Abstract
The Hedgehog pathway drives proliferation and differentiation by activating the Gli/Ci family of zinc finger transcription factors. Gli/Ci proteins form Hedgehog signaling complexes with other signaling components, including the kinesin-like protein Costal-2, the serine-threonine kinase Fused, and Suppressor of Fused [Su(fu)]. In these complexes Gli/Ci proteins are regulated by cytoplasmic sequestration, phosphorylation, and proteolysis. Here we characterize structural and functional determinants of Su(fu) required for Gli regulation and show that Su(fu) contains at least two distinct domains: a highly conserved carboxy-terminal region required for binding to the amino-terminal ends of the Gli proteins and a unique amino-terminal domain that binds the carboxy-terminal tail of Gli1. While each domain is capable of binding to different Gli1 regions independently, interactions between Su(fu) and Gli1 at both sites are required for cytoplasmic tethering and repression of Gli1. Furthermore, we have solved the crystal structure of the amino-terminal domain of human Su(fu)27-268 at 2.65 Å resolution. This domain forms a concave pocket with a prominent acidic patch. Mutation at Asp159 in the acidic patch disrupts Gli1 tethering and repression while not strongly disrupting binding, indicating that the amino-terminal domain of Su(fu) likely impacts Gli binding through a mechanism distinct from that for tethering and repression. These studies provide a structural basis for understanding the function of Su(fu).
The Hedgehog (Hh) family of proteins regulates a wide variety of developmental processes, and as such perturbations in the Hh pathway have been implicated in many developmental disorders and cancer (reviewed extensively in reference 25). Hh proteins are ligands for the 12-transmembrane receptor Patched (Ptc) (13, 27, 37, 63), which negatively regulates the 7-transmembrane protein Smoothened (Smo) (3, 26, 68). Ptc binds to the Hh proteins, resulting in internalization into endosomes and disruption of Ptc-mediated repression. This allows Smo to move from an intracellular compartment to the cell surface, resulting in Smo activation and signal transmission (4, 18, 24, 38, 65, 72). Downstream of Smo, the Hh signal activates the Gli family of zinc finger transcription factors, including Gli1, Gli2, and Gli3 in vertebrates and the Gli homolog Cubitus interruptus (Ci) in the fly (5, 23, 39, 47, 55, 56). Several proteins, including the putative serine-threonine kinase Fused (Fu), protein kinase A (PKA), casein kinase I (CKI), glycogen synthase kinase 3β (GSK3β), the atypical kinesin Costal-2 (Cos2), the F-box/WD40 repeat protein Slimb, and Suppressor of Fused [Su(fu)], have been shown to modulate Ci activity in Drosophila melanogaster (for a review, see reference 25). Many of these proteins have been shown to function within the Hh pathway in vertebrate systems; however, not all vertebrate components have been identified, particularly Cos2.
Gli/Ci proteins are thought to exist as part of Hh signaling complexes (HSCs) consisting of Gli/Ci, Cos2, Fu, Smo, and Su(fu) (35, 45, 54, 61). Smo has only recently been shown to associate with the HSC via Cos2 and Fu (35, 45, 54), suggesting that these proteins preassociate to enable efficient signaling upon Hh stimulation. In contrast, Su(fu) appears to interact mostly with Gli/Ci in a separate complex (35). In the absence of Hh signal, Ci is phosphorylated by PKA at several tandem sites downstream of the zinc finger domains, a feature conserved in the Gli proteins (11, 12, 22, 71). This initial phosphorylation primes Ci for further phosphorylation by the kinases GSK3β and CKI (29, 52), allowing for the proteolysis of Ci. In flies, a Slimb- and proteasome-dependent cleavage event generates an amino-terminal form of Ci (Ci75Rep) that acts as a transcriptional repressor of Hh target genes (7, 40).
The Gli/Ci family of proteins is repressed by Cos2 and Su(fu), both of which tether Gli/Ci in the cytoplasm (19, 33, 41, 53, 58, 64, 69). Su(fu) binds to an amino-terminal region of Gli/Ci proteins containing a highly conserved SYGH motif (20, 64, 69). Unfortunately, several studies attempting to map the comparable interaction site within Su(fu) have resulted in contradictory models (20, 21, 33, 57, 64). Cos2 binds both the carboxy-terminal and amino-terminal ends of Ci (6, 58, 69) and is thought to mediate Ci tethering partly through its interactions with microtubules, since disruption of microtubule networks with nocodazole disrupts Ci cytoplasmic retention (70). Recent evidence shows that Cos2 can compete with Su(fu) for binding at the amino-terminal end of Ci and in so doing may keep Ci in the cytoplasm via a mechanism involving masking of a Ci nuclear localization sequence (NLS) (70). It is not clear how Su(fu) mediates cytoplasmic retention of Gli/Ci proteins; however, Cos2 and Su(fu) are able to interact through Fu, leading to the possibility that these two proteins cooperate (6, 44).
Following Hh stimulation, Gli/Ci proteins relocate to the nucleus (19, 46, 69), suggesting that the ability of Cos2 and Su(fu) to retain Gli/Ci proteins in the cytoplasm is somehow relieved. However, both genetic and biochemical evidence has suggested that Su(fu) is capable of regulating Gli/Ci activity through a second mechanism independent of cytoplasmic tethering (14, 33, 41, 49, 64, 69). This implies that Su(fu) is capable of remaining bound to the Gli/Ci proteins in the nucleus, where it may act to further regulate Gli/Ci activity.
Here we dissect the mechanisms by which Su(fu) binds, tethers, and represses the activity of the Gli proteins. First, we have identified a region in the carboxy-terminal end of Su(fu) that is highly conserved and required for binding to the amino-terminal half of Gli1. In addition, we show that the amino-terminal domain of Su(fu) constitutes a separate Gli binding domain capable of specifically interacting with the carboxy-terminal tail of Gli1. We have identified and solved the crystal structure of a highly conserved amino-terminal domain, consisting of amino acids 27 to 268 of human Su(fu) [hSu(fu)], which is required for stable Gli1 binding, repression, and cytoplasmic retention. This domain contains several prominent features, including a highly charged acidic face and a surface-exposed PKA phosphorylation site that we have functionally evaluated. Taken together, these data show that Su(fu) regulates Gli activity via a mechanism involving the binding of two distinct domains, and they provide a structural framework that helps explain Su(fu)-mediated regulation of the Gli family of transcription factors.
MATERIALS AND METHODS
Construction of Su(fu) truncations and point mutants.
Cloning of hSu(fu)1-484,hSu(fu)1-433, and Gli1 into pRK expression vectors has been described previously (64). Amino-terminal truncation mutants of hSu(fu) lacking the first 100 amino acids were generated by PCR with the 5′ oligonucleotide 5′-CCGGATCCCGATGGGTGACAACAGAGTCCA-3′ and the pRK common 3′ oligonucleotide 5′-GATGCTATTGCTTTATTTGTAACC-3′. Carboxy-terminal glutathione S-transferase (GST) fusion constructs were prepared in a similar fashion by using the following 5′ oligonucleotides containing a BamHI linker (italicized) together with the pRK common 3′ oligonucleotide: hSu(fu)300-forward (5′-GGGGATCCCGATGCTCTCTGGCAAAGACAC-3′), hSu(fu)350-forward (5′-TGGGATCCCTATCGATGGACAGCTCCACGGCC-3′), hSu(fu)380-forward (5′-TGGGATCCCTCATTCCTCTCTGCCTAAGG-3′), and hSu(fu)400-forward (5′-GGGGATCCCGATGATCACAGGTGACATGGC-3′). These constructs were cloned in frame upstream or downstream of GST in the pRK5 vector. Point mutant constructs were generated by using the Transformer site-directed mutagenesis system (Clontech, Inc.) and the QuikChange system (Stratagene, Inc.).
The enhanced green fluorescent protein (eGFP)-Gli1 construct was made by PCR amplifying the eGFP gene containing novel HindIII sites at both the 5′ and 3′ ends and ligating it in frame upstream of a full-length N-Myc-tagged-hGli1 construct. Su(fu)-Anemonia sulcata red fluorescent protein 2 (AsRed2) fusion constructs were generated by PCR amplifying the Su(fu) genes and ligating them in frame to the BamHI-HindIII site in the pAsRed2-N1 construct (Invitrogen, Inc.). The proper sequence and product sizes were verified for all constructs.
Transfections, immunoprecipitations, and Western blot analysis.
COS-7 cells were grown in 50:50 medium (half Ham's F-12-low-glucose medium and half Dulbecco's modified Eagle's medium) containing 10% fetal bovine serum, 2 mM glutamine, and 10 mM HEPES. Transient transfections were carried out with various expression plasmids in 10-cm-diameter plates with Lipofectamine 2000 (Invitrogen, Inc.). Twenty-four hours after transfection, cells were lysed for 20 min at 4°C with rotation in 1 ml of 1% NP-40 lysis buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitors [Complete; Boehringer Mannheim]). Subsequently, samples were centrifuged at 20,000 × g and 4°C for 20 min to remove NP-40 insoluble proteins. Alternatively, constructs were expressed alone, and lysates were mixed after normalization (see Fig. 4 and 5). Reaction mixtures were rotated at 4°C for 4 h to allow for binding, and then immunoprecipitations were performed.
FIG. 4.
