Abstract
Objective
To determine if melanocortin-1 receptor (MC1R) single nucleotide polymorphisms (SNPs) are associated with complicated sepsis after trauma.
Background
Nosocomial infections are an important cause of morbidity and mortality after trauma. Several SNPs in inflammation-related genes have been associated with sepsis. MC1R is an anti-inflammatory mediator that may be involved in the immune response after trauma.
Methods
We genotyped 8 common MC1R SNPs in genomic DNA from subjects enrolled in a previously reported prospective cohort study. Subjects were adult trauma patients admitted to the ICU at a Level I trauma center (2003–2005).
Results
A total of 1,246 subjects were included in the analysis. The majority were male (70%), severely injured (81%), and injured by a blunt mechanism (89%). Forty percent developed sepsis, and 23% developed complicated sepsis, which was defined as sepsis with organ dysfunction. In logistic regression analysis, with adjustments for age, sex, body mass index, injury severity score, red blood cell transfusion requirement, and mechanism of injury, the MC1RR163Q variant (rs885479) was associated with a lower risk of developing complicated sepsis (ORadj=0.48, 95%CI: 0.28–0.81, p=0.006). In a subgroup of 511 subjects with genome-wide SNP data, the association between the MC1RR163Q variant and complicated sepsis remained significant after adjusting for genetic substructure (by principal components) and the above clinical factors (ORadj=0.30, 95%CI: 0.13–0.70, p=0.005).
Conclusions
MC1RR163Q is associated with a lower risk of complicated sepsis after trauma. Therapeutic targeting of MC1R may be beneficial for trauma patients at risk for complicated sepsis.
Keywords: MC1R, SNP, Arg163Gln, rs885479, melanocyte stimulating hormone
Introduction
Sepsis after trauma has a complex pathophysiology that involves both the pathogenic microbe and the patient’s individualized immune response. Several single nucleotide polymorphisms (SNPs) are known to influence a patient’s susceptibility to and recovery from sepsis. Many of these SNPs are in inflammation-related genes(1), such as lipopolysaccharide binding protein(2), interleukin-1(3), CD14(4), toll-like receptors(5, 6), and TNF-β(7). By understanding genetic variation that influences response to injury, we will move closer to personalized, or “precision medicine” - improving our ability to deliver the right treatment to the right patient at the right time.
The melanocortin-1 receptor (MC1R) is an anti-inflammatory mediator(8). It is a G protein-coupled receptor that binds with equal affinity to α-melanocyte stimulating hormone (α-MSH) and adrenocorticotropic hormone (ACTH)(8). The receptor is expressed on numerous cells involved in the inflammatory response, including neutrophils, leukocytes, macrophages, endothelial cells, and melanocytes. Activation of the receptor results in a wide range of anti-inflammatory effects, including attenuation of macrophage cytokine production(9), reduction of leukocyte adhesion and migration(10), and inhibition of neutrophil chemotaxis(11).
The MC1R gene is highly polymorphic, with many functional variants(12). Several MC1R SNPs are associated with diseases such as melanoma(13), non-melanoma skin cancers(14), and multiple sclerosis(15). We previously reported that the MC1RR163Q variant was associated with a higher risk of hypertrophic scarring after burn injury(16). Given the immunologic role of MC1R, we hypothesized that its common functional SNPs (Figure 1) would be associated with the risk of developing complicated sepsis after trauma.
Figure 1. MC1R protein structure.
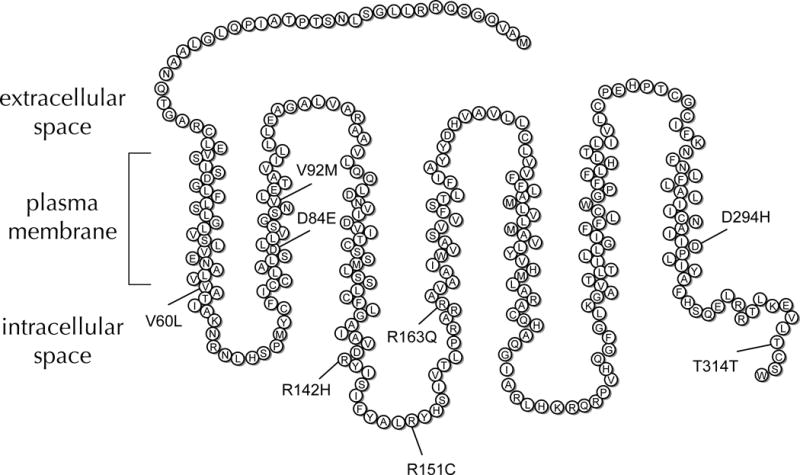
The receptor has 7 transmembrane domains. Eight of its common functional SNPs are indicated by their substituted amino acids and location in the peptide chain.
Methods
Study design, population, and setting
We performed a retrospective cohort study using samples and data from a prior Institutional Review Board (IRB)-approved prospective study that enrolled subjects admitted to the trauma intensive care unit (ICU) at a regional Level 1 trauma center, between 2003 and 2005, as previously described(5, 17). Patients were excluded if they were in the ICU < 48 hours, had an isolated traumatic brain injury, or were expected to die from their injuries. Injury severity score (ISS) was obtained from a prospectively acquired trauma registry. Admission and clinical data were acquired from electronic medical records with IRB approval.
