS-CoV challenge. These results suggest that MERS-CoV RBD in its trimeric form maintains native conformation and induces protective neutralizing antibodies, making it a candidate for further therapeutic development.
Keywords: MERS, MERS-CoV, Spike protein, Receptor-binding domain, Foldon trimerization motif, Neutralization, hDPP4-transgenic mice, Protection
Graphical abstract
Highlights
-
•
A trimeric MERS-CoV protein (RBD-Fd) was constructed by fusing viral RBD with foldon trimerization motif.
-
•
RBD-Fd bound strongly to dipeptidyl peptidase 4 (DPP4), the receptor of MERS-CoV, and RBD-specific neutralizing antibodies.
-
•
RBD-Fd induced robust and long-term neutralizing antibodies, cross-neutralizing MERS pseudovirus of divergent strains.
-
•
RBD-Fd potently protected hDPP4 transgenic mice from lethal MERS-CoV challenge.
1. Introduction
Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV) was first identified in Saudi Arabia in 2012, and since then, it has resulted in increased incidence of human infection (Zaki et al., 2012, Chan et al., 2012, Rha et al., 2015). As of October 04, 2016, a total of 1806 laboratory-confirmed MERS cases, including 643 deaths (mortality rate ~36%), have been reported worldwide (http://www.who.int/emergencies/mers-cov/en/). Bats are regarded as a natural reservoir of MERS-CoV, and the potential mechanisms driving bat-to-human transmission of MERS-CoV are being investigated by comparing MERS-CoV with a closely related bat coronavirus, HKU-4 (Yang et al., 2014, Memish et al., 2013, Ithete et al., 2013, Cui et al., 2013, Wang et al., 2014). Dromedary camels are shown to be an important intermediate host for MERS-CoV, and, as such, they are considered the key to stopping MERS-CoV transmission (Adney et al., 2014, Yusof et al., 2015, Gossner et al., 2016). The severe outbreak of MERS-CoV in South Korea in 2015 demonstrated the ability of this virus to cause human-to-human transmission (Khan et al., 2015, Ki, 2015). The continuing spread of MERS-CoV and increase of MERS cases have highlighted the urgent need to develop effective and safe vaccines against MERS-CoV.
The native spike (S) of MERS-CoV is an envelope glycoprotein presented as a trimer on the virus surface. It is cleaved during virus infection by host cell proteases into S1 and S2 subunits. S1 is responsible for MERS-CoV binding to host cells expressing viral receptor dipeptidyl peptidase 4 (DPP4) via the receptor-binding domain (RBD) (Raj et al., 2013, Lu et al., 2013, Wang et al., 2013, Chen et al., 2013, Li, 2015). Meanwhile, S2 is engaged in fusion between virus and cell surface membranes, thus mediating the entry of MERS-CoV into target cells (Gao et al., 2013, Lu et al., 2014). These activities make MERS-CoV S protein, in particular its RBD, an important vaccine target (Zhang et al., 2014, Du et al., 2013b, Du et al., 2013a, Ma et al., 2014a, Ma et al., 2014b). A number of fragments have been identified as constituents of MERS-CoV RBD, but the fragment containing residues 377–588 in the viral S protein is the critical neutralizing domain (CND) responsible for the induction of neutralizing antibodies in immunized animals (Ma et al., 2014a, Ma et al., 2014b, Zhang et al., 2015). Nevertheless, the RBD in its native trimeric form has never been evaluated for protective efficacy against MERS-CoV infection.
Foldon (Fd), a 27-amino acid phage T4 fibritin, is a trimerization motif which is now being constructed as a fusion partner to guide formation of ultra-stable protein fibers or promote trimerization of recombinant fusion proteins (Bhardwaj et al., 2008, Papanikolopoulou et al., 2004, Sissoeff et al., 2005). For example, influenza virus hemagglutinins (HAs) with an Fd-formed trimerization domain have increased binding affinity to neutralizing antibodies targeting the stalk region of viral HAs (Krammer et al., 2012). In addition, recombinant HIV-1 trimeric envelope glycoproteins induced more efficient and broad-spectrum neutralizing antibodies with increased potency than monomeric gp120 protein against divergent HIV-1 strains (Grundner et al., 2005). These reports reinforce the importance of mimicking native viral trimeric structures in the design of envelope glycoprotein-based subunit vaccines. However, imitating conformational structures of MERS-CoV S protein has never been attempted in MERS vaccine design.
In this study, we expressed a recombinant trimeric RBD protein by fusing the MERS-CoV RBD sequence (residues 377–588) with Fd trimerization motif (RBD-Fd). We then investigated its antigenicity, receptor binding affinity, immunogenicity, neutralization potential, and protective efficacy against MERS-CoV challenge in a human DPP4-transgenic (hDPP4-Tg) mouse model. The outcomes of this study indicate the potential of developing MERS subunit vaccines based on the trimeric RBD of MERS-CoV S protein.
2. Materials and methods
2.1. Ethics statement
Four- to six-week-old female BALB/c mice and four-month-old male and female hDPP4-Tg mice were used in the study. The animal studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocols were approved by the Committee on the Ethics of Animal Experiments of the New York Blood Center (Permit Number: 194.17) and the Beijing Institute of Microbiology and Epidemiology (Permit Number: PMB15-0012).
