Abstract
Necrotizing enterocolitis (NEC) is the most devastating gastrointestinal disease of the premature infant. We have recently shown that NEC development occurs after an increase in proinflammatory CD4+Th17 (Th17) cells and reduced anti-inflammatory Foxp3+ regulatory T cells (Tregs) to the premature small intestine of mice and humans, which can be experimentally reversed in mice by administration of all-trans retinoic acid (ATRA). We have also shown that NEC is characterized by apoptosis of Lgr5-positive intestinal stem cells (ISCs–Lgr5+cells) within the crypts of Lieberkühn, which are subsequently essential for intestinal homeostasis. We now hypothesize that the normal lymphocyte balance within the lamina propria of the intestine can be achieved via administration of ATRA which restores mucosal integrity by preventing the loss of intestinal stem cells. Utilizing both in vivo and in vitro strategies we now demonstrate that Th17 recruitment and Treg depletion lead to increased apoptosis within ISC niches, significantly impairing proliferative capacity and mucosal healing. ATRA exerted its protective effects by preventing T cell imbalance, ultimately leading to the protection of the ISC pool preventing the development of NEC in mice. These findings raise the exciting possibility that dietary manipulations could prevent and treat NEC by modulating lymphocyte balance and the ISC pool within the newborn small intestine.
Keywords: necrotizing enterocolitis, intestinal stem cells, T lymphocyte, retinoic acid, regulatory T cells, Th17 cells
INTRODUCTION
Necrotizing enterocolitis (NEC) is the most frequent and lethal inflammatory disease of the gastrointestinal tract of the premature infant (1, 2). The overall morbidity and mortality caused by the disease remains unabated over the past 30 years (3, 4). We and others have recently describe that the pathogenesis of NEC involves the initiation of a rapid cascade of inflammatory events that originate within the intestinal mucosa of the small bowel, whose net effect is the disruption of the epithelial barrier, translocation of bacteria, multiple organ failure and death (1, 5, 6). In defining the molecular and cellular processes involved, our laboratory has identified a critical role of the lipopolysaccharide receptor Toll-like Receptor 4 (TLR4) in the development of NEC, through effects on mediating the apoptosis of enterocytes and crypt-resident stem cells (7-9). More recently we have shown that intestinal lymphocytes play a critical role in the pathogenesis of NEC (10). In particular, TLR4 signaling on the intestinal epithelium was found to be critical for the STAT3-dependent polarization of T cells toward a proinflammatory CD3+CD4+IL17+ (Th17) profile in the lamina propria of both humans and mice afflicted with NEC (10) along with a reduction in Foxp3+ regulatory T lymphocytes (Tregs) (10). The combined effects of increased Th17 lymphocytes and reduced Treg lymphocytes resulted in NEC development (10). Evidence for a causative role for Th17 in NEC was found the adoptive transfer of intestinal lymphocytes from mice with NEC into naïve mice caused spontaneous intestinal epithelium (10). In addition, inhibition of STAT3 or IL-17 receptor signaling attenuated NEC severity, while the release of IL-17 led to impairment of the mucosal barrier by disruption of enterocyte tight junctions, increased apoptosis and decreased enterocyte proliferation, hallmarks of NEC pathogenesis (1, 10). Interestingly, our previous report provided preliminary evidence suggesting that enteral administration of all-trans retinoid acid (ATRA – a vitamin A metabolite), leads to a significant decrease in the incidence and severity of NEC (10) by restoring lymphocyte balance in the neonatal mucosa towards a Treg phenotype. Based upon these findings identifying a role for lymphocyte imbalance in NEC pathogenesis, along with the central role identified for intestinal stem cells in NEC induction, we now seek to determine whether these processes may be linked. Specifically, we now hypothesize that administration of ATRA improves incidence and severity of NEC by restoring T cell balance, which protects the newborn intestine from intestinal stem cell loss and thus prevents the development of this disease.
In testing this hypothesis, we now report that enteral administration of ATRA prevented the induction of Th17 cells by maintaining a stable pool of regulatory T cells, which in turn leads to the protection of the crypt-based intestinal stem cells, and reducing NEC severity in mice.
MATERIALS AND METHODS
Enteroid culture, antibodies and reagents
Primary intestinal crypt cultures (enteroids) were established and maintained in culture according to the methods published by Sato et al. (11) with a few modifications (9, 12). Enteroids were seeded on Matrigel, allowed to grow for at least 24 h before treatment with recombinant rat IL-17A (Peprotech – 100 ng/mL) or vehicle alone for 6 hours in order to evaluate cell proliferation, differentiation and death by immunohistochemistry (IHC) and confocal microscopy as described in the next section. The following antibodies were used for IHC analysis: BrdU (BRD494 – Novus Biosciences), chromogranin A (ab15160 – Abcam), e-cadherin (AF748 – R&D Systems), Ki67 (ab15580 – Abcam) and mucin glycoprotein 2 (Muc2, sc-15334 – Santa Cruz). Nuclear counterstaining was performed using DAPI (Pierce).