Exposures and outcomes
The exposures of interest were one of eight different MC1R SNP genotypes. Assessed outcomes included a) lower respiratory tract infection, defined as a quantitative protected bronchial culture demonstrating ≥105 colony forming units/ml; b) bacteremia, defined as bacterial growth in a blood culture; c) urinary tract infection, defined as a urine culture with greater than 105 organisms per high power field; d) wound infections, defined by clinical diagnosis with culture confirmation when available; and e) complicated sepsis, defined as severe sepsis or septic shock according to criteria established by The American College of Chest Physicians / Society of Critical Care Medicine Consensus Committee(18).
MC1R Genotyping
DNA was isolated from discarded venous blood with QIAamp® DNA Blood Midi Kits (Qiagen, Valencia, CA). Genotyping was performed on 20 ng DNA samples using pre-designed TaqMan® SNP Genotyping Assays (Qiagen) in 384-well plates with a Viia™7 Real-Time PCR System (Applied Biosystems, Foster City, CA) per manufacturer guidelines. The samples were genotyped for the following MC1R SNPs: MC1RV60L (rs1805005), MC1RD84E (rs1805006), MC1RV92M (rs2228479), MC1RR142H (rs11547464), MC1RR151C (rs1805007), MC1RR163Q (rs885479), MC1RD294H (rs1805009), and MC1RT314T (rs2228478) (Fig 1). SNPs with a minor allele frequency (MAF) ≤1% were excluded from the analysis.
Genome-wide genotyping and principal components analysis
A subgroup of 511 Caucasian subjects had genome-wide genotyping data available from a previous study that investigated the genetic risks for ventilator-associated pneumonia (Wurfel and O’Keefe, manuscript in preparation). These subjects had required mechanical ventilation for ≥ 2 days. We did not test the SNPs from this genome-wide data for associations with complicated sepsis; instead, we used these SNPs as “null markers” of population substructure(19). The “null markers” were transformed into principal components, and the principal components were included as covariates in the regression analyses.
We determined the genotypes for approximately 620,901 polymorphisms. Each chip was stained and imaged on an Illumina Bead Array Reader. Normalized intensity data for each sample were loaded into Illumina Beadstudio 2.0 and genotypes called using the manufacturer’s clustering algorithm. Dataset quality control was performed using SNP and Variation Suite 7.2 (Golden Helix, Bozeman, MT) and PLINK(20). We followed a published protocol for quality control in genetic case-control association studies(21). Samples with gender discordance as determined by X heterozygosity, excess relatedness, and those with an autosomal heterozygosity rate ± 3 standard deviations from the mean were excluded. Genetic markers were then filtered for call rate < 0.95, minor allele frequency < 0.05, and for deviations from Hardy-Weinberg equilibrium in the control population (HWE, p < 0.005). A total of 480,811 SNPs passed quality control filtering. We then performed principal components (PC) analysis to determine eigenvalues.
Statistical analysis
Associations between the MC1R SNP genotypes and outcomes were evaluated with univariate and multivariate logistic regression. We assumed any contribution to the sepsis phenotype from individual MC1R SNPs would be additive. Therefore, genotypes were coded as 0 (common allele homozygous), 1 (heterozygous), or 2 (variant allele homozygous). A univariate and multivariate model was fit for each SNP individually. Multivariate models included the following covariates: age and body mass index (BMI), modeled as continuous variables; sex, injury severity (ISS < 15 or ISS ≥ 15), and red blood cell (PRBC) transfusion requirement (<10 or ≥ 10 units PRBC transfusion during hospitalization) modeled as binary variables; and mechanism of injury (blunt, penetrating, or burn) modeled as a categorical variable. Hardy-Weinberg Equilibrium (HWE) was determined by χ2 test. In the subgroup of subjects with genome-wide data, eigenvalues for the first 10 principal components (see above) were included in the model to adjust for population substructure.
In order to account for multiple testing, all p-value thresholds for significance were adjusted by the Bonferroni correction to control the familywise error rate at 0.05. Logistic regression analysis was performed using Stata® 11.2 statistical software (StataCorp LP, College Station, TX). Haplotype estimation and linkage disequilibrium analyses were performed using the Haplo Stats and LDheatmap packages, respectively, in R version 3.0.2.
Results
Demographics and Clinical Outcomes
Figure 2 diagrams the cohorts we analyzed and that are described below. In total, 1,961 subjects were enrolled (Supplemental Table 1). To minimize confounding by population substructure, only Caucasian subjects with complete clinical and MC1R genotyping data were included in the analysis (n=1,246). The demographics and clinical outcomes of this cohort are detailed in Table 1. The majority were male (70%), severely injured (81%), and injured by a blunt mechanism (89%). Four-hundred and ninety-seven (40%) developed sepsis and 290 (23%) developed complicated sepsis. Overall mortality was 9%, and this was higher among subjects with sepsis (11%) and complicated sepsis (14%).