2.2. Construction, expression and purification of recombinant proteins
The construction, expression and purification of the recombinant proteins were carried out as previously described (Du et al., 2013a; Ma et al., 2014a, Ma et al., 2014b; Du et al., 2013b). Briefly, recombinant RBD-Fd (residues 377–588 of MERS-CoV RBD) containing a C-terminal Fd and a His6 tag was constructed using hIgG-Fc vector (InvivoGen), and expressed in the transfected 293T cell culture supernatants. Recombinant MERS-CoV S1 (residues 18–725) containing a C-terminal His6 (S1-His) was constructed using pJW4303 expression vector, and expressed as described above. Recombinant human DPP4 ectodomain (residues 39–766) containing a C-terminal His6 (sDPP4) was expressed in insect cells using the Bac-to-Bac expression system (Invitrogen), and secreted into cell culture medium (Chen et al., 2013). The aforementioned His-tag proteins were purified using Ni-NTA Superflow (Qiagen).
2.3. SDS-PAGE and western blot
RBD-Fd was analyzed by SDS-PAGE and Western blot as previously described (Ma et al., 2014a; Du et al., 2013b). Briefly, the boiled and non-boiled proteins were separated by 10% Tris-Glycine SDS-PAGE gels and then transferred to nitrocellulose membranes. After blocking overnight at 4 °C with 5% non-fat milk in PBST, the blots were incubated for 1 h at room temperature with MERS-CoV S1-specific antibody (1:1000). After three washes, the blots were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:3000) (GE Healthcare) for 1 h at room temperature, and the signals were visualized using ECL Western blot substrate reagents and Amersham Hyperfilm (GE Healthcare).
2.4. Size-exclusion chromatography with multi-angle light scattering (SEC-MALS)
RBD-Fd protein was concentrated to 2 mg/ml in buffer containing 50 mM PBS and 150 mM NaCl, and analyzed in-line using tandem miniDAWN TREOS MALS and Optilab rEX differential refractive index detectors (Wyatt Technologies) at the PEPCC Facility of Case Western Reserve University. The protein was loaded for molar mass determinations, and the data were analyzed using the ASTRA 6.1 software package (Wyatt Technologies). The size distribution of the protein was measured by DynaPro NanoStar (Wyatt Technologies).
2.5. Co-immunoprecipitation assay
The binding between RBD-Fd protein and DPP4 receptor was carried out by Co-immunoprecipitation (Co-IP) assay and Western blot as previously described (Du et al., 2013a). Briefly, RBD-Fd (10 µg) was incubated with DPP4-expressing Huh-7 cell lysates (5×107/ml) plus Ni-NTA Superflow at 4 °C for 1 h, followed by washing with wash buffer. The eluted proteins were boiled for 10 min and subjected to SDS-PAGE and Western blot analysis to detect the binding using anti-DPP4 antibody (0.2 µg/ml) (R&D Systems) and anti-MERS-CoV RBD antibody (1:5000), respectively.
2.6. Animal immunization and sample collection
BALB/c mice were immunized with RBD-Fd as previously described (Ma et al., 2014a; Ma et al., 2014b). Briefly, mice were subcutaneously (s.c.) vaccinated with RBD-Fd (10 μg/mouse) in the presence of MF59 adjuvant (Schultze et al., 2008) and boosted with the same immunogen and adjuvant at 3 weeks, 6 weeks, and 6 months post-immunization. PBS plus adjuvant was included as the negative control. Sera were collected monthly post-first dose for 6 months to detect MERS-CoV S1-specific antibody responses and/or neutralizing antibodies.
2.7. ELISA
ELISA was carried out to detect the binding between RBD-Fd and sDPP4 proteins as previously described (Du et al., 2013a). Briefly, ELISA plates were precoated with RBD-Fd (2 μg/ml) overnight at 4 °C and blocked with 2% non-fat milk at 37 °C for 2 h. Serially diluted sDPP4 was added to the plates and incubated at 37 °C for 1 h, followed by four washes. Bound antibodies were sequentially incubated with goat anti-DPP4 (0.2 µg/ml) and HRP-conjugated anti-goat IgG (1:5000) (R&D Systems) antibodies at 37 °C for 1 h, followed by addition of substrate 3,3′,5,5′-tetramethylbenzidine (TMB) (Invitrogen) and H2SO4 (1 N) sequentially. The absorbance at 450 nm (A450) was measured by an ELISA plate reader (Tecan).
The binding between RBD-Fd protein and MERS-CoV RBD-specific mouse mAb Mersmab1 and human mAbs m336, m337 and m338 (Du et al., 2014, Ying et al., 2014) was carried out using a protocol similar to that described above, but replacing the primary antibody with serially diluted mAbs. The HRP-conjugated anti-mouse (1:3000) (GE Healthcare) or anti-human (1:5000) (Sigma) IgG antibodies were used as secondary antibodies.
For detecting MERS-CoV S1-specific antibodies in mouse sera, plates were coated with MERS-CoV S1-His protein (1 μg/ml), followed by sequential addition of serially diluted mouse sera and HRP-conjugated anti-mouse IgG (1:3000), IgG1 (1:2000), or IgG2a (1:5000) antibodies (Invitrogen).
2.8. MERS pseudovirus inhibition and neutralization assays
These assays were carried out as previously described with some modifications (Zhao et al., 2013, Du et al., 2010). Briefly, 293T cells were co-transfected with a plasmid encoding Env-defective, luciferase-expressing HIV-1 genome (pNL4-3.luc. RE) and a plasmid encoding S protein of MERS-CoV prototypic strain (EMC-2012), or each of the representing strains isolated in the 2012–2015 outbreaks that carry respective mutations in the RBD (Qiu et al., 2016), using the calcium phosphate method. The pseudovirus-containing supernatants were harvested 72 h post-transfection for single-cycle infection of Huh-7 cells. For the inhibition assay, Huh-7 cells were incubated with serially diluted RBD-Fd protein at 37 °C for 1 h, followed by removal of the protein-containing media and then infection with MERS pseudovirus. For the neutralization assay, MERS pseudovirus was incubated with serially diluted mouse sera at 37 °C for 1 h, followed by addition of the mixture to the cells. The cells were refed with fresh medium 24 h later, lysed at 72 h with cell lysis buffer (Promega), and transferred into 96-well luminometer plates. Luciferase substrate (Promega) was added to the plates, and relative luciferase activity was determined in an Infinite 200 PRO Luminator (Tecan). MERS pseudovirus inhibition and neutralization were calculated as previously described (Chou, 2006), and expressed as 50% inhibition dose (ID50) and neutralizing antibody titer (NT50), respectively.
