Abstract
Background
Prognosticating outcome following cardiac arrest is challenging and requires a multimodal approach. We tested the hypothesis that the combination of initial neurologic examination, quantitative analysis of head computed tomography (CT) and continuous EEG (cEEG) improve outcome prediction after cardiac arrest.
Methods
Review of consecutive patients receiving head CT within 24hrs and cEEG monitoring between April 2010 and May 2013. Initial neurologic examination (Full Outline of UnResponsiveness_Brainstem reflexes (FOUR_B) score and initial Pittsburgh Post-Cardiac Arrest Category (PCAC)), gray matter to white matter attenuation ratio (GWR) on head CT and cEEG patterns were evaluated. The primary outcome was in-hospital mortality.
Results
Of 240 subjects, 70 (29%) survived and 22 (9%) had a good neurologic outcome at hospital discharge. Combined determination of GW ratio and malignant cEEG had an incremental predictive value (AUC: 0.776 for mortality and 0.792 for poor neurologic outcome), with 0% false positive rate when compared with either test alone (AUC of GW ratio: 0.683 for mortality and 0.726 for poor outcome, AUC of malignant cEEG: 0.650 for mortality and 0.647 for poor outcome). Addition of FOUR_B or PCAC to this model improved prediction of mortality (p=0.014 for FOUR_B and 0.001 for PCAC) but not of poor outcome (p=0.786 for FOUR_B and 0.099 for PCAC).
Conclusions
Combining GWR with cEEG was superior to any individual test for predicting mortality and neurologic outcome. Addition of clinical variables further improved prognostication for mortality but not neurologic outcome. These preliminary data support a multi-modal prognostic workup in this population.
Keywords: cardiac arrest, prognostication, examination, imaging, electroencephalography
Introduction
Coma after successful resuscitation from cardiac arrest (CA) is the most common cause of ICU admission, and the main cause of in-hospital mortality is withdrawal of life sustaining treatment for perceived poor neurological prognosis (WLST).1–4 Targeted temperature management (TTM) is considered standard treatment after CA and can influence sedative drug metabolism and may interfere with accurate prognostication.5–8 Moreover, WLST is strongly associated with persistent coma. Therefore, strategies for accurate prediction of neurological outcome after CA are critically needed.
Several prognostic tools such as neurologic examination, somatosensory evoked potential (SSEP), serum biomarkers, electroencephalography (EEG), brain computed tomography (CT) and diffusion weighted magnetic resonance imaging (DW-MRI) have been evaluated as predictors of neurological outcome.9–19 The combination of multiple modalities is recommended because no single test short of physical examination meeting brain death criteria can predict neurologic outcome correctly.20–22 We previously reported that combining the initial neurologic examination with continuous EEG (cEEG) was superior to any individual test for predicting outcome after CA.23 Using a multimodal approach can minimize the risk of erroneous prognostication of poor outcome.
We hypothesized that the combination of initial neurologic examination, quantitative analysis of brain CT and cEEG can improve outcome prediction after CA. We performed a retrospective analysis of data to test whether the combination of initial brain stem reflex examination evaluated using Full Outline of UnResponsiveness Brainstem (FOUR_B) score or Pittsburgh Cardiac Arrest Category (PCAC), quantitative analysis of head CT calculated by gray matter to white matter attenuation ratio (GWR) and cEEG was superior to either test alone for predicting outcome after CA.
Methods
Study design and setting
We conducted a retrospective analysis of prospectively collected data from a single urban teaching hospital between April 2010 and May 2013. This study was approved by the University of Pittsburgh Institutional Review Board. We included patients who received both head CT scan within 24 hrs and cEEG monitoring. All patients receive serial clinical examinations as part of clinical care. Exclusion criteria were as follows: age < 18 yrs, traumatic cardiac arrest, history of cerebrovascular accident, intravenous contrast in brain CT and large artifacts in brain CT.
The patients were managed according to our previously published post-cardiac arrest care protocols.13,23,24 Briefly, TTM at 33°C was induced with rapid infusion of 30 cc/kg of 4°C saline and thermostatically controlled surface cooling devices (Gaymar Industries, Orchard Park, NY; Arctic Sun, Bard Medical Division, Louisville CO) and maintained for 24 hrs. Intravascular cooling is rarely employed after cardiac arrest in our cohort. Propofol was infused to suppress shivering, or midazolam was infused in cases of hypotension. Neuromuscular paralysis was used often during induction period and rarely used during maintenance and rewarming period.