Figure 2. Subject enrollment for MC1R genotyping study.
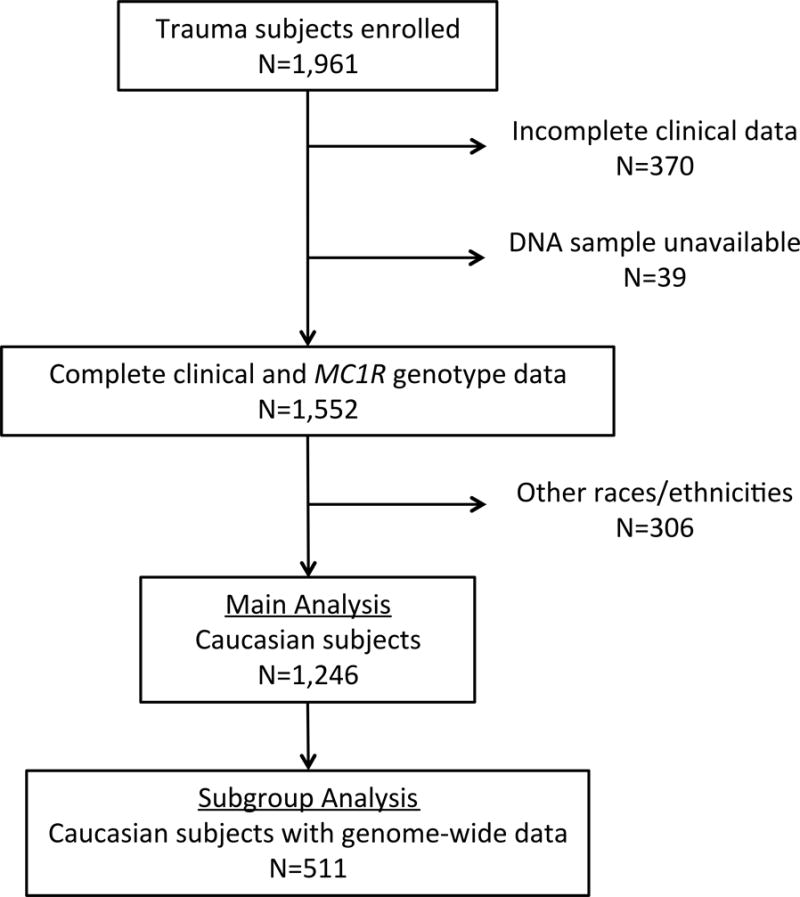
Caucasian subjects with complete clinical and MC1R genotyping data (N=1,246) were included in the main analysis. A subgroup analysis (N=511) was performed on Caucasian subjects who had data available from a previous genome-wide association study (GWAS; Wurfel and O’Keefe, manuscript in preparation).
Table 1.
Subject demographics, characteristics, and outcomes (N = 1,246).
| Characteristics | N (%) or median (IQR) |
|---|---|
| Age, years | 39 (22–53) |
| Male | 869 (70%) |
| Injury severity score ≥ 15 | 1,013 (81%) |
| ≥10 units PRBC transfusion | 176 (14%) |
| BMI, kg/m2 | 26 (22–29) |
| Mechanism of injury | |
| Blunt | 1,107 (89%) |
| Penetrating | 76 (6%) |
| Burn | 63 (5%) |
| Outcomes | N (%) |
| Infections | |
| Any infection | 600 (48%) |
| Urinary tract infection | 237 (19%) |
| Lower respiratory tract infection | 155 (12%) |
| Wound infection | 150 (12%) |
| Bacteremia | 167 (13%) |
| Sepsis | 497 (40%) |
| Complicated sepsis | 290 (23%) |
| Mortality | |
| Overall | 113 (9%) |
| Among subjects with sepsis | 54 (11%) |
| Among subjects with complicated sepsis | 42 (14%) |
Continuous variables are reported as median (IQR) and binary variables are reported as number (%).
Characterization of variation and haplotype structure of the MCR1 gene
The technical genotyping success rate was ≥ 99% for all 8 MC1R SNP genotyping assays. Genotype frequencies and minor allele frequencies (MAF) among Caucasians with complete clinical data (N=1,246) are described in Table 2. MC1RV60L was the most common SNP with a minor allele frequency (MAF) of 12%. MC1RR163Q had a minor allele frequency of 6%. MC1RD84E and MC1RR142H had MAFs ≤ 1%, and were therefore excluded from further analysis, leaving 6 SNPs (MC1RV60L, MC1RV92M, MC1RR151C, MC1RR163Q, MC1RD294H, and MC1RT314T) for association testing. In haplotype analysis only MC1RV92M and MC1RT314T had a relevant degree of linkage disequilibrium (R2=0.74) (Fig 3), with a MC1RV92M/MC1RT314T haplotype frequency of 8.4%.
Table 2.