2.9. Live MERS-CoV neutralization assay
A micro-neutralization assay was carried out to detect neutralizing antibodies against live MERS-CoV infection as previously described (Tao et al., 2013; Ma et al., 2014b). Briefly, mouse sera were diluted at a serial 2-fold in 96-well tissue culture plates, followed by incubation for 1 h at room temperature with ~100 infectious MERS-CoV (EMC-2012) before transferring to Vero E6 cells. After 72 h, cells were observed for the presence or absence of virus-induced cytopathic effect (CPE), and the neutralizing antibody titers were determined as the reciprocal of the highest dilution of sera that completely inhibited virus-induced CPE in at least 50% of the wells (NT50).
2.10. Animal challenge studies
The challenge studies were carried out in hDPP4-Tg mice using our previously optimized protocols (Zhao et al., 2015). Briefly, mice were intramuscularly (i.m.) immunized with RBD-Fd (5 μg/mouse) plus aluminum adjuvant and boosted once at 4 weeks. Immunized mice were challenged with MERS-CoV (EMC-2012 strain, 104 TCID50) 12 weeks later, and observed for 3 weeks post-challenge to detect survival rate, body weight, and pathological changes.
3. Results
3.1. Characterization and antigenicity of RBD-Fd protein
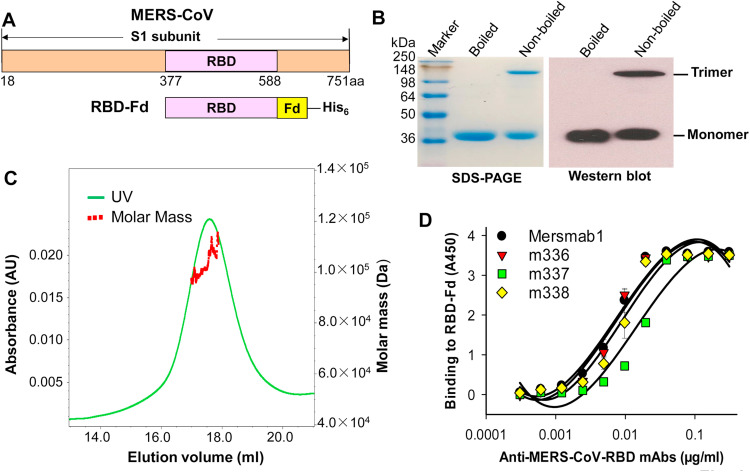
To determine whether MERS-CoV RBD containing the CND (residues 377–588) would form a native trimeric structure, we fused its C-terminus to Fd trimerization motif ( Fig. 1A), expressed RBD-Fd protein in 293T cell culture supernatants, and further characterized the protein by SDS-PAGE and Western blot analysis. A high molecular-weight band (trimer) was shown in the non-denatured (non-boiled) sample, which was 3 times that of the denatured (boiled) protein (monomer) (Fig. 1B, left). The trimeric molecule of the RBD-Fd protein was further confirmed by SEC-MALS analysis, indicating the molar mass of about 1.043×105 (±0.036×105) Da (Fig. 1C). These results demonstrate that recombinant RBD formed a trimeric conformation in the presence of Fd trimerization motif. In addition, RBD-Fd had a strong reactivity with antibodies against a recombinant S1 protein of MERS-CoV (Fig. 1B, right), suggesting that the trimeric RBD is derived from MERS-CoV S protein.
Fig. 1.
Construction, characterization and antigenicity evaluation of MERS-CoV RBD-Fd protein. (A) Schematic structure of MERS-CoV S1 subunit and construction of RBD-Fd. A His6 tag was added at the C-terminus of RBD-Fd for easy purification. (B) SDS-PAGE and Western blot analysis of purified RBD-Fd protein. Denatured (boiled) or non-denatured (non-boiled) samples (5 µg) were subjected to SDS-PAGE (left) and Western blot analysis (right) using MERS-CoV S1-specific antibody. The molecular weight marker (kDa) is indicated on the left. (C) SEC-MALS analysis of purified of RBD-Fd protein. RBD-Fd (2 mg/ml, 100 μl) was subjected to the analysis. The data are presented as mean molar mass (Da). (D) ELISA detection of the binding between RBD-Fd and RBD-specific neutralizing mAbs. RBD-Fd (2 μg/ml)-coated ELISA plates were incubated with serially diluted mAbs for the test.
We further investigated the antigenicity of RBD-Fd protein by testing its binding affinity with neutralizing mAbs specific to the conformational epitopes in MERS-CoV RBD (Ying et al., 2014, Du et al., 2014). ELISA data revealed that RBD-Fd bound strongly to these antibodies and that the binding occurred in a dose-dependent manner (Fig. 1D), suggesting that trimeric MERS-CoV RBD maintains the antigenicity required to recognize RBD-specific antibodies that target conformational epitopes.