Methods of measurement
Initial neurologic examination was routinely assessed using FOUR score and PCAC within the first 6 hours of resuscitation and without sedation and paralysis by one of the post-cardiac arrest service physicians. The FOUR score is composed of Motor, Brainstem, Respiratory and Eye responses. Each domain has a 0–4 score and a higher score indicates greater function. As previously reported, we used the FOUR_B score to stratify patients into three groups; FOUR_B = 0–1, FOUR_B = 2 and FOUR_B = 4.23 We also quantified severity of post-arrest illness using the validated Pittsburgh Cardiac Arrest Category system, where: I) awake, II) coma (not following commands but intact brainstem responses) + mild cardiopulmonary dysfunction (SOFA cardiac + respiratory score <4), III) coma + moderate-severe cardiopulmonary dysfunction (SOFA cardiac + respiratory score ≥4), and IV) coma without brainstem reflexes.3, 25
Our hospital implemented 22-channel digital cEEG recordings for the first 48 h after resuscitation from CA as standard monitoring for all comatose post-cardiac arrest patients in August 2009.13 cEEGs were interpreted during patient care by board certified neurologists, and malignant patterns were defined as follows: non-convulsive status epilepticus (NCSE), convulsive status epilepticus (CSE), myoclonic status epilepticus (MSE) and generalized periodic epileptiform discharges (GPEDs). The definition of each malignant pattern has been described previously.13 Myoclonic status epilepticus was characterized as the presence of myoclonic jerks or facial movements associated with GPEDs or with the bursts in a burst suppression pattern. The presence of reactivity and continuous background was not always provided in the clinical interpretation and was not included in the report of the EEG for this study. Patients with malignant EEG patterns are treated with a bolus of lorazepam followed by levetiracetam and valproic acid. Phenytoin is employed next, followed by either a continuous infusion of midazolam or phenobarbital for refractory cases.
Baseline brain CT scanning in patients presenting comatose after resuscitation is a part of standard care in our hospital. CT scans were obtained on a GE Light Speed VCT scanner (GE Healthcare, Little Chalfont, UK) with 5 mm slices at the time of emergency department admission. GWR was calculated by an investigator blinded to clinical information as previously reported.17 Briefly, Hounsfield Units (HU) were recorded at the basal ganglia level; caudate nucleus, putamen, genu of corpus callosum, and posterior limb of internal capsule and also recorded at the cerebrum level; the medial cortex and medial white matter at the level of the centrum semiovale and high convexity area. Average gray matter to white matter ratio (aGWR) was calculated as the mean of the Basal Ganglia GWR and Cerebrum GWR. Patients were divided into three groups according to their aGWR; severe edema (aGWR < 1.1), mild edema (aGWR 1.1 – 1.2) and no edema (aGWR ≥ 1.2).
Outcome measures
The primary outcome of this study was in-hospital mortality. Functional outcome was assessed at the time of hospital discharge by one of the Post-Cardiac Arrest Service attending physicians using Cerebral Performance Category (CPC). The five categories of the CPC are: CPC 1, conscious and alert with good cerebral performance; CPC 2, conscious and alert with moderate cerebral performance; CPC 3, conscious with severe cerebral disability; CPC 4, comatose or in persistent vegetative state; and CPC 5, brain dead, circulation preserved. We defined a good outcome as a CPC score of 1 or 2.26
Statistical analysis
The data were summarized using means and standard deviations (SD). For patient characteristics and comparisons between groups, we used a parametric and nonparametric analysis of variance for continuous variables and Fisher’s exact test or chi-square test for categorical variables. We determined predictive performance using receiving operating characteristic curves set up with logistic regression models to assess and compare for equality of the area of under the curve (AUC) using the Delong test. First, we determined the AUC of each test using the ROC curves: FOUR_B, PCAC, GWR and cEEG. And then, to test the superiority of the combination of cEEG and GWR than that of either test alone, the AUC of combination of GWR and cEEG were calculated and compared with that of each test. Finally, we added FOUR_B or PCAC into this model and compared with combination of GWR and cEEG. Statistical analyses were performed using SPSS 20.0 (Chicago, IL) and MedCalc 15.2.2 (MedCalc Software, Mariakerke, Belgium). P values ≤ 0.05 were considered statistically significant. The Youden Index was used to determine the optimal cut off point for mortality and poor neurologic outcome.27
Results
Patient demographics
Of the 671 patients seen during the study period, 93 were awake on presentation (PCAC I). In the remaining 578 comatose patients, 314 were excluded due to: lack of CT imaging, CT imaging of poor quality, or imaging completed >24 hrs after arrest. An additional 5 had intracranial hemorrhage and 1 was confounded by IV contrast. An additional 14 subjects did not have cEEG monitoring. Three subjects rapidly awoke and one subject was hypothermic (29°C) on arrival. Thus, 240 subjects met both inclusion and exclusion criteria. Mean age was 56 (SD 17) years and 147 (61%) subjects were male. Ventricular fibrillation was initial rhythm for 66 (27%) subjects. Seventy subjects (29%) survived at hospital discharge and 22 (9%) subjects experienced good neurological outcome. Ninety-three subjects had FOUR_B scores of 0–1, 62 had FOUR-B scores of 2, 76 had FOUR_B scores of 4 and 9 had missing initial FOUR_B score. The distribution of post-arrest illness severity was as follows: PCAC II 25% (n=61), PCAC III 14% (n=33), and PCAC IV 57% (n=137). Malignant cEEG patterns were observed in 75 subjects (31%). [Table 1] Average GWR ranged from 0.899 to 1.534. Thirty-seven subjects exhibited severe brain edema (GWR < 1.1) and 38 subjects had mild brain edema (GWR 1.1 – 1.2).