MC1R SNP genotypes and minor allele frequencies.
| MC1R SNP | Amino acid substitution | rs identifier | Successfully genotyped (N) | Common allele homozygous (N)a | Heterozygous (N)a | Variant allele homozygous (N)a | Minor Allele Frequency (%)b |
|---|---|---|---|---|---|---|---|
| MC1RV60L | Val60Leu | rs1805005 | 1,246 | 962 | 265 | 19 | 12% |
| MC1RD84E | Asp84Glu | rs1805006 | 1,244 | 1,216 | 28 | 0 | 1% |
| MC1RV92M | Val92Met | rs2228479 | 1,246 | 1,042 | 198 | 6 | 8% |
| MC1RR142H | Arg142His | rs11547464 | 1,246 | 1,231 | 15 | 0 | 1% |
| MC1RR151C | Arg151Cys | rs1805007 | 1,244 | 1,057 | 179 | 8 | 8% |
| MC1RR163Q | Arg163Gln | rs885479 | 1,246 | 1,098 | 137 | 11 | 6% |
| MC1RD294H | Asp294His | rs1805009 | 1,245 | 1,204 | 34 | 7 | 2% |
| MC1RT314T | Thr314Thr | rs2228478 | 1,246 | 979 | 258 | 9 | 11% |
Number of subjects with each corresponding genotype.
Minor allele frequency (MAF) was calculated by the equation MAF = ((2 × SNP homozygotes) + (Heterozygotes)) / (2N).
Figure 3. Pair-wise linkage disequilibrium heat map for the six MC1R SNPs included in the haplotype analysis.

R2 is the square of the correlation coefficient between the two SNPs.
The MC1RR163Q variant was associated with a lower risk of complicated sepsis
The incidence of complicated sepsis varied by genotype, ranging from 9% among MC1R163QQ homozygotes to 50% among MC1R151CC homozygotes. Unadjusted and adjusted analyses of associations between the MCR1 SNPs and complicated sepsis are shown in Table 3. Six SNPs were analyzed, so the Bonferroni-adjusted p-value threshold for significance was 0.05/6=0.008. In univariate analysis, the MC1RR163Q variant was associated with a significantly lower risk of complicated sepsis (OR=0.42, 95% CI: 0.26–0.69, p=0.001). This association persisted after adjusting for age, sex, ISS, PRBC transfusion requirement, BMI, and mechanism of injury (ORadj=0.48, 95%CI: 0.28–0.81, p=0.006) (Table 3). There were no associations between the other MC1R SNPs and complicated sepsis in univariate or multivariate analyses. In addition, there were no associations between complicated sepsis and MC1R haplotypes that did not include the MC1RR163Q variant. Notably, the MC1RR163Q variant was not associated with the risk of developing an infection (ORadj=0.78, 95%CI: 0.56–1.09, p=0.142) or sepsis without organ dysfunction (ORadj=0.77, 95%CI: 0.54–1.11, p=0.158).
Table 3.
The MC1RR163Q variant is inversely associated with complicated sepsis.
| Cases of complicated sepsis, N (%)a | Univariate Analysisb | Multivariate Analysisb | |||||
|---|---|---|---|---|---|---|---|
| MC1R SNP | Common allele homozygous | Heterozygous | Minor allele homozygous | OR for complicated sepsis (95% CI) | p-value | ORadjc for complicated sepsis (95% CI) | p-value |
| MC1RV60L | 216 (22%) | 66 (25%) | 8 (42%) | 1.25 (0.95–1.65) | 0.097 | 1.27 (0.94–1.72) | 0.122 |
| MC1RV92M | 247 (24%) | 41 (21%) | 2 (33%) | 0.88 (0.63–1.26) | 0.502 | 0.85 (0.58–1.25) | 0.404 |
| MC1RR151C | 246 (23%) | 39 (22%) | 4 (50%) | 1.05 (0.75–1.48) | 0.765 | 0.99 (0.68–1.44) | 0.947 |
| MC1RR163Q | 273 (25%) | 16 (12%) | 1 (9%) | 0.42 (0.26–0.69) | 0.001d | 0.48 (0.28–0.81) | 0.006d |
| MC1RD294H | 280 (23%) | 9 (27%) | 1 (14%) | 0.98 (0.54–1.79) | 0.956 | 1.01 (0.53–1.96) | 0.968 |
| MC1RT314T | 225 (23%) | 63 (24%) | 2 (22%) | 1.07 (0.79–1.44) | 0.668 | 1.07 (0.77–1.49) | 0.696 |
Number of subjects with complicated sepsis, by genotype (incidence of complicated sepsis among subjects with the corresponding genotype (%)).
Univariate and multivariate logistic regression with MC1R SNP as the independent variable and complicated sepsis as the outcome variable. Each SNP was analyzed individually in the regression model.
Adjusted for age, sex, injury severity score (<15 or ≥ 15), PRBC transfusion requirement (<10 or ≥10 units), BMI, and mechanism of injury (blunt, penetrating, or burn).
Significant association below the Bonferroni-adjusted p-value threshold (p ≤ 0.008).
