Abstract
In just the past five years, dramatic changes have occurred in the clinical management of Tuberous Sclerosis Complex (TSC). Detailed knowledge about the role of the TSC proteins in regulating the activity of the mammalian Target of Rapamycin Complex 1 (mTORC1) underlies this paradigm-shifting progress. Advances continue to be made in understanding the genetic pathogenesis of the different tumours that occur in TSC, including pivotal discoveries using next-generation sequencing (NGS). For example, the pathogenesis of angiofibromas is now known to involve UV-induced mutations, and the pathogenesis of multifocal renal cell carcinoma (RCC) in TSC is now known to result from distinct second-hit mutations. In parallel, the pathological features of TSC-associated tumours, including TSC-associated renal cell carcinoma, continue to be defined, despite the fact that TSC was first described 180 years ago. Here, we review recent discoveries related to the pathologic features and genetic pathogenesis of TSC-associated tumours.
Keywords: Tuberous sclerosis complex (TSC), next generation sequencing (NGS), mosaicism, no mutation identified (NMI), angiofibroma, angiomyolipoma (AML), renal cell carcinoma (RCC), lymphangioleiomyomatosis (LAM)
Introduction
Tuberous sclerosis complex (TSC) is a disease known for its phenotypic heterogeneity [1]. It was first described nearly 180 years ago. In the late nineteenth century, it was identified by simple clinical observations of cortical and dermatological features and has further developed with pathological studies and technological advances of imaging methods during the late twentieth century. The power of next generation sequencing (NGS) allows for in-depth genetic analysis that was never before possible. In parallel, disease phenotypes, such as in renal cell carcinoma (RCC), are being better classified in part because of greater awareness and worldwide collaboration. Major advances are occurring at a more rapid pace now than ever before, from genetic, pathologic, and clinical perspectives. Here, we focus on recent developments in the pathologic features and genetic pathogenesis of tumors in TSC.
Tuberous Sclerosis Complex
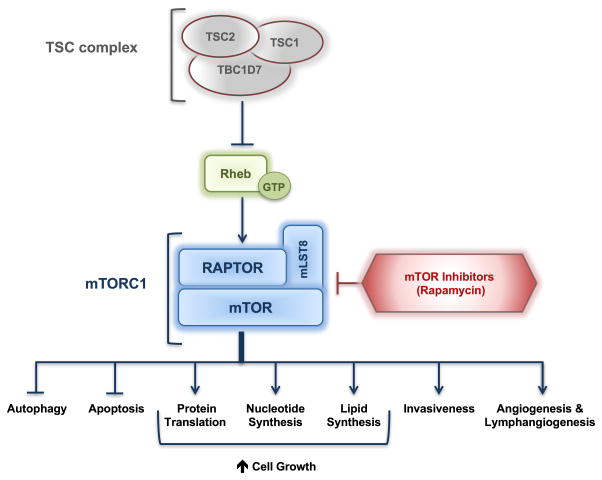
The clinical manifestations of TSC are quite distinctive and include hamartomatous lesions of the brain, skin, heart, lungs and kidneys, in addition to a wide spectrum of neurological features, including seizures, autism, and cognitive disability [2]. The tumours in TSC develop following inactivation of both alleles of either TSC1 or TSC2 genes, consistent with the “two-hit” Knudson tumour suppressor gene hypothesis [3]. The products of these genes, the TSC1 and TSC2 proteins, form a heterodimeric complex with TBC1D7, known as the TSC protein complex [4]. The TSC protein complex acts via the small GTPase Rheb to regulate the activity of the mechanistic or mammalian target of rapamycin complex 1, also known as mTORC1. Tumour cells in tuberous sclerosis have activation of the mTORC1 signalling network. This results in increased protein translation and cell growth, decreased autophagy, and metabolic adaptations that include increased nucleotide synthesis, as recently reviewed elsewhere [4–6]. These findings led to the use of allosteric inhibitors of mTORC1, such as sirolimus (Rapamycin) and everolimus (Afinitor) to restore homeostasis to TSC deficient cells [7] (Figure 1). These agents, referred to as Rapalogs, are now FDA-approved for the treatment of subependymal giant cell astrocytomas (SEGA), facial angiofibromas (in a topical form), lymphangioleiomyomatosis, and angiomyolipomas, following a series of pivotal clinical trials, as recently reviewed [2]. Importantly, Rapalogs have a cytostatic effect on tumours in TSC, with suppression while on therapy and regrowth when therapy is discontinued.
Figure 1. TSC/mTORC1 signalling and rapamycin.
Mutations in TSC1 or TSC2 result in hyperactivation of mTORC1, which in turn results in increased metabolic adaptations that include increased nucleotide synthesis, protein translation and cell growth, in addition to decreased autophagy. Allosteric inhibitors of mTORC1, such as rapamycin are used to restore homeostasis to TSC deficient cells.