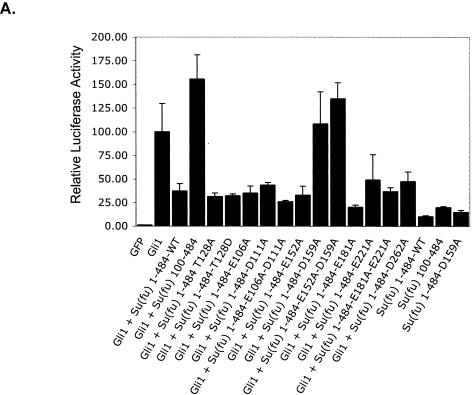
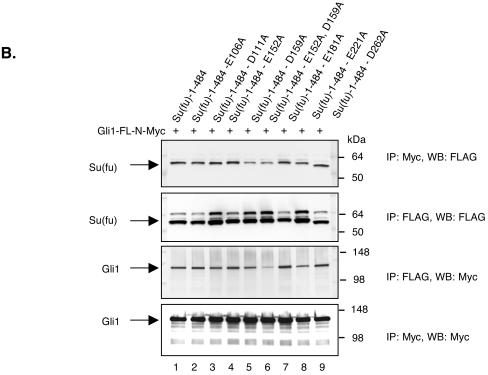
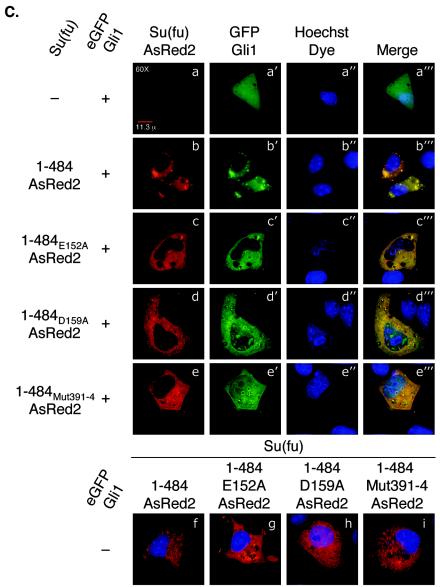
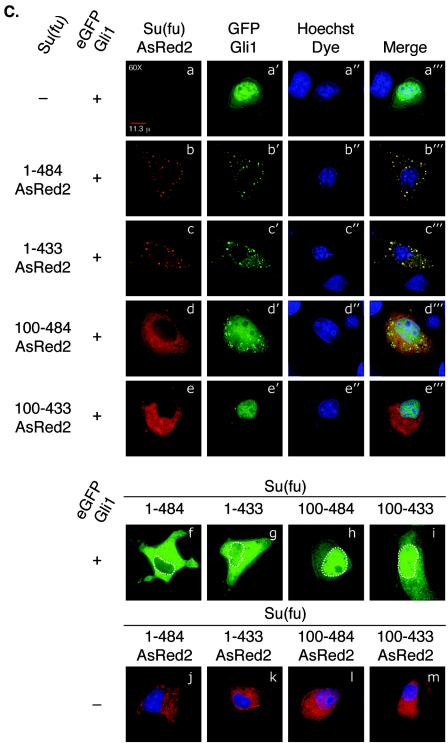
Functional analysis of Su(fu) amino-terminal point mutants. (A) C3H/10T1/2 cells were transfected as described under “Luciferase assays” in Materials and Methods. Equivalent amounts of each Su(fu) variant and hGli1 construct were expressed in each assay. Error bars represent standard deviations of triplicate determinations. (B) COS-7 cells were transfected with the indicated expression vectors encoding Gli1 or various Su(fu) point mutant constructs. Cells were transfected with each construct alone, followed by lysis and normalization for expression. Reaction mixtures were established, and proteins were allowed to mix for 4 h at 4°C, followed by standard coimmunoprecipitation (IP) and Western blotting (WB). Sizes(in kilodaltons) are given on the right. (C) COS-7 cells were plated onto coverslip chamber slides and transfected either with eGFP-Gli1 alone (panels a to a‴) or with the indicated Su(fu) construct, including Su(fu)1-484-AsRed2 (panels b to b‴), Su(fu)1-484-E152A-AsRed2 (panels c to c‴), Su(fu)1-484-D159A-AsRed2 (panels d to d‴), or Su(fu)1-484-Mut391-4-AsRed2 (panels e to e‴). Subcellular localization of each Su(fu) construct in the absence of eGFP-Gli1 is shown (panels f to i). Prior to viewing, cells were counterstained with Hoechst dye and viewed by deconvolution microscopy to determine the subcellular localization of eGFP-Gli1. Images are representative Z-sections chosen to reflect the pattern observed in the majority of cotransfected cells.
FIG. 5.
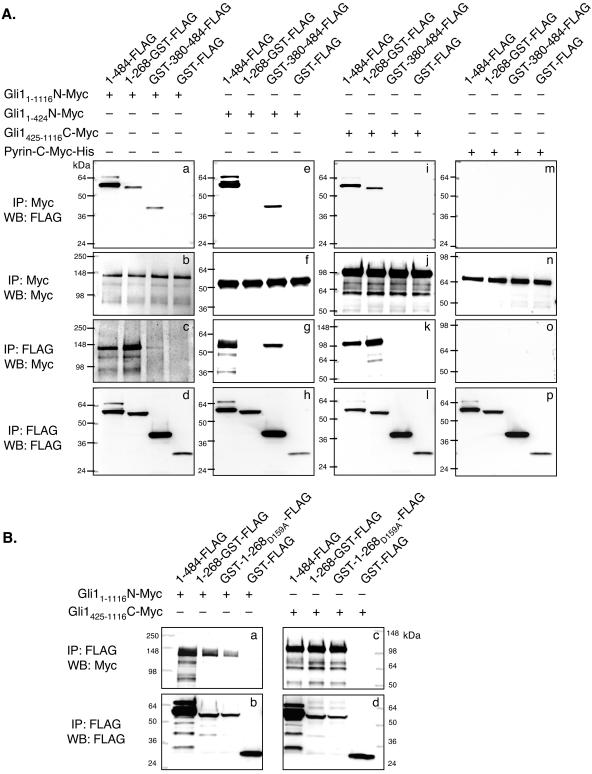
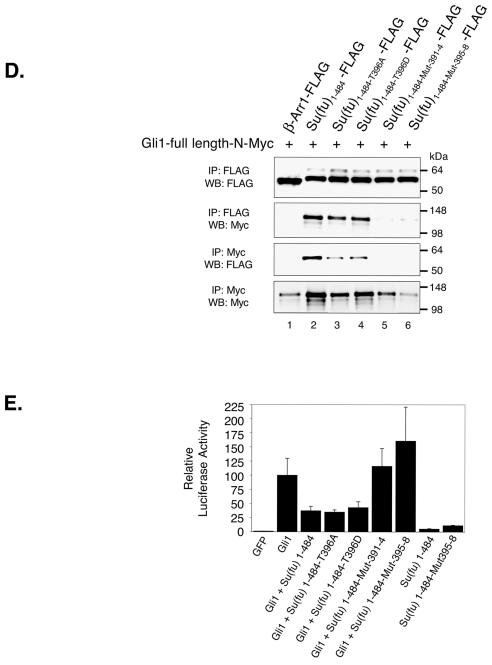
The amino terminus of Su(fu) binds to the carboxy terminus of Gli1. (A) COS-7 cells were transfected with the indicated expression vectors alone, followed by normalization for expression levels and mixing of equivalent volumes of lysates to create the indicated reactions. Gli1 constructs included a full-length construct (hGli1-1-1116-N-Myc) (panels a to d), an amino-terminal construct (hGli1-1-425-N-Myc) (panels e to h), and a carboxy-terminal construct (hGli1-425-1116-C-Myc) (panels i to l). Su(fu) constructs included a full-length construct [Su(fu)1-484-FLAG], an amino-terminal construct [Su(fu)1-268-GST-FLAG], and a carboxy-terminal construct [GST-Su(fu)380-484-FLAG]. GST-FLAG and Pyrin-C-Myc-His (panels m to p) constructs served as negative controls. Interaction between the Gli1 and Su(fu) truncation variants was analyzed by standard coimmunoprecipitation (IP) and Western blotting (WB). Sizes (in kilodaltons) are given to the left of each blot. (B) COS-7 cells were transfected with the indicated expression vectors alone, followed by normalization for expression levels and mixing of equivalent volumes of lysates to createthe indicated reactions. Gli1 constructs included a full-length construct (hGli1-1-1116-N-Myc) (panels a and b) and a carboxy-terminal construct (hGli1-425-1116-C-Myc) (panels c and d). Su(fu) constructs included a full-length construct [Su(fu)1-484-FLAG], an amino-terminal construct [Su(fu)1-268-GST-FLAG], and an amino-terminal construct mutated at Asp159 [Su(fu)1-268-D159A-GST-FLAG]. GST-FLAG served as a negative control. Interaction between Gli1 and Su(fu) truncation variants was analyzed by coimmunoprecipitation followed by Western blotting. Sizes (in kilodaltons) are given on either side.
For immunoprecipitations, lysates were precleared for 1 h at 4°C with 30 μl of protein A-Sepharose (Amersham/Pharmacia Biotechnology, Inc.). Lysates were transferred to a new tube containing 3 μg of an anti-Myc (9E10; Boehringer Mannheim), anti-FLAG-M2 (Sigma), or anti-GST (Amersham/Pharmacia Biotechnology, Inc.) monoclonal antibody and were rotated for 2 h at 4°C. A 30-μl volume of protein A-Sepharose beads was then added to each sample and allowed to rotate for 1 h. The immune-complexed beads were then washed four times with 1 ml of lysis buffer. To ensure that binding was specific, some immunoprecipitates (see Fig. 2) were subjected to two high-salt washes (500 mM NaCl). The immune-complexed beads were resuspended in 30 μl of sodium dodecyl sulfate loading buffer (Invitrogen, Inc.) containing β-mercaptoethanol and boiled at 100°C for 5 min. Samples were analyzed by denaturing sodium dodecyl sulfate-4 to 20% polyacrylamide gel electrophoresis, transferred to 0.2-μm-pore-size nitrocellulose membranes, and blocked for 1 h with 5% milk-2% bovine serum albumin in phosphate-buffered saline (PBS). Membranes were then probed with anti-Myc (9E10)-peroxidase (Boehringer Mannheim, Inc.) at 1:2,500 in blocking solution, anti-FLAG-M2-peroxidase (Sigma) at 1:1,500 in blocking solution, or anti-GST (Amersham/Pharmacia Biotechnology, Inc.) at 1:3,000 in PBS containing 0.05% Tween 20 (PBST) for 1 h at room temperature. Blots were washed four times with 10 ml of PBST, for 5 min each time, followed by chemiluminescent development using Chemiglow West (Alpha Innotech, Inc.).
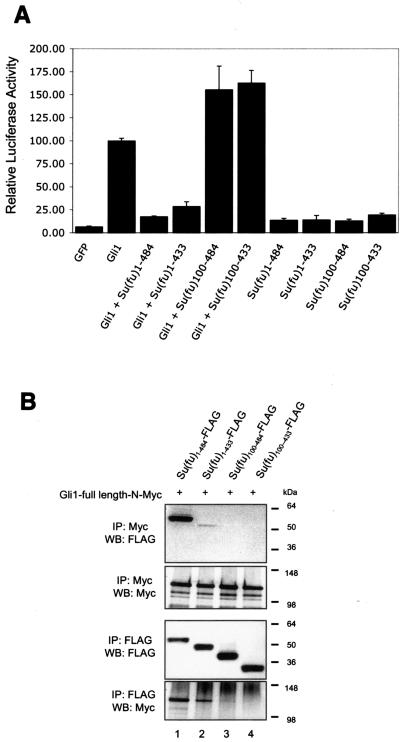
FIG. 2.
