Abstract
Adherence to the 2007 WCRF/AICR cancer prevention recommendations has been associated with lower cancer risk but the underlying biological mechanisms have not been elucidated. We utilized dietary and lifestyle data from 11,342 women in the Nurses’ Health Study and 8,136 men in the Health Professionals Follow-up Study, to investigate associations between adherence scores and markers of inflammation, hormonal and insulin response. Two scores ranging from 0 to 3 were constructed to assess adherence to the energy balance-related recommendations (weight management, physical activity, energy density); and the plant, animal foods and alcohol intake recommendations; with higher scores indicating greater adherence. The following biomarkers were assessed in plasma samples donated by chronic disease-free women (1990) and men (1994): C-reactive protein (CRP), interleukin-6 (IL6), tumor necrosis factor alpha receptor 2 (TNFαR2) and adiponectin for inflammation; estrone and estradiol for hormonal response in women, C-peptide for hyperinsulinemia; and triglycerides/high density lipoprotein-cholesterol (TG/HDL) ratio for insulin resistance. In multivariable-adjusted linear regression analyses, we estimated relative concentrations of biomarkers across adherence categories. There was a significant trend of lower (higher for adiponectin) biomarker concentrations with higher adherence to the energy balance recommendations (all P-trend<0.0001). Comparing the highest (3) to the lowest recommendation category (0–1), the percent difference in relative concentrations of biomarkers was CRP, −69%; IL6, −41%; TNFαR2, −13%; adiponectin, +36; C-peptide, −43%; TG/HDL, −43%; estrone, −31%; and estradiol, −43%; in women; and CRP, −59%; IL6, −42%; TNFαR2, −10%; adiponectin, +22%; C-peptide, −44%; and TG/HDL, −40%; in men. In contrast, associations between adherence to the plant, animal foods and alcohol intake recommendations and biomarker concentrations were weaker, and mostly nonsignificant. The healthier biomarker profile associated with greater adherence to the WCRF/AICR cancer prevention recommendations is driven mainly by adherence to the energy balance-related recommendations.
Keywords: chronic inflammation, hormones, hyperinsulinemia, insulin resistance, cancer prevention, diet, lifestyle, WCRF/AICR recommendations
Introduction
Healthy dietary and lifestyle patterns have been recognized as crucial for the prevention of most chronic diseases including cancer. Part of this recognition is the 2007 World Cancer Research Fund and the American Institute for Cancer Research’s (WCRF/AICR) recommendations for cancer prevention. The recommendations aim to improve individuals’ dietary and lifestyle patterns, including maintaining a lean body mass, participating in moderate physical activity, consuming a primarily plant-based diet and minimizing the consumption of red and processed meats, energy-dense foods and drinks, and alcohol.1 A number of studies have investigated adherence to these recommendations and risk of cancer development, and found higher adherence to be consistently associated with a lower risk of developing cancer,2 especially breast cancer3, 4 and colorectal cancer.5, 6 However, the biological mechanisms through which these recommendations may influence cancer risk are not known. Indeed, only two previous studies have examined associations between adherence to the cancer prevention recommendations and biomarkers of inflammation, oxidative stress and the metabolic syndrome, which could represent potential biological pathways mediating the association between adherence to the WCRF/AICR recommendations and cancer risk.7, 8 In a sample of 275 premenopausal women, adherence to the recommendations was associated with lower concentrations of biomarkers that indicate oxidative stress and inflammation,7 while in a larger sample of 2,092 women diagnosed with invasive breast cancer, women who adhered to more dietary recommendations were less likely to develop metabolic syndrome,8 a risk factor for breast cancer development and recurrence.9, 10
The development of some cancers is associated with states of chronic inflammation, hormonal response, hyperinsulinemia and insulin resistance. It is thus possible that the WCRF/AICR recommendations may influence cancer development through these biological pathways, among other potential pathways. A number of circulating biomarkers of these pathways have been associated with cancer risk. For example, higher concentrations of several inflammation markers including C-reactive protein (CRP),11, 12 interleukin-6 (IL6),13, 14 and tumor necrosis factor alpha receptor 2 (TNFαR2)14, 15 and lower concentrations of adiponectin16, 17 have been associated with higher cancer risk. Adiponectin is thought to play a role in the regulation of glucose and lipid metabolism, and exhibits insulin-sensitizing and anti-inflammatory properties.18, 19 Hormones play a major role in the development of several common cancers, probably due to their effect on cell division, with circulating estrogens linked to higher risk of endometrial, and breast cancers among other cancers.20 Also, higher circulating levels of C-peptide, a marker of insulin secretion, have been positively associated with higher risk of several cancers.21, 22 Studies have also shown insulin resistance to be associated with higher cancer risk,23 and the ratio of triglycerides to high-density lipoprotein-cholesterol (TG/HDL), to be correlated with insulin resistance.24 In this cross-sectional study, we investigated associations between scores of adherence to the WCRF/AICR cancer prevention recommendations and fasting plasma markers of inflammation, hormonal response and insulin response, based on the hypothesis that women and men with higher adherence scores have a healthier biomarker profile driven mainly by adherence to the energy balance-related recommendations.
Materials and Methods
Study population
We used data from two on-going prospective United States cohorts: the Nurses’ Health Study (NHS) enrolled 121,700 female registered nurses, aged 30 to 55 years at baseline in 1976, and the Health Professionals Follow-up Study (HPFS), which enrolled 51,529 male health professionals, aged 40 to 75 years at baseline in 1986. Details of the two cohorts have been described.25 At enrollment in all cohorts, participants completed baseline questionnaires regarding demographic and lifestyle factors, medications, and newly diagnosed diseases. During follow-up, questionnaires are administered every two years to update lifestyle, medical, and other health-related information. The follow-up has been >90% complete for each cohort. Blood samples were collected from subpopulations of the NHS (n=29,611) from 1989 to 1990 and HPFS (n=18,225) from 1993 to 1994. Blood collection was conducted using similar protocols for both cohorts, and blood donors were free from diagnosed major chronic diseases such as cancer, cardiovascular disease and diabetes at the time of blood donation. The procedures, including collection, handling and storage, have been previously summarized.26 In the current study, we used data from previous matched case-control studies nested within each of the two cohorts that measured fasting plasma concentrations of IL6, CRP, TNFαR2, adiponectin, C-peptide, TG, HDL, estrone and estradiol from August 1997 through December 2014. The Institutional Review Boards at Brigham and Women’s Hospital and at Harvard T.H. Chan School of Public Health approved this study.
Biomarker assessment
We assessed inflammation based on four biomarkers: CRP, IL6, TNFαR2, and adiponectin; utilized C-peptide concentrations to assess hyperinsulinemia; ratio of TG/HDL as a marker of insulin resistance and estrone and estradiol as markers of hormonal response in postmenopausal women not using exogenous hormones. Compared to insulin, C-peptide has proven to be a better measure of pancreatic beta-cell secretory activity as it is not extracted by the liver, has a slower metabolic clearance rate, and does not cross-react with antibodies to insulin.27 Studies have shown TG/HDL to be significantly correlated with insulin resistance,24 and a simple and clinically useful way to identify apparently healthy individuals who are insulin resistant,28, 29 though TG/HDL is not a replacement for the homeostasis model assessment-estimated insulin resistance index (HOMA-IR).
All biomarkers were measured in fasting plasma samples and the laboratory procedures have been previously described.30–33 Briefly, concentrations of IL6 and TNFαR2 were measured using enzyme-linked immunosorbent assays (ELISA) (R&D Systems, Minneapolis, USA). CRP was measured using a high sensitivity immunoturbidimetric assay with reagents and calibrators from Denka Seiken Co, Tokyo, Japan. We excluded participants with CRP values >10mg/L (n=308) as this may likely be due to infection or medication use.34 Concentrations of adiponectin were measured using a competitive radioimmunoassay (Linco Research, St. Charles, MO). Estrone and estradiol were measured in the Molecular lab at the Mayo Clinic (Rochester, MN) using the turbulent flow liquid chromatography-tandem mass spectrometry. C-peptide was measured by ELISA (Diagnostic Systems Laboratories/Beckman Coulter, Webster, TX), and HDL-cholesterol and TG were measured by standard methods with reagents from Roche Diagnostics (Indianapolis, IN) and Genzyme (Cambridge, MA).32 The intra-assay coefficient of variation from blinded quality control samples ranged from 1% to 13% for all biomarkers across batches.
