Abstract
Alpha-synuclein is a small, highly charged protein encoded by the synuclein or SNCA gene that is predominantly expressed in central nervous system neurons. Although its physiological function remains enigmatic, alpha-synuclein is implicated in movement disorders such as Parkinson’s disease, multiple system atrophy, and in neurodegenerative diseases such as Dementia with Lewy bodies. Here we have focused on reviewing the existing literature pertaining to wild-type alpha-synuclein structure, its properties, and its potential involvement in regulation of dopamine neurotransmission.
Introduction
Alpha-synuclein is a small, highly charged protein encoded by synuclein or SNCA gene that is predominantly expressed in central nervous system (CNS) neurons [3, 4]. The function of alpha-synuclein under both physiological and pathological conditions remains elusive. Alpha-synuclein is implicated in movement disorders such as Parkinson’s disease (PD), multiple system atrophy (MSA), and in neurodegenerative diseases such as Dementia with Lewy bodies (DLB) [7]. A hallmark of PD is a significant loss of dopaminergic neurons in the substantia nigra pars compacta, with some of the remaining neurons containing alpha-synuclein positive inclusions known as Lewy Bodies. Missense alpha-synuclein point mutations, as well as duplication and triplication of the synuclein gene, have been implicated in familial PD resulting in rapid and early onset of classical symptoms [10, 11]. Postmortem human studies show increased levels of alpha-synuclein in ventral tegmental area (VTA) and substantia nigra dopaminergic neurons of cocaine and methamphetamine addicts that have been linked to early onset of Parkinson’s-like symptoms [3, 4, 10, 14]. Since its discovery, elucidations of physiological and pathological function of alpha-synuclein have been researched by a large number of laboratories. This review examines the existing literature pertaining to wild-type alpha-synuclein structure, its properties, its potential physiological and pathological functions, its interactions with other proteins such as dopamine transporter (DAT), and its role in modulating dopamine neurotransmission.
Structure
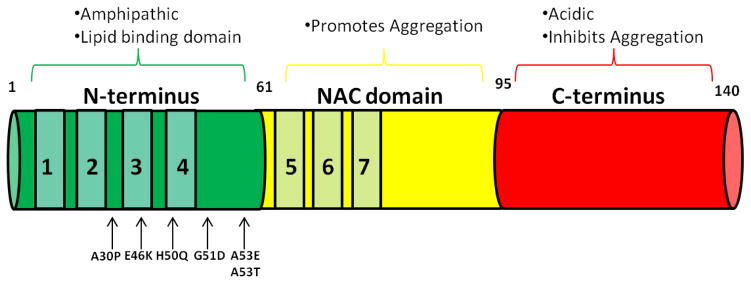
Alpha-synuclein is a small 140 amino acid protein and a member of the synuclein family, which also includes beta and gamma-synuclein [19]. Alpha-synuclein is localized mainly at presynaptic terminals [19, 20] and has been shown to comprise up to ~1% of total proteins in the neuronal cytosol [21]. Alpha-synuclein consists of the amphipathic N-terminus involved in mediating lipid binding properties of the protein, the non-amyloid component (NAC) identified as the aggregation domain, and the acidic C-terminus, which is affiliated with calcium binding and inhibition of protein aggregation [19, 20] (Figure 1).
Figure 1.
Schematic representation of the wild-type full-length alpha-synuclein protein structure. N-terminal missense point mutations are found in the amphipathic and lipid-binding portion of the protein along with 7, 11mer repeats found throughout the N-terminus and Non-Amyloid Component (NAC) domain. The NAC region promotes aggregation while the acidic C-terminus inhibits aggregation of alpha-synuclein.
Despite its role in a number of pathological conditions coined synucleinopathies, which include Parkinson’s disease and Dementia with Lewy Bodies and Multiple Systems Atrophy [4, 20, 22–24], the exact physiological function/s of alpha-synuclein is less understood. Recent reports suggest alpha-synuclein might be involved in regulation of normal cellular function via interaction with a number of binding proteins listed in Table 1, as well as neurotransmitter release, vesicular trafficking and oxidative stress [12, 25–29].
Table 1.
Partner proteins of wild-type alpha-synuclein
| Partner Protein | Overall Finding | Reference |
|---|---|---|
| 14-3-3 | Structural and functional homology to alpha-synuclein and has similar binding partners | [1] |
| Polo-like Kinase 2 (PLK2) | Mediates Ser129 phosphorylation on alpha-synuclein | [2] |
| PKC | Activity inhibited by elevated levels of alpha-synuclein | [1] |
| ERK | Activity inhibited by elevated levels of alpha-synuclein | [5] |
| Phospholipase D2 (PLD2) | Decreased activity by Alpha-synuclein | [4, 6] |
| PKA | Stimulates activity resulting in phosphorylation of tau mediated by alpha-synuclein | [8] |
| Synphilin-1 | May act as an adaptor protein for anchoring of alpha-synuclein to other proteins | [9] |
| Poly Unsaturated Fatty Acids (PUFA) | Upon binding to alpha-synuclein, exhibits significant reduction in membrane fluidity | [12] |
| BDNF | Decrease in expression due to inhibition of required transcription factors mediated by alpha-synuclein | [13] |
| Synaptobrevin | Binding to SNARE complexes inhibited by alpha-synuclein overexpression | [15] |
| Focal Adhesion kinase pp125 | Phosphorylates Tyr136 on alpha-synuclein to improve behavioral performance | [16] |
| Dopamine transporter (DAT) | Increased DAT-mediated inward current leading to membrane depolarization, increased dopamine efflux, and decreased dopamine uptake | [17, 18] |
Alpha-synuclein is implicated in both sporadic and familial forms of PD. In addition to duplication and triplication of the gene that encodes alpha-synuclein (SNCA) [11, 30], N-terminal missense point mutations of alpha-synuclein, namely A53E, A53T, A30P, E46K, H50Q, and G51D, have shown a strong correlation with the autosomal dominant form of PD [31–34]. Alpha-synuclein undergoes a number of post-translational modifications including nitration [35], ubiquitination [36] and C-terminal truncation [37]. Alpha-synuclein inclusions are generally hyperphosphorylated. While Ser129 is the most commonly studied alpha-synuclein phosphorylation site [38, 39], Ser87 [40] and Tyr125 [41] are additional sites that can undergo phosphorylation and have been identified in Lewy bodies.
Animal Models of Wild-type Alpha-synuclein Knockout
To examine the physiological function of alpha-synuclein and its role in synuclein-dependent pathologies multiple lines of alpha-synuclein knockout and alpha-synuclein overexpressing animal models have been generated. In 2000, Abeliovich et al. generated an alpha-synuclein knockout mouse (alpha-Syn−/−) via deletion of the first two exons, which encode amino acids 1–41 and upstream untranslated sequences [25]. These animals were shown to be viable, fertile and possess the capabilities to undergo standard uptake and release of dopamine (DA) in response to electrical stimulus. However, reminiscent to DAT KO animals [42–46], alpha-Syn −/− mice exhibited a reduction in striatal dopamine content and inhibition of dopamine-dependent locomotor response to amphetamine, a psychostimulant known to increase locomotion via a DAT-dependent mechanism [47, 48]. These findings strongly support the hypothesis that alpha-synuclein is involved in regulation of dopamine neurotransmission [17, 18, 49, 50]. Alpha-synuclein regulation of dopaminergic neurons was further studied by Garcia-Reitboeck et al. in mice carrying a spontaneous deletion of the alpha-synuclein locus [51]. They found that deletion of the alpha-synuclein gene resulted in a decrease in the number of dopaminergic neurons in the substantia nigra further supporting the idea that physiological levels of alpha-synuclein may play a role in growth and development of neurons within the brain [51].
