Abstract
Protein phosphatase 2A (PP2A) is a multimeric serine/threonine phosphatase which has multiple functions, including inhibition of the mitogen-activated protein (MAP) kinase pathway. Simian virus 40 small t antigen specifically inhibits PP2A function by binding to the PP2A regulatory subunit, interfering with the ability of PP2A to associate with its cellular substrates. We have reported that the expression of small t antigen inhibits PP2A association with Shc, leading to augmentation of insulin and epidermal growth factor-induced Shc phosphorylation with enhanced activation of the Ras/MAP kinase pathway. However, the potential involvement of PP2A in insulin's metabolic signaling pathway is presently unknown. To assess this, we overexpressed small t antigen in 3T3-L1 adipocytes by adenovirus-mediated gene transfer and found that the phosphorylation of Akt and its downstream target, glycogen synthase kinase 3β, were enhanced both in the absence and in the presence of insulin. Furthermore, protein kinase C λ (PKC λ) activity was also augmented in small-t-antigen-expressing 3T3-L1 adipocytes. Consistent with this result, both basal and insulin-stimulated glucose uptake were enhanced in these cells. In support of this result, when inhibitory anti-PP2A antibody was microinjected into 3T3-L1 adipocytes, we found a twofold increase in GLUT4 translocation in the absence of insulin. The small-t-antigen-induced increase in Akt and PKC λ activities was not inhibited by wortmannin, while the ability of small t antigen to enhance glucose transport was inhibited by dominant negative Akt (DN-Akt) expression and Akt small interfering RNA (siRNA) but not by DN-PKC λ expression or PKC λ siRNA. We conclude that PP2A is a negative regulator of insulin's metabolic signaling pathway by promoting dephosphorylation and inactivation of Akt and PKC λ and that most of the effects of PP2A to inhibit glucose transport are mediated through Akt.
Protein phosphorylation plays a key role in many cellular processes, including insulin signal transduction (24), and the phosphorylation state of a target protein is regulated by opposing kinase and phosphatase activities (24). Thus, the balance of enzyme activity between kinases and phosphatases is critical for the mediation of insulin's effects and, in turn, for the pathogenesis of insulin-resistant states.
Tyrosine phosphorylation is essential for insulin action, and several lines of evidence have demonstrated that protein tyrosine phosphatases can play a role in insulin-resistant states (3, 4). For example, protein tyrosine phosphatase 1B (PTP1B) directly interacts with the activated insulin receptor and exhibits high specific activity for IRS-1 (22, 49). It has been reported previously that hyperglycemia can impair insulin-stimulated tyrosine phosphorylation of the insulin receptor and IRS-1, at least in part because of the increased expression and activity of PTP1B (37, 41), and that overexpression of PTP1B inhibits insulin-stimulated glucose metabolism in 3T3-L1 adipocytes and L6 myocytes (12, 18, 51).
Serine/threonine phosphorylation events are also important to the metabolic actions of insulin. Serine/threonine phosphorylation of either the receptor itself or IRS proteins reduces downstream signaling and can be a cause of insulin resistance (20, 40, 44-46). Furthermore, Akt and protein kinase C λ (PKC λ), both of which are important mediators of insulin-stimulated glucose uptake, are serine/threonine kinases, and their activity states are regulated by serine/threonine phosphorylation (14, 23, 29). However, the phosphatases that catalyze corresponding dephosphorylation events have not been identified.
Protein phosphatase 2A (PP2A) is a ubiquitously expressed cytoplasmic serine/threonine phosphatase that plays an important role in the regulation of a diverse set of cellular proteins, including metabolic enzymes, hormone receptors, kinase cascades, and cell growth (39, 53). Interestingly, PP2A is the target for the simian virus 40 (SV40) small t antigen (42, 48), which associates with the regulatory A subunit of PP2A, inhibiting the association of PP2A with its cellular substrates (38, 63).
Numerous observations suggest that PP2A plays an important role in downregulation of the Ras/mitogen-activated protein (MAP) kinase pathway (39, 53), and the ability of small t antigen to inhibit PP2A activity underlies its mitogenic role during transformation by SV40 (52). For example, it has been previously reported that PP2A associates with Shc and that this association is inhibited by small t antigen, leading to enhanced insulin-, insulin-like growth factor 1-, and epidermal growth factor (EGF)-stimulated Shc phosphorylation with increased Ras/MAP kinase activity (60). It has been suggested that PP2A is also involved in the metabolic actions of insulin. Okadaic acid, an inhibitor of PP2A, can activate glucose transport and GLUT4 translocation (57). Insulin inhibits PP2A activity (56), and the insulin effect on PP2A is abolished in adipocytes from diabetic rats (10). Tumor necrosis factor alpha-induced insulin resistance is accompanied by an elevation of PP2A activity levels in rat skeletal muscle cells (11), and PP2A levels are increased in skeletal muscle samples from insulin-resistant patients with type 2 diabetes (35). Although these results raise the possibility that PP2A can negatively influence insulin action, this outcome has not been directly demonstrated, and the precise role of PP2A in the metabolic actions of insulin is unresolved.
The present data show that PP2A may directly dephosphorylate and inactivate Akt and PKC λ, leading to the attenuation of glucose transport stimulation in 3T3-L1 adipocytes. These results identify a new role for PP2A as a negative regulator of insulin's metabolic signal transduction pathway.
MATERIALS AND METHODS
Materials.
Human insulin was kindly provided by Eli Lilly (Indianapolis, Ind.). Anti-small t antigen antibody was from BD PharMingen (San Diego, Calif.). Phospho-specific MAP kinase antibody, phospho-specific Akt antibody, phospho-specific glycogen synthase kinase 3β (GSK-3β) antibody, phospho-specific PKC ζ/λ antibody, and anti-Akt antibody were from New England Biolabs (Beverly, Mass.). Anti-PP2A antibody, IRS-1 antibody, anti-p85 antibody, anti-PDK-1 antibody, and purified PP2A protein were from Upstate Biotechnology Inc. (Lake Placid, N.Y.). Anti-PP2A A-subunit antibody, anti-GSK-3β antibody, anti-ERK-2 antibody, anti-PKC ζ and -PKC λ antibody, horseradish peroxidase-linked anti-rabbit, anti-mouse, and anti-goat antibodies, and protein A/G-agarose were from Santa Cruz Biotechnology (Santa Cruz, Calif.). Anti-ERK-1 antibody, anti-phosphotyrosine antibody (PY20), and sodium azide-free PP2A antibody were from Transduction Laboratories (Lexington, Ky.). Anti-GLUT4 antibody (1F8) was from Biogenesis Inc. (Brentwood, N.H.). Sheep immunoglobulin G (IgG) and fluorescein isothiocyanate- and tetramethyl rhodamine isothiocyanate-conjugated anti-rabbit, -mouse, -goat, and -sheep IgG antibodies were from Jackson ImmunoResearch Laboratories, Inc. (West Grove, Pa.). All radioisotopes were from ICN (Costa Mesa, Calif.). Dulbecco's modified Eagle's medium, minimal Eagle's medium-α, and fetal calf serum were obtained from Life Technologies (Grand Island, N.Y.). XAR-5 film was obtained from Eastman-Kodak (Rochester, N.Y.). All other reagents and chemicals were purchased from Sigma (St. Louis, Mo.).
Cell culture.
3T3-L1 cells were cultured and differentiated as previously described (17). Prior to experiment, the adipocytes were trypsinized and reseeded in the appropriate culture dishes. The Ad-E1A-transformed human embryonic kidney cell line 293 cells were cultured as described previously (17).
Preparation of recombinant adenovirus and cell treatment.
The adenovirus encoding small t antigen was generated by using an Adeno-X expression system according to the manufacturer's instructions (CLONTECH). The adenovirus encoding the dominant negative (DN) form of Akt (Akt-MAA [K179M, T308A, S473A]) was generated as described previously (28). The adenovirus encoding the DN form of PKC λ (DN-PKC λ) was a gift from Wataru Ogawa (Kobe University, Kobe, Japan) (33). 3T3-L1 adipocytes were infected with adenoviruses at the indicated multiplicity of infection (MOI) for 16 h. Transduced cells were incubated for 48 h at 37°C in an atmosphere of 10% CO2 in Dulbecco's modified Eagle's high-glucose medium with 2% heat-inactivated serum, followed by starvation for 18 h.
Preparation of whole-cell lysates and immunoprecipitation.
Cells were lysed in solubilizing buffer (20 mM Tris, pH 7.5, 1 mM EDTA, 140 mM NaCl, 1% Nonidet P-40, 1 mM sodium vanadate, 50 mM sodium fluoride, 50 U of aprotinin/ml, 1 mM phenylmethylsulfonyl fluoride [PMSF]) for 30 min at 4°C. The cell lysates were centrifuged to remove insoluble materials. For immunoprecipitation, cell lysates were incubated with primary antibody for 6 h at 4°C and with protein A/G-agarose for an additional 2 h. The immunoprecipitates were washed, resuspended in Laemmli sample buffer containing 100 mM dithiothreitol, and heated for 5 min at 100°C.
Immunoblotting.
Whole-cell lysates and antibody immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, Mass.). Membranes were blocked and probed with specified antibodies. Blots were then incubated with horseradish peroxidase-linked second antibody followed by chemiluminescence detection, according to the manufacturer's instructions (Pierce).
PP2A activity.