Identification of an amino-terminal Gli-repressive region in Su(fu). (A) C3H/10T1/2 cells were transfected as described under “Luciferase assays” in Materials and Methods. Equivalent amounts of each Su(fu) variant and hGli1 construct were expressed in each assay. Error bars represent standard deviations of triplicate determinations. (B) COS-7 cells were transfected with each construct alone, followed by lysis and normalization for expression. Reaction mixtures were established, and proteins were allowed to mix for 4 h at 4°C, followed by coimmunoprecipitation and Western blot analysis. A high-stringency (500 mM NaCl) wash used to test for stable interactions between the Su(fu) and Gli proteins. Sizes (in kilodaltons) are given on the right. (C) COS-7 cells were plated onto coverslip chamber slides and transfected either with eGFP-Gli1 alone (panels a to a‴) or with the indicated Su(fu) constructs, including Su(fu)1-484-AsRed2 (panels b to b‴), Su(fu)1-433-AsRed2 (panels c to c‴), Su(fu)100-484-AsRed2 (panels d to d‴), and Su(fu)100-433-AsRed2 (panels e to e‴). Prior to viewing, cells were counterstained with Hoechst dye and analyzed by deconvolution microscopy to determine the subcellular localization of the eGFP-Gli1 proteins. Images are representative Z-sections chosen to reflect the pattern observed in the majority of cotransfected cells. Non-fluorescently tagged versions of the Su(fu) truncation constructs showed functionally equivalent eGFP-Gli1 localization patterns without aggregation (panels f to i). Hoechst staining is outlined by a white dotted line in these panels to clarify eGFP-Gli1 localization. The subcellular localization of the Su(fu)-AsRed2 fusion constructs in the absence of eGFP-Gli1 is shown (panels j to m).
Subcellular localization by microscopy.
Deconvolution microscopy was performed on COS-7 or C3H/10T1/2 cells cotransfected with the eGFP-Gli1 fusion construct and the Su(fu)-AsRed2 constructs or non-fluorescently tagged Su(fu) constructs. Following transfection, cells were split onto coverslip chamber slides and analyzed the following day. Prior to imaging, cells were counterstained with Hoechst dye for 10 min at 37°C to stain the nuclei. Samples were analyzed on a Deltavision deconvolution microscope at ×60. GFP expression was viewed in the fluorescein isothiocyanate channel, AsRed2 was viewed in the phycoerythrin channel, and Hoechst dye was viewed in the 4′,6′-diamidino-2-phenylindole (DAPI) channel.
Luciferase assays.
Hh-responsive C3H/10T1/2 cells were grown in 50:50 medium containing 10% fetal bovine serum, 2 mM glutamine, and 10 mM HEPES. The day before transfection, cells were seeded into 6-well plates at 105 cells/well. DNA transfection cocktails were diluted in Opti-MEM (Invitrogen, Inc.) with the indicated combination of plasmids encoding Gli1, various Su(fu) constructs, and GFP DNA totaling 0.5 μg, in combination with 1 μg of reporter (9xGli-BS-luciferase), 0.0025 μg of pRL-TK (Promega, Inc.), and 0.5 μg of empty carrier vector (pRK5-tk-Neo). Transfections were performed in triplicate in Opti-MEM for 3.5 h by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's recommendations. Cells were then washed twice with 50:50 medium and then grown overnight in 3 ml of 50:50 medium. Reporter gene activity was determined by using the Dual-Luciferase Reporter Assay system (Promega, Inc.). Transfection efficiency was monitored by GFP expression and normalized to the internal control Renilla luciferase. The amounts of Gli1 and Su(fu) constructs used in each assay were determined by titration experiments in order to determine the minimal amount of each construct needed to yield consistent and significant results. Following these guidelines allowed for similar results to be obtained with COS-7 cells; however, the magnitude of Gli1 activation was reduced.
Overexpression, purification, and crystallization.
Su(fu)27-268 was subcloned into pET21a (Novagen, Inc.) and overexpressed in BL21(DE3)pLysS cells at 37°C. Once harvested, the inclusion bodies containing Su(fu)27-268 were resuspended in 6 M urea-25 mM Tris (pH 7.5)-10 mM dithiothreitol (DTT) and centrifuged at 16,270 × g for 30 min. The resulting supernatant was loaded onto a Q-Sepharose fast-flow column (Amersham/Pharmacia Biotechnology, Inc.) and eluted in 0.5 M NaCl-6 M urea-25 mM Tris (pH 7.5)-10 mM DTT. Su(fu)27-268 was further purified by gel filtration and refolded overnight in 2 M urea-25 mM Tris (pH 7.5)-10 mM DTT at 4°C. The HQ tag was removed by using TagZyme (Unizyme Laboratories) and a nickel-nitrilotriacetic acid column.
Su(fu)27-268 was crystallized by the hanging drop method. A 10-μl volume of Su(fu)27-268 (2.5 mg/ml) in 25 mM Tris (pH 7.5) and 10 mM DTT was mixed with 2 μl of reservoir solution and suspended over a reservoir containing 1 M LiCl, 0.1 M sodium citrate (pH 5.0 to 6.0), and 10% (wt/vol) polyethylene glycol 6000 (PEG 6000). A single crystal grew to 0.1 mm3 within 3 weeks.
Data collection and structure determination.
Crystals of Su(fu)27-268 belong to space group R32, with the following unit cell constants: a = b = 172.6 Å; c = 288.4 Å. To obtain the phase information, a single crystal was soaked in a solution containing 1 M NaBr, 0.1 M sodium citrate (pH 5.5), 10% PEG 6000, and 20% PEG 400 for 30 min. The crystal was then flash-cooled by using 20% (vol/vol) PEG 400 to 80% of the reservoir solution, and data were collected at Beamline 9-2 of the Stanford Synchotron Radiation Laboratory, at a wavelength corresponding to f′max (0.919388 Å), by using inverse beam mode. Data were processed by using DENZO and Scalepack (48) (Table 1).
TABLE 1.
Crystallographic statistics for Su(fu)27-268a
| Su(fu)27-268 | Resolution (Å) | No. of measurements | No. unique | Rmergeb | Completeness (%) | I/σ(I) | Redundancy |
|---|---|---|---|---|---|---|---|
| NaBr soak | 99-2.8 (2.9-2.8) | 798,828 | 40,996 | 0.11 (0.398) | 99.8 (98.3) | 6.5 | 19.5 |
| Native | 99-2.65 (2.70-2.65) | 441,505 | 48,643 | 0.094 (0.362) | 99.2 (100.0) | 6.2 | 9.1 |
Values for the high-resolution shell are given in parentheses.
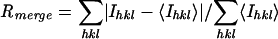 , where Ihkl is the intensity of reflection hkl and <Ihkl> is the average intensity of multiple observations.
, where Ihkl is the intensity of reflection hkl and <Ihkl> is the average intensity of multiple observations.
Thirty Br sites were located by using the SHELX-D program (67); the top 10 of these sites were used for phase refinement with the SHARP program (16). The maps were subjected to solvent flipping by using SOLOMON (1, 15), and the resulting maps were of excellent quality, revealing well-defined α-helices and β-strands (see Fig. 3). Electron density averaging was carried out using the RAVE suite of programs, which had a dramatic effect on the connectivity of the loop regions. The model of Su(fu)27-268 was constructed by using O (31) and associated programs from the RAVE/XUTIL suite (30, 32).
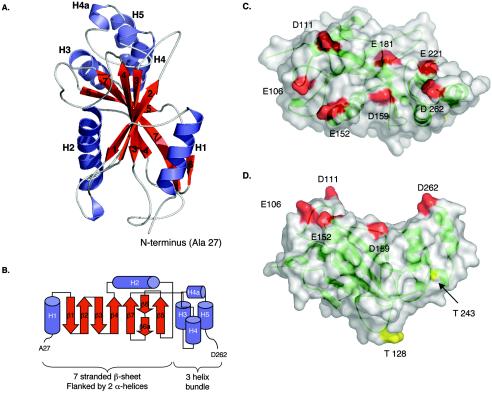
FIG. 3.
Crystal structure of Su(fu)27-268. (A) Ribbon rendering of the structure, viewed perpendicular to the central β-sheet (red). The amino-terminal subdomain consists of the central sheet flanked by two α-helices (blue). The carboxy-terminal subdomain is completely helical. (B) Topology diagram of the structure presented in panel A. (C and D) Surface representation of Su(fu)27-268 (grey), containing a ribbon rendering (green). (C) Shown is an acidic patch, formed by seven negatively charged residues (red), on a concave surface. (D) Two potential PKA phosphorylation sites at Thr128 and Thr243 are shown (in yellow). The residues forming the acidic patch are colored red. Thr243 is largely buried and therefore is not a likely candidate for phosphorylation. The view in panel C is rotated approximately 90° from that in panel D.
Structure refinement.
All crystallographic refinement was performed using CNX (10) (Accelrys). The Rfree (9) test set was generated by using SFTOOLS (15) and consisted of 1,000 reflections from thin shells. The initial model was refined against the Br data set to a resolution of 2.8 Å by maintaining tight noncrystallographic symmetry (NCS) restraints to an Rfactor of 32.1% and an Rfree of 34.0%. A native data set at 2.65 Å resolution, which was isomorphous to the Br data set, was used for further refinement. A total of 2,515 reflections were flagged for the test data set by using the methods described above. To eliminate model bias against the new test set of reflections, the Su(fu)27-268 model was subjected to rigid body refinement and torsion angle molecular dynamics against a maximum-likelihood target function (2, 50). Coordinate and restrained individual B-factor refinement was carried out by using a maximum-likelihood target function and tight NCS restraints over the majority of residues in the four monomers. Residues that displayed significant deviations from NCS were relieved from NCS restraints. Anisotropic B-factor scaling and a “mask”-type bulk solvent model was used throughout the refinement. The present model consists of residues 27 to 262 for all 4 Su(fu)27-268 molecules and 346 water molecules. Electron density was not observed for residues 263 to 268, and these residues have been omitted from the final model. Refinement statistics are reported in Table 2. The atomic coordinates (code 1M1L) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, N.J. (http://www.rscb.org/).
TABLE 2.