In contrast to animal models with deletion of alpha-synuclein, mice with triple synuclein knockout (TKO) (alpha, beta and gamma synuclein) generated by Burre et al. demonstrate age-dependent neurological impairments and decreased SNARE assembly, a cellular processes required for vesicular-mediated neurotransmitter release [15]. Knockdown of alpha, beta and gamma synucleins decreased the life expectancy of these animals [15]. Although initially there were no gross phenotypic differences in TKO alpha-synuclein mice as compared to wild type, aged TKO mice exhibited severe neurological impairments, and 50% of mice died at 500 days [15]. Disruption of SNARE assembly in cultured neurons from the TKO mice was rescued by replacement of exogenous alpha-synuclein [15]. These studies suggest beta and/or gamma synucleins may compensate for some of the physiological properties of alpha-synuclein.
A number of behavioral paradigms including the Morris water maze, rod suspension and open field tests have been used to determine whether alpha-synuclein deletion affects behavioral responses such as locomotion, impulsivity and cognition. For example, Kokhan et al. found complete deletion of the SNCA gene reduces learning working and spatial memory [52]. Pena-Oliver et al. reported a decrease in impulsivity as measured by the 5-choice serial reaction time task in C57BL/6JOIaHsd animals void of SNCA gene [53–55].
Animal Models of Wild-type Alpha-synuclein Overexpression
Developing animal models to recapitulate the pathophysiology of synucleinopathy and characteristic phenotypes of PD-like symptoms has been challenging. To date, more than a dozen alpha-synuclein overexpressing animal models (Table 2) as well as animal models containing alpha-synuclein point mutations have been generated to study synucleinopathy. Over the past two decades, generally two models of alpha-synuclein overexpression have been used: viral-mediated alpha-synuclein overexpression and transgenic animal models. Here, we focus only on transgenic animal models of full-length wild-type alpha-synuclein overexpression. In 2000, Masliah et al. generated a human alpha-synuclein overexpressing model driven by the PDGF-β promoter [56]. At 12 months, these mice had a decrease in tyrosine hydroxylase (TH) activity and levels within the striatum, decrease in TH positive fibers, and exhibited a significant decrease in dopamine levels compared to wild-type controls. Comparable disruptions in dopamine and TH levels and activity were observed in a GFP-tagged alpha-synuclein model generated by Hansen et al. [57]. These results strongly suggest that increased levels of alpha-synuclein result in a direct insult to the dopaminergic system. Additional alpha-synuclein mouse models were generated that displayed similar deficit in the dopaminergic system, but with some variability in the location of the pathology and alterations in phenotype. For example, neither model generated by Kahle et al. nor Matsuoka et al., driven by the mu-Thy-1 and rat TH promoter respectively, showed signs of motor deficits associated with PD [58, 59]. These findings triggered speculation that alpha-synuclein pathology alone might not be sufficient to cause full scale PD pathology. A comparative study by Rockenstein et al. [60] revealed significant differences between animal models driven by the PDGF-β promoter compared to that of the mu Thy-1 promoter. Among the differences, Rockenstein et al. found that alpha-synuclein overexpression driven by PDGF-β was moderate and was found in fewer brain regions as compared to alpha-synuclein expression driven by the mu Thy-1 promoter. This information further highlights the role of promoter specificity in generating transgenic animal models and cloning strategies in general.
Table 2.
Alpha-synuclein overexpressing mouse models (mutant models and viral-mediated alterations in alpha-synuclein overexpression are not included)
| Promoter | DA system disturbances | Brain Region expression | Phenotype | Reference |
|---|---|---|---|---|
| PDGF-β | Decrease in TH activity, levels and fibers; decrease in DA levels. | Neocortex, olfactory bulb and midbrain and glial expression | Motor deficits displayed at 12 months | [42] |
| Mu Thy-1 | N/A | Substantia nigra, striatum, hippocampus, cortex and brainstem | N/A | [44] |
| Rat TH | N/A | Striatum, cerebellum, and olfactory bulb | N/A | [125] |
| Mouse prion | N/A | Spinal cord, brainstem, cerebellum, thalamus, and striatum | Middle age onset, hunched back freezing paralysis | [126] |
| Rat TH | Decrease in DAT density and decrease in DA and metabolite levels | Substantia nigra, locus ceruleus, ventral tegmental area, and striatum | Middle age onset. Decrease in locomotor activity and coordination | [127] |
| Proteolipid | N/A | Cerebellum and mature oligodendricytes | N/A | [128] |
| mu Thy-1 | N/A | Basal ganglia, thalamus and substantia nigra | N/A | [46] |
| Mouse prion | N/A | Olfactory bulb, cortex, striatum, forebrain, hippocampus, midbrain, spinal cord and cerebellum | Decrease in stride length, grip strength, vertical activity and rotarod | [129] |
| Thy-1 | Elevated striatal DA levels at 6 months; at 14 months, decreased TH expression, DA levels in the striatum and abnormal DA modulation of spontaneous EPSCs | Nigrostriatal pathway | Sensory motor deficits at 14 months | [49] |
| Spontaneous autosomal recessive model | Decrease of DA levels in the striatum. Decrease in TH levels in the striatum | Substantia nigra, brainstem, and striatum | 15 days postnatal, rats exhibit tremor, rigidity, spasticity and postural instability | [50] |
| Mouse alpha-synuclein promoter | Reduction in striatal DA release | Substantia nigra, ventral tegmental area, olfactory bulb and neocortex | Reduction in AMPH locomotor response, abulatory movement at7 months and latency to fall at 12 months | [43] |
| Bacterial artificial chromosome. | Early stage decrease in DA release followed by severe loss of dopaminergic neurons at later stages | Caudate putamen, nucleus accumbens, and ventral tegmental area | Reduced latency to fall at 18 months with reduced forepaw stride and poor performance on rotarod | [48] |
Disruptions in dopaminergic signaling have been documented in at least two recent animal models of alpha-synuclein overexpression. In a bacterial artificial chromosome (BAC) alpha-synuclein overexpressing mouse model generated by the Janezic‘s group, early stage decrease in dopamine release was measured via in vivo recording. The measured early stage attenuation of dopamine release in this animal model is attributed to decreased vesicle clustering that precedes the changes in the firing activity of dopamine neurons [61]. Importantly, these changes were only present in the nigrostriatal dopaminergic neurons [61]. Lam et al. generated an overexpressing alpha-synuclein mouse model driven by the Thy1 promoter [50]. These animals exhibit overexpression of alpha-synuclein in the brain regions implicated in PD. At 6 months old, these animals exhibited elevated dopamine levels in striatal tissue and increased open field activity but exhibited no change in dopamine uptake or amphetamine-induced dopamine efflux. However, at 14 months old these animals showed a profound decrease in dopamine and TH levels, indicative of age-dependent degeneration of dopaminergic neurons associated with pathological levels of alpha-synuclein. These findings suggest, prior to substantial neuronal loss and severe motor deficits, there are early, seemingly subtle, disruptions in dopamine neurotransmission. Identifying early stage pathologies and the cellular adaptations following alpha-synuclein elevation before neuronal demise may be the key for early diagnoses and development of effective therapies for the treatment of PD.