3.2. Binding affinity of RBD-Fd protein to DPP4, the receptor of MERS-CoV
Co-IP assay and ELISA were performed to determine the affinity of RBD-Fd binding to cell-associated and recombinant DPP4, respectively. Co-IP results revealed two clear bands in the pull-down samples containing Huh-7 cell lysates and RBD-Fd protein, which correspond to the size of DPP4 and RBD-Fd monomers, and were identified by anti-DPP4 ( Fig. 2A) and anti-MERS-CoV-RBD (Fig. 2B) antibodies, respectively. However, soluble DPP4 (sDPP4) or RBD-Fd protein alone could only be recognized by either anti-DPP4 or anti-MERS-CoV-RBD, but not by both antibodies (Fig. 2A and B). The results from ELISA further revealed a strong binding between RBD-Fd and sDPP4 proteins in a dose-dependent manner (Fig. 2C). These data confirm the binding affinity of the trimeric MERS-CoV RBD protein to the cell-associated and recombinant DPP4 proteins.
Fig. 2.
Functionality of RBD-Fd protein in binding to the DPP4 receptor of MERS-CoV. (A-B) Co-IP and Western blot analysis of the binding between RBD-Fd and cell-associated DPP4. RBD-Fd was incubated with DPP4-expressing Huh-7 cell lysates in the presence of Ni-NTA Superflow, followed by detection of the binding by Western blot using DPP4- (0.2 µg/ml) (A) or MERS-CoV RBD-specific antibody (B) (1:5000). sDPP4 and RBD-Fd proteins only were included as the controls. The molecular weight marker (kDa) is indicated on the left. (C) ELISA measurement of the binding between RBD-Fd and sDPP4 proteins. RBD-Fd (2 μg/ml)-coated ELISA plates were incubated with serially diluted sDPP4 for the test.
3.3. RBD-Fd protein strongly inhibited MERS-CoV infection and induced long-term potent antibody responses with cross-neutralizing activity against MERS-CoV infection
To test the ability of trimeric RBD-Fd to inhibit MERS-CoV infection, we incubated this protein with Huh-7 cells, and calculated the corresponding inhibition of infection as ID50. The data demonstrated that RBD-Fd strongly blocked MERS pseudovirus entry into the target cells, with the ID50 as low as 1.5 μg/ml, whereas the control protein had no inhibitory activity even at the highest concentration ( Fig. 3A). These data suggest the potential application of RBD-Fd as an effective therapeutic agent against MERS-CoV infection.
Fig. 3.
Inhibitory activity of RBD-Fd and its immunogenicity and neutralizing ability in immunized mice. (A) Inhibitory activity of RBD-Fd protein against infections of pseudotyped MERS-CoV (EMC2012) in Huh-7 cells. An unrelated SARS-CoV RBD protein was included as the control. The data are presented as 50% inhibition dose (ID50). (B) MERS-CoV S1-specific IgG antibody titers. Mouse sera from different months were used for the test. MERS-CoV S1-specific IgG1 (C) and IgG2a (D) antibody titers. Mouse sera from 2 and 6 months post-1st immunization were used for the test. The antibody titers are expressed as the endpoint dilution that remains positively detectable, and presented as mean titers ±SD of five mice in each group. RBD-Fd-induced neutralizing antibodies against infections of pseudotyped (E) and live (F) MERS-CoV of EMC2012 strain, as well as pseudotyped MERS-CoV of representative strains isolated from the 2012 (L506F), 2013 (A434V, A431P-A482V, S460F), 2014 (Q522H, T424I), and 2015 (V530L, V534A) outbreaks, respectively (G). Shown here are respective mutations in the RBD of S protein, as compared with those of EMC2012 strain (Qiu et al., 2016). Neutralizing antibody titers were calculated as the reciprocal of the highest dilution of sera that resulted in a complete inhibition of MERS pseudovirus infection (E and G) or MERS-CoV-induced CPE (F) in at least 50% of the wells (NT50). Mouse sera from 2 and 6 months were used for the test, and the data are presented as mean titers ±SD of five mice in each group. For (B)-(G), PBS was included as the control.
To investigate the immunogenicity of trimeric RBD-Fd, we used this protein to s.c. immunize BALB/c mice in the presence of MF59 adjuvant since this protocol has been previously optimized for RBD-based vaccines in the test mouse strain (Zhang et al., 2016). We then measured the generation of MERS-CoV S1-specific IgG antibodies, as well as IgG1 and IgG2a subtypes, using ELISA in the immunized mouse sera. As shown in Fig. 3B, RBD-Fd induced highly potent MERS-CoV S1-specific IgG antibodies, which maintained for at least 6 months during the detection period. As expected, only a background level of IgG antibody responses was induced in the control mice. Evaluation of IgG1 and Ig2a subtypes further demonstrated that both Th1 (IgG2a) and Th2 (IgG1) antibody responses could be elicited by RBD-Fd and for at least the next six months, all maintained titers similar to, or higher than, those induced at 2 months post-immunization (Fig. 3C and D). In contrast, no specific IgG1 and IgG2a antibodies were induced in the control mice injected with PBS. These data confirm high immunogenicity of trimeric MERS-CoV RBD in inducing long-term, strong humoral immune responses in mice.
The neutralizing antibodies in the sera of mice immunized with trimeric RBD-Fd were detected by MERS pseudovirus and live MERS-CoV-based neutralization assays. Indeed, RBD-Fd elicited highly potent neutralizing antibodies against infections of both pseudotyped and live MERS-CoV of prototypic EMC2012 strain in susceptible Huh-7 and Vero E6 cells, respectively, maintaining similar levels at 2 and 6 months. Importantly, these antibodies strongly cross-neutralized infections of MERS pseudoviruses expressing S proteins of divergent strains isolated in the 2012–2015 outbreaks, demonstrating their cross-neutralizing activity. However, no specific neutralizing antibody was induced in the PBS control mice (Fig. 3E–G). The above results suggest that trimeric MERS-CoV RBD has ability to elicit long-term and potent neutralizing antibodies in mice.