Phosphatase activity was measured with para-nitrophenyl phosphate as the substrate, as described previously (60). Infected cells were lysed in lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 1 mM EGTA, 10% glycerol, 1.5 mM magnesium chloride, 1% Triton X-100, 1 μg of leupeptin/ml, 50 U of aprotinin/ml, 1 mM PMSF). Clarified supernatants were incubated with anti-PP2A antibody and protein G agarose for 2 h at 4°C. The washed immunoprecipitates were resuspended in assay buffer (50 mM Tris [pH 7.0], 0.1 mM calcium chloride) containing 2.5 mM nickel chloride and 900 μg of para-nitrophenyl phosphate/ml and incubated for 30 min at 30°C. The amount of para-nitrophenol produced was determined by measuring the absorbance at 405 nm.
PKC λ assay.
PKC λ enzyme activity was determined as described previously (57). Cells were starved for 24 h, stimulated with insulin for 30 min at 37°C, and lysed in buffer A (50 mM Tris [pH 7.5], 150 mM NaCl, 1% Nonidet P-40, 1 mM EGTA, 10 mM 2-glycerol phosphate, 0.1% 2-mercaptoethanol, 1 mM sodium vanadate, 20 mM sodium fluoride, 5 mM sodium pyrophosphate, 0.1 mM PMSF, 10 μg of leupeptin/ml, 20 μg of aprotinin/ml, 10 μM microcystin LR). Clarified supernatants were immunoprecipitated with anti-PKC λ antibody or rabbit IgG and protein A/G-agarose for 4 h at 4°C. Samples were washed twice with buffer A and twice with kinase assay buffer (50 mM Tris [pH 7.5], 5 mM magnesium chloride, 1 mM EGTA, 100 μM sodium vanadate, 100 μM sodium pyrophosphate, 1 mM sodium fluoride, 100 μM PMSF). Suspensions were then incubated with 2 to 4 μCi of [γ-32P]ATP, 50 μM ATP, 4 μg of phosphatidylserine, and 40 μM [159Ser]PKC (amino acids 153 to 164)-NH2 for 10 min at 30°C with gentle agitation. Reactions were stopped by addition of 10 μl of 5% acetic acid. Aliquots of the reaction mixtures were spotted on p81 phosphocellulose paper, washed in 5% acetic acid, and quantitated by liquid scintillation counting.
In vitro dephosphorylation assay.
Cells were stimulated with insulin for 5 min (for Akt) or 30 min (for PKC ζ/λ), and the cell lysates were immunoprecipitated with anti-Akt antibody or anti-PKC ζ/λ antibody and protein A/G-agarose. The immunoprecipitates were incubated with 0.1 to 1 U of purified PP2A protein in buffer (20 mM MOPS [morpholinepropanesulfonic acid], pH 7.5, 60 mM 2-mercaptoethanol, 100 mM NaCl, 0.1 mg of serum albumin/ml) for 30 min at 30°C (1 U is defined as 1 nmol of Pi released from phosphorylase a per min). The immunocomplexes were washed and analyzed by Western blotting with phospho-specific Akt antibody or assayed for PKC ζ and PKC λ activity as described above.
PDK-1 assay.
PDK-1 activity was determined by using exogenous substrates according to the manufacturer's specifications (Upstate Biotechnology Inc.) as described previously (31). Briefly, cells were starved for 24 h, stimulated with insulin for 10 min at 37°C, and lysed in lysis buffer (50 mM Tris [pH 7.5], 1 mM EDTA, 1 mM EGTA, 150 mM NaCl, 1% Triton X-100, 5 mM sodium pyrophosphate, 10 mM 2-glycerolphosphate, 1 mM sodium vanadate, 0.1% 2-mercaptoethanol, 1 μg of leupeptin/ml, 50 U of aprotinin/ml, 1 mM PMSF, 1 μM microcystin LR). Clarified supernatants were immunoprecipitated with anti-PDK-1 antibody or normal sheep IgG and protein A/G-agarose for 2 h at 4°C. Samples were washed three times with lysis buffer and twice with kinase dilution buffer (50 mM Tris [pH 7.5], 0.1 mM EGTA, 0.1 mM EDTA, 0.1% 2-mercaptoethanol, 2.5 μM protein kinase inhibitor, 1 μM microcystin LR, 10 mM magnesium acetate, 100 μM ATP). The washed precipitates were incubated with inactive fusion proteins, SGK1 (500 ng), 100 μM ATP, and 10 μM magnesium acetate for 30 min at 30°C with gentle agitation and then incubated with 66 μM Akt/SGK substrate peptide and 10 μCi of [γ-32P]ATP for 10 min at 30°C with gentle agitation. Samples were transferred onto p81 phosphocellulose squares, washed five times in 0.75% phosphoric acid, washed once with acetone, and quantitated by liquid scintillation counting.
2-Deoxyglucose uptake.
A glucose uptake assay was performed as described previously (25). After 48 h of adenovirus infections, serum- and glucose-deprived 3T3-L1 adipocytes were incubated in minimal Eagle's medium-α in the absence (basal) or presence of insulin for 30 min at 37°C. Glucose uptake was determined in triplicate at each point after the addition of 10 μl of substrate (0.1 μCi of 2-[3H]deoxyglucose or l-[3H]glucose; final concentration, 0.01 mmol/liter) to provide a concentration at which cell membrane transport was rate limiting. The value for l-glucose was subtracted to correct each sample for the contributions of diffusion and trapping.
Microinjection, immunostaining, and immunofluorescence microscopy.
Microinjection of the various reagents was carried out with a semiautomatic Eppendorf microinjection system. Antibodies for microinjection were washed, concentrated to 5 mg/ml in microinjection buffer containing 5 mM sodium phosphate (pH 7.2) and 100 mM KCl, and injected into the cytoplasm, as previously described (25). The duplexes of each small interfering RNA (siRNA), targeting PP2A catalytic-subunit mRNA (target sequence: 5′-GAATCCAACGTTCAAGAGG-3′), PKC λ (target sequence: 5′-TCCTTCAAGTCATGAGAGT-3′), Akt2 (27), and negative control (scrambled sequence), were purchased from Dharmacon Research Inc. (Lafayette, Colo.). The target sequence against PP2A was chosen by the web-based search program, and the lack of homology to any other gene was confirmed by a BLAST search (National Center for Biotechnology Information, National Institutes of Health). The protein level of PP2A or PKC λ after siRNA transfection (Lipofectamine 2000; Invitrogen) in 3T3-L1 fibroblasts was decreased by 38 or 45%, respectively (data not shown). The efficiency of siRNAs against Akt2 was described previously (27). Each siRNA was dissolved at 5 μM in microinjection buffer, including fluorescein-conjugated dextran (Molecular Probes, Eugene, Oreg.) to identify the injected cells. Cells were incubated in complete medium for 48 h after microinjection and then serum starved for 4 h, followed by stimulation with insulin for 20 min. Immunostaining of GLUT4 was performed essentially as described previously (25). The cells were fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature. Following washing, the cells were permeabilized and blocked with 0.1% Triton X-100 and 2% fetal calf serum in PBS for 10 min. The cells were then incubated with anti-GLUT4 antibody in PBS with 2% fetal calf serum overnight at 4°C. After washing, GLUT4 and injected IgG were detected by incubation with tetramethyl rhodamine isothiocyanate-conjugated donkey anti-mouse IgG antibody and fluorescein isothiocyanate-conjugated donkey anti-sheep antibody, respectively, followed by observation under an immunofluorescence microscope. Individual cells were scored as positive or negative for surface GLUT4, and approximately 300 cells per coverslip were counted by an observer blind to the experimental conditions.
RESULTS
Expression of small t antigen in 3T3-L1 adipocytes and its effect on MAP kinase phosphorylation.
Stable lines of HIRc B cells expressing small t antigen were previously established, and it was found that endogenous PP2A activity was inhibited by 60% (60). PP2A associates with Shc, and this association was inhibited by small t antigen, leading to augmented insulin and EGF-induced Shc phosphorylation (60) and subsequent downstream signaling to the Ras/MAP kinase pathway (60). These results demonstrated that PP2A is a negative regulator of insulin's mitogenic signaling pathway.
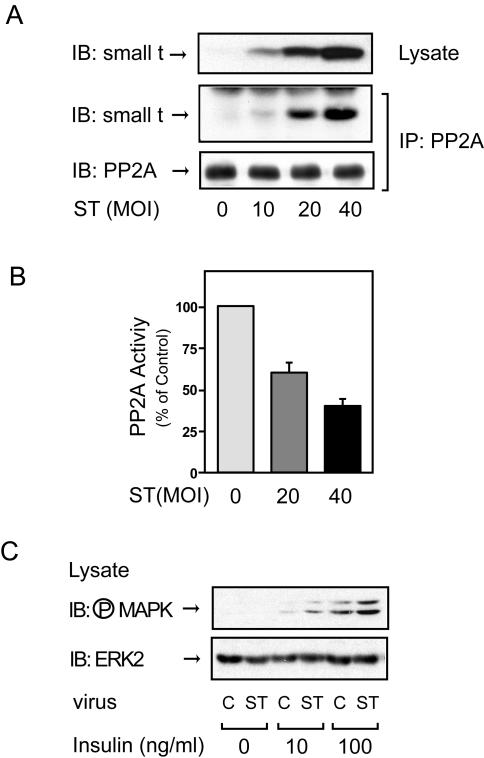
To assess whether PP2A may also be involved in the metabolic actions of insulin, we constructed an adenovirus vector that encodes small t antigen and expressed this construct in 3T3-L1 adipocytes. Small t protein expression was observed in a viral dose-dependent manner (Fig. 1A, top panel), with a subsequent increment in the amount of PP2A associated with small t protein (Fig. 1A, middle panel) and inhibition of endogenous PP2A activity (Fig. 1B). To assess the effect of small t antigen expression on insulin-stimulated MAP kinase activity, lysates from small-t-antigen-expressing cells were analyzed by Western blotting with phospho-specific MAP kinase antibody. Consistent with previous results for HIRc B cells (60), insulin-stimulated MAP kinase phosphorylation was enhanced in 3T3-L1 adipocytes by small t antigen expression without affecting MAP kinase protein levels (Fig. 1C).