The MC1RR163Q variant was associated with a lower risk of complicated sepsis after adjusting for population substructure
All SNPs were in Hardy-Weinberg Equilibrium (HWE) (p>0.05), with the exceptions of MC1RR163Q (p=0.005) and MC1RD294H (p<0.001). The departure from HWE equilibrium raised that possibility that the association between MC1RR163Q and complicated sepsis was an artifact of population substructure. We used principal components to adjust for this potential substructure in a subset of subjects (n=511) who had genome-wide data from a previous study (Wurfel and O’Keefe, manuscript in preparation). The characteristics of this subgroup are detailed in Supplemental Table 1. In regression analysis, principal components did not attenuate the association between the MC1RR163Q variant and lower risk of complicated sepsis (ORadj=0.30, 95%CI: 0.13–0.70, p=0.005).
Discussion
Our results suggest that variation in the MC1R gene is associated with the severity of sepsis after traumatic injury. Subjects with the MC1RR163Q variant had a lower risk of developing complicated sepsis. The variant did not appear to influence the risk of developing an infection, but rather appeared to influence the host response to a nosocomial infection. This is consistent with other studies of the relationship between genetic variation and infection severity(22).
The mechanism for the inverse association between the MC1RR163Q variant and complicated sepsis is unclear, but this SNP may be a causal factor behind the improved outcomes. Several previous studies showed that the melanocortin system mediates inflammation and plays a role in sepsis. In an in vivo study, mice with defective MC1R signaling had more severe vascular dysfunction in response to lipopolysaccharide (LPS) than did wild-type mice(23). In another study, mice treated with α-MSH had less liver damage in response to LPS compared to untreated mice, an effect that was mediated by decreased leukocyte infiltration and cytokine accumulation(24). In mice with sepsis induced by cecal ligation and perforation, an α-MSH analogue decreased hemodynamic failure, acute kidney injury, and mortality(25). Moreover, α-MSH has been associated with the inflammatory response and critical illness in humans(26). In healthy subjects, endotoxin administration resulted in increased serum α-MSH concentrations(27). In critically-ill subjects with sepsis, higher serum α-MSH concentrations were associated with lower serum TNF-α concentrations, and septic patients with persistently low α-MSH concentrations had a higher risk of death(26). Taken together, these studies indicated that the melanocortin system participates in the innate immune response and that experimental genetic variation alters responses. Therefore, it is possible that the association we observed between an MC1R variant and complicated sepsis is due to a functional change in the receptor.
MC1R signaling involves two pathways: a cyclic AMP (cAMP) pathway that is activated by adenylate cyclases(28), and a cAMP-independent pathway involving mitogen-activated protein kinases (MAPK)(29). In general, these two pathways elicit different responses by the immune system. The MAPK pathway, which is also activated by TNF-α receptors and toll-like receptors(30), has pro-inflammatory effects (30, 31), in part by inducing expression of TNF-α(32). The cAMP pathway, on the other hand, elicits an anti-inflammatory response(33), by decreasing expression of pro-inflammatory cytokines, such as TNF-α (34) and macrophage inflammatory protein-1 α (35), and by increasing expression of anti-inflammatory cytokines, such as IL-10(36).
The MC1RR163Q variant, which substitutes a charged arginine residue with a polar glutamine residue in the receptor’s GTPase domain(37), is not a simple loss- or gain-of-function mutation. In an in vitro study using human embryonic kidney cells, Doyle et al.(38) found that the MC1RR163Q variant resulted in a loss of MAPK signaling but preserved cAMP signaling. This is distinct from several other MC1R variants (MC1RR151C, MC1RR160W, MC1RD294H) that had normal MAPK activation but attenuated cAMP signaling(29, 39). Therefore, the MC1RR163Q variant may lower the risk of a severe systemic response to infection by changing the balance between pro- and anti-inflammatory signals from the MC1R receptor.
It is possible that the MC1RR163Q variant is not causally linked to a lower risk of complicated sepsis; rather the observed association may be due to one of three genetic mechanisms. First, the minor allele frequencies of MC1R SNPs differ greatly between populations(12), so any association between them and a phenotype may be an artifact of population substructure; individuals with the MC1RR163Q variant may have a high degree of common ancestry with many shared variants, any of which may be the true causal variant. We adjusted for population substructure among subjects with available genome-wide data and found that the association was not attenuated, which suggested that the association was not due to population substructure.
Second, other haplotypes may have increased the risk of complicated sepsis. Currently there is debate about how genetic variants contribute to common complex diseases, with two main hypotheses. The common disease - common variant (CD-CV) hypothesis proposes that many common variants with low penetrance determine genetic susceptibility to common diseases. Alternatively, the common disease - rare variant (CD-RV) hypothesis proposes that multiple rare variants with relatively higher penetrance determine genetic susceptibility to these diseases. Based on the CD-RV hypothesis, it is possible that rare high-risk variants, which we did not test and may have been multiple, were more common in haplotypes that did not contain the MC1RR163Q variant. In this scenario, the MC1RR163Q variant was not responsible for the lower incidence of complicated sepsis, but served as a marker for individuals who did not have a high risk haplotype. Finally, the MC1RR163Q variant may have tagged a haplotype that included the true causal variant.