Table 1.
Baseline characteristics of subjects
| Cohort N=240 |
Survivors N=70 |
Non-survivors N=170 |
p | |
|---|---|---|---|---|
| Age, year | 56 ± 17 | 54 ± 18 | 58 ± 17 | 0.106 |
| Sex, male | 147 (61%) | 42 (60%) | 105 (62%) | 0.799 |
| Initial Rhythm | < 0.001 | |||
| VF/VT | 66 (27%) | 33 (47%) | 33 (19%) | |
| PEA | 69 (29%) | 16 (23%) | 53 (31%) | |
| Asystole | 65 (27%) | 11 (16%) | 54 (32%) | |
| Unknown | 40 (17%) | 10 (14%) | 30 (18%) | |
| TH | 218 (91%) | 64 (91%) | 154 (91%) | 0.838 |
| LOS, days (IQR) | 5 (3, 12) | 18 (12, 25) | 4 (3, 6) | < 0.001 |
| FOUR_B | < 0.001 | |||
| FOUR_B=0,1 | 93 (39%) | 9 (13%) | 84 (60%) | |
| FOUR_B=2 | 62 (26%) | 21 (30%) | 41 (29%) | |
| FOUR_B=4 | 76 (32%) | 38 (54%) | 38 (27%) | |
| PCAC | < 0.001 | |||
| II | 61 (25%) | 35 (50%) | 26 (19%) | |
| III | 33 (14%) | 16 (23%) | 17 (12%) | |
| IV | 137 (57%) | 17 (24%) | 120 (86%) | |
| cEEG, 48hrs | < 0.001 | |||
| malignant | 75 (31%) | 7 (10%) | 68 (40%) | |
| non-malignant | 165 (69%) | 63 (90%) | 102 (60%) | |
| aGWR | 1.22 ± 0.11 | 1.27 ± 0.09 | 1.20 ± 0.11 | < 0.001 |
Data are expressed mean ± S.D., median (IQR) or percentage.
TH- therapeutic hypothermia; VF/VT- ventricular fibrillation/ventricular tachycardia; PEA- pulseless electrical activity; LOS- length of stay; FOUR- full outline of unresponsiveness; PCAC- Pittsburgh Cardiac Arrest Category; cEEG- continuous EEG; aGWR- Grey-white ratio.
Association between initial FOUR_B and GW ratio
Average GWR was well correlated with initial FOUR_B Score (p < 0.001) [Table 2]. In subjects with GW ratio < 1.2, 43 (57%) had initial FOUR_B score of 0 or 1. Moreover, in subjects with GW ratio < 1.1, 26 (70%) had initial FOUR_B score of 0 or 1. Among subjects with GW ratio > 1.2, 50 (30%) had FOUR_B score of 0 or 1, 49 (30%) had FOUR_B score of 2 and 61 (37%) had FOUR_B score of 4. Even though the majority of subjects with GWR < 1.1 had FOUR_B score of 0 or 1, 6 (16%) subjects had both pupillary light reflex and corneal reflex.
Table 2.
Association between initial neurologic examination and GW ratio.
| FOUR_B 0,1 N=93 |
FOUR_B 2 N=62 |
FOUR_B 4 N=76 |
|
|---|---|---|---|
| Severe edema | 26 (28%) | 2 (3%) | 6 (8%) |
| GW ratio <1.1 | |||
| Mild edema | 17 (18%) | 11 (18%) | 9 (12%) |
| GW ratio 1.1–1.2 | |||
| No edema | 50 (54%) | 49 (79%) | 61 (80%) |
| GW ratio ≥1.2 |
Association between malignant cEEG patterns and GWR
Average GWR was not associated with malignant cEEG patterns (p=0.687) [Table 3]. In subjects with GW ratio < 1.1, only 2 (5%) had malignant cEEG. Both subjects exhibited myoclonic status epilepticus (MSE). Otherwise, in subjects with GW ratio between 1.1 and 1.2, 18 (47%) subjects had malignant cEEG.
Table 3.
Association between GW ratio and malignant EEG
| Severe edema GW ratio <1.1 N=37 |
Mild edema GW ratio 1.1–1.2 N=38 |
No edema GW ratio ≥1.2 N=165 |
|
|---|---|---|---|
| Malignant EEG at 48hrs | 2 (5%) | 18 (47%) | 55 (33%) |
| Non-malignant EEG at 48hrs | 35 (95%) | 20 (53%) | 110 (67%) |
Prognostic value of single modality
Table 4 shows the areas under the ROC curves, sensitivity, specificity, PPV and NPV of each single test. The cutoff value of GW ratio was 1.066 to maintain 0% of FPR for predicting mortality. The cutoff value of GW ratio was 1.077 to maintain 0% of FPR for predicting poor neurologic outcome. [Supplemental Figure 1]
Table 4.