Genetics/ Mosaicism
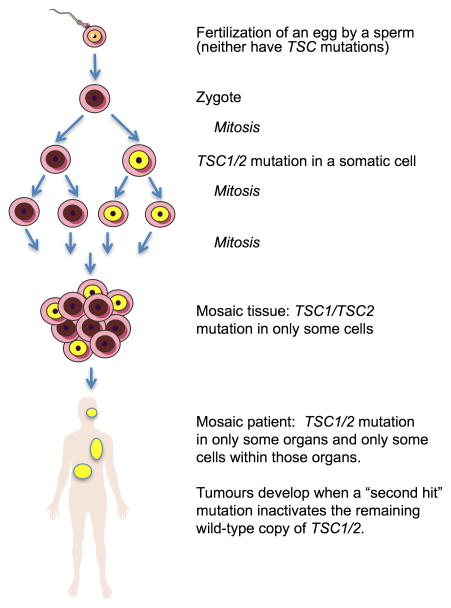
TSC is an autosomal dominant disorder caused by mutations in either the TSC1 or TSC2 gene [2, 8]. Patients with TSC1 mutations develop less frequent and less severe phenotypes than patients with TSC2 mutations [9, 10]. TSC1 is located on chromosome 9q34 (MIM 191100) [11], and has been shown to be mutated in ~20% of patients with a clinical diagnosis. The majority of TSC1 mutations are small truncating nonsense and insertion or deletion mutations, with only a small number of functionally confirmed missense mutations [12]. TSC2 is located on chromosome 16q13 (MIM 191092) [13], and is mutated in ~70% of TSC cases. All mutations in the TSC1 and TSC2 genes are believed to result in loss-of-function of the TSC1 or TSC2 proteins, which are also referred to as hamartin and tuberin, respectively [14–16]. One third of TSC cases are familial and two thirds are sporadic, occurring in the absence of a family history and attributed to de novo mutations [17, 18]. Somatic mosaic mutations have been reported in TSC, and patients showing somatic mosaicism tend to be more mildly affected [19, 20] (Figure 2).
Figure 2. Mosaicism in TSC.
Recent findings demonstrate that patients with no detectable TSC1 or TSC2 mutation in their peripheral blood cells often have somatic mosaicism. The disease phenotype tends to be milder since not all organs or all cells in an organ have the mutation. The mutation can sometimes be detected at very low levels in the blood. A somatic “second hit” mutation inactivating the wild-type allele is required for tumors to develop.
Recent advances
Until recently, it was believed that the 10% of patients with definite clinical features of TSC but who lacked a detectable mutation in TSC1 or TSC2 (no mutation identified: NMI) could have a mutation in a third unknown gene, tentatively designated “TSC3.” However, very recent work indicates that the majority of these patients have either a genetically mosaic form of TSC or an intronic mutation that could not have been detected using conventional screening methods. Two groups have used next generation sequencing (NGS) to analyse the entire genomic extent of TSC1 and TSC2 in TSC NMI patients [21, 22]. Nellist et. al. analysed DNA from 7 NMI patients with definite diagnoses of TSC by clinical parameters using targeted NGS. Nineteen new variants were identified that were missed by conventional genetic testing, with pathogenic mutations confirmed in 43% of NMI patients. A previously missed germline mutation was detected in one individual and mosaic mutations in two individuals with the remaining patients having variants of unconfirmed clinical significance [21]. Similarly, Tyburczy et al. analysed 53 TSC patients who were NMI after prior conventional testing, using blood and saliva DNA samples in addition to biopsies from skin tumours [22]. NGS identified mutations in the majority (85%) of the NMI patients, with 82% detected in TSC2 gene and 18% in TSC1 gene, similar to the general distribution of mutations. Heterozygous non-mosaic mutations in coding exons and consensus splice sites were identified in 11% of the samples and were presumably missed by previous conventional screening. Mutations in the introns, non-coding regions of the genes, were detected in 40% of the NMI samples, 33% of which were mutations which have not been previously reported. Genetic mosaicism was detected in 58% of the samples, thus explaining the majority of the NMI cases in this study. Mosaic mutations consisted of small insertions and deletions, larger genomic deletions, and nonsense, splice site, and missense mutations. Allele frequency of mutations ranged from 0.21–34% and increased allele frequency was detected in the cutaneous tumours compared to the blood and saliva samples. In one particular case of so-called extreme germline mosaicism (meaning that the level of mosaicism appears to be very low) no mutations were detectable in the blood and saliva, while an indel and missense mutation were detected in an angiofibroma at frequencies of 4.6% and 2.7%, respectively. Analysis of additional angiofibromas from the same patient identified the indel mutation at a frequency of 0.86–2.3%, indicative of germline mosaicism for the indel mutation [22]. Together, these findings make the possibility of a third germline TSC-causing gene unlikely. They also highlight the importance of full gene coverage and the challenges of detecting mosaic mutations even when using NGS.
Angiofibromas
Angiofibromas are one of the characteristic skin lesions of TSC, typically occurring on the face. Three or more facial angiofibromas are one of the major diagnostic criteria for TSC [23]. Other skin lesions in TSC include shagreen patches, periungual fibromas, and hypomelanotic macules. Angiofibromas are rarely seen in infants and typically begin to become evident between 3–5 years of age, with increasing severity in teenage years [24]. Angiofibromas are benign tumours composed of fibroblast-like cells, fibrous tissue and vessels, and hair follicles [25, 26].