All-trans retinoic acid (ATRA) was obtained from Sigma-Aldrich, dissolved in DMSO and corn oil (1:1 – final concentration 6 mg/mL, protected from light) and administered daily by gavage to breast-fed and NEC mice (50 μg/mouse) for the duration of the experimental induction of NEC.
Immunohistochemistry
Immunohistochemical analysis of enteroids and intestinal sections was performed as we have previously reported (9) and assessed using a Nikon A1 confocal microscope under oil-immersion objectives. To determine cell proliferation, enteroids were incubated with BrdU-labeling reagent added to the culture media at the time of treatment (6 hours, 10 μL/mL – Invitrogen). The cellular proliferation marker Ki67 was also evaluated by IHC, as we have previously described (12). Cell differentiation was determined by IHC and confocal microscopy using the enteroendocrine marker – chromogranin A, the goblet cell marker mucin glycoprotein 2 – Muc2 and the epithelial cell marker E-cadherin, as described by Shaffiey et al. (12). Cell death was assessed using the Apoptosis/Necrotic Cell Death Detection kit (Promokine Inc.) as we have previously reported (10) and according to the manufacturer's instructions. Apoptotic cells were identified with Annexin V, which binds to phosphatidylserine (PS) exposed on the outer membrane leaflet of cells undergoing apoptosis. Necrotic cells were identified using the nucleic acid probe ethidium homodimer III (EthD-III) to identify cells whose internal organelle and plasma membrane integrity has been lost. Apoptosis was determined in ileum segments (5 μm-thick paraffin sections) by TUNEL staining according to the manufacturer's instructions (Roche Applied Science) as previously described (9). Image analysis and fluorescence intensity quantification was performed using FIJI software (open source project)(13).
Mice and induction of necrotizing enterocolitis
All experiments and procedures were approved by the Johns Hopkins University and the University of Pittsburgh Animal Care and Use committees in accordance to the Guide for the Care and Use of Laboratory Animals (8th Edition, The National Academies Press 2011). C57Bl/6 and B6.129(Cg)-Foxp3tm3(DTR/GFP)Ayr/J (Foxp3+DTR) mice were obtained from the Jackson Laboratory and housed in an specific pathogen-free facility. NEC was induced as we have previously described (7, 9, 14) in 7- to 8-day-old mouse pups by gavage feeding (5 times/day for 4 days) of formula (Similac Advance infant formula – Abbott Nutrition and Esbilac canine milk replacer – PetAg, at a ratio of 2:1) supplemented with enteric bacteria that was isolated from an infant with NEC. In addition, NEC mice were exposed to intermittent hypoxia (5% O2, 95% N2 for 10 min twice daily) for 4 days. As we have previously reported this experimental protocol leads to patchy necrosis of the small intestine (ileum) and upregulation of inflammatory mediators, which resembles the pathologic findings of the human condition (7, 9, 14, 15). Breast-fed control animals were kept with the dam until the time of euthanasia. Pups of both sexes were used in the experiments described. The severity of the disease was determined on histologic sections of the terminal ileum using a scoring system from 0 (normal) to 3 (severe) by a pediatric pathologist blinded to the treatment condition, as we have previously reported (7).
Depletion of regulatory T cells (Tregs) was achieved in Foxp3+DTR mice by administration of diphtheria toxin (DT 100 ng/mouse IP daily for 4 days), this mouse strain selectively express human DT receptor under the Foxp3 gene promoter as described by Mayer et al. (16). Depletion of Foxp3 expressing cells within the lamina propria was confirmed by flow cytometry followed by quantitative real-time PCR analysis as described below. To determine intestinal stem cell proliferative capacity, mice were administered BrdU labeling reagent by gavage (10 μL/g body weight) 24 hours before euthanasia. BrdU incorporation was assessed as described by Neal et al.(8).