Sensitivity analysis for each single test to predicting mortality and poor neurological outcome
| For mortality | |||||
| AUC | Sensitivity | Specificity | PPV | NPV | |
| Malignant EEG | 0.651 (0.586–0.712) |
40 (32.6–47.8) |
90 (80.5–95.9) |
90.7 (81.7–96.2) |
38.2 (30.7–46.1) |
| PCAC > 2 | 0.751 (0.680–0.822) |
84.1 (77.5–89.3) |
51.5 (39.0–63.8) |
80.6 (73.8–86.2) |
57.4 (43.9–70.1) |
| PCAC > 3 | 73.6 (66.2–80.2) |
75.0 (63.0–84.7) |
87.6 (80.8–92.6) |
54.3 (43.7–64.6) |
|
| FOUR_B=0,1 | 0.726 (0.663–0.782) |
51.5 (43.6–59.4) |
86.8 (76.4–93.8) |
90.3 (82.4–95.5) |
42.8 (34.3–51.5) |
| GW Ratio≤1.1 | 0.688 (0.624–0.747) |
20 (14.3–26.8) |
95.7 (88.0–99.1) |
91.9 (78.1–98.3) |
33.0 (26.6–40.0) |
| For poor outcome | |||||
| Sensitivity | Specificity | PPV | NPV | ||
| Malignant EEG | 0.650 (0.584–0.711) |
33.9 (27.7–40.6) |
95.5 (77.2–99.9) |
98.7 (92.7–100) |
12.7 (8.1–18.8) |
| PCAC > 2 | 0.731 (0.627–0.834) |
76.6 (70.2–82.1) |
54.6 (32.2–75.6) |
94.1 (89.4–97.1) |
19.7 (10.6–31.8) |
| PCAC > 3 | 63.6 (56.7–70.2) |
81.8 (59.7–94.8) |
97.1 (92.7–99.2) |
19.1 (11.8–28.6) |
|
| FOUR_B=0,1 | 0.687 (0.623–0.746) |
43.5 (36.7–50.6) |
90.9 (70.8–98.9) |
97.8 (92.4–99.7) |
14.5 (9.1–21.5) |
| GW Ratio≤1.1 | 0.729 (0.667–0.785) |
16.5 (11.8–22.1) |
95.5 (77.2–99.9) |
97.3 (85.6–99.9) |
10.3 (6.5–15.4) |
Prognostic value of combined modality
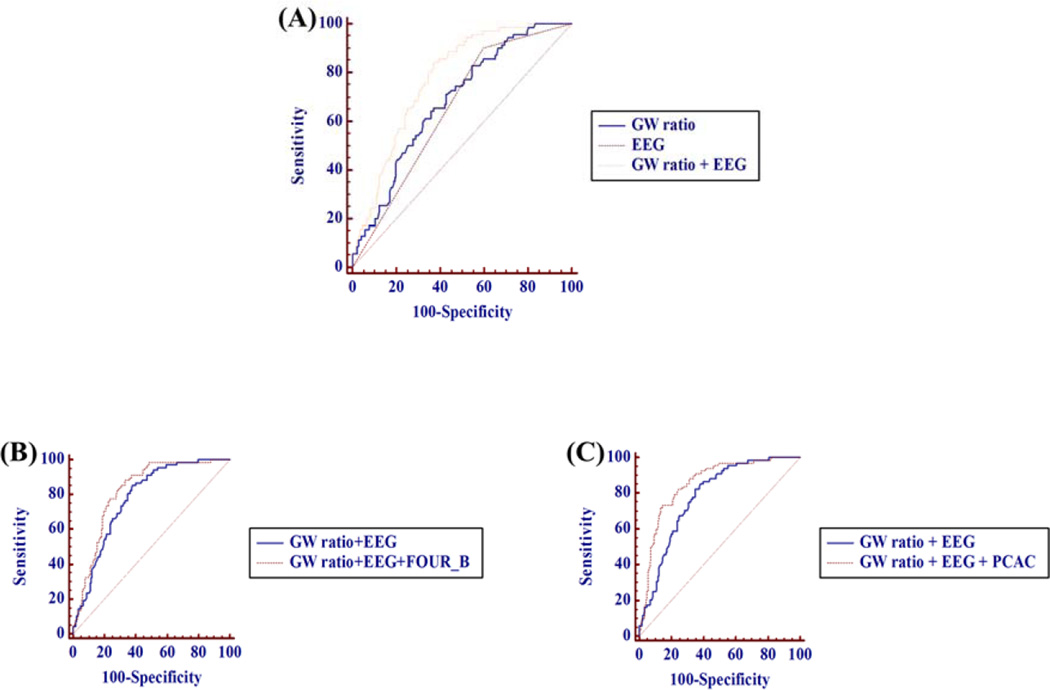
Table 5 shows the areas under the ROC curves for single test. The AUC of combination of GWR and malignant cEEG at 48 hrs to predict mortality is 0.776 (95% C.I. 0.718–0.827) which is superior to either GW ratio alone or malignant cEEG at 48hrs alone (p<0.001 and p<0.001, respectively). When FOUR_B or PCAC is added to this model, the predictive value further improves (AUC of adding FOUR_B: 0.820 (p=0.014), AUC of adding PCAC: 0.855 (p=0.001)) [Fig 1].