Like other tumours in TSC, the lesional cells in angiofibromas undergo a “second-hit” mutation that inactivates the wild-type allele, and loss of function of the TSC1-TSC2 complex, which then activates signalling through the mammalian/mechanistic Target of Rapamycin Complex 1 (mTORC1) [27]. Importantly, in angiofibromas, it is the fibroblast-like cells, and not the vascular elements, that have sustained the genetic second hit events and are believed to be causal, based on the observation that fibroblast-like cells grown from most TSC skin hamartomas showed allelic deletions of TSC2, but no evidence of such second hit mutations in the epidermis of the tumour [28, 29]. It is speculated that these fibroblasts are derived from mesencephalic neural crest [30], thereby explaining the facial distribution of angiofibromas. The mechanisms through which the mesenchymal tumour cells carrying TSC2 mutations influence the wild-type epidermal cells in angiofibromas are beginning to be understood. The release of epiregulin, an epidermal growth factor (EGF)-related growth factor, by fibroblast-like cells may be a key element [28].
Recent advances
In striking contrast to other TSC-associated tumours, the second-hit somatic mutations in angiofibromas are distinctive. Tyburczy et. al.[27] analysed more than 50 skin samples from 22 TSC patients using NGS. The majority (89%) of the second hit mutations that were identified were small insertion/deletions or point mutations, in contrast to the large genomic deletions or recombination events that are typically detected as loss of heterozygosity (LOH), such as in angiomyolipomas, in which ~70% of the second hit events are LOH mutations. Most remarkably, 50% of the somatic point mutations were CC>TT, indicative of sunlight-induced DNA damage [31]. These findings suggest for the first time that sun exposure is the initiating event in the genetic pathogenesis of facial angiofibromas.
Angiomyolipomas
Angiomyolipomas (AMLs) and cysts are the most common renal manifestations of TSC, found in 17% of children with TSC by age 2 and increasing to 65% of children 9–14 years [32, 33]. Approximately 80% of adults with TSC have AMLs, which can progress with age and can rupture, resulting in life threatening haemorrhage [33–35]. AMLs frequently show loss of heterozygosity (LOH) for either TSC1 or TSC2 and activation of mTORC1 is consistently seen in AML tissues, including sporadic AMLs from patients without TSC [36–38]. Interestingly, in AML all three lineages (smooth muscle, fat, and vascular elements) have second hit mutations with inactivation of TSC1/TSC2, as evidenced by LOH, in contrast to angiofibromas where only the fibroblast population has inactivation of both copies of TSC1/TSC2. The mechanisms underlying this tri-lineage differentiation, and the cell-of-origin of AMLs, are not completely understood.
Recent advances
Until recently, it was unknown whether somatic genetic events (in addition to inactivation of TSC1/TSC2) participated in the progression of AMLs; however, a recent whole exome sequencing study found that biallelic loss of TSC1/TSC2 was the primary and sufficient driver of AMLs [39]. Whole exome sequencing was performed to generate a comprehensive genomic landscape of 32 tumours (30 angiomyolipomas and two LAM-associated tumours). There were three TSC patients, one of whom had 16 AMLs. Eleven samples were sporadic AMLs obtained from patients without TSC, including 2 patients with LAM. Biallelic loss of TSC1/TSC2 was found in 30 of the 32 samples. TSC1/TSC2 mutations identified included large deletions, point mutations, indels and copy neutral loss of heterozygosity. For the TSC patient with 16 tumours, most showed distinct second hit events suggesting independent origins for each tumour, although some lesions had the same mutations and presumably had a common clonal ancestry. This analysis revealed an extremely low mutation incidence of an average of 4 nonsynonymous exonic variants/mutations per sample, indicating that the somatic mutation rate is very low in AMLs, especially when compared with malignant tumours. Approximately 38% of mutations were subclonal and 87% were missense mutations, approximately half of which were unlikely to exert any functional effects. Missense mutations of unknown biological relevance were identified in three cancer-associated genes: BAP1, ARHGAP35 and SPEN. These mutations occurred within a single angiomyolipoma each. Distinct mutations were identified in TRIP12 in two different angiomyolipomas. A single loss-of-function mutation in GSK3B was identified with potential relevance to mTOR pathway. This extremely low incidence of mutations suggests that TSC1/TSC2 loss is the primary event supporting the initiation and progression of AMLs [39], although other events including microRNA changes and epigenetic changes have not been excluded.