Lamina propria lymphocyte isolation and flow cytometry
Cells infiltrating the lamina propria were isolated as described by Egan et al. (10) with a few modifications (17). Briefly, samples from small intestine (ileum) were denuded of mesentery, divided longitudinally and minced with fine scissors. Tissue homogenates were incubated in pre-warmed (37°C) DMEM media containing 10% fetal bovine serum (DMEM/FBS) and 1.3 mM EDTA (Quality Biological) for 20 minutes with gentle agitation (220 rpm) followed by centrifugation (10 min 400 × g). The supernatants containing the enterocyte layer were discarded. To isolate the lamina propriainfiltrating leukocytes the remaining tissue was digested at 37°C for 40 minutes by gentle agitation using collagenase (50 U/mL – Sigma-Aldrich) and DNAse (15 μg/mL – Sigma-Aldrich) in DMEM/FBS. Cells were subsequently washed twice with ice-cold 1% BSA-PBS and filtered through 70 μm and 40 μm cell strainers, sequentially.
For flow cytometry analysis, cell viability was determined in single-cell suspensions using the Zombie Aqua dye (BioLegend) following the manufacturer's protocol. Fc receptor binding was blocked using anti-CD16/CD32 (BD Biosciences) in ice-cold FACS buffer (1% BSA, 0.01% NaN3 PBS) solution for 20 minutes at 4°C. Cell surface molecule staining was performed after cells were pelleted by centrifugation (5 minutes 400 × g) and resuspended in optimal concentrations of fluorophore-conjugated antibodies diluted in ice-cold FACS buffer. Staining of intracellular proteins was performed using Foxp3 buffer set. Cells were then washed and at least 100,000 live cells per sample were analyzed using a BD Accuri C6 Flow Cytometer (BD Biosciences). Flow cytometry data analysis was performed using FlowJo software (FlowJo) as previously described (18).
Transcriptional analysis of isolated CD4+ T cells was performed (as described below) using single cell suspensions of lamina propria leukocytes obtained as described above and purified using anti-CD4 magnetic beads per the manufacturer's instructions (Miltenyi Biotec). Cells were washed and separated by positive selection using LS columns and a QuadroMACS separator (Miltenyi Biotec). Cell purity and enrichment was confirmed by cell surface staining of CD4 proteins by flow cytometry. Average purity was consistently above 85%.
Quantitative real-time PCR
Gene expression was determined by quantitative real-time PCR analysis using a Bio-Rad CFX96 Real-Time System (Bio-Rad) as previously described (10, 15) in intestinal samples (ileum) and CD4+ lamina propria T cells obtained from breast-fed controls and mice submitted to experimental NEC. Total RNA was isolated using the RNeasy kit (QIAGEN) and reverse transcribed with the QuantiTect Reverse Transcription Kit (QIAGEN) as we have previously described (19). The relative expression of the genes listed in Table 1 was determined using RPLO as the housekeeping gene.
Table 1.
List of primers used for quantitative real-time PCR.
| Gene | Species | Forward sequence | Reverse sequence | Amplicon Size (bp) |
|---|---|---|---|---|
| Foxp3 | Mouse | AAGTACCACAATATGCGACCC | TCTGAAGTAGGCGAACATGC | 154 |
| IL-17 | Mouse | CCAGCTGATCAGGACGCGCA | TGAGGGATGATCGCTGCTGCC | 115 |
| IL-1β | Mouse | AGTGTGGATCCCAAGCAATACCCA | TGTCCTGACCACTGTTGTTTCCCA | 175 |
| IL-6 | Mouse | CCAATTTCCAATGCTCTCCT | ACCACAGTGAGGAATGTCCA | 182 |
| RPLO | Mouse | GGCGACCTGGAAGTCCAACT | CCATCAGCACCACAGCCTTC | 242 |
Statistical analysis
Determination of statistical significance was performed by 2-tailed Student's t test or ANOVA using Prism 6 software (GraphPad). Statistical significance was set at a p value of < 0.05. All quantitative data is presented as mean ± SD. All experiments were performed at least in triplicate, with at least 5 pups per group for experimental NEC.