Table 5.
Areas of under the receiving operator characteristic curves for each single test and combined modalities to predicting mortality and poor neurological outcome
| Mortality | Poor outcome | |||
|---|---|---|---|---|
| Single test | AUC | 95% CI | AUC | 95% CI |
| FOUR_B | 0.726 | 0.663 – 0.782 | 0.687 | 0.623 – 0.746 |
| PCAC | 0.751 | 0.680 – 0.822 | 0.731 | 0.627 – 0.834 |
| GW ratio | 0.688 | 0.624 – 0.747 | 0.729 | 0.667 – 0.785 |
| Malignant EEG at 48hrs | 0.651 | 0.586 – 0.712 | 0.650 | 0.584 – 0.711 |
| Combined modality | ||||
| FOUR_B + GW ratio | 0.772 | 0.711 – 0.832 | 0.750 | 0.663 – 0.836 |
| PCAC + GW ratio | 0.814 | 0.757 – 0.871 | 0.786 | 0.706 – 0.866 |
| FOUR_B + malignant EEG at 48hrs | 0.787 | 0.724 – 0.850 | 0.762 | 0.675 – 0.849 |
| PCAC + malignant EEG at 48hrs | 0.805 | 0.741 – 0.869 | 0.802 | 0.709 – 0.895 |
| GW ratio + malignant EEG at 48hrs | 0.776 | 0.718 – 0.827 | 0.792 | 0.665 – 0.782 |
| GW ratio + malignant EEG at 48hrs + FOUR_B |
0.820 | 0.765 – 0.868 | 0.822 | 0.742 – 0.850 |
| GW ratio + malignant EEG at 48hrs + PCAC |
0.855 | 0.802 – 0.897 | 0.835 | 0.780 – 0.880 |
Figure 1. Comparison of ROC curve for predicting mortality.
(A) AUC for GW ratio: 0.683, for malignant cEEG: 0.650, for combining GW ratio and malignant cEEG: 0.776 (p<0.001, p<0.001, respectively) (B) AUC for adding FOUR_B: 0.820 (p=0.014) (C) AUC for adding PCAC: 0.855 (p=0.001)
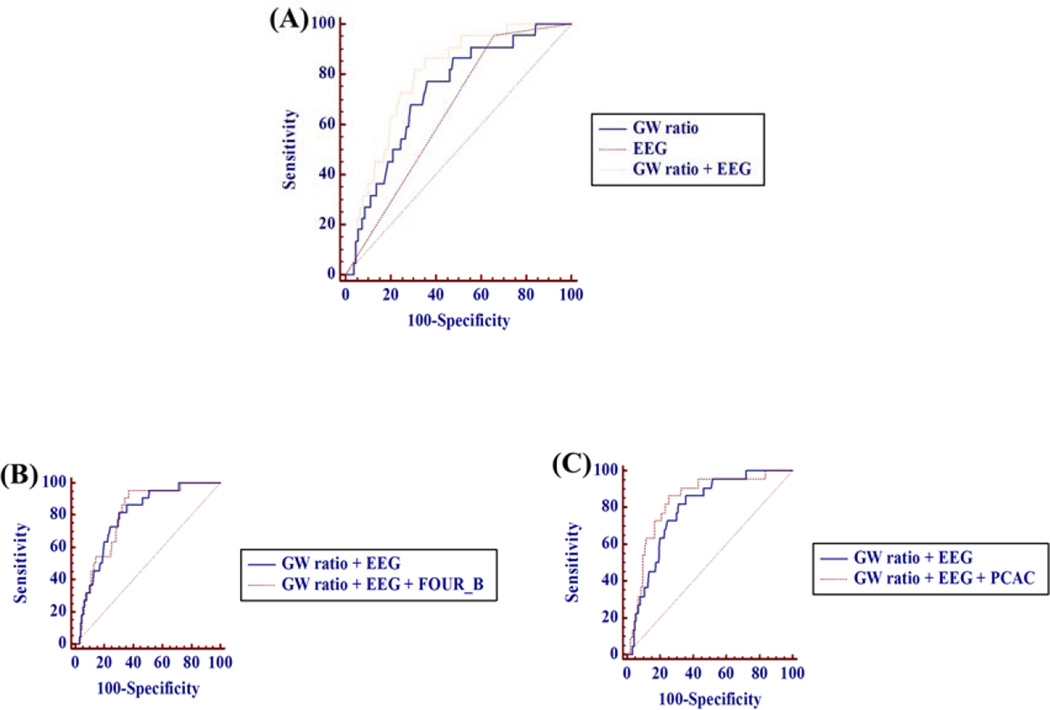
The AUC of combination of GWR and malignant cEEG at 48hrs to predict poor neurological outcome is 0.792 (95% C.I. 0.735–0.841) which is superior to malignant cEEG at 48hrs alone (p<0.001). When FOUR_B or PCAC is added to this model, the predictive value is not further improved (AUC of adding FOUR_B: 0.800 (p=0.786), AUC of adding PCAC: 0.835 (p=0.099)) [Fig 2].