Refinement statistics for Su(fu)27-268
| Statistic | Value |
|---|---|
| Rcrysta | 0.224 |
| Rfreeb | 0.256 |
| No. of protein atoms | 7,486 |
| No. of water molecules | 346 |
| 〈Bprotein〉 | 33.5 Å2 |
| 〈Bwater〉 | 29.8 Å2 |
| RMSD bond lengths | 0.01 Å |
| RMSD angles | 1.5° |
| RMSD bonded B's | 2.9 Å2 |
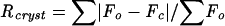 , where Fo and Fc are the observed and calculated structure factor amplitudes, respectively.
, where Fo and Fc are the observed and calculated structure factor amplitudes, respectively.
Rfree is the R value for a randomly selected 8% of reflections, which were not used in the refinement.
RESULTS
Mapping of a Gli-binding region in the carboxy terminus of Su(fu).
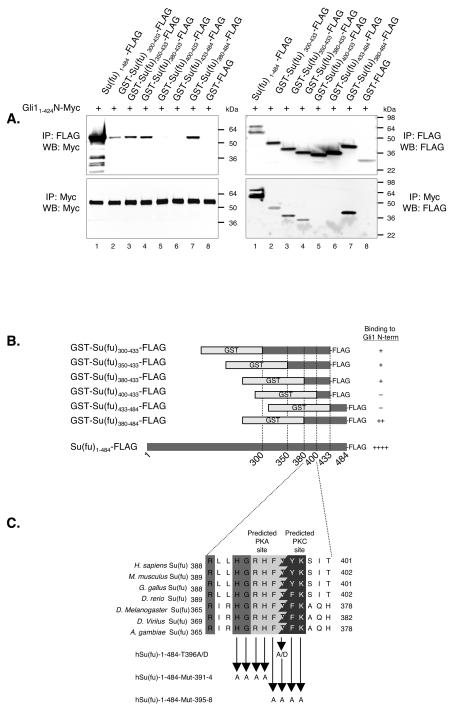
To better define the sequences within the carboxy-terminal region of Su(fu) required for Gli1 binding, several GST fusions of the Su(fu) carboxy-terminal end were generated and tested for the ability to bind to the amino-terminal half of Gli1 [residues 1 to 425, containing a Su(fu) binding domain and all of the zinc finger domains]. To aid in comparison to FLAG epitope-tagged full-length Su(fu), each GST construct also contained a carboxy-terminal FLAG epitope tag. Su(fu) constructs were coexpressed in COS-7 cells with Gli11-425-N-Myc, followed by coimmunoprecipitation with both anti-Myc and anti-FLAG antibodies (Fig. 1A). Full-length Su(fu) and constructs containing residues 300 to 433, 350 to 433, 380 to 433, and 380 to 484 were all capable of coimmunoprecipitating the amino-terminal end of Gli1; however, the truncated Su(fu)-GST fusion proteins bound more weakly than full-length Su(fu) (Fig. 1A; summarized in Fig. 1B). Similar results were obtained when an anti-GST antibody was used to immunoprecipitate the complex (data not shown). These results confirm that sequences in the carboxy-terminal end of Su(fu) are capable of specifically binding to regions within the amino-terminal end of Gli1, and they further define a region between residues 380 and 433 that is sufficient for the ability of Su(fu) to bind to Gli1. These data also show that the central region of Su(fu) between residues 300 and 380 does not contribute significantly to Gli1 binding, since these residues are dispensable for the Su(fu)-Gli1 interaction. While constructs containing only residues 434 to 484 of Su(fu) are incapable of binding to Gli1, it is still formally possible that sequences within this region participate in binding, considering that GST-Su(fu)380-484-FLAG showed stronger binding than GST-Su(fu)380-433-FLAG.
FIG. 1.
Identification of a Gli-binding region in Su(fu). (A) Coimmunoprecipitation (IP) and Western blotting (WB) of FLAG epitope-tagged versions of GST-Su(fu) carboxy-terminal fusion constructs and hGli11-425-N-Myc. Constructs were coexpressed in COS-7 cells, followed by coimmunoprecipitation with both anti-Myc and anti-FLAG antibodies. Sizes (in kilodaltons) are given to the right of the blots. (B) Summary of the constructs used in panel A and the respective binding results. (C) ClustalW sequence alignment of a highly conserved portion of the Gli binding region in humans, mice, chickens, zebra fish, two species of fruit fly, and mosquitoes. Medium shading, conserved residues; light shading, predicted PKA phosphorylation site; dark shading, predicted PKC phosphorylation site. Thr396 is the predicted target residue for both PKA and PKC phosphorylation. The amino acids changed in point mutant constructs are shown below the consensus sequence. (D) IP and WB of Su(fu) point mutants to test binding to cotransfected hGli1-1-1116-N-Myc. (E) C3H/10T1/2 cells were transfected as described under “Luciferase assays” in Materials and Methods. A reporter gene consisting of the firefly luciferase downstream of nine tandem consensus Gli binding sites (9x-Gli-BS-Luciferase) was cotransfected with Gli1 and the indicated Su(fu) constructs. Equal amounts of total DNA were used for each transfection, and transfection efficiencies were visualized by eGFP expression. Data were calculated by dividing the activity of the firefly luciferase by the activity of the internal control Renilla luciferase and were then transformed to reflect the percentage of Gli1 activity alone (set at 100%). Error bars represent standard deviations of triplicate determinations.
The region of highest contiguous conservation within amino acids 380 to 433 of Su(fu) are residues 391 to 398 [RxxHGRHFT(F/Y)K], comprising overlapping PKA and PKC potential phosphorylation sites; Thr396 is the phosphorylation target for both of these kinases (Fig. 1C). To test whether PKA or PKC phosphorylation at Thr396 is necessary for the ability of Su(fu) to bind to Gli1, Su(fu) point mutants were generated, including Thr396 mutated to alanine (T396A) or to aspartic acid (T396D), to mimic phosphorylation at this site (Fig. 1C). These mutant forms of Su(fu) were then coexpressed in COS-7 cells with full-length Gli1 (Gli1-1-1116-N-Myc), and coimmunoprecipitations were performed. Mutation of Thr396 to alanine or aspartic acid did not affect the capacity of Su(fu) to interact with Gli1, indicating that phosphorylation at this site is not required for Gli1 binding (Fig. 1D). Furthermore, mutation of Thr396 to either alanine or aspartic acid did not affect the ability of Su(fu) to repress Gli1 transcription in the 9x-Gli-BS-Luciferase reporter assay using Hh-responsive C3H/10T1/2 cells (44) (Fig. 1E).
Cluster mutagenesis was performed to generate multiple alanine mutants within the RxxHGRHFT(F/Y)K motif (Fig. 1C). These constructs were coexpressed in COS-7 cells along with the Gli1-1-1116-N-Myc construct and tested for the ability to interact by coimmunoprecipitation. Cluster mutagenesis within this conserved site severely disrupted the Su(fu)-Gli1 interaction (Fig. 1D). Furthermore, these constructs [Su(fu)1-484-Mut391-4-FLAG and Su(fu)1-484-Mut395-8-FLAG] were not able to repress Gli1 transcription (Fig. 1E) or to keep Gli1 localized in the cytoplasm (Fig. 4C). Taken together, these data indicate that sequences between residues 380 and 433 of Su(fu), and particularly the highly conserved region between residues 391 and 398, play an essential role in mediating the Su(fu)-Gli1 interaction.
Identification of an amino-terminal region in Su(fu) required for Gli1 repression.
To identify regions of Su(fu) critical for the repression of Gli-mediated transcription, amino and carboxy-terminal truncation mutants of Su(fu) were tested in the 9x-Gli-BS-Luciferase reporter assay. As previously reported, full-length Su(fu)1-484-FLAG effectively blocks Gli1-mediated activation (Fig. 2A). Similarly, coexpression of a construct bearing a truncation of the carboxy-terminal 51 amino acids [Su(fu)1-433-FLAG] also resulted in strong repression of Gli-mediated transcription, indicating that the carboxy-terminal 51 amino acids are largely dispensable for Su(fu)-mediated repression of Gli1 activity in the 9x-Gli-BS-Luciferase reporter system (Fig. 2A). While truncation of the first 27 amino acids from the amino terminus of Su(fu) did not disrupt the capacity of Su(fu) to repress Gli1 activity (data not shown), truncation of the first 100 amino acids [Su(fu)100-484-FLAG and Su(fu)100-433-FLAG] resulted in complete abolition of Su(fu)-mediated repression of Gli1-mediated transcription (Fig. 2A).
To assess the abilities of these Su(fu) constructs to bind Gli1, Gli1-1-1116-N-Myc and the Su(fu)-FLAG truncation constructs were tested for Gli1 binding by coimmunoprecipitation. Initial experiments indicated that the various Su(fu) truncation constructs differed in their abilities to keep Gli1 localized in the cytoplasm, resulting in discrepancies in immunoprecipitation (data not shown). Therefore, to better assess whether each Su(fu) truncation construct was capable of stably interacting with Gli1, each Su(fu) truncation construct was expressed alone, normalized for expression, and then mixed with equal amounts of Gli1-1-1116-C-Myc protein lysates prior to immunoprecipitation. To ensure that Gli1 binding was specific and stable, immune complexes were washed twice with a high-salt wash buffer (500 mM NaCl). As expected, full-length Su(fu)1-484-FLAG maintained an interaction with Gli1 under high-salt conditions (Fig. 2B). Su(fu)1-433-FLAG was also capable of binding Gli1, albeit at a much lower level, again indicating that the final 51 amino acids may contribute to a stable Gli1 interaction. Interestingly, truncation of the first 100 amino acids completely disrupted the ability of Su(fu) to bind to Gli1 under these conditions. These data identify a region at the amino-terminal end of Su(fu) required for the ability of Su(fu) to stably bind and repress Gli1.
Subcellular localization of Gli1 in the presence of Su(fu) truncation constructs.