FIG. 1.
Expression of small t antigen inhibits endogenous PP2A activity and enhances insulin-stimulated MAP kinase phosphorylation in 3T3-L1 adipocytes. (A) 3T3-L1 adipocytes infected with small-t-antigen-encoding adenovirus (ST) at the indicated MOIs, as described in Materials and Methods, were lysed, and whole-cell lysates were analyzed by Western blotting with anti-small t antigen antibody (top panel). The same lysates were immunoprecipitated (IP) with anti-PP2A antibody, followed by immunoblotting (IB) with anti-small t antigen antibody (middle panel) or anti-PP2A antibody (bottom panel). Control studies showed that the antibody (directed against the PP2A C subunit) precipitates intact PP2A. (B) 3T3-L1 adipocytes infected with small-t-antigen-encoding adenovirus at the indicated MOIs were lysed and assayed for PP2A activity. Data are presented as the percentage of phosphatase activity compared to that of uninfected cells and show the mean ± the standard error (SE) of results from four independent experiments. (C) 3T3-L1 adipocytes were infected with small-t-antigen-encoding adenovirus at an MOI of 40 and stimulated with insulin for 5 min, and whole-cell lysates were analyzed by Western blotting with phospho-specific MAP kinase antibody (upper panel) or anti-ERK-2 antibody (lower panel). C, control.
Small t antigen does not affect IRS-1 tyrosine phosphorylation or association with PI 3-kinase.
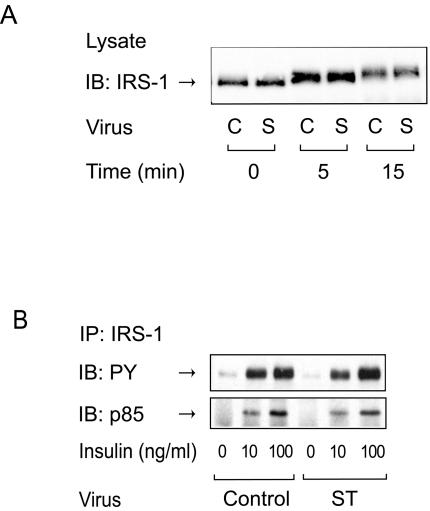
Phosphorylation of serine/threonine residues in IRS-1 can lead to positive or negative modulation of insulin signal transduction (20, 40, 44-46). IRS-1 contains over 30 serine/threonine phosphorylation sites, and certain serine/threonine kinases have been reported to phosphorylate specific serine/threonine residues of IRS-1, leading to an IRS-1 mobility shift, decreased IRS-1 tyrosine phosphorylation, and impaired IRS-1 association with phosphatidylinositol (PI) 3-kinase (20, 40, 44-46). Whereas several serine/threonine kinases that phosphorylate IRS-1 have been studied, phosphatases that act on these sites have not been identified. To assess this action, we analyzed the electrophoretic mobility shift of IRS-1 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis by Western blotting with anti-IRS-1 antibody, since this has been reported to be indicative of serine/threonine phosphorylation (44, 45). As previously reported (40), insulin stimulation increased the apparent molecular mass of IRS-1 in a time-dependent manner, and this effect was not altered in 3T3-L1 adipocytes expressing small t antigen (Fig. 2A). Insulin also stimulates IRS-1 tyrosine phosphorylation and association with the p85 subunit of PI 3-kinase, and, as shown in Fig. 2B, small t antigen expression had no effect on these processes. Consistent with this, we also observed no change in insulin-stimulated IRS-1-associated PI 3-kinase activity (data not shown). These results are consistent with the view that PP2A is not involved in the dephosphorylation of serine/threonine residues that affect the tyrosine phosphorylation, p85 subunit association, or mobility shift of IRS-1.
FIG. 2.
Expression of small t antigen does not affect tyrosine phosphorylation of IRS-1 or its association with PI 3-kinase. (A) 3T3-L1 adipocytes infected with either control virus (C) or small-t-antigen-encoding adenovirus (S) were starved and stimulated with insulin (100 ng/ml) for the indicated periods of time. Whole-cell lysates were analyzed by Western blotting with anti-IRS-1 antibody. (B) 3T3-L1 adipocytes infected with either control virus or small-t-antigen-encoding adenovirus (ST) were starved and stimulated with insulin for 3 min. Whole-cell lysates were prepared and immunoprecipitated (IP) with anti-IRS-1 antibody, followed by immunoblotting (IB) with anti-phosphotyrosine antibody (upper panel) or anti-p85 antibody (lower panel).
Insulin-stimulated Akt and GSK-3β phosphorylation is enhanced in small-t-antigen-expressing cells.
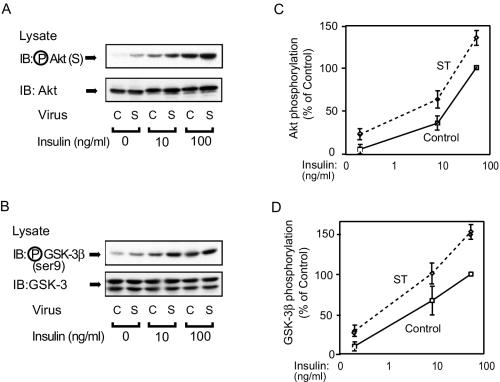
It has been reported that Akt associates with and is dephosphorylated by PP2A (6, 39), and it was previously reported that Akt coimmunoprecipitates with PP2A and that this coimmunoprecipitation was decreased by small t antigen expression in HIRc B fibroblasts (60). To assess the effect of small t antigen expression on Akt in 3T3-L1 adipocytes, we measured Akt phosphorylation in response to insulin. As shown in Fig. 3A and C, both basal and insulin-stimulated Akt phosphorylation were enhanced in small-t-antigen-expressing cells compared to cells infected with control virus. To further investigate the Akt pathway, we measured GSK-3β phosphorylation, which is a downstream target of Akt (14, 15, 23). Again, both basal and insulin-stimulated GSK-3β phosphorylation were enhanced in small-t-antigen-expressing cells (Fig. 3B and D).
FIG. 3.
Akt and GSK-3β phosphorylation is enhanced in small-t-antigen-expressing cells. (A) 3T3-L1 adipocytes were infected with either control virus (C) or small-t-antigen-encoding adenovirus (S). Whole-cell lysates were prepared and analyzed by Western blotting with phospho-specific Akt antibody (upper panel) or anti-Akt antibody (lower panel). (B) The lysates shown in panel A were analyzed by Western blotting with phospho-specific GSK-3β antibody (upper panel) or anti-GSK-3β antibody (lower panel). (C and D) Data are presented as the percentage of Akt or GSK-3β phosphorylation levels compared with that seen with maximally stimulated control cells and represent the mean ± SE of results from three independent experiments.
PDK-1 kinase activity is normal in small-t-antigen-expressing cells.
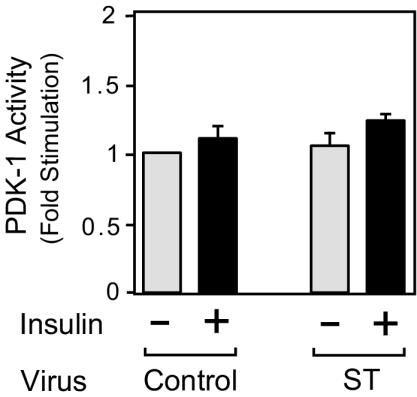
Akt is activated in a PI 3-kinase-dependent manner by PDK-1 (14, 17), and to assess whether PP2A acts upstream of Akt, we measured PDK-1 activity. As previously reported (43), insulin treatment caused only a small increase in PDK-1 activity, and this effect of insulin was not changed by small t antigen expression (Fig. 4). These results indicate that the ability of small t antigen to augment the Akt pathway is at the level of Akt itself.
FIG. 4.
Expression of small t antigen does not affect PDK-1 activity. 3T3-L1 adipocytes were infected with either control virus or small-t-antigen-encoding adenovirus (ST). Whole-cell lysates were prepared and assayed for PDK-1 activity as described in Materials and Methods. Data are presented as the increase in PDK-1 activity (n-fold) compared to unstimulated control cells and represent the mean ± SE of results from three independent experiments.
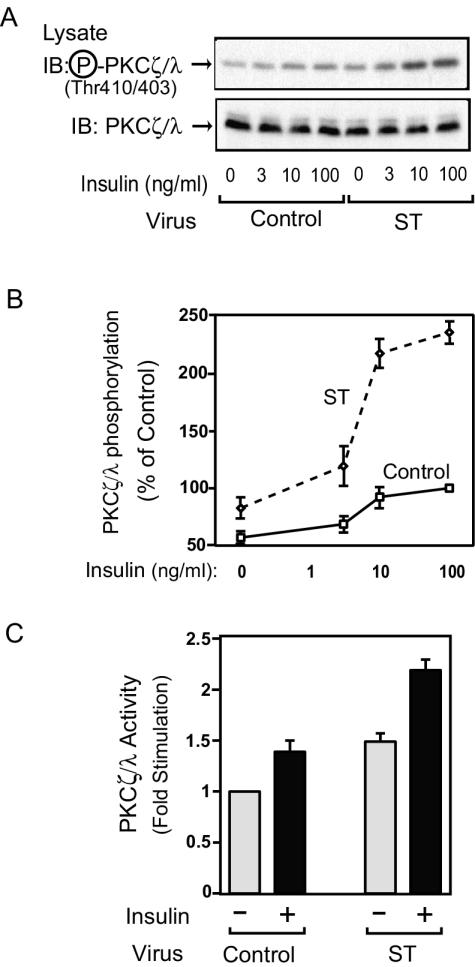
PKC λ phosphorylation and activity are enhanced in small-t-antigen-expressing cells.