Several recent papers have addressed the cell-of-origin of angiomyolipomas. Based on the diversity of cells and markers, multiple origins have been proposed: neural crest progenitor, renal pericytes, and most recently lymphatic endothelial cells (LECs). During development neural crest cells migrate throughout the embryo to form critical mesodermal structures including blood vessels, melanocytes, adipose, membranous bone and connective tissue [30]. Neural crest progenitors give rise to most or all pericytes in the adult. Renal pericytes are also a proposed cell-of-origin as they not only share plasticity with interstitial fibroblasts, but also support microvasculature and accumulate lipids [40]. An alternative hypothesis for AML pathogenesis is that TSC1/TSC2 loss drives the acquisition of plasticity and multipotency and acquisition of hallmark AML features [41]. TSC2-null cells re-expressing TSC2 acquire lymphatic endothelial cells (LEC) markers. These data suggest that AMLs might arise from LECs “reprogrammed” by TSC loss and subsequent mTORC1 hyperactivation [42].
Renal cell carcinoma (RCC)
RCC in TSC patients has been recognized for many years [43], but the precise incidence, histologic features, and prognosis have been challenging to define because it is a relatively uncommon manifestation of TSC [43–45]. Interestingly, there are multiple reports of TSC-associated RCC in children and young adults, and among individuals with TSC who develop RCC, multiple bilateral tumours appear to occur at a higher than expected frequency.
Recent advances
In a recent study, 46 renal epithelial neoplasms from 19 individuals were classified into three TSC-associated RCC types. The average age of the patients at first diagnosis was 30 years, and 12 were women. The largest group of tumours was comprised of 24 lesions with complex papillary architecture designated TSC-associated papillary RCC (PRCC). These tumours showed loss of succinate dehydrogenase subunit B (SDHB) staining, suggesting a role of mitochondrial dysfunction in the pathogenesis of these distinctive tumours. The next largest group comprised 15 tumours morphologically characterized as hybrid oncocytoma/chromophobe tumours (HOCT). A second study of 57 RCCs from 18 patients (13 female and 5 male) with a mean age of 42 years showed 3 major morphologic subtypes of RCC: 30% similar to “renal angiomyoadenomatous tumour” or “RCC with smooth muscle stroma”, 59% similar to chromophobe RCC and 11% with granular eosinophilic-macrocystic morphology [44]. Together, these studies conclusively demonstrate that TSC-associated RCC represent distinctive subtypes of RCC. Much more work is needed to understand the morphologic heterogeneity within these tumours and the relationship between morphology and prognosis. It is clear from these two recent reports of multiple TSC-associated RCC that the phenotypic diversity defies straightforward classification systems that are used for sporadic RCC. Ultimately, a genetic classification system may be preferable, as compared with a histopathological classification. At this point, there is very little information about whether TSC-RCC respond to Rapalogs and/or conventional therapies for RCC. The diagnosis of RCC in TSC patients remains extremely challenging because they are uncommon and often difficult to distinguish from fat-poor angiomyolipomas (AML) using conventional imaging techniques. Needle biopsy is often recommended to distinguish RCC from AMLs and avoid unnecessary surgical procedures.
Important insights into the genetic pathogenesis of TSC-associated RCC were obtained using targeted, next generation sequencing (NGS) of 9 RCC from 2 TSC patients (1 male and 1 female). These analyses revealed that the RCCs arise independently due to distinct second hit events leading to biallelic inactivation of TSC2 [46]. Previously, it was not known whether TSC patients with multiple RCC had intra-renal metastasis of a single tumour clone versus multi-focal tumour development. The mechanisms through which an individual TSC patient would be predisposed to develop many RCC (while the overall incidence of RCC in TSC is relatively low) are completely unknown. Additional whole exome analysis of the TSC-RCC in this study did not identify additional “drivers” of tumour progression, similar to the results in the angiomyolipoma study described above, but even more surprising since these RCC are malignant tumours. Mutations were identified in RASA1 and TACC3, but occurred at a low allele frequency. No mutations were identified in canonical pathways associated with RCC including VHL. Interestingly, no mutations in SDHB were identified suggesting that epigenetic downregulation of SDHB may be responsible for the loss of SDHB protein expression in a subset of TSC-associated RCC.
These data further highlight the unique features of TSC-associated RCC including morphologic heterogeneity and the surprising clinical finding that while RCC are uncommon in TSC, some patients develop multiple bilateral RCC. It seems clear that additional environmental, genetic, epigenetic and metabolic factors must cooperate in the pathogenesis of RCC in TSC, which may have therapeutic implications for TSC-RCC and RCC more generally.
Lymphangioleiomyomatosis (LAM)
LAM is the primary pulmonary manifestation of TSC. LAM is characterized by cystic lung destruction, pneumothorax and chylous pleural effusion [47]. Eighty percent of women with TSC have CT-scan evidence of LAM by age 40, and 10–13% of men with TSC have lung cysts [48], although for both men and women the disease is often asymptomatic. The vast majority of symptomatic LAM occurs in women, but there are a few cases of biopsy-documented male LAM [49]. Symptomatic LAM, occurring in ~5–10% of women with TSC, is characterized by shortness of breath, fatigue, chest pain and can lead to respiratory failure [2]. Symptomatic LAM can occur in individuals with a wide range of mutations that inactivate TSC1 or TSC2, with mutations in TSC2 being more common [50, 51]. LAM tends to progress more rapidly in premenopausal women than in postmenopausal women [52] and there have been reports of worsening of symptoms during pregnancy. These clinical reports, in addition to studies showing LAM cells express oestrogen receptor-α and progesterone receptor [53], suggest that the progression and/or development of LAM might be dependent on these female sex hormones.