Su(fu) is thought to repress Gli activity at least in part via cytoplasmic tethering. We therefore wanted to test whether disruption of the amino-terminal domain affected the subcellular localization of Gli1. Fluorescent protein fusion constructs were made which expressed the various Su(fu) truncations fused to carboxy-terminal AsRed2. These constructs were coexpressed in COS-7 cells with a Gli1 protein fusion construct consisting of eGFP fused to the amino terminus of full-length human Gli1 (eGFP-Gli1). Live cells were then analyzed by deconvolution microscopy to determine the subcellular localization of Su(fu) and Gli1 (Fig. 2C). When expressed alone, the eGFP-Gli1 protein localized predominantly in the nucleus, with a small fraction localizing diffusely in the cytoplasm (94.7% [n = 150 cells] showed mainly nuclear localization), consistent with previous reports showing similar results by immunofluorescence using epitope-tagged versions of Gli1 (Fig. 2C, panels a to a‴). Coexpression of Su(fu)1-484-AsRed2 with eGFP-Gli1 showed that full-length Su(fu) is capable of tethering Gli1 in the cytoplasm (94.3% [n = 140 cells] showed cytoplasmic localization [Fig. 2C, panels b to b‴]). Coexpression of Su(fu)1-484-AsRed2 with eGFP-Gli1 consistently resulted in colocalization of Su(fu)1-484-AsRed2 and eGFP-Gli1 in punctate spots in most cotransfected cells. However, this effect was not observed with all AsRed2 constructs coexpressed with eGFP-Gli1, and it was not observed in Su(fu)-AsRed2 constructs expressed alone (Fig. 2C, panels j to m). Coexpression of eGFP-Gli1 with nonfluorescent versions of the same Su(fu) truncation constructs showed functionally comparable results without the observed aggregate formation (Fig. 2C, panels f to i). Su(fu)1-433-AsRed2 is also capable of maintaining eGFP-Gli1 in the cytoplasm, albeit not as efficiently as full-length Su(fu) (60.3% [n = 136 cells] showed cytoplasmic localization [Fig. 2C, panels c to c‴]). In contrast, loss of the first 100 amino acids of Su(fu) [Su(fu)100-484-AsRed2 and Su(fu)100-433-AsRed2] resulted in localization of eGFP-Gli1 to the nucleus (94.7% [n = 152 cells] and 94.8% [n = 134 cells] showed nuclear localization, respectively [Fig. 2C, panels d to d‴ and e to e‴]), indicating that truncation of the amino terminus of Su(fu) abolishes the ability of Su(fu) to keep Gli1 in the cytoplasm. Interestingly, loss of the first 100 amino acids of Su(fu) also disrupted the ability of Su(fu) to remain strictly cytoplasmic when expressed alone [34.9% (n = 146 cells) of Su(fu)100-484-AsRed2 showed nuclear localization compared to 3.7% (n = 134 cells) for Su(fu)1-484-AsRed2 (Fig. 2C, panels j to m)]. Together these results indicate that the final 51 amino acids contribute to stable Gli1 binding and cytoplasmic tethering. However, these residues do not dramatically affect Gli1 repression in the 9x-Gli-BS-Luciferase system. Furthermore, these results show that the amino terminus of Su(fu) plays a major role in coordinating the stable binding, cytoplasmic tethering, and transcriptional repression of Gli1.
Structural characterization of the amino-terminal domain of Su(fu).
To gain further insight into the regulation of Gli activity, we sought to solve the crystal structure of Su(fu). While attempts at crystallization of full-length Su(fu) failed, crystallization of an amino-terminal fragment of Su(fu) was successful. This domain corresponds to amino acids 27 to 268 [Su(fu)27-268], an area of very high conservation between vertebrate and invertebrate forms of Su(fu). Residues 1 to 268 of hSu(fu) show a 47% average identity and a 62% average similarity to invertebrate homologs (D. melanogaster, Drosophila virilis, mosquito), while residues 269 to 484 show only 22% average identity and 40% average similarity.
Bacterially expressed Su(fu)27-268 was purified, and the crystal structure was determined (see Tables 1 and 2 for crystal structure statistics). Su(fu)27-268 consists of two distinct subdomains (Fig. 3). Residues 27 to 204 comprise a highly curved seven-stranded β-sheet, flanked on each face by an α-helix (Fig. 3A and B). The β-sheet wraps approximately halfway around the amphipathic helix 2 (H2). The first half of the β-sheet consists of β-strands 1 to 4 (β1 to β4), which are arranged in an antiparallel topology. Strand β4 is followed by H2, which continues to β5. Strands β5 to β7 complete the β-sheet from the opposite end, also in an antiparallel topology, with β7 running parallel to β4. Residues 205 to 262 form a compact three-helix bundle, with the loop connecting H4 and H5 interrupted by a short turn of helix (H4a).
The four molecules of Su(fu)27-268 pack in the crystallographic asymmetric unit via two distinct interfaces, both of which are nearly perfect dyads. One interface is composed of helix H2 and buries 1,332 Å2 of solvent-accessible surface area. A second interface is composed primarily of main-chain interactions between β1 of NCS-related molecules. This interface buries a significantly larger surface area (829 Å2 × 2 = 1,658 Å2) and is represented twice in the asymmetric unit. Although Su(fu)27-268 is monomeric in solution, the amount of solvent-accessible surface that is buried by this interface is well within the range of that observed in other stable dimer interfaces (28). However, this model is not favored, because purified full-length Su(fu) interacts with an amino terminal fraction of Gli2 (residues 1 to 225), containing the Su(fu) binding domain, with a 1:1 stoichiometry (data not shown).
Calculation of the electrostatic surface potential of Su(fu)27-268by using GRASP revealed a significant acidic patch on one side of the protein (data not shown). A surface representation of Su(fu)27-268 shows that this acidic patch resides on a concave surface and consists of seven acidic residues (Glu106, Asp111, Glu152, Asp159, Glu181, Glu221, and Asp262) that outline this area (Fig. 3C and D). The remaining surface of Su(fu)27-268 is relatively neutral in comparison to this patch. The concave shape and the high accumulation of acidic residues in this patch raise the possibility that these characteristics are important for a specific protein-protein interaction. Since the Su(fu)27-268 fragment overlaps with the region required for stable Gli1 binding, cytoplasmic tethering, and repression, the Su(fu) acidic patch becomes a reasonable candidate for a functional interface involved in mediating these functions of Su(fu).
Thr128 is one of six potential PKA phosphorylation sites in Su(fu) and one of two within Su(fu)27-268 (Thr128 and Thr243), based on the primary sequence homology (64). Thr128 is quite accessible on the surface of the molecule, on the opposite side from the acidic patch, whereas Thr243 is barely solvent exposed and is unlikely to be phosphorylated (Fig. 3D). In contrast, a regulatory role for Thr121, which has been proposed as a potential PKC phosphorylation site, is unlikely, because it is completely buried in the structure.
Mutational analysis of the amino-terminal domain of Su(fu).
To assess whether the PKA site containing Thr128 is critical for the ability of Su(fu) to mediate the repression of Gli1, Thr128 was mutated to alanine (T128A) or aspartic acid (T128D) in order to mimic phosphorylation at this site, and constructs bearing these mutations were tested for the ability to repress Gli1 activity in a 9x-Gli-BS-Luciferase assay. Mutations at Thr128 did not affect the capacity of Su(fu) to repress Gli1 activity (Fig. 4A), indicating that neither mimicking phosphorylation at Thr128 nor loss of phosphorylation at this residue affects the ability of Su(fu) to repress Gli1.
To test the functionality of the acidic patch within the amino terminus of Su(fu), residues Glu106, Asp111, Glu152, Asp159, Glu181, Glu221, and Asp262 were mutated to alanine individually or in combinations. Constructs bearing mutations within the acidic patch were then tested for the ability to repress Gli1 activity in the 9x-Gli-BS-Luciferase assay. Mutation at residue Glu106, Asp111, Glu152, Glu181, Glu221, or Asp262 within full-length Su(fu) had no effect on the repression of Gli1 activity (Fig. 4A). In contrast, mutation at Asp159, alone or in combination with mutations at other sites, completely disrupted the capacity of Su(fu) to repress Gli1. In agreement with the possibility that Asp159 plays a functionally important role in Gli1 repression, it is the only absolutely conserved residue within the acidic patch of Su(fu) among eight species of Su(fu) (human, mouse, chicken, zebra fish, fugu fish, D. melanogaster, D. virilis, and mosquito).
To address whether the observed loss of Gli1 repression was due to loss of Gli1 binding, each Su(fu) point mutant was assessed for the ability to bind to full-length Gli1 (Gli11-1116-N-Myc) by coimmunoprecipitation in COS-7 cells. Initial experiments in which each construct was coexpressed with full-length Gli1 showed that Su(fu) mutated at Asp159 was unable to maintain high levels of Gli1 in the cytoplasm, resulting in uneven immunoprecipitation of Gli1 (data not shown). To circumvent this problem, each construct was expressed alone in COS-7 cells, normalized for expression, and mixed with equal amounts of Gli11-1116-N-Myc for 4 h at 4°C, and standard coimmunoprecipitations were then performed. All of the Su(fu) acidic patch mutants were able to bind to Gli1 (Fig. 4B). However, mutation of Su(fu) at Asp159 did result in a slight reduction in Gli1 binding. These results indicate that mutations within the acidic patch of Su(fu) do not drastically disrupt Gli1 binding, and they further show that the loss of repression observed for the Su(fu) Asp159 mutants cannot be wholly attributed to a loss of Gli1 binding capacity.
Since truncation of the amino-terminal 100 amino acids resulted in loss of Gli1 cytoplasmic retention, we sought to determine whether mutation of Asp159 to alanine also disrupted Gli1 cytoplasmic retention. Su(fu)-AsRed2 variants were coexpressed with the eGFP-Gli1 construct in COS-7 cells, and the subcellular localization of each fusion protein was analyzed by deconvolution microscopy. As expected, when eGFP-Gli1 was expressed alone, it was visualized predominantly in the nucleus and diffusely in the cytoplasm (Fig. 4C, panels a to a‴). Coexpression of Su(fu)1-484-FLAG with eGFP-Gli1 resulted in the strict cytoplasmic localization of eGFP-Gli1 (Fig. 4C, panels b to b‴). Likewise, tethering of eGFP-Gli1 in the cytoplasm was not disrupted when it was coexpressed with Su(fu) mutated at acidic patch point residues other than Asp159 [for Su(fu)1-484-E152A-AsRed2, 97.4% (n = 114 cells) showed cytoplasmic localization (Fig. 4C, panels c to c‴)]. However, coexpression of eGFP-Gli1 with Su(fu)1-484-D159A-AsRed2 revealed that mutation at Asp159 hindered but did not abolish the ability of Su(fu) to keep eGFP-Gli1 tethered in the cytoplasm (70.8% [n = 130 cells] showed nuclear localization [Fig. 4C, panels d to d‴]). Coexpression of eGFP-Gli1 with Su(fu)1-484-Mut391-4-AsRed2, which is incapable of binding to Gli1, showed that Gli1 entered the nucleus when Su(fu) was incapable of mediating an interaction with Gli1 (93.4% [n = 121 cells] showed nuclear localization [Fig. 4C, panels e to e‴]). Together, these data indicate that while mutation of Su(fu) at Asp159 only moderately affects the capacity to bind Gli1, cytoplasmic tethering and repression of Gli1 are more severely disrupted.