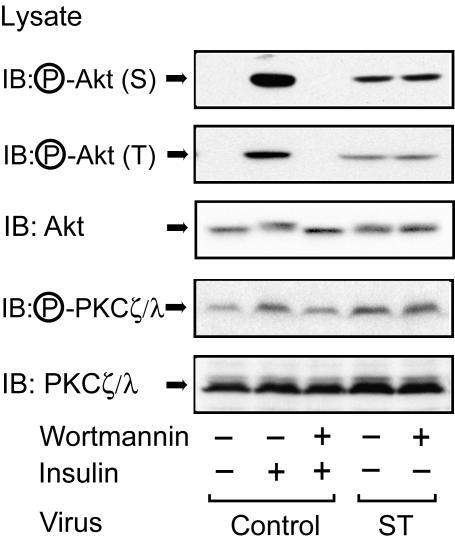
In addition to Akt, PKC λ is another serine/threonine kinase, and its activity is regulated by PDK-1-mediated phosphorylation of threonine 410 and 403 (PKC ζ and PKC λ, respectively) (13, 16, 17, 34). PKC λ can also play an important role in insulin-stimulated GLUT4 translocation and glucose transport (8, 25, 29, 33, 58). Thus, we measured insulin-stimulated PKC λ phosphorylation in 3T3-L1 adipocytes by Western blotting with phospho-specific PKC ζ/λ antibody. As shown in Fig. 5A and B, PKC λ was weakly phosphorylated in the basal state, and this phosphorylation increased after insulin stimulation. Both basal and insulin-stimulated PKC λ phosphorylation were enhanced in small-t-antigen-expressing cells, whereas protein expression was unaltered. To assess the functional importance of PKC λ phosphorylation, we measured actual PKC λ activity in 3T3-L1 adipocytes. As shown in Fig. 5C, both basal and insulin-stimulated PKC λ activity were enhanced in small-t-antigen-expressing cells, consistent with the phosphorylation data. Since Fig. 4 shows that PDK-1 is unaffected in small-t-antigen-expressing cells, these results indicate that PP2A acts directly on PKC λ. In support of this idea, as shown in Fig. 6, pretreatment with wortmannin did not inhibit Akt or PKC λ phosphorylation induced by small t antigen expression, whereas the effect of insulin was completely inhibited.
FIG. 5.
PKC λ phosphorylation and activity are enhanced in small-t-antigen-expressing 3T3-L1 adipocytes. (A) 3T3-L1 adipocytes were infected with either control virus or small-t-antigen-encoding adenovirus (ST), starved, and stimulated with insulin for 30 min. Whole-cell lysates were prepared and analyzed by Western blotting with phospho-specific PKC ζ/λ antibody (upper panel) or anti-PKC ζ/λ antibody (lower panel). (B) Data are shown as the percentage of PKC λ phosphorylation levels compared to that seen in maximally stimulated control cells and represent the mean ± SE of results from four independent experiments. (C) Cells were starved and stimulated with insulin for 30 min. Whole-cell lysates were immunoprecipitated with anti-PKC ζ/λ antibody and assayed for PKC λ activity as described in Materials and Methods. Data are presented as the increase (n-fold) in PKC λ activity compared to unstimulated control cells and represent the mean ± SE of results from three independent experiments.
FIG. 6.
Wortmannin does not inhibit small-t-antigen-induced Akt or PKC λ phosphorylation. 3T3-L1 adipocytes infected with either control virus or small-t-antigen-encoding adenovirus (ST) were starved, pretreated with 100 nM wortmannin for 30 min, and then stimulated with insulin (100 ng/ml) for 5 min (for Akt) or 30 min (for PKC λ). Whole-cell lysates were prepared and analyzed by Western blotting with phospho-specific Akt (serine 473) antibody (top panel), phospho-specific Akt (threonine 308) antibody (second panel from top), or phospho-specific PKC ζ/λ antibody (fourth panel from top). The same lysates were subjected to immunoblotting (IB) with anti-Akt antibody (third panel from top) or anti-PKC ζ/λ antibody (bottom panel).
PP2A dephosphorylates and inactivates Akt and PKC λ in vitro.
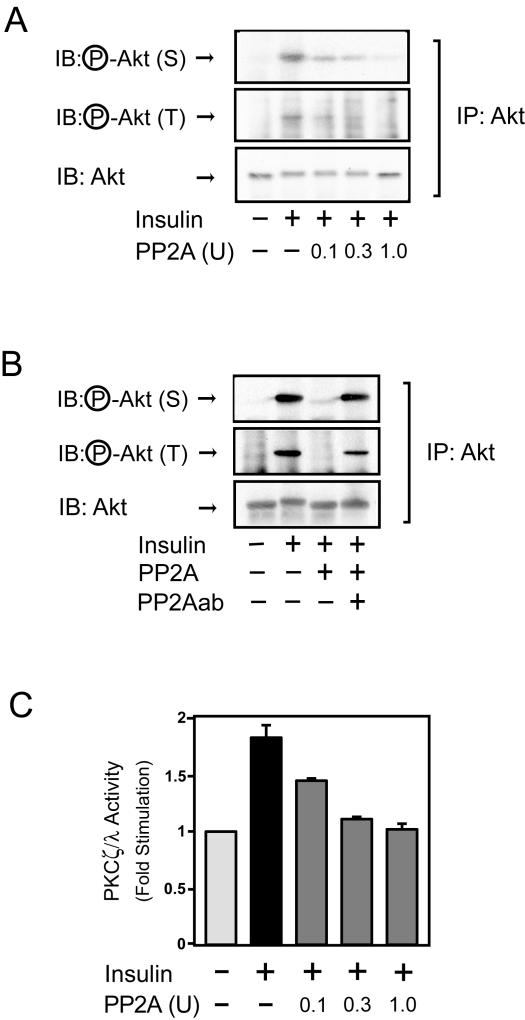
To further verify this idea, we determined whether PP2A could directly dephosphorylate Akt and PKC λ in vitro. As shown in Fig. 7A, recombinant PP2A dephosphorylated both serine 473 and threonine 308 of Akt. Furthermore, when recombinant PP2A protein was preincubated with anti-PP2A antibody for 2 h at 4°C, Akt dephosphorylation was abolished (Fig. 7B), confirming that PP2A activity was responsible for the Akt dephosphorylation. Similarly, recombinant PP2A also inactivated PKC λ.
FIG. 7.
PP2A dephosphorylates and inactivates Akt and PKC λ in vitro. (A) Cells were stimulated with insulin for 5 min. Whole-cell lysates were immunoprecipitated (IP) with anti-Akt antibody, and the immunoprecipitates were incubated with recombinant PP2A or buffer, followed by Western blotting with phospho-specific Akt (serine 473) antibody (top panel), phospho-specific Akt (threonine 308) antibody (middle panel) or anti-Akt antibody (bottom panel). (B) Anti-Akt immunoprecipitates from cells stimulated with insulin were incubated with recombinant PP2A that was preincubated with or without anti-PP2A antibody. The resultant immunoprecipitates were analyzed as described for panel A. (C) Cells were stimulated with insulin for 30 min. Whole-cell lysates were immunoprecipitated with anti-PKC ζ/λ antibody, incubated with recombinant PP2A, and assayed for PKC λ activity as described in Materials and Methods. Data are presented as the increase (n-fold) in PKC λ activity compared to unstimulated control cells and represent the mean ± SE of results from three independent experiments.
Glucose transport and GLUT4 translocation are enhanced in small-t-antigen-expressing cells.