The findings from this study have several potential implications. They further elucidate the role of genetic risk in the pathobiology of sepsis. Genotyping has yet to become a component of clinical practice in trauma care, but increasing our understanding of genetic variants has potential to improve risk stratification and influence clinical decisions(40), such as antibiotic use and resuscitation. In addition, if the functional effects of the MC1RR163Q variant are confirmed, it may help direct future drug development. Currently, customized immunomodulatory therapy for sepsis is lacking: recombinant activated protein C, chimeric antibodies against TNF- α, and glucocorticoids have all failed to show a clear survival benefit. Biased agonists that selectively change the signaling pattern of MC1R(41) may be worthwhile alternatives to investigate. An agonist that manipulates MC1R signaling in a manner similar to the MC1RR163Q variant could reduce the risk of a severe inflammatory response after trauma or infection.
This study had several limitations. Like all candidate gene association studies, it may be confounded by population substructure, which was not fully adjusted for in the main analysis. In keeping with the accepted approach to genetic association studies, we restricted our analyses to one race/ethnicity (in this case Caucasians, our largest cohort) so our findings cannot be generalized to other races/ethnicities. Another potential concern is related to the recently updated definitions of sepsis severity by the Sepsis Definitions Task Force, convened by the Society of Critical Care Medicine and the European Society of Intensive Care Medicine(42). According to the new definition, sepsis is an infection with associated organ dysfunction, defined as an acute change in Sequential Organ Failure Assessment (SOFA) score ≥ 2 points. SIRS criteria were excluded from the definition because they were neither sensitive nor specific to sepsis. Our current study measured organ dysfunction using the Multiple Organ Dysfunction Score rather than the SOFA score. However, our definition of “complicated sepsis” incorporates both sepsis and septic shock as defined in the Sepsis-3 definitions. Finally, our new finding of an association between MC1R and complicated sepsis requires confirmation in an independent sample of trauma patients before using the gene for screening, risk stratification, or therapeutic targeting.
In conclusion, our study suggested that MC1R has a role in the pathophysiology of sepsis after trauma. The MC1RR163Q variant was associated with a lower risk of complicated sepsis in severely injured trauma patients. This SNP has the potential to be used in “precision medicine” for risk stratification and clinical decision making. Finally, therapeutic targeting of the MC1R pathway may be beneficial for trauma patients who are at high risk for developing complicated sepsis.
Supplementary Material
Only Caucasian subjects with complete clinical and MC1R genotyping data (middle column) were included in the main analysis. A subgroup analysis included Caucasian subjects with GWAS data available from a previous study (n=511) (Wurfel and O’Keefe, manuscript in preparation). Continuous variables are reported as median (IQR) and binary variables are reported as number (%).
Acknowledgments
The authors would like to thank Alex Reiner, MD, for reviewing the manuscript. This work was supported by the National Institutes of Health (R01 GM089704 and T32 GM007037), the Jack Ruppelt Endowed Fellowship, and the David and Nancy Auth - Washington Research Foundation Endowed Chair for Restorative Burn Surgery.
Footnotes
The authors have no conflicts of interest.
References
- 1.Hildebrand F, Mommsen P, Frink M, van Griensven M, Krettek C. Genetic predisposition for development of complications in multiple trauma patients. Shock. 2011;35(5):440–448. doi: 10.1097/SHK.0b013e31820e2152. [DOI] [PubMed] [Google Scholar]
- 2.Flores C, Perez-Mendez L, Maca-Meyer N, Muriel A, Espinosa E, Blanco J, Sanguesa R, Muros M, Garcia JG, Villar J. A common haplotype of the lbp gene predisposes to severe sepsis. Crit Care Med. 2009;37(10):2759–2766. doi: 10.1097/CCM.0b013e3181a57b90. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe E, Hirasawa H, Oda S, Shiga H, Matsuda K, Nakamura M, Abe R, Nakada T. Cytokine-related genotypic differences in peak interleukin-6 blood levels of patients with sirs and septic complications. J Trauma. 2005;59(5):1181–1189. doi: 10.1097/00005373-200511000-00025. discussion 1189–1190. [DOI] [PubMed] [Google Scholar]
- 4.Sutherland AM, Walley KR, Russell JA. Polymorphisms in cd14, mannose-binding lectin, and toll-like receptor-2 are associated with increased prevalence of infection in critically ill adults. Crit Care Med. 2005;33(3):638–644. doi: 10.1097/01.ccm.0000156242.44356.c5. [DOI] [PubMed] [Google Scholar]