Similar observations have been made in the rat model of alpha-synuclein overexpression. Stoica et al. showed a decrease in dopamine levels in the striatum and accumulation of alpha-synuclein in multiple brain regions using a spontaneously inherited autosomal recessive rat model (Table 2) [62]. Importantly, none of the existing transgenic animal models of alpha-synuclein overexpression recapitulates all of the pathophysiological conditions associated with PD. This makes it increasingly difficult to pinpoint the specific cellular machinery within the dopamine system that is disrupted by alpha-synuclein overexpression. Over the last decade investigators have examined some of the prototypical cellular and molecular targets involved in neurodegeneration to understand how alpha-synuclein overexpression influences and disrupts neuronal activity before cell death. A few of these cellular responses are reviewed below.
Ca2+ Signaling
Ca2+ regulates multiple signaling mechanisms, neuronal health, and neurotoxicity. Dysregulation of intracellular Ca2+ signaling increases the susceptibility of the neuron to cell death [63–66]. Increased neuronal alpha-synuclein species, including oligomeric and aggregate forms of alpha-synuclein, have been proposed to elicit toxic effects on dopamine neurons and mammalian cell systems via disruption of Ca2+ homeostasis at rest and following electrical or pharmacological stimulation [67–69]. Although the exact role of pathological levels of alpha-synuclein on Ca2+ signaling remains unknown, a growing number of studies have explored the effects of alpha-synuclein on neuronal Ca2+ homeostasis. For example, Adamczyk et al. showed that application of 10 μM of wild-type alpha-synuclein or the NAC domain of alpha-synuclein alone to rat synaptoneurosome preparations induced Ca2+ influx via N-type voltage-dependent Ca2+ channels [68]. Beta-synuclein, which lacks a NAC domain [70], had no effect on Ca2+ influx in this experimental paradigm. These results suggest while the NAC region is mainly associated with protein aggregation, it may also mediate Ca2+ influx. A recent study by Ronzitti et al. suggests that elevation of extracellular monomeric form of alpha-synuclein mediates Ca2+ increase via the Cav2.2 N-type Ca2+ channel in cortical neurons [71], confirming Adamczyk’s report [68]. The alpha-synuclein regulation of Ca2+ channels in striatal slices has been shown to stimulate dopamine release, potentially via both classical neurotransmission mechanism and efflux of dopamine via DAT [18, 71]. Ronzitti et al. postulated that dysregulation of lipid rafts by alpha-synuclein overexpression causes a shift in Cav2.2 channels from cholesterol rich to cholesterol poor membrane microdomains thus altering Cav2.2 function as well as dopamine release. Although the report by Hettiarachchi et al. is not consistent with the above studies, it supports the general idea that abnormal alpha-synuclein levels increase intracellular Ca2+ levels in human neuroblastoma SH-SY5Y overexpressing wild-type alpha-synuclein [69] without any effect on the basal Ca2+ permeability. While only the Ronzitti’s group experimentally identified the monomeric form of alpha-synuclein in their experiments, the studies by Adamyzk et al. and Hettiarachchi et al. did not identify the form of alpha-synuclein involved in increased neuronal Ca2+ influx. An elegant study by Danzer et al. demonstrated alpha-synuclein oligomers, but not monomers, increase the magnitude and duration of Ca2+ mobilization in SH-SY5Y cells and cortical neurons in response to high concentration (6 μM) of ionomycin [67]. Although these studies provide important insights about alpha-synuclein regulation of neuronal Ca2+ homeostasis, several caveats should be noted. First, the existing methodologies for the identification of alpha-synuclein oligomers have yet to address this highly contentious issue; secondly, unpredictable quantities of oligomers penetrate into the cells; and thirdly, the methods for the detection of the oligomers vs. monomers in these studies are derivative at best.
Recent work investigated the role of elevated alpha-synuclein on Ca2+ dynamics in vivo. Reznichenko et al. used two-photon microscopy to examine the spontaneous and stimulus-induced neuronal activity in the barrel cortex of transgenic mice expressing wild-type human alpha-synuclein (see Table 2), alpha-synuclein knockout mice, and wild-type controls [72]. This study revealed that following repetitive electrical stimulation (train of 3 stimuli at 3 Hz) alpha-synuclein overexpression altered both spontaneous and stimulus evoked Ca2+ responses, increased the spontaneous neuronal firing and Ca2+ entry per spike, and disrupted the buffering capacity of intracellular Ca2+ stores by increasing the decay time. Since ATP-dependent pumps and exchangers maintain the steep ionic gradient for Na+, Cl−, K+, and Ca2+ across the plasma membrane, the disruption of neuronal Ca2+ homeostasis can lead to disruption of energy expenditure and therefore neuronal vulnerability, action potential dependent (classical neurotransmission) and action potential independent neurotransmitter release.
Neurotransmitter Release
Numerous studies have demonstrated that alpha-synuclein is localized at synaptic terminals; therefore, investigating the role of alpha-synuclein on synaptic transmission has provided critical information about the role of alpha-synuclein on cellular homeostasis prior to neurodegeneration.
Neurotransmitter release is a highly modulated event that is dependent on the function of SNARE protein complex assembly [73, 74]. Following early studies identifying the potential role of alpha-synuclein in synaptic neurotransmission, Burre et al. demonstrated that alpha-synuclein modulates neurotransmitter release via direct interaction with synaptobrevin-2, a major component of SNARE complex assembly [15]. The observation in triple synuclein knockout mice with a significant decrease in SNARE complex assembly further supports these findings [15]. Studies by Cooper et al. suggest alpha-synuclein may regulate ER-to-golgi trafficking of vesicles via a Rab1 dependent mechanism [26]. A follow up study by Gitler et al. also demonstrated that increased levels of alpha-synuclein decrease ER-to-golgi vesicular trafficking [75]. Abeliovich et al. have shown alpha-synuclein KO mice exhibited exaggerated levels of dopamine release following stimulation, suggesting a modulatory role for alpha-synuclein in dopamine neurotransmission [25]. In another study, Senior et al. reported alpha and gamma-synuclein null mice exhibited an increase in neurotransmitter release by increasing the release probability of synaptic vesicles [76]. In cultured hippocampal neurons, Murphy et al. have shown suppressed expression of alpha-synuclein in distal pools of synaptic vesicles [77]. A subsequent follow-up study did not reproduce these findings [78]. A later study by Larsen et al. found overexpression of alpha-synuclein reduced evoked release of neurotransmitters via inhibition of vesicular priming following the docking of vesicles to the membrane; while Mosharov et al. have shown alpha-synuclein overexpression increases cytosolic catecholamine concentration [79, 80]. Utilizing adeno-associated virus-type vector to overexpress alpha-synuclein in the substantia nigra of rats, Gaugler et al. showed an age-dependent decrease in dopamine release [81]. These findings suggest that the decrease in neurotransmitter release might be due to a decrease in dopamine containing vesicles and/or synaptic contact of these vesicles [81].