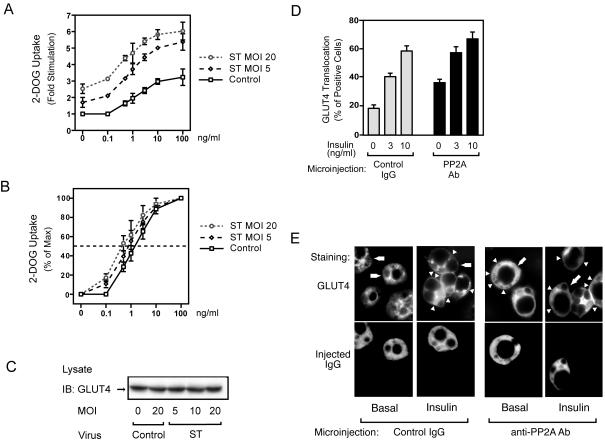
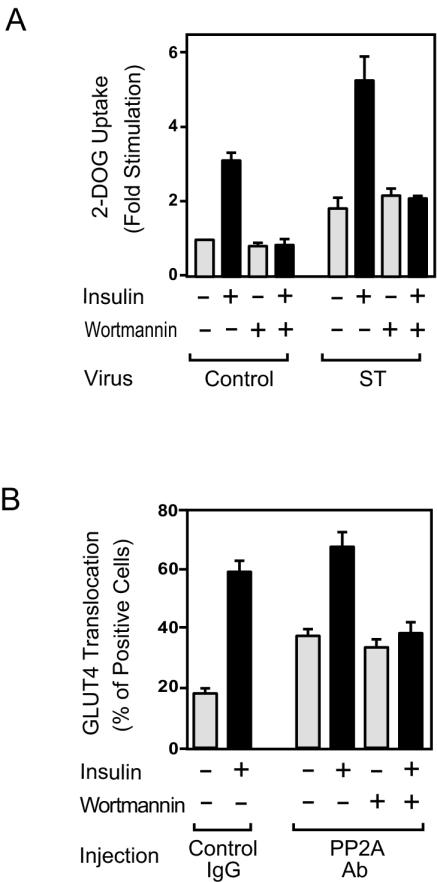
We next examined the effects of small t antigen expression on insulin-stimulated glucose transport in 3T3-L1 adipocytes. As shown in Fig. 8A, glucose transport was increased in the basal state and at all insulin concentrations in small-t-antigen-expressing cells, while GLUT4 protein levels were unchanged (Fig. 8C). Furthermore, expression of small t antigen led to enhanced insulin sensitivity for glucose transport stimulation, as demonstrated by the leftward shift in the dose-response curve in small-t-antigen-expressing cells. Thus, the 50% effective dose (ED50) was threefold greater in control cells than in small-t-antigen-expressing cells (the ED50 was 1.55 ± 0.23 ng/ml in control cells compared to ED50s of 0.8 ± 0.20 ng/ml and 0.44 ± 0.11 ng/ml at MOIs of small-t-antigen-encoding virus of 5 and 20, respectively) (Fig. 8B).
FIG. 8.
Glucose uptake is enhanced in small-t-antigen-expressing 3T3-L1 adipocytes. (A) 3T3-L1 adipocytes were infected with either control virus or small-t-antigen-encoding adenovirus (ST) at the indicated MOI and stimulated with insulin for 30 min. Glucose (2-DOG) uptake was measured as described in Materials and Methods. Data are presented as the increase (n-fold) in glucose uptake compared to that of unstimulated control cells and represent the mean ± SE of results from three independent experiments. (B) Data are presented as the percentage of the maximal response. (C) The expression of GLUT4 protein was unchanged by virus infection. 3T3-L1 adipocytes were infected with either control virus or small-t-antigen-encoding adenovirus (ST) at the indicated MOI. Whole-cell lysates were prepared and analyzed by Western blotting using anti-GLUT4 antibody. (D) 3T3-L1 adipocytes on coverslips were microinjected with anti-PP2A antibody or with sheep IgG as a control. Cells were starved and stimulated with insulin for 20 min. GLUT4 staining was performed as described in Materials and Methods. Data are presented as the percentage of cells positive for GLUT4 translocation, calculated by counting at least 100 cells, and represent the mean ± SE of results from three independent experiments. (E) Representative images of cells depicting GLUT4 staining from the experiment described for panel D. Open triangles indicate the GLUT4 ring, the thin line at cell edges, scored as positive. Lower panels show the injected cells, and the open arrows in the upper panels point to these injected cells.
To further understand the effect of PP2A on glucose transport, we measured insulin-stimulated GLUT4 translocation by immunofluorescence staining of GLUT4 in anti-PP2A antibody-microinjected cells. In the basal state, cells display GLUT4 staining mostly in a perinuclear localization, with some staining distributed in the cytoplasm. After insulin stimulation, GLUT4 staining is seen at the plasma membrane as a circumferential ring, with a concomitant decrease in intracellular distribution (25). As shown in Fig. 8D and E, single-cell microinjection of anti-PP2A antibody led to an increase in GLUT4 translocation in both the basal and the insulin-stimulated states. For these experiments, control studies showed that the anti-PP2A antibody directly inhibited the in vitro phosphatase activity of recombinant PP2A (Fig. 7B and data not shown), further supporting its neutralizing effects in the microinjection experiments.
Small-t-antigen-induced glucose transport is PI 3-kinase independent.
To further analyze the mechanisms of small t antigen action, we examined the effect of wortmannin on glucose transport and GLUT4 translocation. Consistent with the data shown in Fig. 6, small-t-antigen-induced glucose transport was not inhibited by wortmannin, whereas the insulin effect was completely blocked (Fig. 9A). Similarly, GLUT4 translocation induced by microinjection of anti-PP2A antibody was not affected by wortmannin, while insulin's effect was fully inhibited (Fig. 9B). These results suggest that PP2A acts downstream of PI 3-kinase, directly on Akt and/or PKC λ.
FIG. 9.
The stimulatory effects of PP2A inhibition on glucose uptake and GLUT4 translocation are not inhibited by wortmannin. (A) 3T3-L1 adipocytes infected with either control virus or small-t-antigen-encoding adenovirus (ST) were starved, pretreated with 100 nM wortmannin for 30 min, and then stimulated with insulin (100 ng/ml) for 30 min. Glucose (2-DOG) uptake was measured as described in Materials and Methods. Data are presented as the increase (n-fold) in glucose uptake compared to unstimulated control cells and represent the mean ± SE of results from three independent experiments. (B) 3T3-L1 adipocytes on coverslips were microinjected with anti-PP2A antibody (Ab) or with sheep IgG as a control. Cells were starved, pretreated with 100 nM wortmannin for 30 min, and then stimulated with insulin for 20 min. Fixed cells were stained with rabbit anti-GLUT4 antibody, as described in Materials and Methods. The percentage of cells positive for GLUT4 translocation was calculated as described in Materials and Methods. The data shown are the mean ± SE of results from four independent experiments.
Small-t-antigen-induced glucose transport and GLUT4 translocation are dependent on Akt rather than PKC λ.
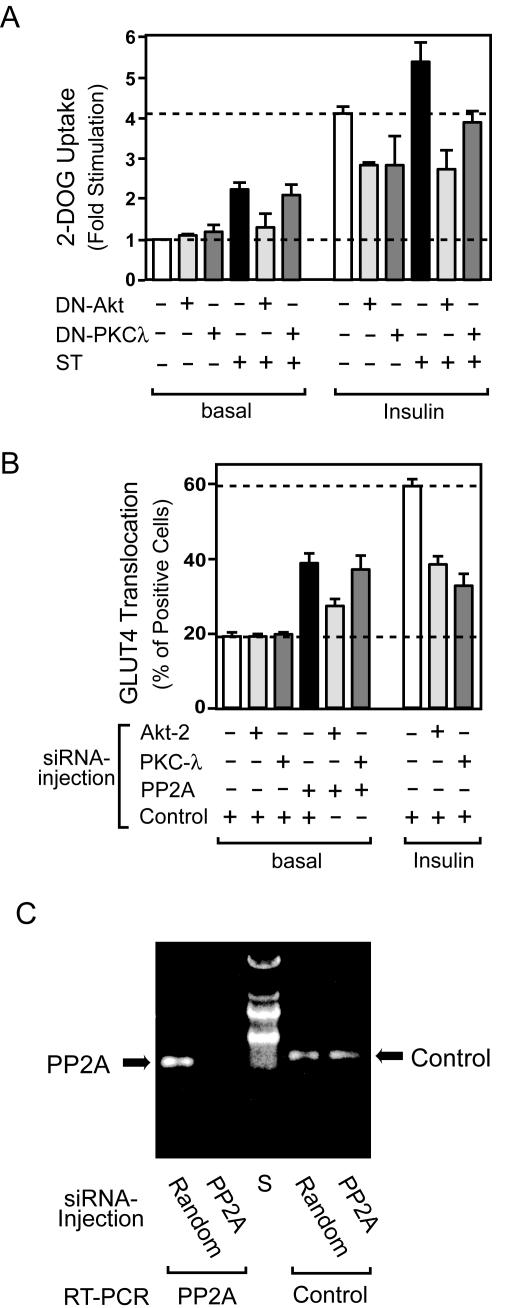
To address the question of whether these effects of small t antigen are mediated through Akt, PKC λ, or both, we coexpressed DN forms of Akt (DN-Akt) or PKC λ (DN-PKC λ) in 3T3-L1 adipocytes. As displayed in Fig. 10A, we found that DN- Akt and DN-PKC λ can inhibit insulin-stimulated glucose transport to the same extent (Fig. 10A, columns 7 to 9). However, DN-Akt, but not DN-PKC λ, inhibited small-t-antigen-induced glucose transport (Fig. 10A, columns 4 to 6 and 10 to 12).
FIG. 10.
The stimulatory effects of PP2A inhibition on glucose (2-DOG) uptake and GLUT4 translocation are dependent on Akt but not on PKC λ. (A) 3T3-L1 adipocytes infected with either control virus or adenovirus encoding DN-Akt, DN-PKC λ, or small t antigen (at MOIs of 50, 80, and 40, respectively) were stimulated with insulin (100 ng/ml) for 30 min. Glucose uptake was measured as described in Materials and Methods. Data are presented as the increase (n-fold) in glucose uptake compared to unstimulated control cells and represent the mean ± SE of results from three independent experiments. (B) 3T3-L1 adipocytes on coverslips were microinjected with each siRNA mixture, which targets Akt2 and negative control (NC), PKC λ and NC, PP2A and NC, Akt2 and PP2A, PKC λ and PP2A, or NC alone. The final concentration of siRNA mixtures is adjusted to 5 μM with or without control siRNA. Cells were starved and stimulated with insulin for 20 min. GLUT4 staining was performed as described in Materials and Methods. Data shown are the mean ± SE of results from three or four independent experiments. (C) The efficiency of siRNA against PP2A during the microinjection experiments was confirmed by reverse transcription (RT)-PCR. Approximately 200 mature 3T3-L1 adipocytes in 3 μl of complete medium were spotted on col-lagen-coated coverslips and incubated for 15 min in a humidified chamber, and the chamber was then filled with complete medium. The next day, all of the cells were microinjected with PP2A or negative control (Random) siRNA. Forty-eight hours after microinjection, cells were scraped, and total RNA was purified with an RNeasy minikit (QIAGEN). The RT-PCR was performed with a PP2A-specific or GRK2 (Control) primer set by using a one-step RT-PCR kit (QIAGEN). A representative image from two independent experiments is shown. S, size marker.