Quality control samples were randomly interspersed among the case-control samples, and laboratory personnel were blinded to quality control and case-control status for all assays. Biomarkers were measured in multiple batches over several years, and there may be differences in mean biomarker levels by batch due to different reagents, technicians, laboratories, or participants’ characteristics in each batch. There were 39 batches for each of the four inflammation markers, 20 for C-peptide, triglycerides, and HDL-cholesterol, and 12 for estradiol and estrone. We therefore used a 3-step method previously described by Rosner et al.35 to recalibrate biomarker concentrations across several batches to the value of an “average batch” accounting for true variability across batches due to different distributions of predictors of the biomarker across batches: i) we constructed a linear regression model with biomarker levels as the dependent variable and batch indicators as well as variables that may vary by batch and are associated with biomarker levels (regular aspirin/non-steroidal anti-inflammatory drugs [NSAID] use, age, smoking status, diabetes, other chronic diseases/conditions, case-control status, BMI, physical activity, and menopausal status, postmenopausal hormone use in women) as the independent variables, ii) next we calculated the average batch beta coefficient (β) by summing the batch indicator βs and dividing by the total number of batches, iii) lastly we calculated the difference between each batch β and average β and recalibrated biomarker concentrations by subtracting this difference from the original biomarker concentration. We recalibrated the data for men and women separately given that these data were pooled separately by cohort. The recalibrated biomarkers were then used in analyses.
Assessment of dietary and nondietary data
Dietary data are updated every four years in the NHS (since 1980) and in the HPFS (since 1986) with a semi quantitative food frequency questionnaire (FFQ) assessing dietary intake in the previous one year. The relative validity of the FFQ has been reported.36, 37 Given that blood was drawn at one time point, we used dietary data from the questionnaires closest to the blood draw, that is, the 1990 FFQ for NHS and the 1994 FFQ for HPFS. Participants with excessive missing items (≥70) on the FFQs or implausibly low or high energy intake (<600 or >3500 kcal/d for women and <800 or >4,200 kcal/d for men) were excluded.
All cohorts have collected nondietary data (e.g., medical history and health practices) since 1976 in the NHS and 1986 in the HPFS, and updated the data through biennial self-administered questionnaires. We calculated participants’ body mass index (BMI – kg/m2) using height (meters) reported at baseline for each cohort, and weight (kg) reported on the questionnaire closest to blood draw. Participants reported smoking status, and we calculated physical activity, expressed in metabolic equivalent (MET)-hours per week by summing the average MET-hours/week for the following activities: tennis/squash/racquetball, rowing, calisthenics, walking, jogging, running, bicycling, and swimming. The validity of the physical activity questionnaire has been evaluated.38, 39 Regular use of aspirin or other NSAID was defined as use of ≥2 standard tablets (325-mg) of aspirin or ≥2 tablets of NSAID per week. We derived a chronic disease comorbidity score by summing the presence=1/absence=0 of the following chronic diseases/conditions: hypercholesterolemia, cancer, diabetes, high blood pressure, heart disease, and rheumatoid/other arthritis, to create the score.
2007 WCRF/AICR score construction
First, given that some recommendations are more related to energy balance than others, we constructed two 3-points scores based on adherence to the 2007 WCRF/AICR cancer prevention recommendations. The first score included the three energy balance-related recommendations (body fatness, physical activity, and foods and drinks that promote weight gain), whereas the second score included plant foods, animal foods, and alcohol consumption. Table 1 describes details of the score operationalization. Briefly, we used the quantitative recommendations as criteria for assigning component (individual recommendations) scores such that participants received 1, 0.5, or 0 point when the recommendation was met, partially met, or not met, respectively. For recommendations with subcomponents such as foods and drinks that promote weight gain, plant foods and animal foods, the subcomponents were scored first (1, 0.5, or 0 point) then the average score was taken. To define intermediate categories, we used a priori cutoffs based on previous publications.5, 6 The overall adherence scores were the sum of the individual recommendation scores, and ranged from a minimum of 0 point (adherence to no recommendation) to a maximum of 3 points (adherence to all 3 recommendations) with 7 levels (0, 0.5, 1, 1.5, 2, 2.5 and 3), and were further categorized into 5 levels by combining levels 0 to 1 since there were few participants in these three levels. Each recommendation contributed equally to the overall score.
Table 1.
2007 WCRF/AICR recommendations for cancer prevention and operationalization in the current study; 1990 for women (n=11,342) and 1994 for men (n=8,136)
| Recommendation category | Description | Operationalization | Points1 | % women | % men |
|---|---|---|---|---|---|
| 1 Body weight | Be as lean as possible within the normal range of body weight | BMI: 18.5 – <25.0 kg/m2 | 1 | 49.6 | 42.2 |
| BMI: 25 to <30 kg/m2 | 0.5 | 31.1 | 46.4 | ||
| BMI ≥30 kg/m2 or <18.5 kg/m2 | 0 | 19.3 | 11.4 | ||
|
| |||||
| 2 Physical activity (PA) | Be physically active as part of your everyday life | ≥210 minutes/week | 1 | 55.3 | 76.1 |
| 120 to <210 minutes/week | 0.5 | 15.9 | 9.7 | ||
| <120 minutes/week | 0 | 28.7 | 14.2 | ||
|
| |||||
| 3 Energy density (ED)2,3 | Limit consumption of energy dense foods; avoid sugary drinks4 | ED ≤125 kcal/100 g/d | 1 | 15.7 | 8.7 |
| ED: 125 to <175 kcal/100 g/d | 0.5 | 8.7 | 15.6 | ||
| ED ≥175 kcal/100 g/d | 0 | 75.6 | 75.7 | ||
| Sugary drinks intake 0 g/d | 1 | 23.0 | 5.8 | ||
| Sugary drinks intake >0 g to ≤370 g/d | 0.5 | 63.2 | 70.9 | ||
| Sugary drinks intake >370 g/d | 0 | 13.8 | 23.3 | ||
|
| |||||
| 4 Plant foods2 | Eat mostly foods of plant origin | F&V ≥400 g/d | 1 | 23.5 | 50.8 |
| F&V 200 to <400 g/d | 0.5 | 22.7 | 38.0 | ||
| F&V <200 g/d | 0 | 53.8 | 11.2 | ||
| Dietary fiber intake ≥25 g/d | 1 | 11.0 | 33.1 | ||
| Dietary fiber intake of 12.5 to <25 g/d | 0.5 | 79.3 | 63.0 | ||
| Dietary fiber intake <12.5 g/d | 0 | 9.7 | 3.9 | ||
|
| |||||
| 5 Animal foods2 | Limit intake of red meat | Red meat <500 g/wk | 1 | 90.3 | 67.3 |
| Red meat 500 to <1000 g/wk | 0.5 | 1.5 | 25.9 | ||
| Red meat ≥1000 g/wk | 0 | 8.2 | 6.8 | ||
| Avoid processed meat | Processed meat ≤3 g/d | 1 | 76.9 | 37.9 | |
| Processed meat >3 to <27 g/d | 0.5 | 22.0 | 57.7 | ||
| Processed meat ≥27 g/d | 0 | 1.1 | 4.4 | ||
|
| |||||
| 6 Alcoholic drinks5 | Limit alcoholic drinks | Ethanol intake ≤20 g/d (men); ≤10 g/d (women) | 1 | 81.4 | 79.9 |
| Ethanol intake >20 to ≤30 g/d (men); >10 to ≤20 g/d (women) | 0.5 | 11.8 | 7.3 | ||
| Ethanol intake >30 g/d (men); >20 g/d (women) | 0 | 6.8 | 12.8 | ||
Quantitative recommendations were used as criteria for assigning component (individual recommendations) scores such that participants received 1, 0.5, or 0 point when the recommendation was met, partially met, or not met, respectively. For recommendations with subcomponents such as foods and drinks that promote weight gain, plant foods and animal foods, the subcomponents were scored first (1, 0.5, or 0 point) then the average score was taken. To define intermediate categories, we used a priori cutoffs based on previous publications (ref #4 and 5);
Score was the average score of the subcomponents of this recommendation;
ED was calculated as total non-beverage energy (kcal) from foods divided by the total weight (g) of the non-beverage foods.;
Sugary drinks also included fruit juices;
An alcoholic drink corresponds to 10g ethanol;
F&V=fruits and vegetables;
Second, we constructed two additional scores: a 5-points score that included five recommendations with the exception of body weight, and a 6-points score that included all six recommendations. The 5-point score was meant to be used in models to additionally adjust for BMI under the extreme assumption that the associations were confounded by BMI. The overall adherence scores ranged from 0 (no adherence to any recommendation) to 5 or 6 (adherence to all five or six recommendations). We further categorized the continuous scores into five or six categories as follows: category 1 (0 to ≤2), category 2 (>2 to ≤3), category 3 (>3 to ≤4), category 4 ((>4 to ≤5) for the 5-points score, and category 5 (>5 to 6) for the 6-points score. The recommendations on breast feeding, food preservation, processing, and preparation were not included in this score because of insufficient data.