- 5.Thompson CM, Holden TD, Rona G, Laxmanan B, Black RA, O’Keefe GE, Wurfel MM. Toll-like receptor 1 polymorphisms and associated outcomes in sepsis after traumatic injury: A candidate gene association study. Ann Surg. 2014;259(1):179–185. doi: 10.1097/SLA.0b013e31828538e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen KH, Gu W, Zeng L, Jiang DP, Zhang LY, Zhou J, Du DY, Hu P, Liu Q, Huang SN, et al. Identification of haplotype tag snps within the entire tlr2 gene and their clinical relevance in patients with major trauma. Shock. 2011;35(1):35–41. doi: 10.1097/SHK.0b013e3181eb45b3. [DOI] [PubMed] [Google Scholar]
- 7.Riese J, Woerner K, Zimmermann P, Denzel C, Hohenberger W, Haupt W. Association of a tnfbeta gene polymorphism with complications after major abdominal operations. Shock. 2003;19(1):1–4. doi: 10.1097/00024382-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. 2003;284(3):E468–474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- 9.Taherzadeh S, Sharma S, Chhajlani V, Gantz I, Rajora N, Demitri MT, Kelly L, Zhao H, Ichiyama T, Catania A, et al. Alpha-msh and its receptors in regulation of tumor necrosis factor-alpha production by human monocyte/macrophages. Am J Physiol. 1999;276(5 Pt 2):R1289–1294. doi: 10.1152/ajpregu.1999.276.5.R1289. [DOI] [PubMed] [Google Scholar]
- 10.Leoni G, Voisin MB, Carlson K, Getting S, Nourshargh S, Perretti M. The melanocortin mc(1) receptor agonist bms-470539 inhibits leucocyte trafficking in the inflamed vasculature. Br J Pharmacol. 2010;160(1):171–180. doi: 10.1111/j.1476-5381.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catania A, Rajora N, Capsoni F, Minonzio F, Star RA, Lipton JM. The neuropeptide alpha-msh has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides. 1996;17(4):675–679. doi: 10.1016/0196-9781(96)00037-x. [DOI] [PubMed] [Google Scholar]
- 12.Rana BK, Hewett-Emmett D, Jin L, Chang BH, Sambuughin N, Lin M, Watkins S, Bamshad M, Jorde LB, Ramsay M, et al. High polymorphism at the human melanocortin 1 receptor locus. Genetics. 1999;151(4):1547–1557. doi: 10.1093/genetics/151.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy C, ter Huurne J, Berkhout M, Gruis N, Bastiaens M, Bergman W, Willemze R, Bavinck JN. Melanocortin 1 receptor (mc1r) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol. 2001;117(2):294–300. doi: 10.1046/j.0022-202x.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- 14.Box NF, Duffy DL, Irving RE, Russell A, Chen W, Griffyths LR, Parsons PG, Green AC, Sturm RA. Melanocortin-1 receptor genotype is a risk factor for basal and squamous cell carcinoma. J Invest Dermatol. 2001;116(2):224–229. doi: 10.1046/j.1523-1747.2001.01224.x. [DOI] [PubMed] [Google Scholar]
- 15.Strange RC, Ramachandran S, Zeegers MP, Emes RD, Abraham R, Raveendran V, Boggild M, Gilford J, Hawkins CP. The multiple sclerosis severity score: Associations with mc1r single nucleotide polymorphisms and host response to ultraviolet radiation. Mult Scler. 2010;16(9):1109–1116. doi: 10.1177/1352458510373784. [DOI] [PubMed] [Google Scholar]
- 16.Sood RF, Hocking AM, Muffley LA, Ga M, Honari S, Reiner AP, Rowhani-Rahbar A, Gibran NS. Race and melanocortin 1 receptor polymorphism r163q are associated with post-burn hypertrophic scarring: A prospective cohort study. J Invest Dermatol. 2015;135(10):2394–2401. doi: 10.1038/jid.2015.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shalhub S, Junker CE, Imahara SD, Mindrinos MN, Dissanaike S, O’Keefe GE. Variation in the tlr4 gene influences the risk of organ failure and shock posttrauma: A cohort study. J Trauma. 2009;66(1):115–122. doi: 10.1097/TA.0b013e3181938d50. discussion 122–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 sccm/esicm/accp/ats/sis international sepsis definitions conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 19.Balding DJ. A tutorial on statistical methods for population association studies. Nat Rev Genet. 2006;7(10):781–791. doi: 10.1038/nrg1916. [DOI] [PubMed] [Google Scholar]
- 20.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, et al. Plink: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, Zondervan KT. Data quality control in genetic case-control association studies. Nat Protoc. 2010;5(9):1564–1573. doi: 10.1038/nprot.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukaszewicz AC, Payen D. The future is predetermined in severe sepsis, so what are the implications? Crit Care Med. 2010;38(10 Suppl):S512–517. doi: 10.1097/CCM.0b013e3181f23dc4. [DOI] [PubMed] [Google Scholar]
- 23.Rinne P, Ahola-Olli A, Nuutinen S, Koskinen E, Kaipio K, Eerola K, Juonala M, Kahonen M, Lehtimaki T, Raitakari OT, et al. Deficiency in melanocortin 1 receptor signaling predisposes to vascular endothelial dysfunction and increased arterial stiffness in mice and humans. Arterioscler Thromb Vasc Biol. 2015;35(7):1678–1686. doi: 10.1161/ATVBAHA.114.305064. [DOI] [PubMed] [Google Scholar]