3.4. RBD-Fd protein elicited long-term protective immunity in hDPP4-Tg mice against lethal infection of MERS-CoV
To investigate the ability of trimeric RBD-Fd to induce protective immunity against MERS-CoV challenge, we utilized this protein to i.m. immunize hDPP4-Tg mice, a small animal model on the C57BL/6 background that is susceptible to MERS-CoV infection (Zhao et al., 2015), in the presence of aluminum adjuvant. The above immunization protocol was selected because that it is optimized for hDPP4-Tg mice (unpublished data), and that aluminum is an approved adjuvant for using with human vaccines (Lee and Nguyen, 2015). We then challenged the immunized mice with MERS-CoV at a lethal dose, and evaluated for their survivals, weight, and pathological changes.
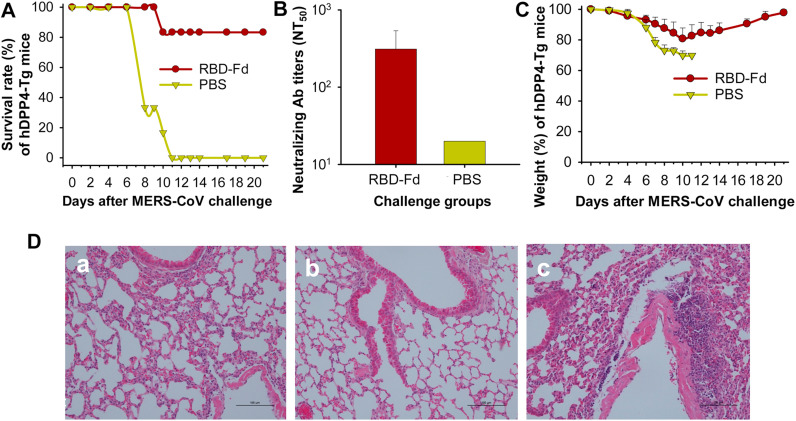
The data demonstrated that 83% of the RBD-Fd-immunized mice survived from lethal MERS-CoV infection ( Fig. 4A). These mice had neutralizing antibody titers ranging from 1:100 to 1:673 (average 1:312) against live MERS-CoV infection before virus challenge (Fig. 4B). Although the challenged mice slightly reduced weight during day 8–14 after MERS-CoV challenge, they rapidly recovered to the normal weight (Fig. 4C). In contrast, the control mice with a background level of neutralizing antibodies in their sera kept continual reduction of the weight after virus challenge, and all mice (100%) died at day 11 (Fig. 4A–C). Observation of pathological changes in the challenged mouse lungs revealed almost normal lung tissues in the RBD-Fd-immunized mice (Fig. 4Da). These lung tissues were similar to those of normal mice (Fig. 4Db), with only slightly thickened alveolar wall (Fig. 4Da). By comparison, the PBS control mice challenged with MERS-CoV had marked interstitial pneumonia, including inflammatory cell infiltration, alveolar septal thickening, focal exudation, and hemorrhage (Fig. 4Dc). The hDPP4-Tg mice were immunized with RBD-Fd at 4 months old, boosted once 4 weeks later, challenged with MERS-CoV at 12 weeks post-boost, and survived for 3 weeks post-challenge before being sacrificed for evaluating pathological changes. Thus, the above results demonstrate the ability of RBD-Fd to induce long-term protective immunity in aging mice against MERS-CoV infection.
Fig. 4.
Protective efficacy of RBD-Fd in hDPP4-Tg mice. Groups of 6 mice were challenged with MERS-CoV at 12 weeks post-last dose and then observed for 3 weeks for survival rate (A) and body weight changes (C). The data are presented as mean with SD. (B) Neutralizing antibodies were detected in the sera of immunized mice before MERS-CoV challenge. Neutralizing antibody titers are presented as mean NT50 ±SD of six mice in each group. For (A)–(C), PBS was included as the control. (D) Evaluation of pathological changes in lung tissues of challenged mice. Representative images from mice immunized with RBD-Fd (a) and PBS (c) challenged with MERS-CoV (EMC2012) and normal mice (b) are shown. Lung tissue sections were stained with hematoxylin and eosin (H&E) and observed under light microscopy (100×magnification).
4. Discussion
Rapid development of a safe and efficacious vaccine with high potency has become the first priority to prevent continuous infection of MERS-CoV in humans. We have previously demonstrated that a RBD fragment containing residues 377–588 of MERS-CoV S protein is a critical neutralizing domain (CND) capable of inducing neutralizing antibodies against MERS-CoV infection (Ma et al., 2014b; Du et al., 2013a, 2016) and, hence, an important target for the development of subunit vaccines. It is known that native MERS-CoV S protein forms a trimer and is cleaved to form functional S1 and S2 subunits (Wang et al., 2013, Lu et al., 2013, Du et al., 2016), but it is still unknown whether the trimeric form of RBD (CND) of MERS-CoV S1 protein can induce protective immunity against MERS-CoV infection.
Fd, a trimerization motif, has been previously fused with recombinant proteins, such as influenza virus HA and HIV-1 glycoproteins and promotes them to form trimeric structures, subsequently stabilizing the proteins to broaden and increase their immunogenicity against infections from divergent virus strains (Grundner et al., 2005; G. Gao et al., 2013; Du et al., 2013c, Nkolola et al., 2010; Li et al., 2013). Using MERS-CoV RBD fused with Fd (RBD-Fd) as a model antigen, this study attempts to generate a MERS-CoV RBD trimer, and then evaluate its antigenicity, receptor binding affinity, immunogenicity, neutralizing potential, and protective efficacy against MERS-CoV infection.