Statistical analyses
The distributions of biomarkers were skewed and we transformed all biomarkers using natural log transformation prior to using them in analyses. We then back transformed (i.e., ex, where x was the natural log transformed biomarker concentration) biomarker concentrations before presentation of results. Descriptive statistics for continuous variables were summarized as means ± standard deviation (or geometric mean ± coefficient of variation40), and categorical variables were summarized using proportions; according to categories of the 6-points adherence score. We used linear regression analyses to estimate the percent changes in relative concentrations of biomarkers in categories of the overall adherence score, comparing higher categories with the lowest (reference) category (i.e., the percent differences in biomarker concentration between higher adherence categories and the lowest category). We constructed multivariable-adjusted linear regression models separately for the energy balance-related adherence score and the plants, animal foods and alcohol intake adherence score, to estimate the percent changes in relative concentrations of each biomarker in categories of the scores. To determine the independent effects of each of the six recommendations on biomarker concentrations, we estimated the relative concentration of biomarkers in categories (1, 0.5 and 0) of the individual recommendations adjusting for multiple covariates that included adherence scores to all the other five component recommendations. Next, to determine the influence of body weight on the association between the overall adherence score and each biomarker, we additionally controlled for BMI (as a continuous variable) in the multivariable-adjusted models of the 5-points score. Lastly, we examined associations between the 6-points score and biomarker concentrations, with the expectation that associations will be similar to those of the 3-points energy balance-related recommendations score.
All multivariable-adjusted models included the following covariates as potential confounding variables: age at blood draw (continuous, years), smoking status (never, former, current), regular aspirin/NSAID use (yes/no), race (white, nonwhite), case-control status, chronic disease comorbidity score and additionally for menopausal status and postmenopausal hormone use in women. Linear trends were assessed using the continuous score values adjusted for multiple covariates. We examined associations between adherence scores and estrone and estradiol concentrations only among postmenopausal women not using menopausal hormones. All tests were 2-sided and 95% confidence intervals not including 0 were considered statistically significant. All analyses were conducted by using SAS software, version 9.4 for UNIX (SAS Institute, Cary, NC).
Results
The average age at blood draw was 56.5±6.9 years for women, and 62.1±8.7 years for men, and increased slightly with higher adherence scores in women. The average BMI at blood draw was 26.0±5.0 kg/m2 among women and 26.0±3.5 kg/m2 among men. Similar proportions of women and men met the body weight and alcohol consumption recommendations and a higher proportion of men than women met the physical activity and plant foods recommendations. In contrast, a higher proportion of women than men met the recommendations for foods and drinks that promote weight gain and for red and processed meat intake (Table 1). A higher proportion of women than men were current smokers; and among both women and men the proportion of current smokers decreased with higher adherence scores (Table 2). BMI values as well as concentrations of CRP, IL6, TNFαR2, C-peptide and TG/HDL ratio were highest among the least adherers compared to the most adherent women and men, while concentrations of adiponectin were highest among adherers, decreasing monotonically towards the least adherent women and men The majority of women were postmenopausal and the highest proportion (65%) of postmenopausal hormone users was among the most adherent women. The proportion of women and men with ≥3 chronic diseases/conditions decreased with higher adherence scores (Table 2). All biomarkers were moderately inversely (positively for adiponectin) correlated with adherence scores (r=0.10 to 0.25) except for TNFαR2 in men. Adherence scores and biomarkers were correlated with BMI (r=0.16 to 0.55) except for TNFαR2 in men. There were also moderate to high correlations among the biomarkers, except for estrone and estradiol that were highly correlated (r= 0.83) but both were not significantly correlated with adiponectin and TNFαR2, and IL6 was not correlated with estrone (Supplemental Table 1).
Table 2.
Distribution of participant characteristics across WCRF/AICR score categories
| Nurses’ Health Study; 1990 | |||||
|---|---|---|---|---|---|
|
| |||||
| Characteristic | 0 to ≤2 (least adherent) (n1=211) | >2 to ≤3 (n1=2,007) | >3 to ≤4 (n1=4,619) | >4 to ≤5 (n1=3,992) | >5 to 6 (most adherent) (n1=513) |
| Plasma biomarkers [geometric mean ± CV]2 | |||||
| C-reactive protein (mg/L) | 3.3 ± 1.0 | 2.2 ± 1.3 | 1.6 ± 1.4 | 1.2 ± 1.4 | 1.1 ± 1.3 |
| Interleukin-6 (pg/mL) | 2.0 ± 1.2 | 1.5 ± 1.0 | 1.3 ± 1.0 | 1.1 ± 1.1 | 1.1 ± 1.0 |
| Tumor necrosis factor alpha receptor 2 (ng/ml) | 2.7 ± 0.6 | 2.7 ± 0.6 | 2.5 ± 0.6 | 2.5 ± 0.6 | 2.5 ± 0.6 |
| Adiponectin (μg/mL) | 10.0 ± 0.8 | 9.0 ± 0.8 | 10.0 ± 0.8 | 11.0 ± 0.8 | 12.2 ± 0.8 |
| C-peptide (ng/mL) | 3.0 ± 1.0 | 2.7 ± 0.9 | 2.2 ± 0.9 | 1.8 ± 0.9 | 1.8 ± 0.8 |
| TG/HDL ratio | 3.0 ± 1.1 | 2.5 ± 1.1 | 2.2 ± 1.0 | 1.8 ± 0.9 | 1.6 ± 1.0 |
| Estrone (pg/mL)3 | 30.0 ± 0.8 | 30.0 ± 0.8 | 24.5 ± 0.8 | 24.5 ± 0.8 | 22.2 ± 0.8 |
| Estradiol (pg/mL)3 | 10.0 ± 0.9 | 8.2 ± 1.0 | 6.7 ± 1.0 | 6.0 ± 0.9 | 5.5 ± 0.9 |
| Age at blood draw (years) | 55.1 ± 6.6 | 55.5 ± 6.8 | 56.1 ± 6.8 | 56.6 ± 6.8 | 58.1 ± 6.3 |
| WCRF/AICR score components [mean ± SD] | |||||
| Body mass index (kg/m2) | 31.1 ± 6.1 | 29.5 ± 6.0 | 26.3 ± 4.8 | 23.9 ± 3.1 | 22.6 ± 2.0 |
| Physical activity (MET-hrs/week) | 2.9 ± 3.6 | 6.1 ± 10.4 | 16.8 ± 19.3 | 28.2 ± 23.2 | 35.6 ± 38.1 |
| Energy density (Kcal/100g/d) | 788 ± 620 | 593 ± 361 | 520 ± 424 | 339 ± 301 | 153 ± 109 |
| Sugary drinks (g/d) | 534 ± 1326 | 380 ± 1066 | 330 ± 1006 | 302 ± 936 | 232 ± 752 |