Surprisingly, few studies exist that have directly examined dopamine releases following alpha-synuclein overexpression. In vivo amperometry studies by Lunblad et al. revealed a decrease in dopamine release following KCl pulses and progressive axonal damage in 3-week-old triple synuclein knockout mice that amplified with age. These animals developed classical motor deficits seen in Parkinson’s disease [82]. Although these studies are highly significant, they cannot isolate the action potential-dependent vs. action potential-independent dopamine release as they were performed in the absence of dopamine transporter blockade, known to mediate dopamine release via a reverse transport mechanism [48]. In addition to regulating dopamine neurotransmission, alpha-synuclein has also been implicated in glutamatergic neurotransmission. Diogenes et al. has found treating hippocampal slices with the oligomeric form of alpha-synuclein affects glutamatergic synaptic transmission in the CA1 region of hippocampus decreasing long-term potentiation [83].
Alpha-synuclein Regulation of Dopamine Synthesis, Storage, Clearance and Efflux
Alpha-synuclein Modulation of Tyrosine Hydroxylase
The influence of alpha-synuclein on the disruption of the dopamine system has been extensively studied. The clinical efficacy of levodopa, a dopamine precursor, suggests that one of the core symptoms of PD is related to alteration in dopamine neurotransmission. Although the literature supports the idea that decreases in the number of tyrosine hydroxylase (TH) positive neurons in the substantia nigra is associated with PD [84], it is important to note that a large number of reports have shown dysregulation of TH, DAT, and vesicular monoamine transporter function/levels/activity occurs prior to neuronal death. Increased wild-type alpha-synuclein level affects dopamine neurotransmission at multiple levels: synthesis, storage, recycling, reuptake and efflux of dopamine. Increased alpha-synuclein levels have been shown to decrease dopamine synthesis by decreasing the synthesis and activity of the rate-limiting enzyme TH via a direct interaction in dopamine neurons or cells expressing both proteins [85]. In addition, in MN9D cells alpha-synuclein overexpression has been shown to reduce TH activity by stabilizing TH in its inactive unphosphorylated form [86]. Alpha-synuclein decreases TH phosphorylation at Ser40 by inhibiting protein phosphatase 2A [86]. In addition, human and primate studies support an inverse relationship between increased levels of alpha-synuclein and TH expression [87], which further supports the idea that alpha-synuclein overexpression may affect dopamine production in the dopamine neuron.
Alpha-synuclein Modulation of VMAT2
Following its synthesis, dopamine is stored in the synaptic vesicles via vesicular monoamine transporter 2 (VMAT2), a function that is thought to reduce the harmful oxidative properties of dopamine metabolites [88]. The disruption of dopamine sequestration into the synaptic vesicles is thought to be one of the early events in degeneration of dopamine neurons [89–92]. Disruption in the expression or activity of VMAT2 has shown to increase oxidative stress [49]. Alpha-synuclein overexpression has been linked to modulation of VMAT2 levels and activity [93]. Supporting this idea, Ulusoy et al., found a decrease in vesicular dopamine storage in the nigral neurons followed by increased degeneration of dopamine neurons [94]. Consistently, knockdown of alpha-synuclein increased the amount of VMAT2 while overexpression of alpha-synuclein attenuated VMAT2 activity [94]. The idea that oxidation of un-sequestered cytosolic dopamine and its metabolites increase degeneration of dopaminergic neurons [95, 96] has led to the hypothesis that enhancing dopamine sequestration by VMAT2 may decrease alpha-synuclein toxicity. While this concept may hold true in the animal models of PD or in vitro models of synucleinopathy, more than half a century of clinical data have shown levodopa, which increases cytosolic and vesicular dopamine levels in patients with PD, does not decrease or accelerate the progression of the disease [97–100]. Therefore, alpha-synuclein mediated increase in cytosolic concentrations of dopamine alone may not be a significant source of alpha-synuclein-mediated neurotoxicity.
Alpha-synuclein modulation of Dopamine Transporter (DAT) Trafficking
Changes in constitutive DAT trafficking have been shown to regulate dopamine neurotransmission in the brain. A limited number of studies have explored whether increased levels of alpha-synuclein affect constitutive or regulated DAT trafficking. DAT trafficking has not been studied in alpha-synuclein knockout animals. The existing information about alpha-synuclein regulation of DAT trafficking is contradictory. For example, while Lee et al. demonstrated an increase in surface DAT levels following overexpression of alpha-synuclein in Ltk− cells [101], Wersinger et al. showed a decrease in cell surface distribution of DAT when alpha-synuclein was overexpressed [102]. Similar findings were recapitulated by Oaks et al. in HEK293 and SK-N-MC cells showing sequestration of DAT in the ER-golgi pathway following alpha-synuclein overexpression [103]. In a follow up study, Wersingler et al. claimed trypsin rescues cell surface distribution of DAT in cells overexpressing wild-type alpha-synuclein [104]. Contrary to these studies, Swant et al. reported no change in membrane distribution DAT following alpha-synuclein overexpressing [17]. These conflicting results could be due to a number of reasons including but not limited to 1) the amount of alpha-synuclein in the cell, 2) alpha-synuclein conformation and 3) the model system used.
Alpha-synuclein modulation of Dopamine Clearance
Neurotransmitter uptake by transporter proteins is a major mechanism for terminating synaptic transmission. High affinity transport systems have been identified for the neurotransmitters dopamine, norepinephrine, serotonin, GABA, glutamate, and glycine [105–107]. DAT is a presynaptic membrane protein critical to dopamine homeostasis, as evidenced by transporter knockout studies, where long-term decrease in dopamine uptake (dopamine recycling) leads to hypo-dopamine state [42, 43, 108–110]. Similar to alpha-synuclein, DAT is implicated in neurological disorders such as Parkinson’s disease [111, 112], schizophrenia [113, 114], psychostimulant abuse [115–118], and attention deficit hyperactivity (ADHD) [119–121]. The importance of DAT activity on synaptic dopamine levels was revealed in DAT-KO mice [42, 43, 118]. The DAT KO animals exhibit a profound increases in extracellular dopamine and significant depletion of intra-neuronal concentrations of dopamine [43, 118, 122]. Therefore, long-term decrease in DAT activity can lead to lower dopamine content, where the rate of synthesis determines the dopamine content.
It is well established that alpha-synuclein co-immunoprecipitates with DAT in transfected cells, rat primary mesencephalic dopaminergic neurons, and striatal tissue [18, 102, 123]; however, the consequence of this interaction on dopamine uptake via DAT is less understood. While Chadchankar’s group reported a decrease in dopamine uptake in the dorsal striatum of alpha-synuclein knockout mice, in vitro and in vivo studies suggest increased alpha-synuclein levels decreased DAT-mediated dopamine uptake [17, 102]. In vitro studies by Wersinger et al., and Swant et al. have shown alpha-synuclein overexpression decreases dopamine uptake or uptake of a fluorescent substrate of DAT, respectively [17, 102]. Similarly, Pelkonen et al. have shown that microinjection of alpha-synuclein into the dorsal striatum of alpha-synuclein KO or wild-type mice decreases dopamine uptake six days after the microinjection without effecting acute dopamine uptake [124]. These results contradict a report by Lee et al., where they have shown that alpha-synuclein increases dopamine uptake [123]. Since DAT operates in multiple modes (i.e., forward, reverse, and channel mode), an increase in dopamine efflux may affect the measured net dopamine uptake. Recently two different studies have shown that increased alpha-synuclein at or near the surface membrane enhances the interaction between DAT and alpha-synuclein, thus altering the ionic conductance of the transporter that can uniquely affect the excitability of dopaminergic neurons and thus dopamine neurotransmission [17, 18].