Indeed, RBD-Fd protein formed a trimeric structure that bound strongly to MERS-CoV RBD-specific, conformational epitope-targeting mAbs, as well as MERS-CoV's receptor DPP4, demonstrating its capacity to elicit highly efficacious IgG antibodies, along with Th2-based IgG1 and Th1-based IgG2 antibody responses. These results suggest that the trimeric MERS-CoV RBD maintains potent antigenicity, strong receptor-binding affinity, and high immunogenicity to induce robust antibody responses in the immunized animals. Particularly, RBD-Fd protein was able to induce long-term and highly potent neutralizing antibodies against infections of live and pseudotyped MERS-CoV of divergent strains isolated in the 2012–2015 outbreaks, possibly because RBD-Fd forms a trimeric structure to mimic the native conformation of the CND in MERS-CoV S protein, thus eliciting vigorous cross-neutralizing antibodies.
Due to the low expression of RBD protein monomer in the mammalian cell expression system, we were unable to obtain sufficient monomeric RBD protein to include it as a control in this study. Nevertheless, 83% of the survival rate and minimal pathological changes in the RBD-Fd-immunized hDPP4-Tg mice demonstrate its high protective efficacy against lethal MERS-CoV challenge. To further improve its protective immunity, RBD-trimer could be optimized by stabilizing its trimeric structure with disulfide bonds, adding other trimeric motifs, such as GCN4, adjusting linker sequences between the RBD and Fd, or combining these approaches, as reported for other viral proteins (Yang et al., 2000a, Yang et al., 2000b, Ringe et al., 2015). Since RBD-Fd only induced a background level of T cell responses (data not shown), its protective efficacy is largely associated with neutralizing antibodies. This is evidenced by that all of the mice survived from MERS-CoV infection had relatively high serum neutralizing antibody titers, suggesting that neutralizing antibodies, rather than cellular immune responses, may play a key role to prevent MERS-CoV infection in trimeric RBD-based subunit vaccines.
Overall, this report confirms the capability of MERS-CoV RBD's critical neutralizing epitopes to form a trimeric structure, resulting in the induction of strong humoral immune responses, potent neutralizing antibodies, and significant protective efficacy. It further emphasizes the importance of maintaining the native conformational structure of S protein in developing MERS subunit vaccines. In this context, our study provides valuable guidance for the rational design of efficacious subunit vaccines against MERS-CoV and other viruses with class I membrane fusion proteins.
Conflict of interest statement
The authors declared no conflict of interest.
Acknowledgments
This study was supported by China National Program of Infectious Disease Fund (2014ZX10004001004) to YZ, and NIH Grants (R01AI098775, U01AI124260, and R21AI109094) to LD and SJ. We thank Drs. Dimiter S. Dimitrov and Tianlei Ying at the National Institutes of Health for providing m336, m337 and m338 mAbs.
Contributor Information
Shibo Jiang, Email: sjiang@nybc.org.
Lanying, Du, Email: ldu@nybc.org.
Yusen Zhou, Email: yszhou@bmi.ac.cn.
References
- Adney D.R., van D.N., Brown V.R., Bushmaker T., Scott D., de W.E., Bowen R.A., Munster V.J. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerg. Infect. Dis. 2014;20:1999–2005. doi: 10.3201/eid2012.141280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj A., Walker-Kopp N., Wilkens S., Cingolani G. Foldon-guided self-assembly of ultra-stable protein fibers. Protein Sci. 2008;17:1475–1485. doi: 10.1110/ps.036111.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Li K.S., To, Cheng K.K., Chen V.C., Yuen H., K. Y Is the discovery of the novel human betacoronavirus 2c EMC/2012 (HCoV-EMC) the beginning of another SARS-like pandemic? J. Infect. 2012;65:477–489. doi: 10.1016/j.jinf.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Rajashankar K.R., Yang Y., Agnihothram S.S., Liu C., Lin Y.L., Baric R.S., Li F. Crystal structure of the receptor-binding domain from newly emerged Middle East respiratory syndrome coronavirus. J. Virol. 2013;87:10777–10783. doi: 10.1128/JVI.01756-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- Cui J., Eden J.S., Holmes E.C., Wang L.F. Adaptive evolution of bat dipeptidyl peptidase 4 (dpp4): implications for the origin and emergence of Middle East respiratory syndrome coronavirus. Virol. J. 2013;10:304. doi: 10.1186/1743-422X-10-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Zhao G., Zhang X., Liu Z., Yu H., Zheng B.J., Zhou Y., Jiang S. Development of a safe and convenient neutralization assay for rapid screening of influenza HA-specific neutralizing monoclonal antibodies. Biochem. Biophys. Res. Commun. 2010;397:580–585. doi: 10.1016/j.bbrc.2010.05.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Kou Z., Ma C., Tao X., Wang L., Zhao G., Chen Y., Yu F., Tseng C.T., Zhou Y., Jiang S. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: implication for developing therapeutics and vaccines. PLoS One. 2013;8:e81587. doi: 10.1371/journal.pone.0081587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Zhao G., Kou Z., Ma C., Sun S., Poon V.K., Lu L., Wang L., Debnath A.K., Zheng B.J., Zhou Y., Jiang S. Identification of a receptor-binding domain in the S protein of the novel human coronavirus Middle East respiratory syndrome coronavirus as an essential target for vaccine development. J. Virol. 