| Fruits and vegetables (g/d) | 187 ± 749 | 244 ± 626 | 327 ± 702 | 489 ± 784 | 956 ± 847 |
| Dietary fiber (g/d) | 13.3 ± 3.8 | 16.2 ± 4.2 | 17.8 ± 4.7 | 20.2 ± 5.5 | 25.0 ± 7.2 |
| Red meat (g/wk) | 807 ± 1650 | 735 ± 1761 | 626 ± 1625 | 523 ± 1526 | 362 ± 1286 |
| Processed meat (g/d) | 7.0 ± 20.9 | 4.3 ± 17.8 | 3.6 ± 22.5 | 2.4 ± 19.5 | 0.5 ± 2.2 |
| Alcohol (g/d) | 21.8 ± 18.8 | 8.3 ± 13.6 | 5.6 ± 9.5 | 3.1 ± 5.0 | 1.9 ± 2.7 |
| Smoking status (%) | |||||
| Never | 29.9 | 39.5 | 42.5 | 47.5 | 49.5 |
| Former | 37.9 | 42.2 | 42.7 | 41.7 | 43.3 |
| Current | 32.2 | 18.3 | 14.8 | 10.9 | 7.2 |
| Regular aspirin/NSAID user (%) | 38.9 | 40.7 | 37.8 | 33.8 | 29.0 |
| Chronic disease/conditions comorbidity score4 | |||||
| no chronic disease/condition | 38.9 | 35.9 | 41.1 | 43.3 | 42.3 |
| 1 chronic disease/condition | 28.9 | 29.8 | 31.0 | 33.0 | 32.4 |
| 2 chronic diseases/conditions | 19.4 | 21.1 | 17.9 | 16.6 | 18.1 |
| ≥3 chronic diseases/conditions | 12.8 | 13.3 | 10.1 | 7.0 | 7.2 |
| Postmenopausal women (%) | 84.4 | 84.0 | 85.2 | 87.2 | 91.8 |
| Postmenopausal hormone user (%) | 41.7 | 46.8 | 52.4 | 56.9 | 64.7 |
|
| |||||
| Health Professionals Follow-up Study; 1994 | |||||
|
| |||||
| 0 to ≤2 (least adherent) (n1=235) | >2 to ≤3 (n1=749) | >3 to ≤4 (n1=2,296) | >4 to ≤5 (n1=3,831) | >5 to 6 (most adherent) (n1=1,025) | |
|
| |||||
| Plasma biomarkers [geometric mean ± CV]2 | |||||
| C-reactive protein (mg/L) | 1.3 ± 1.5 | 1.2 ± 1.3 | 1.1 ± 1.6 | 0.8 ± 1.4 | 0.7 ± 1.4 |
| Interleukin-6 (pg/mL) | 2.0 ± 1.0 | 1.5 ± 1.1 | 1.5 ± 1.1 | 1.2 ± 1.4 | 1.2 ± 1.1 |
| tumor necrosis factor alpha receptor 2 (ng/ml) | 2.7 ± 0.6 | 2.7 ± 0.6 | 2.7 ± 0.6 | 2.7 ± 0.6 | 2.7 ± 0.6 |
| Adiponectin (μg/mL) | 5.5 ± 0.8 | 6.0 ± 0.8 | 6.0 ± 0.8 | 6.0 ± 0.8 | 6.7 ± 0.8 |
| C-peptide (ng/mL) | 3.0 ± 0.9 | 2.7 ± 0.9 | 2.5 ± 0.9 | 2.2 ± 0.9 | 1.8 ± 0.9 |
| TG/HDL ratio | 3.0 ± 1.1 | 3.0 ± 1.1 | 3.0 ± 1.1 | 2.7 ± 1.1 | 2.2 ± 1.0 |
| Age at blood draw (years) | 62.5 ± 9.1 | 61.8 ± 8.5 | 61.8 ± 8.5 | 62.1 ± 8.8 | 62.8 ± 8.9 |
| WCRF/AICR score components [mean ± SD] | |||||
| Body mass index (kg/m2) | 28.6 ± 5.5 | 28.0 ± 4.2 | 27.1 ± 3.6 | 25.4 ± 8.8 | 23.4 ± 1.7 |
| Physical activity (MET-hrs/week) | 23.0 ± 38.7 | 23.6 ± 38.9 | 29.4 ± 35.1 | 43.0 ± 42.3 | 48.5 ± 38.4 |
| Energy density (Kcal/100g/d) | 331 ± 185 | 330 ± 206 | 306 ± 182 | 276 ± 159 | 206 ± 107 |
| Sugary drinks (g/d) | 441 ± 418 | 391 ± 374 | 313 ± 325 | 249 ± 257 | 192 ± 178 |
| Fruits and vegetables (g/d) | 353 ± 219 | 357 ± 186 | 397 ±221 | 461 ± 261 | 605 ± 269 |
| Dietary fiber (g/d) | 15.8 ± 4.7 | 18.8 ± 5.6 | 21.2 ± 6.5 | 23.8 ± 7.1 | 26.3 ± 8.0 |
| Red meat (g/wk) | 671 ± 392 | 552 ± 449 | 491 ± 389 | 382 ± 324 | 275 ± 241 |
| Processed meat (g/d) | 12.5 ± 14.6 | 10.0 ± 10.6 | 8.6 ± 10.8 | 6.7 ± 9.4 | 4.3 ± 6.2 |
| Alcohol (g/d) | 25.4 ± 22.8 | 23.5 ± 22.6 | 16.2 ± 18.3 | 8.5 ± 10.4 | 6.0 ± 6.0 |
| Smoking status (%) | |||||
| Never | 33.2 | 37.0 | 39.5 | 49.3 | 54.9 |
| Former | 54.9 | 52.7 | 53.4 | 46.1 | 41.5 |
| Current | 11.9 | 10.3 | 7.2 | 4.6 | 3.6 |
| Regular aspirin/NSAID user (%) | 17.0 | 13.5 | 16.3 | 12.7 | 11.7 |
| Chronic disease/conditions comorbidity score4 | |||||
| no chronic disease/condition | 34.9 | 37.3 | 38.5 | 42.4 | 45.9 |
| 1 chronic disease/condition | 30.6 | 30.7 | 34.3 | 31.5 | 30.8 |
| 2 chronic diseases/conditions | 20.4 | 20.2 | 16.5 | 17.4 | 14.7 |
| ≥3 chronic diseases/conditions | 14.0 | 11.9 | 10.7 | 8.7 | 8.6 |
Biomarker sample sizes vary: in NHS, n=3,550 for all four inflammatory markers, n=5,834 for C-peptide, n=3,826 for TG/HDL ratio, n=1,217 for estrone, n=1,254 for estradiol; and in HPFS, n=5,157 for C-reactive protein, n=3,044 for interleukin-6, n=4,072 for tumor necrosis factor alpha receptor 2, n=4,348 for adiponectin, n=3,955 for C-peptide and n=3,575 for TG/HDL ratio in HPFS; TG=triglycerides, HDL=high density lipoprotein cholesterol. Sample size for all other characteristics is the total sample size;
Hormornes (estrone and estradiol) were examined only among postmenopausal women not using exogenous hormones
Geometric means (coefficient of variation, CV) are presented for biomarkers because all biomarkers were log transformed prior to analyses; The Quan-Zhang formula, CV=(eSD-1)1/2 was used to calculate CVs (ref #40);
Chronic diseases/conditions included in the score: hypercholesterolemia, cancer, diabetes, high blood pressure, heart disease, ulcerative colitis and rheumatoid/other arthritis.
TG=triglycerides, HDL=high density lipoprotein cholesterol.
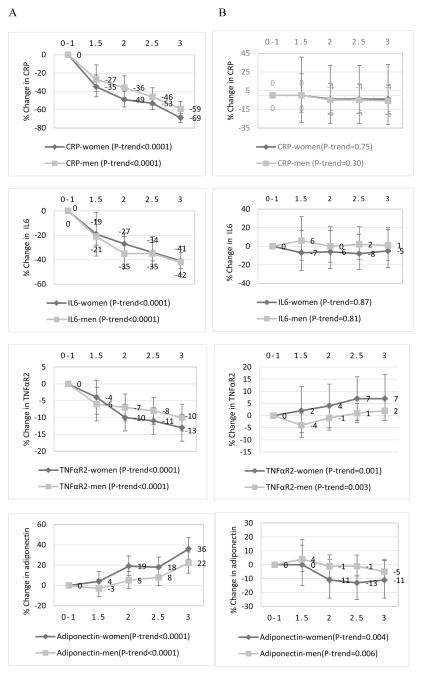
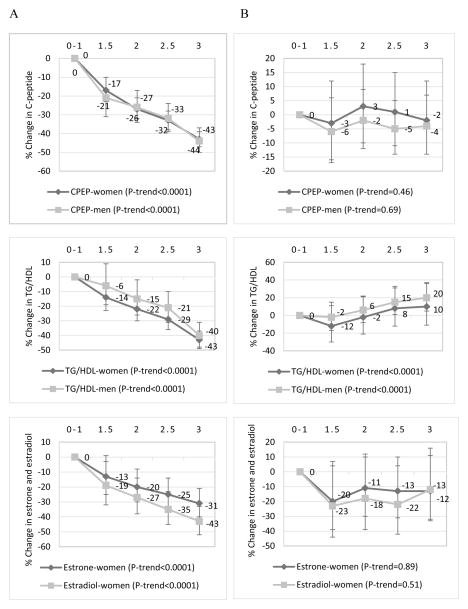
In both women and men, there was a significantly decreasing trend of biomarker concentrations with increasing adherence to the energy balance-related recommendations (P-trend for all eight biomarkers <0.0001) (Figures 1 and 2). The percent change in the relative concentrations of biomarkers in women was CRP, −69%; IL6, −41%; TNFαR2, −13%; adiponectin, +36%; C-peptide, −43%; TG/HDL, −43%; estrone, −31% and estradiol, −43%, comparing the highest (3 recommendations) with the lowest (0–1 recommendation) adherence categories. Corresponding percent changes in men were: CRP, −59%; IL6, −42%; TNFαR2, −10%; adiponectin, +22%; C-peptide, −44%, and TG/HDL, −40%. In contrast, associations between adherence to the plant/animal foods/alcohol intake recommendations and biomarker concentrations were weaker than for the energy balance-related recommendations, and mostly nonsignificant (Figures 1 and 2). An examination of the independent associations of the individual recommendations with each of the eight biomarkers of inflammation, hormonal and insulin response revealed significant differences in biomarker concentrations between non-adherers and partial adherers compared to adherers, for the body weight and physical activity recommendations among both women and men. While most of the associations for the alcohol recommendation were not statistically significant, moderate alcohol intake did not appear to be favorable for TNFαR2, adiponectin and C-peptide concentrations in women and men (Table 3). When we additionally adjusted for BMI in models for the 5-points adherence score, relative concentrations of all biomarkers were highly attenuated and most were nonsignificant (Supplemental Table 2).
Figure 1.