Alpha-synuclein modulation of DAT-mediated dopamine efflux
Dopamine can be released by two mechanisms: 1) vesicular release, the classic mechanism for neurotransmitter release, and 2) transporter-mediated dopamine efflux. These two mechanisms are differentially affected by inhibitors of DAT [125]. Like other neurotransmitters, dopamine is released from synaptic vesicles fused with the plasma membrane following an action potential [125–127]. Alpha-synuclein regulation of action potential dependent neurotransmitter release is described above. Efflux of dopamine, on the other hand, is mediated through DAT in an action potential independent process [42, 125, 128, 129]. The reverse transport of dopamine or dopamine efflux is critical for maintenance of tonic dopamine signaling and salient motivational states [130, 131]. Since, DAT-mediated dopamine efflux is regulated by intracellular Ca2+ [132] it is possible that alpha-synuclein induced increase in intracellular Ca2+ regulates the action potential independent release of dopamine via DAT. Consistent with this idea, Lam et al. have shown mice overexpressing alpha-synuclein exhibit an elevated extracellular dopamine concentration in the striatum that precedes dopamine loss in the striatum [50]. The increase in extracellular dopamine levels following alpha-synuclein overexpression might be due to decreased dopamine uptake, increased dopamine efflux, or combination of both mechanisms. Swant et al. have shown elevated alpha-synuclein decreases the initial slope of dopamine uptake and then within five minutes reaches the uptake level of cells not expressing alpha-synuclein [17]. Therefore, the alpha-synuclein mediated decrease in dopamine uptake alone, might not be the sole underlying mechanism for the elevated extracellular dopamine levels. In a recent study, Butler et al. have shown alpha-synuclein overexpression increases the DAT-mediated, nomifensine-sensitive basal dopamine efflux at resting membrane potential [18]. These reports are in agreement with the emerging consensus that alpha-synuclein regulates dopamine neurotransmission by influencing the action potential independent dopamine release via DAT.
Conclusions
Although multiplication of alpha-synuclein gene in human results in a seemingly modest increase in the amount of alpha-synuclein protein, this is still sufficient for the development of PD and/or dementia with lewy bodies [11]. While progressive decline in vulnerable substantia nigra pars compacta neurons are reported independently of overt protein aggregation, which supports a toxic role for non-aggregate (potentially monomeric) forms of alpha-synuclein [133–135], similar number of reports suggest that fibrillar alpha-synuclein inclusions damage dopamine neurons in the substantia nigra pars compacta [136]. This is still a highly debated issue. What is clear, however, is that overexpression of wild-type alpha-synuclein decreases dopamine neurotransmission. Alpha-synuclein overexpression decreases TH and VMAT2 expression and activity, decreases DAT-mediated dopamine uptake and increases DAT-mediated dopamine efflux; thus, long-term alpha-synuclein overexpression decreases intracellular and extracellular levels of dopamine ultimately decreases dopaminergic neurotransmission in the brain.
Acknowledgments
Funding source: DA026947, NS071122, OD020026, DA026947S1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Ostrerova N, et al. alpha-Synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci. 1999;19(14):5782–91. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oueslati A, et al. Polo-like kinase 2 regulates selective autophagic alpha-synuclein clearance and suppresses its toxicity in vivo. Proc Natl Acad Sci U S A. 2013;110(41):E3945–54. doi: 10.1073/pnas.1309991110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276(5321):2045–7. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 4.Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 5.Hanin I, PL, Ostrerova N, Farrer M, Mehta N, Jeal H. alpha-synuclein attenuates ERK/ELK signalling. Soc Neurosci. 1999;25(27) [Google Scholar]
- 6.Jenco JM, et al. Regulation of phospholipase D2: selective inhibition of mammalian phospholipase D isoenzymes by alpha- and beta-synucleins. Biochemistry. 1998;37(14):4901–9. doi: 10.1021/bi972776r. [DOI] [PubMed] [Google Scholar]
- 7.Kruger R, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18(2):106–8. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 8.Jensen PH, et al. alpha-synuclein binds to Tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356. J Biol Chem. 1999;274(36):25481–9. doi: 10.1074/jbc.274.36.25481. [DOI] [PubMed] [Google Scholar]
- 9.Engelender S, et al. Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nat Genet. 1999;22(1):110–4. doi: 10.1038/8820. [DOI] [PubMed] [Google Scholar]
- 10.Tong J, et al. Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson's disease and progressive supranuclear palsy: a comparative investigation. Brain. 2010;133(Pt 1):172–88. doi: 10.1093/brain/awp282. [DOI] [PubMed] [Google Scholar]
- 11.Singleton AB, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 12.Ben Gedalya T, et al. Alpha-synuclein and polyunsaturated fatty acids promote clathrin-mediated endocytosis and synaptic vesicle recycling. Traffic. 2009;10(2):218–34. doi: 10.1111/j.1600-0854.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan Y, et al. Overexpression of alpha-synuclein down-regulates BDNF expression. Cell Mol Neurobiol. 2010;30(6):939–46. doi: 10.1007/s10571-010-9523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mash DC, et al. Cocaine abusers have an overexpression of alpha-synuclein in dopamine neurons. J Neurosci. 2003;23(7):2564–71. doi: 10.1523/JNEUROSCI.23-07-02564.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burre J, et al. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663–7. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi HS, et al. Phosphorylation of alpha-synuclein is crucial in compensating for proteasomal dysfunction. Biochem Biophys Res Commun. 2012;424(3):597–603. doi: 10.1016/j.bbrc.2012.06.159. [DOI] [PubMed] [Google Scholar]
- 17.Swant J, et al. alpha-Synuclein stimulates a dopamine transporter-dependent chloride current and modulates the activity of the transporter. J Biol Chem. 2011;286(51):43933–43. doi: 10.1074/jbc.M111.241232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler B, et al. Dopamine Transporter Activity Is Modulated by alpha-Synuclein. J Biol Chem. 2015;290(49):29542–54. doi: 10.1074/jbc.M115.691592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21(6):249–54. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- 20.Ueda K, et al. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(23):11282–6. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefanis L. alpha-Synuclein in Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2(2):a009399. doi: 10.1101/cshperspect.a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Recchia A, et al. Alpha-synuclein and Parkinson's disease. FASEB J. 2004;18(6):617–26. doi: 10.1096/fj.03-0338rev. [DOI] [PubMed] [Google Scholar]
- 23.Tu PH, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44(3):415–22. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- 24.Baba M, et al. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152(4):879–84. [PMC free article] [PubMed] [Google Scholar]