Su(fu) interacts with Gli1 via a dual binding mechanism.
The results obtained with the Su(fu) truncation constructs raise the possibility that the amino-terminal domain of Su(fu) is also involved in binding to Gli. Based on our previous data, a Su(fu)-Gli interaction is mediated between the carboxy-terminal end of Su(fu) and the amino-terminal Su(fu) binding domain of the Gli proteins. This interaction is disrupted by mutation of the RxxHGRHFT(F/Y)K motif in Su(fu) or by mutation of the SYGH motif in Gli proteins (20). However, based on the disruption in binding observed for amino-terminally truncated Su(fu) constructs, it is possible that the Su(fu) amino-terminal domain is either cooperating or directly interacting with the Gli proteins.
To test this hypothesis, GST fusion constructs were generated consisting of Su(fu)1-268 or Su(fu)380-484 fused up or downstream of GST, respectively. Each construct was also tagged at the carboxy terminus with a FLAG epitope [Su(fu)1-268-GST-FLAG, GST-Su(fu)380-484-FLAG] to allow for easier comparison with the Su(fu)1-484-FLAG construct. Each construct was expressed alone in COS-7 cells, normalized for expression, and mixed with equal amounts of Gli11-1116-N-Myc or Gli1-1-425-N-Myc for 4 h at 4°C, and standard coimmunoprecipitations were then performed. As expected, Su(fu)1-484-FLAG and GST-Su(fu)380-484-FLAG were both capable of binding to Gli1-1-1116-N-Myc and Gli1-1-425-N-Myc (Fig. 5A, panels a to d and e to h, respectively). Surprisingly, Su(fu)1-268-GST-FLAG was also capable of interacting with Gli1-1-1116-N-Myc but not with Gli1-1-425-N-Myc (Fig. 5A, panels a to d and e to h).
We next tested whether Su(fu)1-268-GST-FLAG was capable of interacting with the carboxy-terminal region of Gli1 following the DNA-binding domain (Gli1-425-1116-C-Myc). Su(fu)1-484-FLAG was capable of interacting with Gli1-425-1116-C-Myc (Fig. 5A, panels i to l). In contrast, GST-Su(fu)380-484-FLAG was incapable of interacting with the carboxy-terminal tail, while Su(fu)1-268-GST-FLAG interacted strongly with Gli1-425-1116-C-Myc. Neither of the control Myc or FLAG epitope-tagged proteins (Pyrin-C-Myc-His and GST-FLAG) was capable of binding to Su(fu) or Gli1, respectively (Fig. 5). These data suggest that Su(fu) mediates an interaction with Gli via a dual binding mechanism in which Su(fu) interacts with the Gli proteins via amino- and carboxy-terminal sequences. Su(fu)1-268-GST-FLAG was capable of binding to full-length hGli1 and mouse Gli2 (mGli2) but showed only weak binding to hGli3 (data not shown), showing that this Su(fu)-Gli interaction is common among multiple Gli family members.
Since mutation at Asp159 affects the capacity of Su(fu) to tether and repress Gli1, we tested whether mutation at Asp159 could disrupt the ability of Su(fu)1-268-GST-FLAG to bind to Gli1-425-1116-C-Myc. Su(fu)1-484-FLAG, Su(fu)1-268-GST-FLAG, Su(fu)1-268-D159A-GST-FLAG, and GST-FLAG were each expressed alone in COS-7 cells, normalized for expression, and then mixed with equal amounts of either Gli11-1116-N-Myc or Gli1-425-1116-C-Myc, followed by standard coimmunoprecipitations. Surprisingly, mutation at Asp159 of Su(fu) did not dramatically disrupt the capacity of the amino-terminal domain to bind to the carboxy-terminal tail of Gli1 (Fig. 5B). These data suggest that elements other than Asp159 in the amino-terminal domain of Su(fu) mediate the interaction with the carboxy-terminal tail of Gli1. Furthermore, these data suggest that mutation of Asp159 fundamentally alters the functional integrity of Su(fu), not simply the structural or binding capacity of the domain.
DISCUSSION
We have shown here that Su(fu) binds and regulates Gli1 through two distinct regions. The first interaction involves the carboxy-terminal end of Su(fu) containing a highly conserved RxxHGRHFT(F/Y)K motif between residues 388 and 398 that is required for binding the amino-terminal end of Gli1. Mutation of this motif is sufficient to disrupt all Su(fu)-Gli1 binding, tethering, and repression, indicating that this region is essential to all Su(fu) function. The second interaction occurs between the amino-terminal domain of Su(fu) and the carboxy-terminal tail of Gli1. When isolated, these regions are capable of binding to their respective targets independently, implying that each interaction may be distinctly regulated. However, the fact that point mutants in the RxxHGRHFT(F/Y)K motif disrupt all Su(fu) function suggests that the amino-terminal domain cannot tether or functionally repress Gli1 activity on its own.
Interaction between the amino-terminal domain of Su(fu) and the carboxy-terminal end of the Gli proteins is supported by several observations. First, mSu(fu) interacts with the carboxy-terminal half of mGli1 (residues 225 to 1111) and represses both full-length and amino-terminally deleted mGli1 in reporter gene assays (19). Second, yeast two-hybrid studies have found that mSu(fu)13-325, but not mSu(fu)13-109, is capable of interacting with full-length hGli1 and hGli3 (49). Third, weak interactions between chick Su(fu)-GST fusions and a carboxy-terminal construct of hGli3 have been observed by GST pulldown (51). Fourth, in the present study, truncation within the amino-terminal domain of Su(fu) disrupts stable binding to Gli1. And last, the intact amino-terminal domain of Su(fu) (residues 1 to 268) is capable of binding to the carboxy-terminal end of the Gli proteins. This interaction is likely to be direct, because interactions at both the amino- and carboxy-terminal sites are required for binding and repression of full-length Gli1. Furthermore, Su(fu) purified from Escherichia coli is capable of interacting directly with Gli made by in vitro transcription and translation (64), and Su(fu) has also been shown to interact with Gli by yeast-two hybrid analysis (17, 42, 43, 49). These findings are consistent with the recent observation that Su(fu) is present in a separate complex with Ci alone (60). However, the possibility that the interaction between the amino-terminal domain of Su(fu) and the carboxy-terminal end of Gli1 is mediated through an endogenous protein, such as Fu or Cos2, cannot be ruled out.
The crystal structure of the amino-terminal domain of Su(fu) reveals a completely novel structure composed of two separate subdomains. The first 178 residues (amino acids 27 to 204) of the structure comprise a highly twisted seven-stranded β-sheet sandwiched between two amphipathic α-helices, while the remaining 50 amino acids fold into a compact three-helix bundle. Despite the presence of two distinct structural subdomains, it is possible that together these constitute a single structural unit, as the loop from H4 and H4a clearly penetrates between β4 and β7. Database searches for functional homologs of the amino-terminal domain reveal only other Su(fu) homologs in animals. Interestingly, the first subdomain of the Su(fu) amino-terminal domain shows homology with several hypothetical bacterial proteins of unknown function (see supplemental data at http://share.gene.com). A search of the RCSB database using the TOP program (34) revealed only limited similarity to a number of β-sheet structures. The only similarity in architecture that is of interest was found between Su(fu)27-268 and the Ran-binding protein Mog1p (PDB code 1eq6), notably in the placement of two helices on each side of a twisted β-sheet (62) (unpublished). This similarity is functionally noteworthy, because Mog1p is involved in nuclear transport through its interactions with Ran (8, 36, 59).
Drosophila Su(fu) is phosphorylated following Hh stimulation in a Cos2-Fu-dependent manner (35), indicating that phosphorylation may regulate Su(fu) function. To investigate the role of phosphorylation in Su(fu) function, we mutated phosphorylation sites of potential interest. The crystal structure revealed a prominently exposed PKA phosphorylation site at Thr128. However, mutation of Thr128 to alanine or aspartic acid had no effect on Gli1 binding or repression, indicating that phosphorylation of this residue is unlikely to play a major role in the regulation of Gli1. Likewise, when Thr396, a predicted PKA/PKC phosphorylation site within the RxxHGRHFT(F/Y)K motif, was mutated, no effects on Gli1 activity were observed, despite the fact that this motif is required for Gli1 binding. More work must be done to determine the nature of Su(fu) phosphorylation following Hh stimulation and how it alters Su(fu) function.
The function of the acidic patch in the amino-terminal domain of Su(fu) is dominated by the effects of Asp159 on Su(fu) function. Asp159 maps to the middle of the concave face of Su(fu)27-268 on a long exposed loop between H2 and β5 juxtaposed to the second subdomain of Su(fu)27-268 (unpublished). Interactions between Asp159 and Cys156 help form the sharp turn in this long loop, also aligning portions of the first subdomain with the three-helix bundle. Mutation of Asp159 may disrupt the structural balance in this region, leading to the observed effects on Su(fu) function. Indeed, Asp159, or the long loop it helps form, may represent a structural element that is targeted downstream of Hh signaling to negatively regulate Su(fu) function. Mutation of Asp159 in Su(fu) results in slightly weakened Gli1 binding; however, it severely affects Gli1 cytoplasmic retention and repression, implying that this residue impacts Su(fu) functions more specifically than through a structural defect alone. One possibility is that Asp159, or structures it helps form, are involved in mediating the cytoplasmic tethering of Gli1 through interactions with other proteins in the cytoplasm. Additionally, Asp159 and the surrounding acidic residues may serve a distinct role in binding corepressors in the nucleus. A recent report of yeast two-hybrid studies indicates that Su(fu) acts as a promiscuous binding partner of multiple nuclear regulatory factors, and it has been shown to have transcriptional repression activity through recruitment of histone deacetylase activity (14, 49).