Multivariable-adjusted percent changes in the relative concentrations of plasma inflammation markers (95% confidence intervals) across adherence categories of (A) the energy balance-related recommendations (BMI, physical activity and energy density), and (B) the combined plant/animal food/alcohol intake recommendations in the Nurses’ Health Study (women), 1990; and Health Professional Follow-up Study (men), 1994. 0–1 was the lowest or least adherent category (reference) while 3 was the highest or most adherent category. CRP=C-reactive protein, IL6=interleukin-6, TNFαR2=tumor necrosis factor alpha receptor 2. Biomarker concentrations were adjusted for regular aspirin/NSAID use, age at blood draw, smoking status, physical activity, case-control status, postmenopausal status, postmenopausal hormone use, and chronic diseases/conditions. The following chronic diseases/conditions (yes=1/no=0) were included in the score: hypercholesterolemia, cancer, diabetes, high blood pressure, heart disease, and rheumatoid/other arthritis). The P-value for trend was the P-value of the combined recommendation score as a continuous variable adjusted for all covariates previously listed. Biomarker sample sizes were different: in women, n=3,550 for all four inflammatory markers. In men; CRP, n=5,157; IL6, n=3,044; TNFαR2, n=4,072; and adiponectin, n=4,348.
Figure 2.
Multivariable-adjusted percent changes in the relative concentrations of plasma markers of insulin response and hormonal response (95% confidence intervals) across adherence categories of (A) the energy balance-related recommendations (BMI, physical activity and energy density), and (B) the combined plant/animal food/alcohol intake recommendations in the Nurses’ Health Study (women), 1990; and Health Professional Follow-up Study (men), 1994. 0–1 was the lowest or least adherent category (reference) while 3 was the highest or most adherent category. CPEP=C-peptide, TG/HDL=triglyceride/high density lipoprotein ratio. Estrone and estradiol were examined only among postmenopausal women not using exogenous hormones. Biomarker concentrations were adjusted for regular aspirin/NSAID use, age at blood draw, smoking status, physical activity, case-control status, postmenopausal status, postmenopausal hormone use, and chronic diseases/conditions. The following chronic diseases/conditions (yes=1/no=0) were included in the score: hypercholesterolemia, cancer, diabetes, high blood pressure, heart disease, and rheumatoid/other arthritis). The P-value for trend was the P-value of the combined recommendation score as a continuous variable adjusted for all covariates previously listed. Biomarker sample sizes were different: in women; CPEP, n=5,834; TG/HDL, n=3,826; estrone, n=1,217; and estradiol, n=1,254. In men; CPEP, n=3,955; and TG/HDL, n=3,575.
Table 3.
Percent change in the relative concentrations (95% CI) of plasma markers of inflammation, hormonal and insulin response across categories of each of the six WCRF/AICR recommendations in women and men1,2
| Nurses’ Health Study | Health Professionals Follow-up Study | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| WCRF/AICR score categories | ||||||
|
| ||||||
| Biomarker | 0 (not adherent) reference | 0.5 | 1 (adherent) | 0 (not adherent) reference | 0.5 | 1 (adherent) |
| Body weight | ||||||
| CRP | 1 | −37% (−43%, −30%) | −62% (−66%, −59%) | 1 | −39% (−45%, −32%) | −58% (−62%,−53%) |
| IL6 | 1 | −18% (−23%, −11%) | −34% (−38%, −29%) | 1 | −26% (−41%, −16%) | −33% (−41%, −24%) |
| TNFαR2 | 1 | −8% (−11%, −6%) | −14% (−16%, −11%) | 1 | −6% (−9%, −4%) | 8% (−10%, −5%) |
| ADIPO | 1 | +12% (+7%, +19%) | +35% (+29%, +42%) | 1 | +10% (+5%, +16%) | +31% (+24%, +38%) |
| CPEP | 1 | −21% (−25%, −17%) | −41% (−43%, −38%) | 1 | −23% (−28%, −18%) | −40% (−44%, −35%) |
| TG/HDL | 1 | −10% (−16%, −3%) | −36% (−40%, −31%) | 1 | −18% (−24%, −11%) | −42% (−47%, −37%) |
| Estrone | 1 | −22% (−29%, −15%) | −35% (−40%, −29%) | NA | NA | NA |
| Estradiol | 1 | −26% (−33%, −17%) | −46% (−52%, −40%) | NA | NA | NA |
|
| ||||||
| Physical activity | ||||||
| CRP | 1 | −10% (−19%, +1%) | −16% (−23%, −8%) | 1 | −12% (−23%, +1%) | −17% (−25%, −8%) |
| IL6 | 1 | −5% (13%, +2%) | −10% (−15%, −5%) | 1 | −10% (−23%, +5%) | −15% (−24%, −5%) |
| TNFαR2 | 1 | 0% (−3%, +3%) | −1% (−4%, +1%) | 1 | +1% (−3%, +4%) | −2% (−5%, +1%) |
| ADIPO | 1 | 0% (−4%, +6%) | +2% (−2%, +6%) | 1 | +2 (−4%, +9%) | +3% (−2%, +7%) |
| CPEP | 1 | −5% (−10%, −1%) | −7% (−11%, −4%) | 1 | −6% (−14%, +3%) | −12% (−17%, −6%) |
| TG/HDL | 1 | −9% (−16%, −2%) | −11% (−16%, −6%) | 1 | −4% (−14%, +7%) | 8% (−15%, −1%) |
| Estrone | 1 | +4 (−6%, +15%) | −2% (−9%, +5%) | NA | NA | NA |
| Estradiol | 1 | −4% (−15%, +8%) | −4% (−12%, +5%) | NA | NA | NA |
|
| ||||||
| Energy density | ||||||
| CRP | 1 | −7% (−18%, +6%) | +8 (−22%, +8%) | 1 | −3% (−11%, +8%) | +2% (−13%, +20%) |
| IL6 | 1 | −7% (−15%, +1%) | −4% (−14%, +7%) | 1 | −3% (−11%, +8%) | +1% (−14%, +20%) |
| TNFαR2 | 1 | −1% (−5%, +2%) | +1% (−4%, +5%) | 1 | 0% (−2%, +2%) | 0% (−4%, +4%) |
| ADIPO | 1 | +4% (−2%, +11%) | +9% (0%, +18%) | 1 | +1% (−4%, +5%) | +1% (−6%, +9%) |
| CPEP | 1 | −5% (−11%, 0%) | −4% (−10%, +3%) | 1 | +1% (−5%, +6%) | +3% (−6%, +14%) |
| TG/HDL | 1 | −7% (−15%, +2%) | −10% (−19%, +1%) | 1 | −2% (−9%, +6%) | +3% (−9%, +17%) |
| Estrone | 1 | +2% (−8%, +14%) | +3% (−11%, +18%) | NA | NA | NA |
| Estradiol | 1 | 0% (−12%, +14%) | +4% (−11%, +23%) | NA | NA | NA |
|
| ||||||
| Plant foods | ||||||
| CRP | 1 | −15% (−26%, −3%) | −17% (−28%, −3%) | 1 | −5% (−45%, +65%) | −11% (−48%, +55%) |
| IL6 | 1 | −11% (−19%, −3%) | −7% (−16%, +3%) | 1 | −42% (−69%, +7%) | −43% (−69%, +5%) |
| TNFαR2 | 1 | −1% (−5%, +3%) | −1% (−5%, +3%) | 1 | −3% (−17%, +14%) | −3% (−17%, +13%) |
| ADIPO | 1 | −2% (−8%, +5%) | −1% (−8%, +7%) | 1 | 0% (−22%, +27%) | −1% (−22%, +26%) |
| CPEP | 1 | −4% (−10%, +1%) | −6% (−12%, 0%) | 1 | 1% (−23%, +32%) | −1% (−24%, +30%) |
| TG/HDL | 1 | −6% (−14%, +4%) | −2% (−11%, +9%) | 1 | +27% (−15%, +88%) | +27% (−15%, +89%) |
| Estrone | 1 | +1% (−10%, +13%) | +1% (−11%, +14%) | NA | NA | NA |
| Estradiol | 1 | −5% (−17%, +9%) | −6% (−19%, +10%) | NA | NA | NA |
|
| ||||||
| Animal foods | ||||||
| CRP | 1 | +21% (−34%, +120%) | +32% (−27%, +139%) | 1 | −21% (−43%, +9%) | −24% (−45%, +5%) |
| IL6 | 1 | +15% (−24%, +75%) | +18% (−22%, +78%) | 1 | +12% (−21%, +57%) | +9% (−22%, +53%) |
| TNFαR2 | 1 | −1% (−16%, +18%) | −1% (−16%, +17%) | 1 | −2% (−10%, +7%) | −2% (−10%, +7%) |
| ADIPO | 1 | −7% (−31%, +25) | −5% (−29%, +27%) | 1 | +5% (−9%, +21%) | +3% (−10%, +19%) |
| CPEP | 1 | +8% (−16%, +38%) | 0% (−22%, +27%) | 1 | −7% (−23%, +11%) | −5% (−21%, +13%) |
| TG/HDL | 1 | +17% (−20%, +72%) | +10% (−25%, +60%) | 1 | +10% (−14%, +40%) | +9% (−14%, +39%) |
| Estrone | 1 | +21% (−21%, +85%) | +11% (−27%, +68%) | NA | NA | NA |
| Estradiol | 1 | +21% (−28%, +103%) | +13% (−32%, +18%) | NA | NA | NA |
|
| ||||||
| Alcohol intake | ||||||
| CRP | 1 | −9% (−23%, +8%) | −5% (−17%, +10%) | 1 | −11% (−24%, +5%) | 0% (−10%, +11%) |
| IL6 | 1 | −6% (−17%, +6%) | −5% (−14%, +5%) | 1 | −4% (−20%, +14%) | 0% (−11%, +12%) |
| TNFαR2 | 1 | +2% (−2%, +8%) | +7% (+3%, +11%) | 1 | 0% (−4%, +5%) | +6% (+3%, +9%) |
| ADIPO | 1 | −7% (−14%, +2%) | −11% (−18%, −5%) | 1 | +3% (−4%, +12%) | −5% (−9%, 0%) |
| CPEP | 1 | −2% (−9%, +5%) | +1% (−5%, +7%) | 1 | −4% (−12%, +5%) | +1% (−5%, +7%) |
| TG/HDL | 1 | +6% (−6%, +20%) | +22% (+10%, +35%) | 1 | +3% (−10%, +17%) | +23% (+13%, +34%) |
| Estrone | 1 | −2% (−16%, +13%) | −5% (−16%, +8%) | NA | NA | NA |
| Estradiol | 1 | −8% (−23%, +10%) | −11% (−24%, +3%) | NA | NA | NA |
Linear regression models were used to estimate the percent change in the relative concentrations of biomarkers, i.e., differences in biomarker concentrations between higher dietary index quintiles relative to quintile 1 as the reference (e.g. biomarker concentration in Q5 minus concentration in Q1). 95%CI not including 0 are statistically significant. Multivariable models were adjusted for age, smoking status (never, former, current), regular use of aspirin/non-steroidal anti-inflammatory drugs (yes/no), race (white, nonwhite), case-control status, and chronic disease comorbidity score. Chronic diseases/conditions included in the score: hypercholesterolemia, cancer, diabetes, high blood pressure, heart disease, ulcerative colitis and rheumatoid/other arthritis;
Biomarker sample sizes vary: in NHS, n=3,550 for all four inflammatory markers, n=5,834 for C-peptide and n=3,826 for TG/HDL ratio, n=1,217 for estrone, n=1,254 for estradiol. Estrone and estradiol were examined only among postmenopausal women not using exogenous hormones; and in HPFS, n=5,157 for C-reactive protein, n=3,044 for interleukin-6, n=4,072 for tumor necrosis factor alpha receptor 2, n=4,348 for adiponectin, n=3,955 for C-peptide and n=3,575 for TG/HDL ratio in HPFS;