- 24.Chiao H, Foster S, Thomas R, Lipton J, Star RA. Alpha-melanocyte-stimulating hormone reduces endotoxin-induced liver inflammation. J Clin Invest. 1996;97(9):2038–2044. doi: 10.1172/JCI118639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doi K, Hu X, Yuen PS, Leelahavanichkul A, Yasuda H, Kim SM, Schnermann J, Jonassen TE, Frokiaer J, Nielsen S, et al. Ap214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int. 2008;73(11):1266–1274. doi: 10.1038/ki.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catania A, Cutuli M, Garofalo L, Airaghi L, Valenza F, Lipton JM, Gattinoni L. Plasma concentrations and anti-l-cytokine effects of alpha-melanocyte stimulating hormone in septic patients. Crit Care Med. 2000;28(5):1403–1407. doi: 10.1097/00003246-200005000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Catania A, Suffredini AF, Lipton JM. Endotoxin causes release of alpha-melanocyte-stimulating hormone in normal human subjects. Neuroimmunomodulation. 1995;2(5):258–262. doi: 10.1159/000097204. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez CI, Setaluri V. Cyclic amp (camp) signaling in melanocytes and melanoma. Arch Biochem Biophys. 2014;563:22–27. doi: 10.1016/j.abb.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Herraiz C, Journe F, Abdel-Malek Z, Ghanem G, Jimenez-Cervantes C, Garcia-Borron JC. Signaling from the human melanocortin 1 receptor to erk1 and erk2 mitogen-activated protein kinases involves transactivation of ckit. Mol Endocrinol. 2011;25(1):138–156. doi: 10.1210/me.2010-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13(9):679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 31.Liang Y, Li X, Zhang X, Li Z, Wang L, Sun Y, Liu Z, Ma X. Elevated levels of plasma tnf-alpha are associated with microvascular endothelial dysfunction in patients with sepsis through activating the nf-kappab and p38 mitogen-activated protein kinase in endothelial cells. Shock. 2014;41(4):275–281. doi: 10.1097/SHK.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmeyer A, Grosse-Wilde A, Flory E, Neufeld B, Kunz M, Rapp UR, Ludwig S. Different mitogen-activated protein kinase signaling pathways cooperate to regulate tumor necrosis factor alpha gene expression in t lymphocytes. J Biol Chem. 1999;274(7):4319–4327. doi: 10.1074/jbc.274.7.4319. [DOI] [PubMed] [Google Scholar]
- 33.Wall EA, Zavzavadjian JR, Chang MS, Randhawa B, Zhu X, Hsueh RC, Liu J, Driver A, Bao XR, Sternweis PC, et al. Suppression of lps-induced tnf-alpha production in macrophages by camp is mediated by pka-akap95-p105. Sci Signal. 2009;2(75):ra28. doi: 10.1126/scisignal.2000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N. Elevated cyclic amp inhibits nf-kappab-mediated transcription in human monocytic cells and endothelial cells. J Biol Chem. 1996;271(34):20828–20835. doi: 10.1074/jbc.271.34.20828. [DOI] [PubMed] [Google Scholar]
- 35.Pozo D, Guerrero JM, Calvo JR. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit lps-stimulated mip-1alpha production and mrna expression. Cytokine. 2002;18(1):35–42. doi: 10.1006/cyto.2002.1024. [DOI] [PubMed] [Google Scholar]
- 36.Platzer C, Meisel C, Vogt K, Platzer M, Volk HD. Up-regulation of monocytic il-10 by tumor necrosis factor-alpha and camp elevating drugs. Int Immunol. 1995;7(4):517–523. doi: 10.1093/intimm/7.4.517. [DOI] [PubMed] [Google Scholar]
- 37.Ibarrola-Villava M, Pena-Chilet M, Llorca-Cardenosa MJ, Oltra S, Cadenas CM, Bravo J, Ribas G. Modeling mc1r rare variants: A structural evaluation of variants detected in a mediterranean case-control study. J Invest Dermatol. 2014;134(4):1146–1149. doi: 10.1038/jid.2013.469. [DOI] [PubMed] [Google Scholar]
- 38.Doyle JR, Fortin JP, Beinborn M, Kopin AS. Selected melanocortin 1 receptor single-nucleotide polymorphisms differentially alter multiple signaling pathways. J Pharmacol Exp Ther. 2012;342(2):318–326. doi: 10.1124/jpet.112.194548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herraiz C, Jimenez-Cervantes C, Zanna P, Garcia-Borron JC. Melanocortin 1 receptor mutations impact differentially on signalling to the camp and the erk mitogen-activated protein kinase pathways. FEBS Lett. 2009;583(19):3269–3274. doi: 10.1016/j.febslet.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Villar J, Maca-Meyer N, Perez-Mendez L, Flores C. Bench-to-bedside review: Understanding genetic predisposition to sepsis. Crit Care. 2004;8(3):180–189. doi: 10.1186/cc2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montero-Melendez T, Gobbetti T, Cooray SN, Jonassen TE, Perretti M. Biased agonism as a novel strategy to harness the proresolving properties of melanocortin receptors without eliciting melanogenic effects. J Immunol. 2015;194(7):3381–3388. doi: 10.4049/jimmunol.1402645. [DOI] [PubMed] [Google Scholar]
- 42.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Only Caucasian subjects with complete clinical and MC1R genotyping data (middle column) were included in the main analysis. A subgroup analysis included Caucasian subjects with GWAS data available from a previous study (n=511) (Wurfel and O’Keefe, manuscript in preparation). Continuous variables are reported as median (IQR) and binary variables are reported as number (%).