- 25.Abeliovich A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–52. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 26.Cooper AA, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313(5785):324–8. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M, et al. Effect of the overexpression of wild-type or mutant alpha-synuclein on cell susceptibility to insult. J Neurochem. 2001;76(4):998–1009. doi: 10.1046/j.1471-4159.2001.00149.x. [DOI] [PubMed] [Google Scholar]
- 28.Giasson BI, et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290(5493):985–9. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 29.Lee HJ, et al. Human alpha-synuclein modulates vesicle trafficking through its interaction with prenylated Rab acceptor protein 1. Biochem Biophys Res Commun. 2011;412(4):526–31. doi: 10.1016/j.bbrc.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 30.Ibanez P, et al. Causal relation between alpha-synuclein gene duplication and familial Parkinson's disease. Lancet. 2004;364(9440):1169–71. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 31.Kiely AP, et al. alpha-Synucleinopathy associated with G51D SNCA mutation: a link between Parkinson's disease and multiple system atrophy? Acta Neuropathol. 2013;125(5):753–69. doi: 10.1007/s00401-013-1096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarranz JJ, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–73. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 33.Appel-Cresswell S, et al. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson's disease. Mov Disord. 2013;28(6):811–3. doi: 10.1002/mds.25421. [DOI] [PubMed] [Google Scholar]
- 34.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4(11):1318–20. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 35.Paxinou E, et al. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci. 2001;21(20):8053–61. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nonaka T, Iwatsubo T, Hasegawa M. Ubiquitination of alpha-synuclein. Biochemistry. 2005;44(1):361–8. doi: 10.1021/bi0485528. [DOI] [PubMed] [Google Scholar]
- 37.Li W, et al. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson's disease-linked mutations. Proc Natl Acad Sci U S A. 2005;102(6):2162–7. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson JP, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281(40):29739–52. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 39.Hara S, et al. Serine 129 phosphorylation of membrane-associated alpha-synuclein modulates dopamine transporter function in a G protein-coupled receptor kinase-dependent manner. Mol Biol Cell. 2013;24(11):1649–60. S1–3. doi: 10.1091/mbc.E12-12-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paleologou KE, et al. Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions. J Neurosci. 2010;30(9):3184–98. doi: 10.1523/JNEUROSCI.5922-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Y, et al. Phosphorylation of alpha-Synuclein at Y125 and S129 alters its metal binding properties: implications for understanding the role of alpha-Synuclein in the pathogenesis of Parkinson's Disease and related disorders. ACS Chem Neurosci. 2011;2(11):667–75. doi: 10.1021/cn200074d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giros B, et al. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–12. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 43.Pifl C, Giros B, Caron MG. The dopamine transporter. The cloned target site of parkinsonism-inducing toxins and of drugs of abuse. Adv Neurol. 1996;69:235–8. [PubMed] [Google Scholar]
- 44.Caron MG. Images in neuroscience. Molecular biology, II. A dopamine transporter mouse knockout. Am J Psychiatry. 1996;153(12):1515. doi: 10.1176/ajp.153.12.1515. [DOI] [PubMed] [Google Scholar]
- 45.Jones SR, et al. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2(7):649–55. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- 46.Jaber M, et al. The dopamine transporter: a crucial component regulating dopamine transmission. Mov Disord. 1997;12(5):629–33. doi: 10.1002/mds.870120502. [DOI] [PubMed] [Google Scholar]
- 47.Pierce RC, Kalivas PW. Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J Pharmacol Exp Ther. 1995;275(2):1019–29. [PubMed] [Google Scholar]
- 48.Goodwin JS, et al. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284(5):2978–89. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen MK, et al. VMAT2 and dopamine neuron loss in a primate model of Parkinson's disease. J Neurochem. 2008;105(1):78–90. doi: 10.1111/j.1471-4159.2007.05108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lam HA, et al. Elevated tonic extracellular dopamine concentration and altered dopamine modulation of synaptic activity precede dopamine loss in the striatum of mice overexpressing human alpha-synuclein. J Neurosci Res. 2011;89(7):1091–102. doi: 10.1002/jnr.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Reitboeck P, et al. Endogenous alpha-synuclein influences the number of dopaminergic neurons in mouse substantia nigra. Exp Neurol. 2013;248:541–5. doi: 10.1016/j.expneurol.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kokhan VS, Afanasyeva MA, Van'kin GI. alpha-Synuclein knockout mice have cognitive impairments. Behav Brain Res. 2012;231(1):226–30. doi: 10.1016/j.bbr.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 53.Pena-Oliver Y, et al. Alpha-synuclein deletion decreases motor impulsivity but does not affect risky decision making in a mouse Gambling Task. Psychopharmacology (Berl) 2014;231(12):2493–506. doi: 10.1007/s00213-013-3416-y. [DOI] [PubMed] [Google Scholar]
- 54.Pena-Oliver Y, V, Buchman L, Stephens DN. Lack of involvement of alpha-synuclein in unconditioned anxiety in mice. Behav Brain Res. 2010;209(2):234–40. doi: 10.1016/j.bbr.2010.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pena-Oliver Y, et al. Deletion of alpha-synuclein decreases impulsivity in mice. Genes Brain Behav. 2012;11(2):137–46. doi: 10.1111/j.1601-183X.2011.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masliah E, et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287(5456):1265–9. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 57.Hansen C, et al. A novel alpha-synuclein-GFP mouse model displays progressive motor impairment, olfactory dysfunction and accumulation of alpha-synuclein-GFP. Neurobiol Dis. 2013;56:145–55. doi: 10.1016/j.nbd.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 58.Kahle PJ, et al. Subcellular localization of wild-type and Parkinson's disease-associated mutant alpha -synuclein in human and transgenic mouse brain. J Neurosci. 2000;20(17):6365–73. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kahle PJ, et al. Physiology and pathophysiology of alpha-synuclein. Cell culture and transgenic animal models based on a Parkinson's disease-associated protein. Ann N Y Acad Sci. 2000;920:33–41. doi: 10.1111/j.1749-6632.2000.tb06902.x. [DOI] [PubMed] [Google Scholar]
- 60.Rockenstein E, et al. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68(5):568–78. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- 61.Taylor TN, et al. Region-specific deficits in dopamine, but not norepinephrine, signaling in a novel A30P alpha-synuclein BAC transgenic mouse. Neurobiol Dis. 2014;62:193–207. doi: 10.1016/j.nbd.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stoica G, et al. Potential role of alpha-synuclein in neurodegeneration: studies in a rat animal model. J Neurochem. 2012;122(4):812–22. doi: 10.1111/j.1471-4159.2012.07805.x. [DOI] [PubMed] [Google Scholar]
- 63.Wojda U, Salinska E, Kuznicki J. Calcium ions in neuronal degeneration. IUBMB Life. 2008;60(9):575–90. doi: 10.1002/iub.91. [DOI] [PubMed] [Google Scholar]