2013;87:9939–9942. doi: 10.1128/JVI.01048-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Zhao G., Sun S., Zhang X., Zhou X., Guo Y., Li Y., Zhou Y., Jiang S. A critical HA1 neutralizing domain of H5N1 influenza in an optimal conformation induces strong cross-protection. PLoS One. 2013;8:e53568. doi: 10.1371/journal.pone.0053568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Zhao G., Yang Y., Qiu H., Wang L., Kou Z., Tao X., Yu H., Sun S., Tseng C.T., Jiang S., Li F., Zhou Y. A conformation-dependent neutralizing monoclonal antibody specifically targeting receptor-binding domain in Middle East respiratory syndrome coronavirus spike protein. J. Virol. 2014;88:7045–7053. doi: 10.1128/JVI.00433-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Tai W., Zhou Y., Jiang S. Vaccines for the prevention against the threat of MERS-CoV. Expert. Rev. Vaccin. 2016;15:1123–1134. doi: 10.1586/14760584.2016.1167603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Wieczorek L., Peachman K.K., Polonis V.R., Alving C.R., Rao M., Rao V.B. Designing a soluble near full-length HIV-1 gp41 trimer. J. Biol. Chem. 2013;288:234–246. doi: 10.1074/jbc.M112.424432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Lu G., Qi J., Li Y., Wu Y., Deng Y., Geng H., Li H., Wang Q., Xiao H., Tan W., Yan J., Gao G.F. Structure of the fusion core and inhibition of fusion by a heptad-repeat peptide derived from the S protein of MERS-CoV. J. Virol. 2013;87:13134–13140. doi: 10.1128/JVI.02433-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossner C., Danielson N., Gervelmeyer A., Berthe F., Faye B., Kaasik A.K., Adlhoch C., Zeller H., Penttinen P., Coulombier D. Human-dromedary camel interactions and the risk of acquiring zoonotic Middle East respiratory syndrome coronavirus infection. Zoonoses Public Health. 2016;63:1–9. doi: 10.1111/zph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundner C., Li Y., Louder M., Mascola J., Yang X., Sodroski J., Wyatt R. Analysis of the neutralizing antibody response elicited in rabbits by repeated inoculation with trimeric HIV-1 envelope glycoproteins. Virology. 2005;331:33–46. doi: 10.1016/j.virol.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Ithete N.L., Stoffberg S., Corman V.M., Cottontail V.M., Richards L.R., Schoeman M.C., Drosten C., Drexler J.F., Preiser W. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg. Infect. Dis. 2013;19:1697–1699. doi: 10.3201/eid1910.130946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Farooqui A., Guan Y., Kelvin D.J. Lessons to learn from MERS-CoV outbreak in South Korea. J. Infect. Dev. Ctries. 2015;9:543–546. doi: 10.3855/jidc.7278. [DOI] [PubMed] [Google Scholar]
- Ki M. 2015 MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol. Health. 2015;37 doi: 10.4178/epih/e2015033. (e2015033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F., Margine I., Tan G.S., Pica N., Krause J.C., Palese P. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS One. 2012;7:e43603. doi: 10.1371/journal.pone.0043603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Nguyen M.T. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015;15:51–57. doi: 10.4110/in.2015.15.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J. Virol. 2015;89:1954–1964. doi: 10.1128/JVI.02615-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Du L., Qiu H., Zhao G., Wang L., Zhou Y., Jiang S., Gao J. A recombinant protein containing highly conserved hemagglutinin residues 81-122 of influenza H5N1 induces strong humoral and mucosal immune responses. Biosci. Trends. 2013;7:129–137. [PubMed] [Google Scholar]
- Lu G., Hu Y., Wang Q., Qi J., Gao F., Li Y., Zhang Y., Zhang W., Yuan Y., Bao J., Zhang B., Shi Y., Yan J., Gao G.F. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Liu Q., Zhu Y., Chan K.H., Qin L., Li Y., Wang Q., Chan J.F., Du L., Yu F., Ma C., Ye S., Yuen K.Y., Zhang R., Jiang S. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat. Commun. 2014;5:3067. doi: 10.1038/ncomms4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Li Y., Wang L., Zhao G., Tao X., Tseng C.T., Zhou Y., Du L., Jiang S. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: implication for designing novel mucosal MERS vaccines. Vaccine. 2014;32:2100–2108. doi: 10.1016/j.vaccine.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Wang L., Tao X., Zhang N., Yang Y., Tseng C.T., Li F., Zhou Y., Jiang S., Du L. Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments – the importance of immunofocusing in subunit vaccine design. Vaccine. 2014;32:6170–6176. doi: 10.1016/j.vaccine.2014.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish Z.A., Mishra N., Olival K.J., Fagbo S.F., Kapoor V., Epstein J.H., Alhakeem R., Durosinloun A., Al A.M., Islam A., Kapoor A., Briese T., Daszak P., Al Rabeeah A.A., Lipkin W.I. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg. Infect. Dis. 2013;19:1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkolola J.P., Peng H., Settembre E.C., Freeman M., Grandpre L.E., Devoy C., Lynch D.M., La P.A., Simmons N.L., Bradley R., Montefiori D.C., Seaman M.S., Chen B., Barouch D.H. Breadth of neutralizing antibodies elicited by stable, homogeneous clade A and clade C HIV-1 gp140 envelope trimers in guinea pigs. J. Virol. 2010;84:3270–3279. doi: 10.1128/JVI.02252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikolopoulou K., Forge V., Goeltz P., Mitraki A. Formation of highly stable chimeric trimers by fusion of an adenovirus fiber shaft fragment with the foldon domain of bacteriophage t4 fibritin. J. Biol. Chem. 2004;279:8991–8998. doi: 10.1074/jbc.M311791200. [DOI] [PubMed] [Google Scholar]