To extend these findings, we utilized the previously reported approach of siRNA microinjection (61). Accordingly, we measured GLUT4 translocation after microinjection of siRNA and found that siRNA against Akt and PKC λ inhibited insulin-stimulated translocation by 52 and 68%, respectively (Fig. 10B, columns 7 to 9). Injection of PP2A siRNA enhanced GLUT4 translocation ca. twofold, consistent with the results of small t antigen expression (Fig. 8A) and anti-PP2A antibody injection (Fig. 8D), and Fig. 10C shows that the PP2A siRNA largely depleted PP2A mRNA. In keeping with the glucose transport data in Fig. 10A, Akt siRNA inhibited small-t-antigen-induced GLUT4 translocation by 58%, whereas PKC λ siRNA had no effect (Fig. 10B, columns 4 to 6). Furthermore, although microinjection of anti-PKC λ antibody inhibited insulin-stimulated GLUT4 translocation (25), it did not affect small-t-antigen-induced translocation (data not shown). Taken together, these results show that the abilities of small t antigen to augment GLUT4 translocation and glucose transport are primarily mediated by direct actions of PP2A to dephosphorylate and inhibit Akt.
DISCUSSION
One of the major metabolic actions of insulin involves stimulation of GLUT4 translocation and glucose transport. The PI 3-kinase dependency of this stimulation has been clearly demonstrated in a variety of insulin-responsive tissues and cell lines (29, 50). The molecular events subsequent to PI 3-kinase, which cause glucose transport stimulation, have also been extensively studied. Akt and PKC λ are serine/threonine kinases activated downstream of PI 3-kinase, and both have been suggested to be mediators of glucose transport stimulation (14, 23, 29). PDK-1 is a PI 3-kinase-dependent serine/threonine kinase which is upstream of Akt and PKC λ, and the ability of PDK-1 to phosphorylate and activate Akt and PKC λ has been extensively studied (13, 14, 16, 17). Although phosphorylation clearly activates these kinases, the process of dephosphorylation and deactivation is less well understood.
PP2A is a multimeric ubiquitously expressed serine/threonine phosphatase consisting of scaffolding A, regulatory B, and catalytic C subunits. SV40-derived small t antigen inhibits PP2A activity by interfering with the regulatory B subunit recognition of PP2A target substrates, and it was previously shown that small t antigen expression augments insulin's mitogenic effects (60).
In the present study, we demonstrate that the inhibition of PP2A, either by expression of small t antigen or by microinjection of anti-PP2A antibody or PP2A siRNA, caused increased GLUT4 translocation and glucose transport in 3T3-L1 adipocytes. These changes in glucose transport were accompanied by the elevation of Akt and PKC λ phosphorylation and activity levels, and these effects (small-t-antigen-induced Akt and PKC λ activation, glucose transport, and GLUT4 translocation) were not inhibited by wortmannin. Furthermore, in vitro, recombinant PP2A was able to dephosphorylate and inactivate Akt and PKC λ, and this effect was inhibited by preincubating the recombinant PP2A with anti-PP2A antibody. Thus, these results indicate that the augmentation of glucose transport by inhibition of PP2A is due to direct actions of PP2A downstream of PI 3-kinase, most likely at the level of Akt and PKC λ.
The precise roles of Akt and PKC λ in glucose transport are incompletely understood. With respect to Akt, a number of previous studies report both positive and negative evidence for its role in glucose transport (7, 27, 28, 30, 32, 59, 62). The expression of two different types of constitutively active forms of Akt augments glucose transport both in the basal state and after insulin stimulation (32, 59) in 3T3-L1 adipocytes and L6 myotubes, and the expression of a constitutively active form of PKC λ showed similar results in L6 myotubes (8), 3T3-L1 adipocytes (33), and rat adipocytes (58). Furthermore, the expression of DN forms of Akt (28, 30, 32, 59, 62) and PKC λ (8, 9, 25, 29, 33, 58) partially inhibited glucose uptake in 3T3-L1 adipocytes and L6 myotubes. Interestingly, the DN constructs completely inhibited endogenous enzymatic activities, even though glucose transport was only partially blocked (28, 30, 33, 62). Thus, it is quite possible that both Akt and PKC λ contribute to insulin-stimulated glucose transport and that both are necessary for full activation of this process. Furthermore, the relative importance of these molecules may be cell context dependent.
Both Akt and PKC λ activities are elevated in the basal and insulin-stimulated states in small-t-antigen-expressing cells, and this result parallels the glucose transport results. Each of these kinases may contribute to insulin-stimulated glucose transport, because both DN-Akt and DN-PKC λ, as well as Akt and PCK λ siRNA, partially inhibited the effect of insulin on GLUT4 translocation and glucose transport. However, whereas the inhibition of Akt and PKC λ decreased insulin-stimulated glucose uptake to the same extents, when PP2A was suppressed by small t antigen or PP2A siRNA, only Akt inhibition reversed the effects of PP2A suppression on glucose transport. These results indicate that PP2A exerts its inhibitory effects on glucose transport at the level of Akt and not PKC λ. While Akt activation is an important component of glucose transport stimulation, these studies do not rule out a role for PKC λ in this process. Indeed, DN-PKC λ and PKC λ siRNA both inhibited insulin-stimulated GLUT4 translocation and glucose transport, and it is possible that the magnitude of PKC λ activation induced by PP2A inhibition was insufficient to generate a glucose transport stimulatory signal.
While the Akt activation mechanism has been extensively investigated, the inactivation mechanisms are less well understood. We found that the inhibition of PP2A led to an elevation of the Akt phosphorylation level without affecting PI 3-kinase or PDK-1 activity, and these results suggest that PP2A may directly modulate Akt function. In support of this idea, it has been reported that Akt can associate with PP2A and is dephosphorylated by PP2A (6, 47). PP2A was eluted in the same fraction as Akt by ion-exchange chromatography (47), and the coexpression of Akt with the catalytic subunit of PP2A inhibits Akt activity (26). In fact, it was previously reported that Akt was coimmunoprecipitated with PP2A and that this coimmunoprecipitation was decreased by small t antigen expression, a finding consistent with the idea that small t antigen displaces the PP2A regulatory B subunit, inhibiting the ability of PP2A to associate with its cellular targets (60). Thus, we conclude that PP2A is a physiologically relevant Akt phosphatase.
Interestingly, GSK-3 is one of the cellular targets of Akt (14, 15, 23), and Akt phosphorylates GSK-3α and GSK-3β at specific serine residues (serine 21 and serine 8, respectively), leading to enzyme inactivation (15, 19). Our studies showed that small t antigen expression resulted in enhanced GSK-3β phosphorylation, consistent with the idea that PP2A can function as an Akt phosphatase.
Although PKC λ activity is enhanced in small-t-antigen-expressing cells, the present studies do not definitively identify a mechanism for this effect. PKC λ is directly activated by PDK-1-mediated phosphorylation at threonine 410 (13, 16). The expression of small t antigen did not affect PDK-1 activity in our study, suggesting that the effects of PP2A on PKC λ may be direct. On the other hand, we did not observe coimmunoprecipitation of PKC λ with PP2A (data not shown). However, these negative results are inconclusive, since coimmunoprecipitation cannot detect all interactions of cellular proteins. Thus, it remains possible that PP2A directly dephosphorylates and inactivates PKC λ. Interestingly, it has been reported that expression of small t antigen led to increased PKC ζ activity in CV-1 cells and that small-t-antigen-induced MEK activation and cell growth were inhibited by the coexpression of a DN form of PKC ζ (55). However, all of these small t antigen effects were PI 3-kinase dependent in CV-1 cells (55), and these disparate results suggest that small t antigen can modulate PKC ζ/λ activity by different mechanisms in a cell context-specific manner. Similarly, several different mechanisms whereby PP2A negatively regulates the Ras/MAP kinase pathway have been reported (39). In EGF-treated adipocytes or PC12 cells, PP2A may directly dephosphorylate MAP kinase (5). On the other hand, in small-t-antigen-transfected CV-1 cells, the activation of MEK and MAP kinase by inhibition of PP2A was dependent on PI 3-kinase and PKC ζ (55). Whereas small t antigen did not affect Raf-1 activity in CV-1 cells (54), it has been reported that PP2A can associate with Raf-1 and that it positively regulates its activity in COS7 cells (1). Furthermore, it has been demonstrated that PP2A associates with Shc and inhibits its tyrosine phosphorylation in HIRc B cells (60). These different results may be explained by unique PP2A subunits. There are multiple targeting B subunits for PP2A, and different forms of this trimeric protein may be responsible for its diverse actions, since each B subunit has unique substrate specificity and displays tissue- and cell type-specific distribution (39, 53). Thus, it is possible that PP2A can act at multiple points, not only in the Ras/MAP kinase pathway, but also on the PI 3-kinase pathway, depending on cell context.