Figure 2. Comparison of ROC curve for predicting poor neurological outcome.
(A) AUC for GW ratio: 0.726, for malignant cEEG: 0.647, for combining GW ratio and malignant cEEG: 0.792 (p=0.067, p<0.001, respectively) (B) AUC for adding FOUR_B: 0.800 (p=0.786) (C) AUC for adding PCAC: 0.835 (p=0.099)
For prediction of mortality, the cutoff value of GW ratio was 1.066 to maintain 0% of FPR (sensitivity 16.47%, 95% CI 11.2–22.9) and FPR of malignant cEEG was 10%. [Supplemental Table 2] For prediction of poor neurologic outcome, the cutoff value of GW ratio was 1.077 to maintain 0% of FPR (sensitivity 15.6%, 95% CI 11–21.1) and FPR of malignant cEEG was 4.5%. Among patients with malignant cEEG, the cutoff value of GW ratio was 1.213 to maintain 0% of FPR (sensitivity 39.71%, 95% CI 28–52.3) for prediction of mortality. Among patients with non-malignant cEEG, the cutoff value of GW ratio was 1.066 to maintain 0% of FPR for prediction of mortality. But the sensitivity (26.47%, 95% CI 18.2–36.1) was higher than that of GW ratio alone (16.47%, 95%CI 11.2–22.9). And among patients with non-malignant cEEG, the cutoff value of GW ratio was 1.077 to maintain 0% of FPR for prediction of poor neurologic outcome. However, the sensitivity (22.22%, 95% CI 15.7–29.9) was higher than that of GW ratio alone (15.6%, 95%CI 11–21.1).
Discussion
We demonstrate that combining the GWR with cEEG was superior to either test alone for predicting in-hospital mortality and neurological outcome after resuscitation from CA. Addition of FOUR_B or PCAC to the above model further improved prediction of mortality. This study quantifies the incremental benefit of each modality in this multimodal approach to prognostication. These findings support current guidelines recommending such an approach and quantify the additive benefit in this population.20–22
Prior work has examined prognostic tests such as neurologic examination, SSEP, biomarkers, EEG, brain CT, and MRI in isolation.9–18 Recent work has tested predictive value of multiple modalities, but the ideal combination is not known.28–32 Importantly, not all tests are necessary for all patients and some facilities may not offer all of these modalities. Some authors propose a stepwise approach to avoid premature WLST.33 Recent published guidelines also highlighted a multimodal strategy to minimize erroneous prognostication21–22. The European Resuscitation Council and European Society of Intensive Care Medicine suggested using a prognostication algorithm in all comatose patients with an absent or extensor motor response to pain at ≥72 h from ROSC21. We propose that such approaches should be tested prospectively.
One novel finding of our study is that malignant EEG patterns are more frequent in patients demonstrating mild edema according to their aGWR than in severe edema patients. We have recently demonstrated heterogeneity in MRI findings between those with malignant and non-malignant cEEG patterns.34 These data suggest that patients with diffuse cerebral edema may lack the neuronal substrate necessary to generate malignant EEG patterns. Severe edema is more likely to result in profound suppression of EEG. While seizures are common after CA and associated with poor outcome, some patients with seizures will awaken and survive.35–36 To date, there are no studies in the TTM era evaluating prophylactic antiepileptic medications after CA.
There are several limitations in this study. Generalizability is limited given the single site evaluated. Only subjects receiving both head CT scans and cEEG monitoring were included. While it is our protocol for comatose patients to CT imaging and cEEG monitoring, a large proportion were excluded due to CT image quality, timing of CT, and rapid awakening.17 This rate of exclusion is similar to prior work.17 These exclusions may be one reason for the low sensitivity seen for predicting poor outcome and the lower good neurologic outcome rate found in the PCAC II cohort. Another limitation is the lack of standard definition of malignant cEEG. The cEEG studies in this study were completed prior to publication of the new definitions. Similarly, data on reactivity or the presence of a continuous background are not available for this entire cohort. Such an approach provides data commonly available to clinicians treating this population. Results of prognostication tests were available to the treating physicians, potentially creating a self-fulfilling prophecy. Our median length of stay of 4 (IQR 3, 6) days for non-survivors indicates that early WLST may be limited in this cohort. We did not analyze the other prognostic modalities such as SSEP, biomarker and brain DWI. Future studies are needed to determine the added value of combining these modalities. Finally, the short-term outcome of survival to hospital discharge does not address the full magnitude of recovery, which frequently requires up to one year.37
Conclusion
Combining the GWR with cEEG was superior to either individual test alone for predicting in-hospital mortality and neurological outcome after cardiac arrest. Addition of FOUR_B or PCAC to this model improved prediction of mortality but not of poor outcome. These data support a multi-modal approach that incorporates clinical, radiographic, and electrophysiologic data to improve prognostic accuracy for this population. Large, multicenter studies should verify these findings.