- Qiu H., Sun S., Xiao H., Feng J., Guo Y., Tai W., Wang Y., Du L., Zhao G., Zhou Y. Single-dose treatment with a humanized neutralizing antibody affords full protection of a human transgenic mouse model from lethal Middle East respiratory syndrome (MERS)-coronavirus infection. Antivir. Res. 2016;132:141–148. doi: 10.1016/j.antiviral.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V.S., Mou H., Smits S.L., Dekkers D.H., Muller M.A., Dijkman R., Muth D., Demmers J.A., Zaki A., Fouchier R.A., Thiel V., Drosten C., Rottier P.J., Osterhaus A.D., Bosch B.J., Haagmans B.L. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rha B., Rudd J., Feikin D., Watson J., Curns A.T., Swerdlow D.L., Pallansch M.A., Gerber S.I. Update on the epidemiology of Middle East respiratory syndrome coronavirus (MERS-CoV) infection, and guidance for the public, clinicians, and public health authorities - January 2015. Morb. Mortal. Wkly. Rep. 2015;64:61–62. [PMC free article] [PubMed] [Google Scholar]
- Ringe R.P., Yasmeen A., Ozorowski G., Go E.P., Pritchard L.K., Guttman M., Ketas T.A., Cottrell C.A., Wilson I.A., Sanders R.W., Cupo A., Crispin M., Lee K.K., Desaire H., Ward A.B., Klasse P.J., Moore J.P. Influences on the design and purification of soluble, recombinant native-like HIV-1 envelope glycoprotein trimers. J. Virol. 2015;89:12189–12210. doi: 10.1128/JVI.01768-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze V., D’Agosto V., Wack A., Novicki D., Zorn J., Hennig R. Safety of MF59 adjuvant. Vaccine. 2008;26:3209–3222. doi: 10.1016/j.vaccine.2008.03.093. [DOI] [PubMed] [Google Scholar]
- Sissoeff L., Mousli M., England P., Tuffereau C. Stable trimerization of recombinant rabies virus glycoprotein ectodomain is required for interaction with the p75NTR receptor. J. Gen. Virol. 2005;86:2543–2552. doi: 10.1099/vir.0.81063-0. [DOI] [PubMed] [Google Scholar]
- Tao X., Hill T.E., Morimoto C., Peters C.J., Ksiazek T.G., Tseng C.T. Bilateral entry and release of Middle East respiratory syndrome coronavirus induces profound apoptosis of human bronchial epithelial cells. J. Virol. 2013;87:9953–9958. doi: 10.1128/JVI.01562-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Shi X., Jiang L., Zhang S., Wang D., Tong P., Guo D., Fu L., Cui Y., Liu X., Arledge K.C., Chen Y.H., Zhang L., Wang X. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23:986–993. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Qi J., Yuan Y., Xuan Y., Han P., Wan Y., Ji W., Li Y., Wu Y., Wang J., Iwamoto A., Woo P.C., Yuen K.Y., Yan J., Lu G., Gao G.F. Bat origins of MERS-CoV supported by bat coronavirus HKU4 usage of human receptor CD26. Cell Host Microbe. 2014;16:328–337. doi: 10.1016/j.chom.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Farzan M., Wyatt R., Sodroski J. Characterization of stable, soluble trimers containing complete ectodomains of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 2000;74:5716–5725. doi: 10.1128/jvi.74.12.5716-5725.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Florin L., Farzan M., Kolchinsky P., Kwong P.D., Sodroski J., Wyatt R. Modifications that stabilize human immunodeficiency virus envelope glycoprotein trimers in solution. J. Virol. 2000;74:4746–4754. doi: 10.1128/jvi.74.10.4746-4754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Du L., Liu C., Wang L., Ma C., Tang J., Baric R.S., Jiang S., Li F. Receptor usage and cell entry of bat coronavirus HKU4 provide insight into bat-to-human transmission of MERS coronavirus. Proc. Natl. Acad. Sci. USA. 2014;111:12516–12521. doi: 10.1073/pnas.1405889111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying T., Du L., Ju T.W., Prabakaran P., Lau C.C., Lu L., Liu Q., Wang L., Feng Y., Wang Y., Zheng B.J., Yuen K.Y., Jiang S., Dimitrov D.S. Exceptionally potent neutralization of Middle East respiratory syndrome coronavirus by human monoclonal antibodies. J. Virol. 2014;88:7796–7805. doi: 10.1128/JVI.00912-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusof M.F., Eltahir Y.M., Serhan W.S., Hashem F.M., Elsayed E.A., Marzoug B.A., Abdelazim A.S., Bensalah O.K., Al Muhairi S.S. Prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels in Abu Dhabi Emirate, United Arab Emirates. Virus Genes. 2015;50:509–513. doi: 10.1007/s11262-015-1174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van B.S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhang N., Jiang S., Du L. Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert. Rev. Vaccin. 2014;13:761–774. doi: 10.1586/14760584.2014.912134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Tang J., Lu L., Jiang S., Du L. Receptor-binding domain-based subunit vaccines against MERS-CoV. Virus Res. 2015;202:151–159. doi: 10.1016/j.virusres.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Channappanavar R., Ma C., Wang L., Tang J., Garron T., Tao X., Tasneem S., Lu L., Tseng C.T., Zhou Y., Perlman S., Jiang S., Du L. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell Mol. Immunol. 2016;13:180–190. doi: 10.1038/cmi.2015.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Du L., Ma C., Li Y., Li L., Poon V.K., Wang L., Yu F., Zheng B.J., Jiang S., Zhou Y. A safe and convenient pseudovirus-based inhibition assay to detect neutralizing antibodies and screen for viral entry inhibitors against the novel human coronavirus MERS-CoV. Virol. J. 2013;10:266. doi: 10.1186/1743-422X-10-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Jiang Y., Qiu H., Gao T., Zeng Y., Guo Y., Yu H., Li J., Kou Z., Du L., Tan W., Jiang S., Sun S., Zhou Y. Multi-organ damage in human dipeptidyl peptidase 4 transgenic mice infected with Middle East respiratory syndrome-coronavirus. PLoS One. 2015;10:e0145561. doi: 10.1371/journal.pone.0145561. [DOI] [PMC free article] [PubMed] [Google Scholar]