CRP=C-reactive protein, IL6=interleukin-6, TNFαR2=tumor necrosis factor alpha receptor 2, ADIPO=adiponectin, CPEP=C-peptide, TG=triglycerides, HDL=high density lipoprotein cholesterol;
Results for the 6-points adherence score were similar to those for the energy balance-related adherence score. There was a significant trend of lower (higher for adiponectin) concentrations of biomarkers of inflammation, hormonal and insulin response across adherence categories with higher adherence to the cancer prevention recommendations (all P-trend <0.0001) except for TNFαR2 in men (P-trend =0.09, Supplemental Table 3). Significant reductions in mean CRP, IL6 and C-peptide concentrations comparing higher categories of adherence to the lowest category were evident after adhering to >2 recommendations among women and >3 recommendations among men. In both women and men, adhering to at least four of the six recommendations was associated with significant decreases (increases for adiponectin) in biomarker concentrations (Supplemental Table 3).
The proportion of current smokers was >4.5 times higher among the least adherent women compared to the most adherent women, and 3 times higher for least adherent men compared to the most adherent men (Table 1). We therefore stratified analyses by smoking status and findings showed stronger associations between adherence scores and biomarker concentrations mainly among never and former smokers in both women and men (Supplemental Table 4).
Discussion
In this large study of women and men, greater adherence to the 2007 WCRF/AICR diet and lifestyle recommendations for cancer prevention was associated with a healthier profile of plasma markers of inflammation (CRP, IL6, TNFαR2 and adiponectin), hormonal response (estrone and estradiol) and insulin response (C-peptide and TG/HDL). The healthier biomarker profile was driven mainly by adherence to the three energy balance-related recommendations (body weight, physical activity and energy density of foods), especially by adherence to the body weight recommendation. Overall, adherence to the energy balance-related recommendations was associated with a percent decrease (or increase for adiponectin) in biomarker concentrations that ranged from 13% (TNFαR2) to 69% (CRP) in women and from 10% (TNFαR2) to 59% (CRP) in men. Also, mainly body weight (to a large extent) and physical activity were associated with concentrations of biomarkers independent of the adherence level to the other five recommendations.
Our finding that adherence to the energy balance-related recommendations was more strongly associated with concentrations of all eight biomarkers compared to adherence to the plant/animal foods/alcohol intake recommendations, suggests that the type of foods consumed may be less important in determining biomarker concentrations than the excess body weight from poor dietary patterns. Measurement error could account for this finding given that it is easier to measure body weight than dietary intake. However, this is unlikely given the highly attenuated and mostly nonsignificant associations of the 5-points adherence score and biomarker concentrations when additionally adjusted for BMI; suggesting that body weight may play a more mediating than confounding role. The role of body weight as a mediator of biomarker profile was also evident in the study by Morimoto et al.7 The investigators examined associations between adherence scores and BMI status and found that results were similar to the associations between adherence scores and biomarkers of inflammation and oxidative stress.7
However, this finding does not imply that the inflammation, hormonal or insulinemic potential of diet is not an important determinant of future disease risk. Indeed, studies have found robust associations between the inflammation potential of diet and concentrations of circulating biomarker of inflammation41, 42 and with risk of developing cancer43 or of dying from cancer44. These previous studies have mostly used quantile cutpoints and/or continuous scores of dietary indices developed to assess dietary inflammation potential. It is therefore possible that the absence of an association between the combined score for plants/animal foods and alcohol intake recommendations, or with these three recommendations individually, may be related to the cutpoints used to define adherence to these recommendations. In addition, information on the quality of foods is not incorporated into the scores; e.g., foods such as white bread and potatoes may contribute to a better score because they include some fiber but in contrast may have some adverse effects on TG/HDL or C-peptide concentrations.
In the other previous adherence study, Bruno et al constructed adherence scores using five of the six recommendations (except body weight) included in the current study, and examined associations with prevalence of metabolic syndrome among a large sample of breast cancer survivors. They reported a lower prevalence of metabolic syndrome with higher adherence to the recommendations.8 Insulin resistance (assessed in the current study by the TG/HDL ratio) is one of the criteria for determining the metabolic syndrome.45 Though the WCRF/AICR recommendations were issued for cancer prevention, studies have found that greater adherence to the recommendations is associated with lower risk of death from cancer-related2 and other causes,46, 47 which suggests that the recommendations may be relevant for cancer progression.
Our study is limited by the cross-sectional design and self-reported dietary and lifestyle measures, and therefore some measurement error is inevitable. However, the studies that evaluated questionnaire validity showed reasonably good correlations between FFQ and diet records, and longitudinal studies using data from the NHS and HPFS have observed high correlations between biannually assessed lifestyle measures across several years. This suggests that dietary and lifestyle assessment is generally well conducted in our cohorts.36, 37 Additionally, study participants in both cohorts are mostly Caucasian health professionals, though the distributions of most participant characteristics are generally similar to that of the larger US multi-racial/ethnic population. Also, our findings align with findings from the Morimoto et al study that used data from multi-ethnic populations.7 Though we adjusted for a large number of potential confounding variables including a history of cancer and other chronic diseases/conditions, these variables were self-reported, thus allowing the possibility of residual confounding. Another study limitation is that we had only one measurement of biomarkers which may underestimate associations with cancer prevention recommendations adherence scores.48 Also, differences in biomarker concentrations may be due to multiple batch measurements, different technicians and laboratories, but we recalibrated biomarker concentrations to an average batch to account for these potential sources of variation.35
Though we may not completely discern etiological pathways using cross-sectional designs, the substantial inter-correlation among some of these biomarkers makes it difficult to disentangle underlying etiological pathways irrespective of study design;49 therefore it is not clear whether these biomarkers act independently or through overlapping mechanisms. For example, circulating adiponectin is inversely correlated with circulating insulin and is reduced in individuals with insulin-resistant conditions such as obesity and type 2 diabetes,50 while insulin resistance has been linked to obesity, inflammation and type 2 diabetes.51, 52 Hyperinsulinemia and insulin resistance are associated with obesity, a state of low grade chronic inflammation, and have been directly associated with inflammatory cytokines (TNFαR2, IL6) and adipokines (adiponectin and leptin).
In summary, adherence to the 2007 WCRF/AICR cancer prevention recommendations is associated with a healthier profile of plasma markers of inflammation, hormonal and insulin response. Our findings provide insights on the biological mechanisms underlying associations between these dietary and lifestyle recommendations and cancer risk, while emphasizing the dominant role of adherence to the energy balance-related recommendations especially the recommendation on body weight. Prospective studies are warranted to investigate whether adherence to the recommendations is associated with changes in biomarker concentrations over time and/or with biomarker patterns that incorporate multiple biomarkers simultaneously.