The finding that Su(fu) interacts with Gli proteins at both the amino- and carboxy-terminal ends reconciles observations made in a number of studies and has interesting implications for the nature of the HSCs observed in vivo (20, 21, 33, 57, 64, 66). In fly embryos, Su(fu) does not stably interact with the Cos2-Fu-Ci HSCs that are associated with vesicles but rather forms its own complex with Ci in the cytoplasm (60). Cos2 has recently been shown to bind to Ci at both the carboxy-terminal end of Ci, through the Cos2-responsive domain, and the amino-terminal end, via the CDN (Cos2 domain N-terminal) (70). While the CDN does not map to the same region known to interact with Su(fu), overexpression of Cos2 competes with Su(fu) for Ci binding (70). Binding of Cos2 to both ends of Ci may mask an NLS within Ci, since expression of an NLS in a more accessible location within Ci leads to nuclear accumulation of Ci (70). While the present work was done using mammalian cells, where a Cos2 homolog has yet to be identified, it is tempting to hypothesize a model where Su(fu) and Cos2 compete for binding at either end of Gli/Ci, thereby resulting in the interchange of Gli/Ci proteins between distinct HSCs. In either complex [Su(fu)-Gli/Ci or Cos2-Fu-Gli/Ci] the Gli/Ci proteins would be bound at both the amino- and carboxy-terminal ends, possibly resulting in the masking of a Gli/Ci NLS. Intermediate complexes could also exist, where Su(fu) would bind the amino-terminal end of the Gli/Ci proteins and Cos2 would bind the Cos2-responsive domain in the carboxy-terminal end of the Gli/Ci proteins, consistent with the observation of these proteins in a tetrameric complex (61). Su(fu) and Cos2 may therefore work either together or alone to regulate Gli/Ci proteins in distinct HSCs. Whether these HSCs represent functionally distinct complexes is not clear. The Cos2-Fu-Gli/Ci complex may be the only HSC functionally capable of transmitting the Hh signal due to the potential association between Cos2 and the cytoplasmic tail of Smo. In this model, the Su(fu)-Gli/Ci complex may represent a latent pool of Gli/Ci where Su(fu) stabilizes the otherwise labile Gli/Ci protein in an inactive conformation while also maintaining cytoplasmic localization and possibly recruitment into the Cos2-Fu complex. The Su(fu)-Gli/Ci complexes may also feed into the production of truncated, repressive forms of Gli/Ci.
In conclusion, we have identified two distinct regions within Su(fu) required for the stable binding and regulation of Gli activity: the RxxHGRHFT(F/Y)K motif at the carboxy-terminal end and the amino-terminal domain (residues 27 to 268) of Su(fu). Mutation of the amino-terminal domain by truncation or point mutation at Asp159 results in the loss of Su(fu) activity, indicating that this domain plays a major role in Su(fu)-mediated repression, both by mediating a second interaction with the carboxy-terminal end of Gli1 and through mechanisms independent of Gli binding. The structural elements identified here may constitute entry sites for Hh signals to alter Su(fu)-mediated regulation of the Gli proteins and will require further investigation for full understanding.
Acknowledgments
We thank J. Yin for help with constructs; S. Palmieri, S. Scales, and W. Mallet for help with microscopy; M. Evangelista and F. Bazan for help with the figures and manuscript preparation; and the Genentech assay and automation technology, oligonucleotide synthesis, and DNA sequencing groups.
REFERENCES
- 1.Abrahams, J. P., and A. G. W. Leslie. 1996. Methods used in the structure determination of bovine mitochondrial Fl ATPase. Acta Crystallogr. D 52:30-42. [DOI] [PubMed] [Google Scholar]
- 2.Adams, P. D., N. S. Pannu, R. J. Read, and A. T. Brunger. 1997. Cross-validated maximum likelihood enhances crystallographic simulated annealing refinement. Proc. Natl. Acad. Sci. USA 94:5018-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcedo, J., M. Ayzenzon, T. Von Ohlen, M. Noll, and J. E. Hooper. 1996. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell 86:221-232. [DOI] [PubMed] [Google Scholar]
- 4.Alcedo, J., Y. Zou, and M. Noll. 2000. Post-transcriptional regulation of smoothened is part of a self-correcting mechanism in the Hedgehog signaling system. Mol. Cell 6:457-465. [DOI] [PubMed] [Google Scholar]
- 5.Alexandre, C., A. Jacinto, and P. W. Ingham. 1996. Transcriptional activation of hedgehog target genes in Drosophila is mediated directly by the cubitus interruptus protein, a member of the GLI family of zinc finger DNA-binding proteins. Genes Dev. 10:2003-2013. [DOI] [PubMed] [Google Scholar]
- 6.Ascano, M., Jr., K. E. Nybakken, J. Sosinski, M. A. Stegman, and D. J. Robbins. 2002. The carboxyl-terminal domain of the protein kinase fused can function as a dominant inhibitor of hedgehog signaling. Mol. Cell. Biol. 22:1555-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aza-Blanc, P., F. A. Ramirez-Weber, M. P. Laget, C. Schwartz, and T. B. Kornberg. 1997. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell 89:1043-1053. [DOI] [PubMed] [Google Scholar]
- 8.Baker, R. P., M. T. Harreman, J. F. Eccleston, A. H. Corbett, and M. Stewart. 2001. Interaction between Ran and Mog1 is required for efficient nuclear protein import. J. Biol. Chem. 276:41255-41262. [DOI] [PubMed] [Google Scholar]
- 9.Brünger, A. T. 1992. The free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature 355:472-474. [DOI] [PubMed] [Google Scholar]
- 10.Brünger, A. T., P. D. Adams, G. M. Clore, W. L. DeLano, P. Gros, R. W. Grosse-Kunstleve, J.-S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. 1998. Crystallography & NMR System: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54:905-921. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Y., J. R. Cardinaux, R. H. Goodman, and S. M. Smolik. 1999. Mutants of cubitus interruptus that are independent of PKA regulation are independent of hedgehog signaling. Development 126:3607-3616. [DOI] [PubMed] [Google Scholar]
- 12.Chen, Y., N. Gallaher, R. H. Goodman, and S. M. Smolik. 1998. Protein kinase A directly regulates the activity and proteolysis of cubitus interruptus. Proc. Natl. Acad. Sci. USA 95:2349-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, Y., and G. Struhl. 1996. Dual roles for patched in sequestering and transducing Hedgehog. Cell 87:553-563. [DOI] [PubMed] [Google Scholar]
- 14.Cheng, S. Y., and J. M. Bishop. 2002. Suppressor of Fused represses Gli-mediated transcription by recruiting the SAP18-mSin3 corepressor complex. Proc. Natl. Acad. Sci. USA 99:5442-5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collaborative Computational Project, number 4. 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50:760-763. [DOI] [PubMed] [Google Scholar]
- 16.De La Fortelle, E., and G. Bricogne. 1997. Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods Enzymol. 276:472-494. [DOI] [PubMed] [Google Scholar]
- 17.Delattre, M., S. Briand, M. Paces-Fessy, and M. F. Blanchet-Tournier. 1999. The Suppressor of fused gene, involved in Hedgehog signal transduction in Drosophila, is conserved in mammals. Dev. Genes Evol. 209:294-300. [DOI] [PubMed] [Google Scholar]
- 18.Denef, N., D. Neubuser, L. Perez, and S. M. Cohen. 2000. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell 102:521-531. [DOI] [PubMed] [Google Scholar]
- 19.Ding, Q., S. Fukami, X. Meng, Y. Nishizaki, X. Zhang, H. Sasaki, A. Dlugosz, M. Nakafuku, and C. Hui. 1999. Mouse suppressor of fused is a negative regulator of sonic hedgehog signaling and alters the subcellular distribution of Gli1. Curr. Biol. 9:1119-1122. [DOI] [PubMed] [Google Scholar]
- 20.Dunaeva, M., P. Michelson, P. Kogerman, and R. Toftgard. 2003. Characterization of the physical interaction of Gli proteins with SUFU proteins. J. Biol. Chem. 278:5116-5122. [DOI] [PubMed] [Google Scholar]
- 21.Grimm, T., S. Teglund, D. Tackels, E. Sangiorgi, F. Gurrieri, C. Schwartz, and R. Toftgard. 2001. Genomic organization and embryonic expression of Suppressor of Fused, a candidate gene for the split-hand/split-foot malformation type 3. FEBS Lett. 505:13-17. [DOI] [PubMed] [Google Scholar]
- 22.Hammerschmidt, M., M. J. Bitgood, and A. P. McMahon. 1996. Protein kinase A is a common negative regulator of Hedgehog signaling in the vertebrate embryo. Genes Dev. 10:647-658. [DOI] [PubMed] [Google Scholar]
- 23.Hynes, M., D. M. Stone, M. Dowd, S. Pitts-Meek, A. Goddard, A. Gurney, and A. Rosenthal. 1997. Control of cell pattern in the neural tube by the zinc finger transcription factor and oncogene Gli-1. Neuron 19:15-26. [DOI] [PubMed] [Google Scholar]
- 24.Incardona, J. P., J. Gruenberg, and H. Roelink. 2002. Sonic hedgehog induces the segregation of patched and smoothened in endosomes. Curr. Biol. 12:983-995. [DOI] [PubMed] [Google Scholar]
- 25.Ingham, P. W., and A. P. McMahon. 2001. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15:3059-3087. [DOI] [PubMed] [Google Scholar]
- 26.Ingham, P. W., S. Nystedt, Y. Nakano, W. Brown, D. Stark, M. van den Heuvel, and A. M. Taylor. 2000. Patched represses the Hedgehog signalling pathway by promoting modification of the Smoothened protein. Curr. Biol. 10:1315-1318. [DOI] [PubMed] [Google Scholar]
- 27.Ingham, P. W., A. M. Taylor, and Y. Nakano. 1991. Role of the Drosophila patched gene in positional signalling. Nature 353:184-187. [DOI] [PubMed] [Google Scholar]
- 28.Janin, J., and C. Chothia. 1990. The structure of protein-protein recognition sites. J. Biol. Chem. 264:16027-16030. [PubMed] [Google Scholar]
- 29.Jia, J., K. Amanai, G. Wang, J. Tang, B. Wang, and J. Jiang. 2002. Shaggy/GSK3 antagonizes Hedgehog signalling by regulating Cubitus interruptus. Nature 416:548-552. [DOI] [PubMed] [Google Scholar]
- 30.Jones, T. A. 1992. A, yaap, asap, @#*? A set of averaging programs, p. 91-105. In E. J. Dodson, S. Gover, and W. Wolf (ed.), Molecular replacement. SERC Daresbury Laboratory, Warrington, United Kingdom.