- 64.Mattson MP. Cellular signaling mechanisms common to the development and degeneration of neuroarchitecture. A review. Mech Ageing Dev. 1989;50(2):103–57. doi: 10.1016/0047-6374(89)90010-9. [DOI] [PubMed] [Google Scholar]
- 65.Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34(4–5):325–37. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 66.Peggion C, Bertoli A, Sorgato MC. Possible role for Ca2+ in the pathophysiology of the prion protein? Biofactors. 2011;37(3):241–9. doi: 10.1002/biof.161. [DOI] [PubMed] [Google Scholar]
- 67.Danzer KM, et al. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27(34):9220–32. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adamczyk A, Strosznajder JB. Alpha-synuclein potentiates Ca2+ influx through voltage-dependent Ca2+ channels. Neuroreport. 2006;17(18):1883–6. doi: 10.1097/WNR.0b013e3280115185. [DOI] [PubMed] [Google Scholar]
- 69.Hettiarachchi NT, et al. alpha-Synuclein modulation of Ca2+ signaling in human neuroblastoma (SH-SY5Y) cells. J Neurochem. 2009;111(5):1192–201. doi: 10.1111/j.1471-4159.2009.06411.x. [DOI] [PubMed] [Google Scholar]
- 70.Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345(1):27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- 71.Ronzitti G, et al. Exogenous alpha-synuclein decreases raft partitioning of Cav2.2 channels inducing dopamine release. J Neurosci. 2014;34(32):10603–15. doi: 10.1523/JNEUROSCI.0608-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reznichenko L, et al. In vivo alterations in calcium buffering capacity in transgenic mouse model of synucleinopathy. J Neurosci. 2012;32(29):9992–8. doi: 10.1523/JNEUROSCI.1270-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bennett MR. The concept of neurotransmitter release. Adv Second Messenger Phosphoprotein Res. 1994;29:1–29. doi: 10.1016/s1040-7952(06)80004-2. [DOI] [PubMed] [Google Scholar]
- 74.Bennett MK. Molecular mechanisms of neurotransmitter release. Ann N Y Acad Sci. 1994;733:256–65. doi: 10.1111/j.1749-6632.1994.tb17275.x. [DOI] [PubMed] [Google Scholar]
- 75.Gitler AD, et al. The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci U S A. 2008;105(1):145–50. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Senior SL, et al. Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both alpha-synuclein and gamma-synuclein. Eur J Neurosci. 2008;27(4):947–57. doi: 10.1111/j.1460-9568.2008.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murphy DD, et al. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20(9):3214–20. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cabin DE, et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22(20):8797–807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Larsen KE, et al. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26(46):11915–22. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mosharov EV, et al. Alpha-synuclein overexpression increases cytosolic catecholamine concentration. J Neurosci. 2006;26(36):9304–11. doi: 10.1523/JNEUROSCI.0519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gaugler MN, et al. Nigrostriatal overabundance of alpha-synuclein leads to decreased vesicle density and deficits in dopamine release that correlate with reduced motor activity. Acta Neuropathol. 2012;123(5):653–69. doi: 10.1007/s00401-012-0963-y. [DOI] [PubMed] [Google Scholar]
- 82.Lundblad M, et al. Impaired neurotransmission caused by overexpression of alpha-synuclein in nigral dopamine neurons. Proc Natl Acad Sci U S A. 2012;109(9):3213–9. doi: 10.1073/pnas.1200575109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Diogenes MJ, et al. Extracellular alpha-synuclein oligomers modulate synaptic transmission and impair LTP via NMDA-receptor activation. J Neurosci. 2012;32(34):11750–62. doi: 10.1523/JNEUROSCI.0234-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Damier P, et al. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122(Pt 8):1437–48. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 85.Perez RG, et al. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22(8):3090–9. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peng X, et al. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci. 2005;118(Pt 15):3523–30. doi: 10.1242/jcs.02481. [DOI] [PubMed] [Google Scholar]
- 87.Chu Y, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson's disease? Neurobiol Dis. 2007;25(1):134–49. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, et al. Preferential localization of a vesicular monoamine transporter to dense core vesicles in PC12 cells. J Cell Biol. 1994;127(5):1419–33. doi: 10.1083/jcb.127.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller GW, et al. Immunochemical analysis of vesicular monoamine transporter (VMAT2) protein in Parkinson's disease. Exp Neurol. 1999;156(1):138–48. doi: 10.1006/exnr.1998.7008. [DOI] [PubMed] [Google Scholar]
- 90.Gonzalez-Hernandez T, et al. Expression of dopamine and vesicular monoamine transporters and differential vulnerability of mesostriatal dopaminergic neurons. J Comp Neurol. 2004;479(2):198–215. doi: 10.1002/cne.20323. [DOI] [PubMed] [Google Scholar]
- 91.Yamamoto S, et al. Positive immunoreactivity for vesicular monoamine transporter 2 in Lewy bodies and Lewy neurites in substantia nigra. Neurosci Lett. 2006;396(3):187–91. doi: 10.1016/j.neulet.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 92.Henry JP, et al. Biochemistry and molecular biology of the vesicular monoamine transporter from chromaffin granules. J Exp Biol. 1994;196:251–62. doi: 10.1242/jeb.196.1.251. [DOI] [PubMed] [Google Scholar]
- 93.Guo JT, et al. Inhibition of vesicular monoamine transporter-2 activity in alpha-synuclein stably transfected SH-SY5Y cells. Cell Mol Neurobiol. 2008;28(1):35–47. doi: 10.1007/s10571-007-9227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ulusoy A, et al. Dysregulated dopamine storage increases the vulnerability to alpha-synuclein in nigral neurons. Neurobiol Dis. 2012;47(3):367–77. doi: 10.1016/j.nbd.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 95.Testa CM, Sherer TB, Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res. 2005;134(1):109–18. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 96.Chen L, et al. Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. J Neurosci. 2008;28(2):425–33. doi: 10.1523/JNEUROSCI.3602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simuni T, Stern MB. Does levodopa accelerate Parkinson's disease? Drugs Aging. 1999;14(6):399–408. doi: 10.2165/00002512-199914060-00001. [DOI] [PubMed] [Google Scholar]
- 98.Rajput AH, et al. Is levodopa toxic to human substantia nigra? Mov Disord. 1997;12(5):634–8. doi: 10.1002/mds.870120503. [DOI] [PubMed] [Google Scholar]
- 99.Quinn N, et al. Preservation of the substantia nigra and locus coeruleus in a patient receiving levodopa (2 kg) plus decarboxylase inhibitor over a four-year period. Mov Disord. 1986;1(1):65–8. doi: 10.1002/mds.870010109. [DOI] [PubMed] [Google Scholar]
- 100.Maier Hoehn MM. Parkinsonism treated with levodopa: progression and mortality. J Neural Transm Suppl. 1983;19:253–64. [PubMed] [Google Scholar]
- 101.Lee FJ, et al. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001;15(6):916–26. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- 102.Wersinger C, Sidhu A. Differential cytotoxicity of dopamine and H2O2 in a human neuroblastoma divided cell line transfected with alpha-synuclein and its familial Parkinson's disease-linked mutants. Neurosci Lett. 2003;342(1–2):124–8. doi: 10.1016/s0304-3940(03)00212-x. [DOI] [PubMed] [Google Scholar]