Supplementary Material
Appendix
The Post Cardiac Arrest Service
Clifton W. Callaway, MD, PhD
Jon C. Rittenberger, MD, MS
Francis X. Guyette, MD, MS
Ankur A. Doshi, MD
Jonathan Elmer, MD
Cameron Dezfulian, MD
Josh Reynolds, MD
Adam Frisch, MD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflict of interest to report.
References
- 1.Laver S, Farrow C, Turner D, et al. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30(11):2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 2.Mulder M, Gibbs HG, Smith SW, Dhaliwal R, Scott NL, Sprenkle MD, Geocadin RG. Awakening and withdrawal of life-sustaining treatment in cardiac arrest survivors treated with therapeutic hypothermia. Crit Care Med. 2014;42(12):2493–2499. doi: 10.1097/CCM.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rittenberger JC, Tisherman SA, Holm MB, et al. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation. 2011;82:1399–1404. doi: 10.1016/j.resuscitation.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmer J, Torres C, Aufderheide TP, Austin MA, Callaway CW, Golan E, Herren H, Jasti J, Kudenchuk PJ, Scales DC, Stub D, Richardson DK, Zive DM Resuscitation Outcomes Consortium. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016 Feb 3; doi: 10.1016/j.resuscitation.2016.01.016. pii: S0300-9572(16)00042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 6.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen N, Wetterslev J, Cronberg T, et al. TTM Trial Investigators. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369(23):2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 8.Samaniego EA, Mlynash M, Caulfield AF, et al. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit Care. 2011;15:113–119. doi: 10.1007/s12028-010-9412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouwes A, Binnekade JM, Kuiper MA, Bosch FH, Zandstra DF, Toornvliet AC, Biemond HS, Kors BM, Koelman JH, Verbeek MM, Weinstein HC, Hijdra A, Horn J. Prognosis of coma after therapeutic hypothermia: a prospective cohort study. Ann Neurol. 2012;71:206–212. doi: 10.1002/ana.22632. [DOI] [PubMed] [Google Scholar]
- 10.Fugate JE, Wijdicks EF, Mandrekar J, Claassen DO, Manno EM, White RD, Bell MR, Rabinstein AA. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol. 2010;68:907–914. doi: 10.1002/ana.22133. [DOI] [PubMed] [Google Scholar]
- 11.Rittenberger JC, Sangl J, Wheeler M, et al. Association between clinical examination and outcome after cardiac arrest. Resuscitation. 2010;81:1128–1132. doi: 10.1016/j.resuscitation.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouwes A, Binnekade JM, Zandstra DF, Koelman JH, van Schaik IN, Hijdra A, Horn J. Somatosensory evoked potentials during mild hypothermia after cardiopulmonary resuscitation. Neurology. 2009;73:1457–1461. doi: 10.1212/WNL.0b013e3181bf98f4. [DOI] [PubMed] [Google Scholar]
- 13.Rittenberger JC, Popescu A, Brenner RP, et al. Frequency and timing of nonconvulsive status epilepticus in comatose post-cardiac arrest subjects treated with hypothermia. Neurocrit Care. 2012;16:114–122. doi: 10.1007/s12028-011-9565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmeijer J, Beernink TM, Bosch FH, Beishuizen A, Tjepkema-Cloostermans MC, van Putten MJ. Early EEG contributes to multimodal outcome prediction of postanoxic coma. Neurology. 2015;85(2):137–143. doi: 10.1212/WNL.0000000000001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee BK, Jeung KW, Song KH, Jung YH, Choi WJ, Kim SH, Youn CS, Cho IS, Lee DH Korean Hypothermia Network Investigators. Prognostic values of gray matter to white matter ratios on early brain computed tomography in adult comatose patients after out-of-hospital cardiac arrest of cardiac etiology. Resuscitation. 2015;96:46–52. doi: 10.1016/j.resuscitation.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Choi SP, Park KN, Youn CS, Oh SH, Choi SM. Early brain computed tomography findings are associated with outcome in patients treated with therapeutic hypothermia after out-of-hospital cardiac arrest. Scand J Trauma Resusc Emerg Med. 2013;21:57. doi: 10.1186/1757-7241-21-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metter RB, Rittenberger JC, Guyette FX, Callaway CW. Association between a quantitative CT scan measure of brain edema and outcome after cardiac arrest. Resuscitation. 2011;82:1180–1185. doi: 10.1016/j.resuscitation.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youn CS, Park KN, Kim JY, Callaway CW, Choi SP, Rittenberger JC, Kim SH, Oh SH, Kim YM. Repeated diffusion weighted imaging in comatose cardiac arrest patients with therapeutic hypothermia. Resuscitation. 2015;96:1–8. doi: 10.1016/j.resuscitation.