Multifocal micronodular pneumocyte hyperplasia (MMPH), another pulmonary manifestation of TSC, can occur in both men and women and is usually asymptomatic [50, 54, 55]. MMPH presents with multiple diffuse pulmonary nodules that pathologically show proliferation of alveolar type II cells [56, 57]. The overall incidence of MMPH in tuberous sclerosis is not well defined. The genetic mechanisms of MMPH and the role of somatic inactivation of TSC1/TSC2 are not yet well understood.
TSC-associated LAM is caused by the diffuse, bilateral proliferation of so-called “LAM cells” that carry bi-allelic inactivation of TSC1 or TSC2. LAM can also occur in a sporadic form, in women who do not have TSC. It was previously shown that LAM cells from women with sporadic LAM carry bi-allelic somatic TSC mutations [58], and circulating LAM cells with loss of heterozygosity (LOH) for the region of chromosome 16p13 containing TSC2 have been detected in the blood of majority of women with sporadic LAM [59–61], consistent with the Knudson’s “two-hit” hypothesis [62]; In addition to blood, circulating LAM cells have been isolated from urine, chyle and bronchoalveolar lavage fluid (BALF) of LAM patients [60, 61, 63] and have been documented in the uterus [64]. LAM cells have also been identified in the lungs of some LAM patients after transplantation [65].
Recent Advances
In a study by Cai et. al., two cell surface proteins were identified that could be used to isolate circulating LAM cells, namely, CD44v6 and CD9 [60]. Upon analysis of these isolated cells from blood, chyle, urine or BALF of sporadic LAM patients, it was shown that they have identical TSC2 LOH patterns in the majority of cases, again supporting the hypothesized metastatic nature of LAM cells through the blood and/or lymphatic circulatory systems [66]. In a small group of sporadic LAM patients, LAM cells showed both genetic and phenotypic heterogeneity [60]. In a another recent study [67], careful microdissection of LAM nodules from women with sporadic LAM revealed inactivating TSC2 mutations in 8/10 cases, but surprisingly these mutations were present in only 4–60% of the microdissected cells, which were selected based on smooth muscle actin (SMA) and HMB-45 positivity. This suggests that the LAM nodules are genetically heterogeneous, containing both TSC2-mutant cells and wild-type cells. The origin of these wild-type cells and their role in the pathogenesis of LAM is not yet well understood. Furthermore, the study showed that two of the cases had no detected mutations in either TSC1 or TSC2, which might indicate that an alternative genetic mechanism is contributing to pathogenesis of sporadic LAM. If another mTOR-independent mechanism is involved in a subset of LAM, this might help explain why some sporadic LAM patients have continued lung function decline during treatment with the mTORC1 inhibitor sirolimus/rapamycin, while the majority of patients have a response to rapamycin [52].
Conclusions
The recent clinical progress in TSC has been stunning, with effective therapy (mTORC1 inhibition) now available to decrease the size of SEGAs, angiofibromas and angiomyolipomas, and delay the loss of lung function in LAM. mTORC1 inhibitors result in a cytostatic response, with tumour regrowth upon discontinuation. Therefore, continuous therapy is required. Complete, durable responses, and ultimately prevention and/or cure for TSC-associated tumours, are the next goals. Many critical questions remain unanswered. What is the cell-of-origin for the unusual tumour types in TSC, including LAM and angiomyolipomas? Are genetic second hits present in all TSC tumours, including MMPH and renal cysts? Given that the number of additional mutations in angiomyolipomas appear to be extremely low, yet these tumours can grow to a large size, are there other cooperating events not detected by whole exome analyses, such as epigenetic changes or microRNA alterations, that participate in the initiation and/or progression of individual tumours? Are there germline alterations in modifying genes that influence the number and size of tumours in TSC? Which pathways and mechanisms are common to all tumours and which are organ-specific? Understanding the genetics and pathobiology of tumours in TSC is the key to future therapeutic progress and prevention.
Acknowledgments
Work in the Henske Laboratory is supported by grants from the NHLBI, the NIDDK, the US Department of Defense Tuberous Sclerosis Medical Research Program, the Adler Foundation, and the Engles Program in TSC and LAM Research. HCL is a LAM Foundation Postdoctoral Fellow.
Footnotes
Statement of author contribution. All three authors participated in the design and writing of this review.