Another important issue concerning the involvement of serine/threonine phosphatases in insulin's metabolic actions involves phosphorylation of IRS proteins. In the basal state, IRS-1 is highly phosphorylated on serine and threonine residues and becomes more heavily phosphorylated after insulin stimulation. Reports have shown that serine/threonine phosphorylation of IRS-1 can serve as either a positive or a negative modulator of insulin signal transduction (20, 40, 44-46). Several serine/threonine kinases responsible for phosphorylation of IRS-1 have been reported, including Akt (45), PKC ζ (36, 46), JNK (2), IKKβ (21), and GSK-3 (20). Treatment of cells with okadaic acid increases IRS-1 serine/threonine phosphorylation and attenuates insulin action (40). Thus, phosphatases must be involved in the regulation of serine/threonine phosphorylation of IRS-1, but a specific phosphatase has not been identified. In this study, we did not observe any change in IRS-1 function in small-t-antigen-expressing cells, which suggests that PP2A does not modulate serine/threonine residues that affect IRS-1 tyrosine phosphorylation or PI 3-kinase association.
In summary, our results show that the inhibition of PP2A by small t antigen expression stimulates GLUT4 translocation and glucose transport, most likely by causing increased Akt phosphorylation and activation. These effects of small t antigen are PI 3-kinase independent, suggesting that PP2A acts directly on Akt. These results indicate that the serine/threonine phosphatase PP2A is a physiologic negative regulator of insulin's metabolic signaling pathway, which exerts its effects by dephosphorylating and inactivating Akt. As such, this result raises the possibility that abnormalities in PP2A function may be a cause of insulin resistance.
Acknowledgments
We thank Marc C. Mumby, University of Texas Southwestern Medical Center, for providing pCMV5-small t antigen; Wataru Ogawa, Kobe University, for providing DN-PKC λ adenovirus; and Elizabeth Hansen for editorial assistance.
This work was supported by a research grant from the National Institutes of Health (DK 33651), the Hilblom Foundation, and the Whittier Diabetes Institute. This work was supported in part by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to H.M.).
REFERENCES
- 1.Abraham, D., K. Podar, M. Pacher, M. Kubicek, N. Welzel, B. A. Hemmings, S. M. Dilworth, H. Mischak, W. Kolch, and M. Baccarini. 2000. Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J. Biol. Chem. 275:22300-22304. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre, V., E. D. Werner, J. Giraud, Y. H. Lee, S. E. Shoelson, and M. F. White. 2002. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 277:1531-1537. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad, F., R. V. Considine, T. L. Bauer, J. P. Ohannesian, C. C. Marco, and B. J. Goldstein. 1997. Improved sensitivity to insulin in obese subjects following weight loss is accompanied by reduced protein-tyrosine phosphatases in adipose tissue. Metabolism 46:1140-1145. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad, F., and B. J. Goldstein. 1995. Increased abundance of specific skeletal muscle protein-tyrosine phosphatases in a genetic model of insulin-resistant obesity and diabetes mellitus. Metabolism 44:1175-1184. [DOI] [PubMed] [Google Scholar]
- 5.Alessi, D. R., N. Gomez, G. Moorhead, T. Lewis, S. M. Keyse, and P. Cohen. 1995. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr. Biol. 5:283-295. [DOI] [PubMed] [Google Scholar]
- 6.Andjelkovic, M., T. Jakubowicz, P. Cron, X. F. Ming, J. W. Han, and B. A. Hemmings. 1996. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc. Natl. Acad. Sci. USA 93:5699-5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae, S. S., H. Cho, J. Mu, and M. J. Birnbaum. 2003. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J. Biol. Chem. 278:49530-49536. [DOI] [PubMed] [Google Scholar]
- 8.Bandyopadhyay, G., Y. Kanoh, M. P. Sajan, M. L. Standaert, and R. V. Farese. 2000. Effects of adenoviral gene transfer of wild-type, constitutively active, and kinase-defective protein kinase C-lambda on insulin-stimulated glucose transport in L6 myotubes. Endocrinology 141:4120-4127. [DOI] [PubMed] [Google Scholar]
- 9.Bandyopadhyay, G., M. L. Standaert, L. Zhao, B. Yu, A. Avignon, L. Galloway, P. Karnam, J. Moscat, and R. V. Farese. 1997. Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J. Biol. Chem. 272:2551-2558. [DOI] [PubMed] [Google Scholar]
- 10.Begum, N., and L. Ragolia. 1998. Altered regulation of insulin signaling components in adipocytes of insulin-resistant type II diabetic Goto-Kakizaki rats. Metabolism 47:54-62. [DOI] [PubMed] [Google Scholar]
- 11.Begum, N., L. Ragolia, and M. Srinivasan. 1996. Effect of tumor necrosis factor-alpha on insulin-stimulated mitogen-activated protein kinase cascade in cultured rat skeletal muscle cells. Eur. J. Biochem. 238:214-220. [DOI] [PubMed] [Google Scholar]
- 12.Byon, J. C., A. B. Kusari, and J. Kusari. 1998. Protein-tyrosine phosphatase-1B acts as a negative regulator of insulin signal transduction. Mol. Cell. Biochem. 182:101-108. [PubMed] [Google Scholar]
- 13.Chou, M. M., W. Hou, J. Johnson, L. K. Graham, M. H. Lee, C. S. Chen, A. C. Newton, B. S. Schaffhausen, and A. Toker. 1998. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr. Biol. 8:1069-1077. [DOI] [PubMed] [Google Scholar]
- 14.Coffer, P. J., J. Jin, and J. R. Woodgett. 1998. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 335:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 16.Dong, L. Q., R. B. Zhang, P. Langlais, H. He, M. Clark, L. Zhu, and F. Liu. 1999. Primary structure, tissue distribution, and expression of mouse phosphoinositide-dependent protein kinase-1, a protein kinase that phosphorylates and activates protein kinase Czeta. J. Biol. Chem. 274:8117-8122. [DOI] [PubMed] [Google Scholar]
- 17.Egawa, K., H. Maegawa, K. Shi, T. Nakamura, T. Obata, T. Yoshizaki, K. Morino, S. Shimizu, Y. Nishio, E. Suzuki, and A. Kashiwagi. 2002. Membrane localization of 3-phosphoinositide-dependent protein kinase-1 stimulates activities of Akt and atypical protein kinase C but does not stimulate glucose transport and glycogen synthesis in 3T3-L1 adipocytes. J. Biol. Chem. 277:38863-38869. [DOI] [PubMed] [Google Scholar]
- 18.Egawa, K., H. Maegawa, S. Shimizu, K. Morino, Y. Nishio, M. Bryer-Ash, A. T. Cheung, J. K. Kolls, R. Kikkawa, and A. Kashiwagi. 2001. Protein-tyrosine phosphatase-1B negatively regulates insulin signaling in l6 myocytes and Fao hepatoma cells. J. Biol. Chem. 276:10207-10211. [DOI] [PubMed] [Google Scholar]
- 19.Eldar-Finkelman, H., G. M. Argast, O. Foord, E. H. Fischer, and E. G. Krebs. 1996. Expression and characterization of glycogen synthase kinase-3 mutants and their effect on glycogen synthase activity in intact cells. Proc. Natl. Acad. Sci. USA 93:10228-10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eldar-Finkelman, H., and E. G. Krebs. 1997. Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc. Natl. Acad. Sci. USA 94:9660-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao, Z., D. Hwang, F. Bataille, M. Lefevre, D. York, M. J. Quon, and J. Ye. 2002. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J. Biol. Chem. 277:48115-48121. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein, B. J., A. Bittner-Kowalczyk, M. F. White, and M. Harbeck. 2000. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J. Biol. Chem. 275:4283-4289. [DOI] [PubMed] [Google Scholar]
- 23.Hajduch, E., G. J. Litherland, and H. S. Hundal. 2001. Protein kinase B (PKB/Akt)—a key regulator of glucose transport? FEBS Lett. 492:199-203. [DOI] [PubMed] [Google Scholar]
- 24.Hunter, T. 1995. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell 80:225-236. [DOI] [PubMed] [Google Scholar]
- 25.Imamura, T., P. Vollenweider, K. Egawa, M. Clodi, K. Ishibashi, N. Nakashima, S. Ugi, J. W. Adams, J. H. Brown, and J. M. Olefsky. 1999. G alpha-q/11 protein plays a key role in insulin-induced glucose transport in 3T3-L1 adipocytes. Mol. Cell. Biol. 19:6765-6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivaska, J., L. Nissinen, N. Immonen, J. E. Eriksson, V.-M. Kahari, and J. Heino. 2002. Integrin α2β1 promotes activation of protein phosphatase 2A and dephosphorylation of Akt and glycogen synthase kinase 3β. Mol. Cell. Biol. 22:1352-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang, Z. Y., Q. L. Zhou, K. A. Coleman, M. Chouinard, Q. Boese, and M. P. Czech. 2003. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc. Natl. Acad. Sci. USA 100:7569-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katome, T., T. Obata, R. Matsushima, N. Masuyama, L. C. Cantley, Y. Gotoh, K. Kishi, H. Shiota, and Y. Ebina. 2003. Use of RNA interference-mediated gene silencing and adenoviral overexpression to elucidate the roles of AKT/protein kinase B isoforms in insulin actions. J. Biol. Chem. 278:28312-28323. [DOI] [PubMed] [Google Scholar]