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 19.Elmer J, Gianakas JJ, Rittenberger JC, Baldwin ME, Faro J, Plummer C, Shutter LA, Wassel CL, Callaway CW Fabio A and the Pittsburgh Post-Cardiac Arrest Service. Group-Based Trajectory Modeling of Suppression Ratio After Cardiac Arrest. Neurocrit Care. 2016 Mar 31; doi: 10.1007/s12028-016-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM, Zimmerman JL. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S465–S482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandroni C, Cariou A, Cavallaro F, Cronberg T, Friberg H, Hoedemaekers C, Horn J, Nolan JP, Rossetti AO, Soar J. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Resuscitation. 2014;85:1779–1789. doi: 10.1016/j.resuscitation.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, Bottiger BW, Friberg H, Sunde K, Sandroni C. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation. 2015;95:202–222. doi: 10.1016/j.resuscitation.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Youn CS, Callaway CW, Rittenberger JC Post Cardiac Arrest Service. Combination of initial neurologic examination and continuous EEG to predict survival after cardiac arrest. Resuscitation. 2015;94:73–79. doi: 10.1016/j.resuscitation.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Rittenberger JC, Guyette FX, Tisherman SA, et al. Outcomes of a hospital-wide plan to improve care of comatose survivors of cardiac arrest. Resuscitation. 2008;79:198–204. doi: 10.1016/j.resuscitation.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coppler PJ, Calderon L, Sabedra A, Doshi A, Callaway CW, Rittenberger JC, Dezfulian C. Validation of the Pittsburgh cardiac arrest category. Resuscitation. 2015;89:86–92. doi: 10.1016/j.resuscitation.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rittenberger JC, Raina K, Kim YJ, et al. Association between cerebral performance category, modified ranking scale, and discharge disposition after cardiac arrest. Resuscitation. 2011;82:1036–1040. doi: 10.1016/j.resuscitation.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Oddo M, Rossetti AO. Early multimodal outcome prediction after cardiac arrest in patients treated with hypothermia. Crit Care Med. 2014;42(6):1340–1347. doi: 10.1097/CCM.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 29.Cronberg T, Horn J, Kuiper MA, Friberg H, Nielsen N. A structured approach to neurologic prognostication in clinical cardiac arrest trials. Scand J Trauma Resusc Emerg Med. 2013;21:45. doi: 10.1186/1757-7241-21-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friberg H, Rundgren M, Westhall E, Nielsen N, Cronberg T. Continuous evaluation of neurological prognosis after cardiac arrest. Acta Anaesthesiologica Scandinavica. 2013;57:6–15. doi: 10.1111/j.1399-6576.2012.02736.x. [DOI] [PubMed] [Google Scholar]
- 31.Hofmeijer J, Beernink TM, Bosch FH, Beishuizen A, Tjepkema-Cloostermans MC, van Putten MJ. Early EEG contributes to multimodal outcome prediction of postanoxic coma. Neurology. 2015;85(2):137–143. doi: 10.1212/WNL.0000000000001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elmer J, Jeong K, Abebe KZ, Guyette FX, Murugan R, Callaway CW, Rittenberger JC on behalf of the Pittsburgh Post-Cardiac Arrest Service. Serum Neutrophil gelatinase-associated lipocalin predicts survival after resuscitation from cardiac arrest. Crit Care Med. 2016 Jan;44(1):111–119. doi: 10.1097/CCM.0000000000001357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossetti AO, Rabinstein AA, Oddo M. Neurological prognostication of outcome in patients in coma after cardiac arrest. Lancet Neurol. 2016 Mar 23; doi: 10.1016/S1474-4422(16)00015-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Mettenburg JM, Agarwal V, Baldwin M, Rittenberger JC. Discordant observation of brain injury by MRI and malignant EEG patterns in comatose survivors of cardiac arrest. AJNR. 2016 Jun 16; doi: 10.3174/ajnr.A4839. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amorim E, Rittenberger JC, Baldwin ME, Callaway CW, Popescu A Post Cardiac Arrest Service. Malignant EEG patterns in cardiac arrest patients treated with targeted temperature management who survive to hospital discharge. Resuscitation. 2015;90:127–132. doi: 10.1016/j.resuscitation.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dragancea I, Backman S, Westhall E, Rundgren M, Friberg H, Cronberg T. Outcome following postanoxic status epilepticus in patients with targeted temperature management after cardiac arrest. Epilepsy Behav. 2015 Aug;49:173–177. doi: 10.1016/j.yebeh.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 37.Raina KD, Rittenberger JC, Holm MB, Callaway CW. Functional Outcomes: one year after a cardiac arrest. Biomed Research International. 2015;2015:283608. doi: 10.1155/2015/283608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.