Conflict of interest statement: No potential conflicts of interest to disclose
References
- 1.Gomez MR. History of the tuberous sclerosis complex. Brain Dev. 1995;17(Suppl):55–57. doi: 10.1016/0387-7604(94)00130-8. [DOI] [PubMed] [Google Scholar]
- 2.Henske EP, Jozwiak S, Kingswood JC, et al. Tuberous sclerosis complex. Nat Rev Dis Primers. 2016;2:16035. doi: 10.1038/nrdp.2016.35. [DOI] [PubMed] [Google Scholar]
- 3.Henske EP, Neumann HP, Scheithauer BW, et al. Loss of heterozygosity in the tuberous sclerosis (TSC2) region of chromosome band 16p13 occurs in sporadic as well as TSC-associated renal angiomyolipomas. Genes Chromosomes Cancer. 1995;13:295–298. doi: 10.1002/gcc.2870130411. [DOI] [PubMed] [Google Scholar]
- 4.Kwiatkowski DJ, Manning BD. Molecular basis of giant cells in tuberous sclerosis complex. N Engl J Med. 2014;371:778–780. doi: 10.1056/NEJMcibr1406613. [DOI] [PubMed] [Google Scholar]
- 5.Dibble CC, Cantley LC. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015;25:545–555. doi: 10.1016/j.tcb.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19:373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 9.Au KS, Williams AT, Roach ES, et al. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med. 2007;9:88–100. doi: 10.1097/gim.0b013e31803068c7. [DOI] [PubMed] [Google Scholar]
- 10.Dabora SL, Jozwiak S, Franz DN, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet. 2001;68:64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 12.Kwiatkowska J, Jozwiak S, Hall F, et al. Comprehensive mutational analysis of the TSC1 gene: observations on frequency of mutation, associated features, and nonpenetrance. Ann Hum Genet. 1998;62:277–285. doi: 10.1046/j.1469-1809.1998.6240277.x. [DOI] [PubMed] [Google Scholar]
- 13.European Chromosome 16 Tuberous Sclerosis C. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 14.Niida Y, Lawrence-Smith N, Banwell A, et al. Analysis of both TSC1 and TSC2 for germline mutations in 126 unrelated patients with tuberous sclerosis. Hum Mutat. 1999;14:412–422. doi: 10.1002/(SICI)1098-1004(199911)14:5<412::AID-HUMU7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Jones AC, Shyamsundar MM, Thomas MW, et al. Comprehensive mutation analysis of TSC1 and TSC2-and phenotypic correlations in 150 families with tuberous sclerosis. Am J Hum Genet. 1999;64:1305–1315. doi: 10.1086/302381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Au KS, Rodriguez JA, Finch JL, et al. Germ-line mutational analysis of the TSC2 gene in 90 tuberous-sclerosis patients. Am J Hum Genet. 1998;62:286–294. doi: 10.1086/301705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:125–127. doi: 10.1111/j.1749-6632.1991.tb37754.x. [DOI] [PubMed] [Google Scholar]
- 18.Sampson JR, Yates JR, Pirrit LA, et al. Evidence for genetic heterogeneity in tuberous sclerosis. J Med Genet. 1989;26:511–516. doi: 10.1136/jmg.26.8.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verhoef S, Vrtel R, van Essen T, et al. Somatic mosaicism and clinical variation in tuberous sclerosis complex. Lancet. 1995;345:202. doi: 10.1016/s0140-6736(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 20.Sampson JR, Maheshwar MM, Aspinwall R, et al. Renal cystic disease in tuberous sclerosis: role of the polycystic kidney disease 1 gene. Am J Hum Genet. 1997;61:843–851. doi: 10.1086/514888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nellist M, Brouwer RW, Kockx CE, et al. Targeted Next Generation Sequencing reveals previously unidentified TSC1 and TSC2 mutations. BMC Med Genet. 2015;16:10. doi: 10.1186/s12881-015-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyburczy ME, Dies KA, Glass J, et al. Mosaic and Intronic Mutations in TSC1/TSC2 Explain the Majority of TSC Patients with No Mutation Identified by Conventional Testing. PLoS Genet. 2015;11:e1005637. doi: 10.1371/journal.pgen.1005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng JM, Cowen EW, Wataya-Kaneda M, et al. Dermatologic and dental aspects of the 2012 International Tuberous Sclerosis Complex Consensus Statements. JAMA Dermatol. 2014;150:1095–1101. doi: 10.1001/jamadermatol.2014.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb DW, Clarke A, Fryer A, et al. The cutaneous features of tuberous sclerosis: a population study. Br J Dermatol. 1996;135:1–5. [PubMed] [Google Scholar]
- 25.Nickel WR, Reed WB. Tuberous sclerosis. Special reference to the microscopic alterations in the cutaneous hamartomas. Arch Dermatol. 1962;85:209–226. doi: 10.1001/archderm.1962.01590020049006. [DOI] [PubMed] [Google Scholar]
- 26.Reed RJ, Ackerman AB. Pathology of the adventitial dermis. Anatomic observations and biologic speculations. Hum Pathol. 1973;4:207–217. doi: 10.1016/s0046-8177(73)80008-5. [DOI] [PubMed] [Google Scholar]