- 29.Khan, A. H., and J. E. Pessin. 2002. Insulin regulation of glucose uptake: a complex interplay of intracellular signalling pathways. Diabetologia 45:1475-1483. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura, T., W. Ogawa, H. Sakaue, Y. Hino, S. Kuroda, M. Takata, M. Matsumoto, T. Maeda, H. Konishi, U. Kikkawa, and M. Kasuga. 1998. Requirement for activation of the serine-threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol. Cell. Biol. 18:3708-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi, T., M. Deak, N. Morrice, and P. Cohen. 1999. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem. J. 344:189-197. [PMC free article] [PubMed] [Google Scholar]
- 32.Kohn, A. D., S. A. Summers, M. J. Birnbaum, and R. A. Roth. 1996. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 271:31372-31378. [DOI] [PubMed] [Google Scholar]
- 33.Kotani, K., W. Ogawa, M. Matsumoto, T. Kitamura, H. Sakaue, Y. Hino, K. Miyake, W. Sano, K. Akimoto, S. Ohno, and M. Kasuga. 1998. Requirement of atypical protein kinase Cλ for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol. Cell. Biol. 18:6971-6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Good, J. A., W. H. Ziegler, D. B. Parekh, D. R. Alessi, P. Cohen, and P. J. Parker. 1998. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281:2042-2045. [DOI] [PubMed] [Google Scholar]
- 35.Levin, K., H. Daa Schroeder, F. P. Alford, and H. Beck-Nielsen. 2001. Morphometric documentation of abnormal intramyocellular fat storage and reduced glycogen in obese patients with type II diabetes. Diabetologia 44:824-833. [DOI] [PubMed] [Google Scholar]
- 36.Liu, Y. F., K. Paz, A. Herschkovitz, A. Alt, T. Tennenbaum, S. R. Sampson, M. Ohba, T. Kuroki, D. LeRoith, and Y. Zick. 2001. Insulin stimulates PKCzeta-mediated phosphorylation of insulin receptor substrate-1 (IRS-1). A self-attenuated mechanism to negatively regulate the function of IRS proteins. J. Biol. Chem. 276:14459-14465. [DOI] [PubMed] [Google Scholar]
- 37.Maegawa, H., R. Ide, M. Hasegawa, S. Ugi, K. Egawa, M. Iwanishi, R. Kikkawa, Y. Shigeta, and A. Kashiwagi. 1995. Thiazolidine derivatives ameliorate high glucose-induced insulin resistance via the normalization of protein-tyrosine phosphatase activities. J. Biol. Chem. 270:7724-7730. [DOI] [PubMed] [Google Scholar]
- 38.Mateer, S. C., S. A. Fedorov, and M. C. Mumby. 1998. Identification of structural elements involved in the interaction of simian virus 40 small tumor antigen with protein phosphatase 2A. J. Biol. Chem. 273:35339-35346. [DOI] [PubMed] [Google Scholar]
- 39.Millward, T. A., S. Zolnierowicz, and B. A. Hemmings. 1999. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 24:186-191. [DOI] [PubMed] [Google Scholar]
- 40.Mothe, I., and E. Van Obberghen. 1996. Phosphorylation of insulin receptor substrate-1 on multiple serine residues, 612, 632, 662, and 731, modulates insulin action. J. Biol. Chem. 271:11222-11227. [DOI] [PubMed] [Google Scholar]
- 41.Obata, T., H. Maegawa, A. Kashiwagi, T. S. Pillay, and R. Kikkawa. 1998. High glucose-induced abnormal epidermal growth factor signaling. J. Biochem. (Tokyo) 123:813-820. [DOI] [PubMed] [Google Scholar]
- 42.Pallas, D. C., L. K. Shahrik, B. L. Martin, S. Jaspers, T. B. Miller, D. L. Brautigan, and T. M. Roberts. 1990. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell 60:167-176. [DOI] [PubMed] [Google Scholar]
- 43.Park, J., M. M. Hill, D. Hess, D. P. Brazil, J. Hofsteenge, and B. A. Hemmings. 2001. Identification of tyrosine phosphorylation sites on 3-phosphoinositide-dependent protein kinase-1 and their role in regulating kinase activity. J. Biol. Chem. 276:37459-37471. [DOI] [PubMed] [Google Scholar]
- 44.Paz, K., R. Hemi, D. LeRoith, A. Karasik, E. Elhanany, H. Kanety, and Y. Zick. 1997. A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J. Biol. Chem. 272:29911-29918. [DOI] [PubMed] [Google Scholar]
- 45.Paz, K., Y. F. Liu, H. Shorer, R. Hemi, D. LeRoith, M. Quan, H. Kanety, R. Seger, and Y. Zick. 1999. Phosphorylation of insulin receptor substrate-1 (IRS-1) by protein kinase B positively regulates IRS-1 function. J. Biol. Chem. 274:28816-28822. [DOI] [PubMed] [Google Scholar]
- 46.Ravichandran, L. V., D. L. Esposito, J. Chen, and M. J. Quon. 2001. Protein kinase C-zeta phosphorylates insulin receptor substrate-1 and impairs its ability to activate phosphatidylinositol 3-kinase in response to insulin. J. Biol. Chem. 276:3543-3549. [DOI] [PubMed] [Google Scholar]
- 47.Resjo, S., O. Goransson, L. Harndahl, S. Zolnierowicz, V. Manganiello, and E. Degerman. 2002. Protein phosphatase 2A is the main phosphatase involved in the regulation of protein kinase B in rat adipocytes. Cell. Signal. 14:231-238. [DOI] [PubMed] [Google Scholar]
- 48.Rundell, K. 1987. Complete interaction of cellular 56,000- and 32,000-Mr proteins with simian virus 40 small-t antigen in productively infected cells. J. Virol. 61:1240-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seely, B. L., P. A. Staubs, D. R. Reichart, P. Berhanu, K. L. Milarski, A. R. Saltiel, J. Kusari, and J. M. Olefsky. 1996. Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes 45:1379-1385. [DOI] [PubMed] [Google Scholar]
- 50.Shepherd, P. R., D. J. Withers, and K. Siddle. 1998. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem. J. 333:471-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimizu, S., H. Maegawa, K. Egawa, K. Shi, M. Bryer-Ash, and A. Kashiwagi. 2002. Mechanism for differential effect of protein-tyrosine phosphatase 1B on Akt versus mitogen-activated protein kinase in 3T3-L1 adipocytes. Endocrinology 143:4563-4569. [DOI] [PubMed] [Google Scholar]
- 52.Sleigh, M. J., W. C. Topp, R. Hanich, and J. F. Sambrook. 1978. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell 14:79-88. [DOI] [PubMed] [Google Scholar]
- 53.Sontag, E. 2001. Protein phosphatase 2A: the Trojan horse of cellular signaling. Cell. Signal. 13:7-16. [DOI] [PubMed] [Google Scholar]
- 54.Sontag, E., S. Fedorov, C. Kamibayashi, D. Robbins, M. Cobb, and M. Mumby. 1993. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the MAP kinase pathway and induces cell proliferation. Cell 75:887-897. [DOI] [PubMed] [Google Scholar]
- 55.Sontag, E., J. M. Sontag, and A. Garcia. 1997. Protein phosphatase 2A is a critical regulator of protein kinase C zeta signaling targeted by SV40 small t to promote cell growth and NF-kappaB activation. EMBO J. 16:5662-5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srinivasan, M., and N. Begum. 1994. Regulation of protein phosphatase 1 and 2A activities by insulin during myogenesis in rat skeletal muscle cells in culture. J. Biol. Chem. 269:12514-12520. [PubMed] [Google Scholar]
- 57.Standaert, M. L., G. Bandyopadhyay, M. P. Sajan, L. Cong, M. J. Quon, and R. V. Farese. 1999. Okadaic acid activates atypical protein kinase C (zeta/lambda) in rat and 3T3/L1 adipocytes. An apparent requirement for activation of Glut4 translocation and glucose transport. J. Biol. Chem. 274:14074-14078. [DOI] [PubMed] [Google Scholar]
- 58.Standaert, M. L., L. Galloway, P. Karnam, G. Bandyopadhyay, J. Moscat, and R. V. Farese. 1997. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J. Biol. Chem. 272:30075-30082. [DOI] [PubMed] [Google Scholar]
- 59.Ueki, K., R. Yamamoto-Honda, Y. Kaburagi, T. Yamauchi, K. Tobe, B. M. Burgering, P. J. Coffer, I. Komuro, Y. Akanuma, Y. Yazaki, and T. Kadowaki. 1998. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J. Biol. Chem. 273:5315-5322. [DOI] [PubMed] [Google Scholar]
- 60.Ugi, S., T. Imamura, W. Ricketts, and J. M. Olefsky. 2002. Protein phosphatase 2A forms a molecular complex with Shc and regulates Shc tyrosine phosphorylation and downstream mitogenic signaling. Mol. Cell. Biol. 22:2375-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Usui, I., T. Imamura, J. Huang, H. Satoh, and J. M. Olefsky. 2003. Cdc42 is a Rho GTPase family member that can mediate insulin signaling to glucose transport in 3T3-L1 adipocytes. J. Biol. Chem. 278:13765-13774. [DOI] [PubMed] [Google Scholar]
- 62.Wang, Q., R. Somwar, P. J. Bilan, Z. Liu, J. Jin, J. R. Woodgett, and A. Klip. 1999. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol. Cell. Biol. 19:4008-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang, S. I., R. L. Lickteig, R. Estes, K. Rundell, G. Walter, and M. C. Mumby. 1991. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol. Cell. Biol. 11:1988-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]