- 103.Oaks AW, et al. Synucleins antagonize endoplasmic reticulum function to modulate dopamine transporter trafficking. PLoS One. 2013;8(8):e70872. doi: 10.1371/journal.pone.0070872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wersinger C, Vernier P, Sidhu A. Trypsin disrupts the trafficking of the human dopamine transporter by alpha-synuclein and its A30P mutant. Biochemistry. 2004;43(5):1242–53. doi: 10.1021/bi035308s. [DOI] [PubMed] [Google Scholar]
- 105.Giros B, et al. Cloning and functional characterization of a cocaine-sensitive dopamine transporter. FEBS Lett. 1991;295(1–3):149–54. doi: 10.1016/0014-5793(91)81406-x. [DOI] [PubMed] [Google Scholar]
- 106.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279(5348):227–30. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 107.DeFelice LJ, Galli A. Electrophysiological analysis of transporter function. Adv Pharmacol. 1998;42:186–90. doi: 10.1016/s1054-3589(08)60724-3. [DOI] [PubMed] [Google Scholar]
- 108.Perona MT, et al. Animal models of depression in dopamine, serotonin, and norepinephrine transporter knockout mice: prominent effects of dopamine transporter deletions. Behav Pharmacol. 2008;19(5–6):566–74. doi: 10.1097/FBP.0b013e32830cd80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berlanga ML, et al. Multiscale imaging characterization of dopamine transporter knockout mice reveals regional alterations in spine density of medium spiny neurons. Brain Res. 2011;1390:41–9. doi: 10.1016/j.brainres.2011.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spielewoy C, et al. Increased rewarding properties of morphine in dopamine-transporter knockout mice. Eur J Neurosci. 2000;12(5):1827–37. doi: 10.1046/j.1460-9568.2000.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pimoule C, et al. Decrease in [3H]cocaine binding to the dopamine transporter in Parkinson's disease. Eur J Pharmacol. 1983;95(1–2):145–6. doi: 10.1016/0014-2999(83)90281-9. [DOI] [PubMed] [Google Scholar]
- 112.Varrone A, et al. [(123)I]beta-CIT SPECT imaging demonstrates reduced density of striatal dopamine transporters in Parkinson's disease and multiple system atrophy. Mov Disord. 2001;16(6):1023–32. doi: 10.1002/mds.1256. [DOI] [PubMed] [Google Scholar]
- 113.Maier W, et al. Genetic relationship between dopamine transporter gene and schizophrenia: linkage and association. Schizophr Res. 1996;20(1–2):175–80. doi: 10.1016/0920-9964(95)00083-6. [DOI] [PubMed] [Google Scholar]
- 114.Markota M, et al. Reduced dopamine transporter expression in the amygdala of subjects diagnosed with schizophrenia. Schizophr Bull. 2014;40(5):984–91. doi: 10.1093/schbul/sbu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sandoval V, et al. Methamphetamine-induced rapid and reversible changes in dopamine transporter function: an in vitro model. J Neurosci. 2001;21(4):1413–9. doi: 10.1523/JNEUROSCI.21-04-01413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pizzo AB, et al. Amphetamine-induced behavior requires CaMKII-dependent dopamine transporter phosphorylation. Mol Psychiatry. 2014;19(3):279–81. doi: 10.1038/mp.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rocha BA, et al. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1(2):132–7. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- 118.Jones SR, et al. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18(6):1979–86. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miller GM, et al. Single nucleotide polymorphisms distinguish multiple dopamine transporter alleles in primates: implications for association with attention deficit hyperactivity disorder and other neuropsychiatric disorders. Mol Psychiatry. 2001;6(1):50–8. doi: 10.1038/sj.mp.4000809. [DOI] [PubMed] [Google Scholar]
- 120.Krause KH, et al. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neurosci Lett. 2000;285(2):107–10. doi: 10.1016/s0304-3940(00)01040-5. [DOI] [PubMed] [Google Scholar]
- 121.Dresel S, et al. Attention deficit hyperactivity disorder: binding of [99mTc]TRODAT-1 to the dopamine transporter before and after methylphenidate treatment. Eur J Nucl Med. 2000;27(10):1518–24. doi: 10.1007/s002590000330. [DOI] [PubMed] [Google Scholar]
- 122.Pifl C, Giros B, Caron MG. Dopamine transporter expression confers cytotoxicity to low doses of the parkinsonism-inducing neurotoxin 1-methyl-4-phenylpyridinium. J Neurosci. 1993;13(10):4246–53. doi: 10.1523/JNEUROSCI.13-10-04246.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Volles MJ, et al. Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson's disease. Biochemistry. 2001;40(26):7812–9. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- 124.Pelkonen A, Kallunki P, Yavich L. Effects of exogenous alpha-synuclein on stimulated dopamine overflow in dorsal striatum. Neurosci Lett. 2013;554:141–5. doi: 10.1016/j.neulet.2013.08.072. [DOI] [PubMed] [Google Scholar]
- 125.Sulzer D, Galli A. Dopamine transport currents are promoted from curiosity to physiology. Trends Neurosci. 2003;26(4):173–6. doi: 10.1016/S0166-2236(03)00063-8. [DOI] [PubMed] [Google Scholar]
- 126.Mundorf ML, et al. Vesicular Ca(2+) participates in the catalysis of exocytosis. J Biol Chem. 2000;275(13):9136–42. doi: 10.1074/jbc.275.13.9136. [DOI] [PubMed] [Google Scholar]
- 127.Mundorf ML, et al. Catecholamine release and uptake in the mouse prefrontal cortex. J Neurochem. 2001;79(1):130–42. doi: 10.1046/j.1471-4159.2001.00554.x. [DOI] [PubMed] [Google Scholar]
- 128.Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend. 1998;51(1–2):87–96. doi: 10.1016/s0376-8716(98)00068-4. [DOI] [PubMed] [Google Scholar]
- 129.Amara SG, et al. Molecular physiology and regulation of catecholamine transporters. Adv Pharmacol. 1998;42:164–8. doi: 10.1016/s1054-3589(08)60718-8. [DOI] [PubMed] [Google Scholar]
- 130.Borre L, et al. The second sodium site in the dopamine transporter controls cation permeation and is regulated by chloride. J Biol Chem. 2014;289(37):25764–73. doi: 10.1074/jbc.M114.574269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hansen FH, et al. Missense dopamine transporter mutations associate with adult parkinsonism and ADHD. J Clin Invest. 2014;124(7):3107–20. doi: 10.1172/JCI73778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gnegy ME, et al. Intracellular Ca2+ regulates amphetamine-induced dopamine efflux and currents mediated by the human dopamine transporter. Mol Pharmacol. 2004;66(1):137–43. doi: 10.1124/mol.66.1.137. [DOI] [PubMed] [Google Scholar]
- 133.Hodara R, et al. Functional consequences of alpha-synuclein tyrosine nitration: diminished binding to lipid vesicles and increased fibril formation. J Biol Chem. 2004;279(46):47746–53. doi: 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- 134.Zhou M, et al. Highly neurotoxic monomeric alpha-helical prion protein. Proc Natl Acad Sci U S A. 2012;109(8):3113–8. doi: 10.1073/pnas.1118090109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Janezic S, et al. Deficits in dopaminergic transmission precede neuron loss and dysfunction in a new Parkinson model. Proc Natl Acad Sci U S A. 2013;110(42):E4016–25. doi: 10.1073/pnas.1309143110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Greenbaum EA, et al. The E46K mutation in alpha -synuclein increases amyloid fibril formation. J Biol Chem. 2005;280(9):7800–7807. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]