- 27.Tyburczy ME, Wang JA, Li S, et al. Sun exposure causes somatic second-hit mutations and angiofibroma development in tuberous sclerosis complex. Hum Mol Genet. 2014;23:2023–2029. doi: 10.1093/hmg/ddt597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Takeuchi F, Wang JA, et al. Mesenchymal-epithelial interactions involving epiregulin in tuberous sclerosis complex hamartomas. Proc Natl Acad Sci U S A. 2008;105:3539–3544. doi: 10.1073/pnas.0712397105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S, Thangapazham RL, Wang JA, et al. Human TSC2-null fibroblast-like cells induce hair follicle neogenesis and hamartoma morphogenesis. Nat Commun. 2011;2:235. doi: 10.1038/ncomms1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarnat HB, Flores-Sarnat L. Embryology of the neural crest: its inductive role in the neurocutaneous syndromes. J Child Neurol. 2005;20:637–643. doi: 10.1177/08830738050200080101. [DOI] [PubMed] [Google Scholar]
- 31.Ikehata H, Ono T. The mechanisms of UV mutagenesis. J Radiat Res. 2011;52:115–125. doi: 10.1269/jrr.10175. [DOI] [PubMed] [Google Scholar]
- 32.Jozwiak S, Schwartz RA, Janniger CK, et al. Usefulness of diagnostic criteria of tuberous sclerosis complex in pediatric patients. J Child Neurol. 2000;15:652–659. doi: 10.1177/088307380001501003. [DOI] [PubMed] [Google Scholar]
- 33.Ewalt DH, Sheffield E, Sparagana SP, et al. Renal lesion growth in children with tuberous sclerosis complex. J Urol. 1998;160:141–145. [PubMed] [Google Scholar]
- 34.Bernstein J, Robbins TO. Renal involvement in tuberous sclerosis. Ann N Y Acad Sci. 1991;615:36–49. doi: 10.1111/j.1749-6632.1991.tb37746.x. [DOI] [PubMed] [Google Scholar]
- 35.Dixon BP, Hulbert JC, Bissler JJ. Tuberous sclerosis complex renal disease. Nephron Exp Nephrol. 2011;118:e15–20. doi: 10.1159/000320891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henske EP, Wessner LL, Golden J, et al. Loss of tuberin in both subependymal giant cell astrocytomas and angiomyolipomas supports a two-hit model for the pathogenesis of tuberous sclerosis tumors. Am J Pathol. 1997;151:1639–1647. [PMC free article] [PubMed] [Google Scholar]
- 37.Robb VA, Karbowniczek M, Klein-Szanto AJ, et al. Activation of the mTOR signaling pathway in renal clear cell carcinoma. J Urol. 2007;177:346–352. doi: 10.1016/j.juro.2006.08.076. [DOI] [PubMed] [Google Scholar]
- 38.Karbowniczek M, Yu J, Henske EP. Renal angiomyolipomas from patients with sporadic lymphangiomyomatosis contain both neoplastic and non-neoplastic vascular structures. Am J Pathol. 2003;162:491–500. doi: 10.1016/S0002-9440(10)63843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giannikou K, Malinowska IA, Pugh TJ, et al. Whole Exome Sequencing Identifies TSC1/TSC2 Biallelic Loss as the Primary and Sufficient Driver Event for Renal Angiomyolipoma Development. PLoS Genet. 2016;12:e1006242. doi: 10.1371/journal.pgen.1006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siroky BJ, Yin H, Dixon BP, et al. Evidence for pericyte origin of TSC-associated renal angiomyolipomas and implications for angiotensin receptor inhibition therapy. Am J Physiol Renal Physiol. 2014;307:F560–570. doi: 10.1152/ajprenal.00569.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel B, Patel J, Cho JH, et al. Exosomes mediate the acquisition of the disease phenotypes by cells with normal genome in tuberous sclerosis complex. Oncogene. 2016;35:3027–3036. doi: 10.1038/onc.2015.358. [DOI] [PubMed] [Google Scholar]
- 42.Yue M, Pacheco G, Cheng T, et al. Evidence Supporting a Lymphatic Endothelium Origin for Angiomyolipoma, a TSC2(−) Tumor Related to Lymphangioleiomyomatosis. Am J Pathol. 2016;186:1825–1836. doi: 10.1016/j.ajpath.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bjornsson J, Short MP, Kwiatkowski DJ, et al. Tuberous sclerosis-associated renal cell carcinoma. Clinical, pathological, and genetic features. Am J Pathol. 1996;149:1201–1208. [PMC free article] [PubMed] [Google Scholar]
- 44.Guo J, Tretiakova MS, Troxell ML, et al. Tuberous sclerosis-associated renal cell carcinoma: a clinicopathologic study of 57 separate carcinomas in 18 patients. Am J Surg Pathol. 2014;38:1457–1467. doi: 10.1097/PAS.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 45.De Waele L, Lagae L, Mekahli D. Tuberous sclerosis complex: the past and the future. Pediatr Nephrol. 2015;30:1771–1780. doi: 10.1007/s00467-014-3027-9. [DOI] [PubMed] [Google Scholar]