Supplementary Material
What’s new?
Data from two large United States cohorts showed that women and men with greater adherence to the 2007 WCRF/AICR cancer prevention recommendations, especially the recommendations related to energy balance, have healthier profiles of plasma markers of inflammation, hormonal and insulin response. Findings also suggest that the type of foods consumed may be less important in determining biomarker concentrations than the excess body weight from poor dietary patterns.
Acknowledgments
Dr. Jorge E. Chavarro was supported by National Institutes of Health (NIH) grants P30DK046200 and U54 CA155426. The HPFS and NHS cohorts are supported by the following NIH grants: UM1 CA 167552 and UM1 CA 176726 respectively. We would like to thank the participants and staff of the NHS and HPFS cohorts for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Footnotes
Conflict of interest
All authors declare no conflict of interest.
References
- 1.World Cancer Research Fund / American Institute for Cancer Research. WCRF/AICR Expert Report: Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. American Institute for Cancer Research; 2007. [Google Scholar]
- 2.Kohler LN, Garcia DO, Harris RB, Oren E, Roe DJ, Jacobs ET. Adherence to diet and physical activity cancer prevention guidelines and cancer outcomes: a systematic review. Cancer Epidemiology Biomarkers & Prevention. 2016 doi: 10.1158/1055-9965.EPI-16-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fanidi A, Ferrari P, Biessy C, Ortega C, Angeles-Llerenas A, Torres-Mejia G, Romieu I. Adherence to the World Cancer Research Fund/American Institute for Cancer Research cancer prevention recommendations and breast cancer risk in the Cancer de Màma (CAMA) study. Public Health Nutrition. 2015;18:3337–48. doi: 10.1017/S1368980015000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hastert TA, Beresford SA, Patterson RE, Kristal AR, White E. Adherence to WCRF/AICR Cancer Prevention Recommendations and Risk of Postmenopausal Breast Cancer. Cancer Epidemiology Biomarkers & Prevention. 2013;22:1498–508. doi: 10.1158/1055-9965.EPI-13-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romaguera D, Vergnaud AC, Peeters PH, van Gils CHCD, Ferrari P, Romieu I, Jenab M, Slimani N, Clavel-Chapelon F, Fagherazzi G, Perquier F, Kaaks R, Teucher B, Boeing H, von Rüsten A, Tjønneland A, Olsen A, Dahm CC, Overvad K, Quirós JR, Gonzalez CA, Sánchez MJ, Navarro C, Barricarte A, Dorronsoro M, Khaw KT, Wareham NJ, Crowe FL, Key TJ, Trichopoulou A, Lagiou P, Bamia C, Masala G, Vineis P, Tumino R, Sieri S, Panico S, May AM, Bueno-de-Mesquita HB, Büchner FL, Wirfält E, Manjer J, Johansson I, Hallmans G, Skeie G, Benjaminsen Borch K, Parr CL, Riboli E, Norat T. Is Concordance with World Cancer Research Fund/American Institute for Cancer Research Guidelines for Cancer Prevention Related to Subsequent Risk of Cancer? Results from the EPIC Study. The American Journal of Clinical Nutrition. 2012;96:150–63. doi: 10.3945/ajcn.111.031674. [DOI] [PubMed] [Google Scholar]
- 6.Cerhan JR, Potter JD, Gilmore JM, Janney CA, Kushi LH, Lazovich D, Anderson KE, Sellers TA, Folsom AR. Adherence to the AICR Cancer Prevention Recommendations and Subsequent Morbidity and Mortality in the Iowa Women’s Health Study Cohort. Cancer Epidemiology Biomarkers & Prevention. 2004;13:1114–20. [PubMed] [Google Scholar]
- 7.Morimoto Y, Beckford F, Cooney RV, Franke AA, Maskarinec G. Adherence to cancer prevention recommendations and antioxidant and inflammatory status in premenopausal women. British Journal of Nutrition. 2015;114:134–43. doi: 10.1017/S0007114515001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruno E, Gargano G, Villarini A1, Traina A, Johansson H, Mano MP, Santucci De Magistris M, Simeoni M, Consolaro E, Mercandino A, Barbero M, Galasso R, Bassi MC, Zarcone M, Zagallo E, Venturelli E, Bellegotti M, Berrino F, Pasanisi P. Adherence to WCRF/AICR cancer prevention recommendations and metabolic syndrome in breast cancer patients. International Journal of Cancer. 2016;138:237–44. doi: 10.1002/ijc.29689. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. The American Journal of Clinical Nutrition. 2007;86:836S–42S. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 10.Stocks T, Bjørge, Tone, Ulmer, Hanno, Manjer J, Häggström C, Nagel G, Engeland A, Johansen D, Hallmans G, Selmer R, Concin H, Tretli S, Jonsson H, Stattin P. Metabolic risk score and cancer risk: pooled analysis of seven cohorts. International Journal of Epidemiology. 2015;44:1353–63. doi: 10.1093/ije/dyv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Lee IM, Tworoger SS, Buring JE, Ridker PM, Rosner B, Hankinson SE. Plasma C-Reactive Protein and Risk of Breast Cancer in Two Prospective Studies and a Meta-analysis. Cancer Epidemiology Biomarkers & Prevention. 2015;24:1199–206. doi: 10.1158/1055-9965.EPI-15-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prizment A, Anderson KE, Visvanathan K, Folsom AR. Association of inflammatory markers with colorectal cancer incidence in the Atherosclerosis Risk in Communities (ARIC) study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:297–307. doi: 10.1158/1055-9965.EPI-10-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waldner MJ, Foersch S, Neurath MF. Interleukin-6 - A Key Regulator of Colorectal Cancer Development. International Journal of Biological Sciences. 2012;8:1248–53. doi: 10.7150/ijbs.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Il’yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB. Circulating Levels of Inflammatory Markers and Cancer Risk in the Health Aging and Body Composition Cohort. Cancer Epidemiology Biomarkers & Prevention. 2005;14:2413–18. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 15.Al-Lamki RS, Sadler TJ, Wang J, Reid MJ, Warren AY, Movassagh M, Lu W, Mills IG, Neal DE, Burge J, Vandenebeele P, Pober JS, Bradley JR. Tumor Necrosis Factor Receptor Expression and Signaling in Renal Cell Carcinoma. The American Journal of Pathology. 2010;177:943–54. doi: 10.2353/ajpath.2010.091218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inamura K, Song M, Jung S, Nishihara R, Yamauchi M, Lochhead P, Qian ZR, Kim SA, Mima K, Sukawa Y, Masuda A, Imamura Y, Zhang X, Pollak MN, Mantzoros CS, Harris CC, Giovannucci E, Fuchs CS, Cho E, Chan AT, Wu K, Ogino S. Prediagnosis Plasma Adiponectin in Relation to Colorectal Cancer Risk According to KRAS Mutation Status. Journal of the National Cancer Institute. 2016:108. doi: 10.1093/jnci/djv363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao Y, Giovannucci EL, Kraft P, Stampfer MJ, Ogino S, Ma J, Buring JE, Sesso HD, Lee IM, Gaziano JM, Rifai N, Pollak MN, Cochrane BB, Kaklamani V, Lin JH, Manson JE, Fuchs CS, Wolpin BM. A Prospective Study of Plasma Adiponectin and Pancreatic Cancer Risk in Five US Cohorts. JNCI Journal of the National Cancer Institute. 2013;105:95–103. doi: 10.1093/jnci/djs474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Badri M, Zantout MS, Azar ST. The role of adipokines in gestational diabetes mellitus. Therapeutic Advances in Endocrinology and Metabolism. 2015;6:103–08. doi: 10.1177/2042018815577039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinalski M, Telejko B, Kuźmicki M, Kretowski A, Kinalska I. Tumor necrosis factor alpha system and plasma adiponectin concentration in women with gestational diabetes. Horm Metab Res. 2005;37:450–4. doi: 10.1055/s-2005-870238. [DOI] [PubMed] [Google Scholar]
- 20.Lukanova A, Kaaks R. Endogenous Hormones and Ovarian Cancer: Epidemiology and Current Hypotheses. Cancer Epidemiology Biomarkers & Prevention. 2005;14:98–107. [PubMed] [Google Scholar]
- 21.Ahern TP, Hankinson SE, Willett WC, Pollak MN, Eliassen AH, Tamimi RM. Plasma c-peptide, mammographic breast density, and risk of invasive breast cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22 doi: 10.1158/055-9965.EPI-13-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai GY, Helzlsouer KJ, Clipp SL, Rifai N, Platz EA. Association between C-peptide concentration and prostate cancer incidence in the CLUE II cohort study. Cancer prevention research (Philadelphia, Pa) 2010;3:1334–41. doi: 10.1158/1940-6207.CAPR-10-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arcidiacono B, Iiritano S, Nocera A, Possidente K, Nevolo MT, Ventura V, Foti D, Chiefari E, Brunetti A. Insulin Resistance and Cancer Risk: An Overview of the Pathogenetic Mechanisms. Experimental Diabetes Research. 2012;2012:789174. doi: 10.1155/2012/789174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson K, Hendricks B, Murdock DK. The triglyceride to HDL ratio and its relationship to insulin resistance in pre- and postpubertal children: observation from the Wausau SCHOOL Project. Cholesterol. 2012;2012:794252. doi: 10.1155/2012/794252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colditz G, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–96. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 26.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, Longcope C, Speizer FE. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87:1297–302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 27.Bonser A, Garcia-Webb P. C-Peptide Measurement: Methods and Clinical Utility. Critical Reviews in Clinical Laboratory Sciences. 1984;19:297–352. doi: 10.3109/10408368409165766. [DOI] [PubMed] [Google Scholar]