- 31.Jones, T. A., J. Y. Zou, S. W. Cowan, and M. Kjeldgaard. 1991. Improved methods for building protein models in electron density maps and location of errors in these models. Acta Crystallogr. A 47:110-119. [DOI] [PubMed] [Google Scholar]
- 32.Kleywegt, G. J. 1996. Making the most of your search model. CCP4/ESF-EACBM Newsl. Protein Crystallogr. 32:32-36. [Google Scholar]
- 33.Kogerman, P., T. Grimm, L. Kogerman, D. Krause, A. B. Unden, B. Sandstedt, R. Toftgard, and P. G. Zaphiropoulos. 1999. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat. Cell Biol. 1:312-319. [DOI] [PubMed] [Google Scholar]
- 34.Lu, G. 2000. TOP: a new method for protein structure comparisons and similarity searches. J. Appl. Crystallogr. 33:176-183. [Google Scholar]
- 35.Lum, L., C. Zhang, S. Oh, R. K. Mann, D. P. von Kessler, J. Taipale, F. Weis-Garcia, R. Gong, B. Wang, and P. A. Beachy. 2003. Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol. Cell 12:1261-1274. [First published 27 October 2003; 10.1016/S1097-2765(03)00426-X.] [DOI] [PubMed] [Google Scholar]
- 36.Marfatia, K. A., M. T. Harreman, P. Fanara, P. M. Vertino, and A. H. Corbett. 2001. Identification and characterization of the human MOG1 gene. Gene 266:45-56. [DOI] [PubMed] [Google Scholar]
- 37.Marigo, V., R. A. Davey, Y. Zuo, J. M. Cunningham, and C. J. Tabin. 1996. Biochemical evidence that patched is the Hedgehog receptor. Nature 384:176-179. [DOI] [PubMed] [Google Scholar]
- 38.Martin, V., G. Carrillo, C. Torroja, and I. Guerrero. 2001. The sterol-sensing domain of Patched protein seems to control Smoothened activity through Patched vesicular trafficking. Curr. Biol. 11:601-607. [DOI] [PubMed] [Google Scholar]
- 39.Methot, N., and K. Basler. 2001. An absolute requirement for Cubitus interruptus in Hedgehog signaling. Development 128:733-742. [DOI] [PubMed] [Google Scholar]
- 40.Methot, N., and K. Basler. 1999. Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell 96:819-831. [DOI] [PubMed] [Google Scholar]
- 41.Methot, N., and K. Basler. 2000. Suppressor of fused opposes hedgehog signal transduction by impeding nuclear accumulation of the activator form of Cubitus interruptus. Development 127:4001-4010. [DOI] [PubMed] [Google Scholar]
- 42.Monnier, V., F. Dussillol, G. Alves, C. Lamour-Isnard, and A. Plessis. 1998. Suppressor of fused links fused and Cubitus interruptus on the hedgehog signalling pathway. Curr. Biol. 8:583-586. [DOI] [PubMed] [Google Scholar]
- 43.Monnier, V., K. S. Ho, M. Sanial, M. P. Scott, and A. Plessis. 2002. Hedgehog signal transduction proteins: contacts of the Fused kinase and Ci transcription factor with the kinesin-related protein Costal2. BMC Dev. Biol. 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murone, M., S. M. Luoh, D. Stone, W. Li, A. Gurney, M. Armanini, C. Grey, A. Rosenthal, and F. J. de Sauvage. 2000. Gli regulation by the opposing activities of fused and suppressor of fused. Nat. Cell Biol. 2:310-312. [DOI] [PubMed] [Google Scholar]
- 45.Ogden, S. K., M. Ascano, Jr., M. A. Stegman, L. M. Suber, J. E. Hooper, and D. J. Robbins. 2003. Identification of a functional interaction between the transmembrane protein Smoothened and the kinesin-related protein Costal2. Curr. Biol. 13:1998-2003. (First published 7 October 2003; 10.1016/j.cub.2003.10.004.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohlmeyer, J. T., and D. Kalderon. 1998. Hedgehog stimulates maturation of Cubitus interruptus into a labile transcriptional activator. Nature 396:749-753. [DOI] [PubMed] [Google Scholar]
- 47.Orenic, T. V., D. C. Slusarski, K. L. Kroll, and R. A. Holmgren. 1990. Cloning and characterization of the segment polarity gene cubitus interruptus Dominant of Drosophila. Genes Dev. 4:1053-1067. [DOI] [PubMed] [Google Scholar]
- 48.Otwinowski, Z., and W. Minor. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276:307-326. [DOI] [PubMed] [Google Scholar]
- 49.Paces-Fessy, M., D. Boucher, E. Petit, S. Paute-Briand, and M. F. Blanchet-Tournier. 2004. The negative regulator of Gli, Suppressor of fused (Sufu), interacts with SAP18, Galectin3 and other nuclear proteins. Biochem. J. 378:353-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pannu, N. S., and R. J. Read. 1996. Improved structure refinement through maximum likelihood. Acta Crystallogr. A 52:659-668. [Google Scholar]
- 51.Pearse, R. V., II, L. S. Collier, M. P. Scott, and C. J. Tabin. 1999. Vertebrate homologs of Drosophila suppressor of fused interact with the gli family of transcriptional regulators. Dev. Biol. 212:323-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price, M. A., and D. Kalderon. 2002. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell 108:823-835. [DOI] [PubMed] [Google Scholar]
- 53.Robbins, D. J., K. E. Nybakken, R. Kobayashi, J. C. Sisson, J. M. Bishop, and P. P. Therond. 1997. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein Costal2. Cell 90:225-234. [DOI] [PubMed] [Google Scholar]
- 54.Ruel, L., R. Rodriguez, A. Gallet, L. Lavenant-Staccini, and P. P. Therond. 2003. Stability and association of Smoothened, Costal2 and Fused with Cubitus interruptus are regulated by Hedgehog. Nat. Cell Biol. 5:907-913. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz i Altaba, A. 1998. Combinatorial Gli gene function in floor plate and neuronal inductions by Sonic hedgehog. Development 125:2203-2212. [DOI] [PubMed] [Google Scholar]
- 56.Sasaki, H., Y. Nishizaki, C. Hui, M. Nakafuku, and H. Kondoh. 1999. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 126:3915-3924. [DOI] [PubMed] [Google Scholar]
- 57.Simon-Chazottes, D., M. Paces-Fessy, C. Lamour-Isnard, J. L. Guenet, and M. F. Blanchet-Tournier. 2000. Genomic organization, chromosomal assignment, and expression analysis of the mouse suppressor of fused gene (Sufu) coding a Gli protein partner. Mamm. Genome 11:614-621. [DOI] [PubMed] [Google Scholar]
- 58.Sisson, J. C., K. S. Ho, K. Suyama, and M. P. Scott. 1997. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell 90:235-245. [DOI] [PubMed] [Google Scholar]
- 59.Steggerda, S. M., and B. M. Paschal. 2001. Identification of a conserved loop in Mog1 that releases GTP from Ran. Traffic 11:804-811. [DOI] [PubMed] [Google Scholar]
- 60.Stegman, M. A., J. A. Goetz, M. Ascano, Jr., S. K. Ogden, K. E. Nybakken, and D. J. Robbins. 2004. The kinesin-related protein Costal2 associates with membranes in a Hedgehog-sensitive, Smoothened-independent manner. J. Biol. Chem. 279:7064-7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stegman, M. A., J. E. Vallance, G. Elangovan, J. Sosinski, Y. Cheng, and D. J. Robbins. 2000. Identification of a tetrameric hedgehog signaling complex. J. Biol. Chem. 275:21809-21812. [DOI] [PubMed] [Google Scholar]
- 62.Stewart, M., and R. P. Baker. 2000. 1.9 Å resolution crystal structure of the Saccharomyces cerevisiae Ran-binding protein Mog1p. J. Mol. Biol. 299:213-223. [DOI] [PubMed] [Google Scholar]
- 63.Stone, D. M., M. Hynes, M. Armanini, T. A. Swanson, Q. Gu, R. L. Johnson, M. P. Scott, D. Pennica, A. Goddard, H. Phillips, M. Noll, J. E. Hooper, F. de Sauvage, and A. Rosenthal. 1996. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 384:129-134. [DOI] [PubMed] [Google Scholar]
- 64.Stone, D. M., M. Murone, S. Luoh, W. Ye, M. P. Armanini, A. Gurney, H. Phillips, J. Brush, A. Goddard, F. J. de Sauvage, and A. Rosenthal. 1999. Characterization of the human suppressor of fused, a negative regulator of the zinc-finger transcription factor Gli. J. Cell Sci. 112:4437-4448. [DOI] [PubMed] [Google Scholar]
- 65.Strutt, D. I., V. Wiersdorff, and M. Mlodzik. 1995. Regulation of furrow progression in the Drosophila eye by cAMP-dependent protein kinase A. Nature 373:705-709. [DOI] [PubMed] [Google Scholar]
- 66.Taylor, M. D., L. Liu, C. Raffel, C. C. Hui, T. G. Mainprize, X. Zhang, R. Agatep, S. Chiappa, L. Gao, A. Lowrance, A. Hao, A. M. Goldstein, T. Stavrou, S. W. Scherer, W. T. Dura, B. Wainwright, J. A. Squire, J. T. Rutka, and D. Hogg. 2002. Mutations in SUFU predispose to medulloblastoma. Nat. Genet. 31:306-310. [DOI] [PubMed] [Google Scholar]
- 67.Uson, I., and G. M. Sheldrick. 1999. Advances in direct methods for protein crystallography. Curr. Opin. Struct. Biol. 9:643-648. [DOI] [PubMed] [Google Scholar]
- 68.van den Heuvel, M., and P. W. Ingham. 1996. smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature 382:547-551. [DOI] [PubMed] [Google Scholar]
- 69.Wang, G., K. Amanai, B. Wang, and J. Jiang. 2000. Interactions with Costal2 and suppressor of fused regulate nuclear translocation and activity of cubitus interruptus. Genes Dev. 14:2893-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang, G., and J. Jiang. 2004. Multiple Cos2/Ci interactions regulate Ci subcellular localization through microtubule dependent and independent mechanisms. Dev. Biol. 268:493-505. (First published 20 February 2004; 10.1016/j.ydbio. 2004.01.008.) [DOI] [PubMed] [Google Scholar]
- 71.Wang, G., B. Wang, and J. Jiang. 1999. Protein kinase A antagonizes Hedgehog signaling by regulating both the activator and repressor forms of Cubitus interruptus. Genes Dev. 13:2828-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu, A. J., L. Zheng, K. Suyama, and M. P. Scott. 2003. Altered localization of Drosophila Smoothened protein activates Hedgehog signal transduction. Genes Dev. 17:1240-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]