- 46.Tyburczy ME, Jozwiak S, Malinowska IA, et al. A shower of second hit events as the cause of multifocal renal cell carcinoma in tuberous sclerosis complex. Hum Mol Genet. 2015;24:1836–1842. doi: 10.1093/hmg/ddu597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henske EP, McCormack FX. Lymphangioleiomyomatosis - a wolf in sheep’s clothing. J Clin Invest. 2012;122:3807–3816. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adriaensen ME, Schaefer-Prokop CM, Duyndam DA, et al. Radiological evidence of lymphangioleiomyomatosis in female and male patients with tuberous sclerosis complex. Clin Radiol. 2011;66:625–628. doi: 10.1016/j.crad.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 49.Aubry MC, Myers JL, Ryu JH, et al. Pulmonary lymphangioleiomyomatosis in a man. Am J Respir Crit Care Med. 2000;162:749–752. doi: 10.1164/ajrccm.162.2.9911006. [DOI] [PubMed] [Google Scholar]
- 50.Franz DN, Brody A, Meyer C, et al. Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis. Am J Respir Crit Care Med. 2001;164:661–668. doi: 10.1164/ajrccm.164.4.2011025. [DOI] [PubMed] [Google Scholar]
- 51.Strizheva GD, Carsillo T, Kruger WD, et al. The spectrum of mutations in TSC1 and TSC2 in women with tuberous sclerosis and lymphangiomyomatosis. Am J Respir Crit Care Med. 2001;163:253–258. doi: 10.1164/ajrccm.163.1.2005004. [DOI] [PubMed] [Google Scholar]
- 52.McCormack FX, Inoue Y, Moss J, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Logginidou H, Ao X, Russo I, et al. Frequent estrogen and progesterone receptor immunoreactivity in renal angiomyolipomas from women with pulmonary lymphangioleiomyomatosis. Chest. 2000;117:25–30. doi: 10.1378/chest.117.1.25. [DOI] [PubMed] [Google Scholar]
- 54.Hayashi T, Kumasaka T, Mitani K, et al. Loss of heterozygosity on tuberous sclerosis complex genes in multifocal micronodular pneumocyte hyperplasia. Mod Pathol. 2010;23:1251–1260. doi: 10.1038/modpathol.2010.114. [DOI] [PubMed] [Google Scholar]
- 55.von Ranke FM, Zanetti G, JLES, et al. Tuberous Sclerosis Complex: State-of-the-Art Review with a Focus on Pulmonary Involvement. Lung. 2015;193:619–627. doi: 10.1007/s00408-015-9750-6. [DOI] [PubMed] [Google Scholar]
- 56.Muir TE, Leslie KO, Popper H, et al. Micronodular pneumocyte hyperplasia. Am J Surg Pathol. 1998;22:465–472. doi: 10.1097/00000478-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Lantuejoul S, Ferretti G, Negoescu A, et al. Multifocal alveolar hyperplasia associated with lymphangioleiomyomatosis in tuberous sclerosis. Histopathology. 1997;30:570–575. doi: 10.1046/j.1365-2559.1997.4600811.x. [DOI] [PubMed] [Google Scholar]
- 58.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai X, Pacheco-Rodriguez G, Haughey M, et al. Sirolimus decreases circulating lymphangioleiomyomatosis cells in patients with lymphangioleiomyomatosis. Chest. 2014;145:108–112. doi: 10.1378/chest.13-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cai X, Pacheco-Rodriguez G, Fan QY, et al. Phenotypic characterization of disseminated cells with TSC2 loss of heterozygosity in patients with lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2010;182:1410–1418. doi: 10.1164/rccm.201003-0489OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crooks DM, Pacheco-Rodriguez G, DeCastro RM, et al. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2004;101:17462–17467. doi: 10.1073/pnas.0407971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knudson AG. Hereditary cancer: two hits revisited. J Cancer Res Clin Oncol. 1996;122:135–140. doi: 10.1007/BF01366952. [DOI] [PubMed] [Google Scholar]
- 63.Kumasaka T, Seyama K, Mitani K, et al. Lymphangiogenesis-mediated shedding of LAM cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am J Surg Pathol. 2005;29:1356–1366. doi: 10.1097/01.pas.0000172192.25295.45. [DOI] [PubMed] [Google Scholar]
- 64.Tobino K, Hirai T, Johkoh T, et al. Differentiation between Birt-Hogg-Dube syndrome and lymphangioleiomyomatosis: quantitative analysis of pulmonary cysts on computed tomography of the chest in 66 females. Eur J Radiol. 2012;81:1340–1346. doi: 10.1016/j.ejrad.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 65.Karbowniczek M, Astrinidis A, Balsara BR, et al. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med. 2003;167:976–982. doi: 10.1164/rccm.200208-969OC. [DOI] [PubMed] [Google Scholar]
- 66.Henske EP. Metastasis of benign tumor cells in tuberous sclerosis complex. Genes Chromosomes Cancer. 2003;38:376–381. doi: 10.1002/gcc.10252. [DOI] [PubMed] [Google Scholar]
- 67.Badri KR, Gao L, Hyjek E, et al. Exonic mutations of TSC2/TSC1 are common but not seen in all sporadic pulmonary lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2013;187:663–665. doi: 10.1164/ajrccm.187.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]