- 28.Murguía-Romero M, Jiménez-Flores JR, Sigrist-Flores SC, Espinoza-Camacho MA, Jiménez-Morales M, Piña E, Méndez-Cruz AR, Villalobos-Molina R, Reaven GM. Plasma triglyceride/HDL-cholesterol ratio, insulin resistance, and cardiometabolic risk in young adults. Journal of Lipid Research. 2013;54:2795–99. doi: 10.1194/jlr.M040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salazar M, Carbajal HA, Espeche WG, Leiva Sisnieguez CE, March CE, Balbín E, Dulbecco CA, Aizpurúa M, Marillet AG, Reaven GM. Comparison of the abilities of the plasma triglyceride/high-density lipoprotein cholesterol ratio and the metabolic syndrome to identify insulin resistance. Diabetes and Vascular Disease Research. 2013;10:346–52. doi: 10.1177/1479164113479809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pai J, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. New England Journal of Medicine. 2004;351:2599–610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 31.Song M, Zhang X, Wu K, Ogino S, Fuchs CS, Giovannucci EL, Chan AT. Plasma Adiponectin and Soluble Leptin Receptor and Risk of Colorectal Cancer: A Prospective Study. Cancer Prevention Research. 2013;6:875–85. doi: 10.1158/1940-6207.CAPR-13-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shai I, Rimm EB, Hankinson SE, Curhan G, Manson JE, Rifai N, Stampfer MJ, Ma J. Multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women: potential implications for clinical guidelines. Circulation. 2004;110:2824–30. doi: 10.1161/01.CIR.0000146339.57154.9B. [DOI] [PubMed] [Google Scholar]
- 33.Lin J, Zhang SM, Rexrode KM, Manson JE, Chan AT, Wu K, Tworoger SS, Hankinson SE, Fuchs C, Gaziano JM, Buring JE, Giovannucci E. Association between sex hormones and colorectal cancer risk in men and women. Clinical Gastroenterology and Hepatology. 2013;11:419–24. e1. doi: 10.1016/j.cgh.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F Centers for Disease Control and Prevention; American Heart Association. Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: A Statement for Healthcare Professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 35.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. American Journal of Epidemiology. 2008;167:653–66. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- 36.Willett W, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. American Journal of Epidemiology. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 37.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. Journal of the American Dietetic Association. 1993;93:790–96. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 38.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and Validity of a Self-Administered Physical Activity Questionnaire for Male Health Professionals. Epidemiology. 1996;7:81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and Validity of a Self-Administered Physical Activity Questionnaire. International Journal of Epidemiology. 1994;23:991–99. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 40.Quan H, Zhang J. Estimate of standard deviation for a log-transformed variable using arithmetic means and standard deviations. Statistics in Medicine. 2003;22:2723–36. doi: 10.1002/sim.1525. [DOI] [PubMed] [Google Scholar]
- 41.Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, Hou L, Hurley TG, Hingle M, Jiao L, Martin LW, Millen EA, Park HL, Rosal CM, Shikany JM, Shivappa N, Ockene JK, Hebert JR. Construct validation of the dietary inflammatory index among postmenopausal women. Annals of Epidemiology. 2015;25:398–405. doi: 10.1016/j.annepidem.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs SF, Hu FB, Chan AT, Willett WC, Giovannucci EL. Development and validation of an empirical index of dietary inflammatory potential. The Journal of Nutrition. 2016;146:1560–70. doi: 10.3945/jn.115.228718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabung FK, Steck SE, Ma Y, Liese AD, Zhang J, Caan B, Hou L, Johnson KC, Mossavar-Rahmani Y, Shivappa N, Wactawski-Wende J, Ockene JK, Hebert JR. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the Women’s Health Initiative. Cancer Causes & Control. 2015;26:399–408. doi: 10.1007/s10552-014-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shivappa N, Steck SE, Hussey JR, Ma Y, Hebert JR. Inflammatory potential of diet and all-cause, cardiovascular, and cancer mortality in National Health and Nutrition Examination Survey III Study. European Journal of Nutrition. 2015:1–10. doi: 10.1007/s00394-015-1112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C Participants ftC. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–38. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 46.Vergnaud AC, Romaguera D, Peeters PH, van Gils CHCD, Romieu I, Freisling H, Ferrari P, Clavel-Chapelon F, Fagherazzi G, Dartois L, Li K, Tikk K, Bergmann MM, Boeing H, Tjønneland A, Olsen A, Overvad K, Dahm CC, Redondo ML, Agudo A, Sánchez MJ, Amiano P, Chirlaque MD, Ardanaz E, Khaw KT, Wareham NJ, Crowe F, Trichopoulou A, Orfanos P, Trichopoulos D, Masala G, Sieri S, Tumino R, Vineis P, Panico S, Bueno-de-Mesquita HB, Ros MM, May A, Wirfält E, Sonestedt E, Johansson I, Hallmans G, Lund E, Weiderpass E, Parr CL, Riboli E, Norat T. Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: results from the European Prospective Investigation into Nutrition and Cancer cohort study. The American Journal of Clinical Nutrition. 2013;97:1107–20. doi: 10.3945/ajcn.112.049569. [DOI] [PubMed] [Google Scholar]
- 47.Romaguera DWH, Wark PA, Vergnaud ACPP, van Gils CH, Ferrari P, Fedirko V, Jenab M, Boutron-Ruault MC, Dossus L, Dartois L, Hansen CP, Dahm CC, Buckland G, Sánchez MJ, Dorronsoro M, Navarro C, Barricarte A, Key TJ, Trichopoulou A, Tsironis C, Lagiou P, Masala G, Pala V, Tumino R, Vineis P, Panico S, Bueno-de-Mesquita HB, Siersema PD, Ohlsson B, Jirström K, Wennberg M, Nilsson LM, Weiderpass E, Kühn T, Katzke V, Khaw KT, Wareham NJ, Tjønneland A, Boeing H, Quirós JR, Gunter MJ, Riboli E, Norat T. Pre-diagnostic concordance with the WCRF/AICR guidelines and survival in European colorectal cancer patients: a cohort study. BMC Medicine. 2015;13:107. doi: 10.1186/s12916-015-0332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perrier F, Giorgis-Allemand Li, Slama R, Philippat C. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology. 2016;27:378–88. doi: 10.1097/EDE.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aleksandrova K, Jenab M, Bueno-de-Mesquita HB, Fedirko V, Kaaks R, Lukanova A, van Duijnhoven FJ, Jansen E, Rinaldi S, Romieu I, Ferrari P, Murphy N, Gunter MJ, Riboli E, Westhpal S, Overvad K, Tjønneland A, Halkjær J, Boutron-Ruault MC, Dossus L, Racine A, Trichopoulou A, Bamia C, Orfanos P, Agnoli C, Palli D, Panico S, Tumino R, Vineis P, Peeters PH, Duell EJ, Molina-Montes E, Quirós JR, Dorronsoro M, Chirlaque MD, Barricarte A, Ljuslinder I, Palmqvist R, Travis RC, Khaw KT, Wareham N, Pischon T, Boeing H. Biomarker patterns of inflammatory and metabolic pathways are associated with risk of colorectal cancer: results from the European Prospective Investigation into Cancer and Nutrition (EPIC) European Journal of Epidemiology. 2014;29:261–75. doi: 10.1007/s10654-014-9901-8. [DOI] [PubMed] [Google Scholar]
- 50.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 51.Fernández-Veledo S, Nieto-Vazquez I, Vila-Bedmar R, Garcia-Guerra L, Alonso-Chamorro M, Lorenzo M. Molecular mechanisms involved in obesity-associated insulin resistance: therapeutical approach. Archives of Physiology and Biochemistry. 2009;115:227–39. doi: 10.1080/13813450903164330. [DOI] [PubMed] [Google Scholar]
- 52.Reaven GM. Pathophysiology of insulin resistance in human disease. Physiological Reviews. 1995;75:473–86. doi: 10.1152/physrev.1995.75.3.473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.