RESULTS
Enteral administration of retinoic acid prevents the development of necrotizing enterocolitis
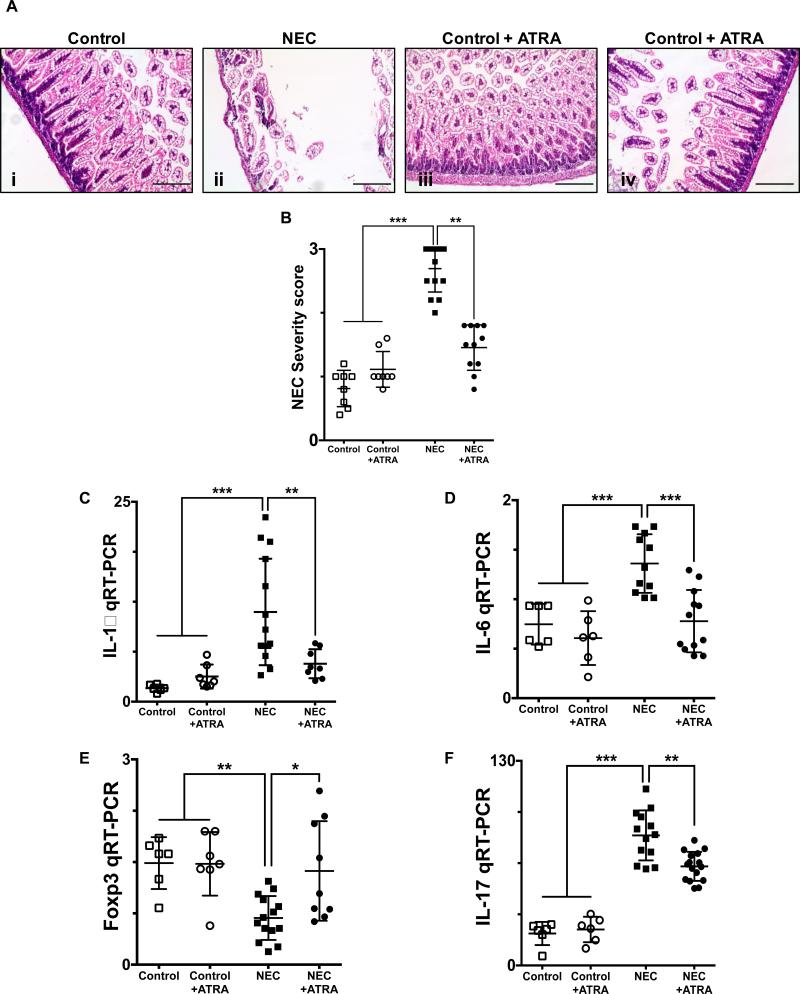
We first sought to determine the effect of all-trans retinoic acid (ATRA) supplementation of the feeding formula on the development and severity of NEC. As depicted in Fig. 1A, ATRA had a protective effect on the ileum of mice exposed to experimental NEC, evidenced by preservation of the normal histological architecture of the intestinal mucosa and decreased injury severity (Fig. 1B) consistent with our earlier findings (10). Furthermore, ATRA prevented the characteristic NEC-induced proinflammatory response within the ileum, which was evidenced by the decreased expression of the inflammatory cytokines interleukin (IL)-1β and IL-6 (Fig. 1, C and D, respectively). To further determine the effects of ATRA on the development of NEC we analyzed the lymphocyte infiltration of the lamina propria, which we have recently described to characterize the disease (10). Our analysis demonstrated that ATRA treatment was associated with preserved levels of regulatory T lymphocytes (Tregs), as evidenced by the transcriptional profile of the CD4+ T cells isolated from the lamina propria demonstrating an enrichment of Foxp3 expressing cells (Fig. 1E). On the other hand, ATRA led to decreased induction of Th17 cells, as evidenced by the low levels of IL-17 expression compared to experimental NEC alone (Fig. 1F), consistent with our earlier studies(10).
Fig. 1. Enteral administration of retinoic acid prevents the development of necrotizing enterocolitis.
A, H&E sections of the terminal ileum obtained from control mice that did not (i) or did (iii) receive ATRA (50 μg/mouse orally per day) and mice submitted to experimental NEC in the absence (ii) or presence (iv) of ATRA. Histological evaluation demonstrates a preserved intestinal mucosa in mice that received ATRA during the course of induction of experimental NEC. B, Injury severity score as determined by a pathologist blinded to the experimental conditions, demonstrates a significant decrease in the incidence and severity of experimental NEC in the presence of ATRA intervention. Gene expression analysis of the terminal ileum (C – D) or lamina propria isolated CD4+ T cells (E – F), obtained from control mice and mice submitted to experimental NEC in the absence or presence of ATRA. Inflammatory mediators IL-1β (C) and IL-6 (D) demonstrate a significant decrease in the inflammatory response associated with NEC in animals that received ATRA. Furthermore, CD4+ T cell gene expression profile was evaluated using Foxp3 (E) and IL-17 (F) expression; demonstrating preserved levels of Tregs (E) and absence of Th17 induction (F) among animals that were exposed to NEC and received oral ATRA. Scale bar = 50μm. Data depicted is presented as mean ± SD and represents 3 independent experiments with at least 10 mice per group. *P ≤ 0.05, **P ≤ 0.001, ***P ≤ 0.0001 determined by ANOVA followed by Tukey's multiple comparisons test.
Enteral administration of retinoic acid prevents the induction of crypt apoptosis and preserves the proliferative capacity of the crypt-based intestinal stem cells in reducing the severity of experimental NEC
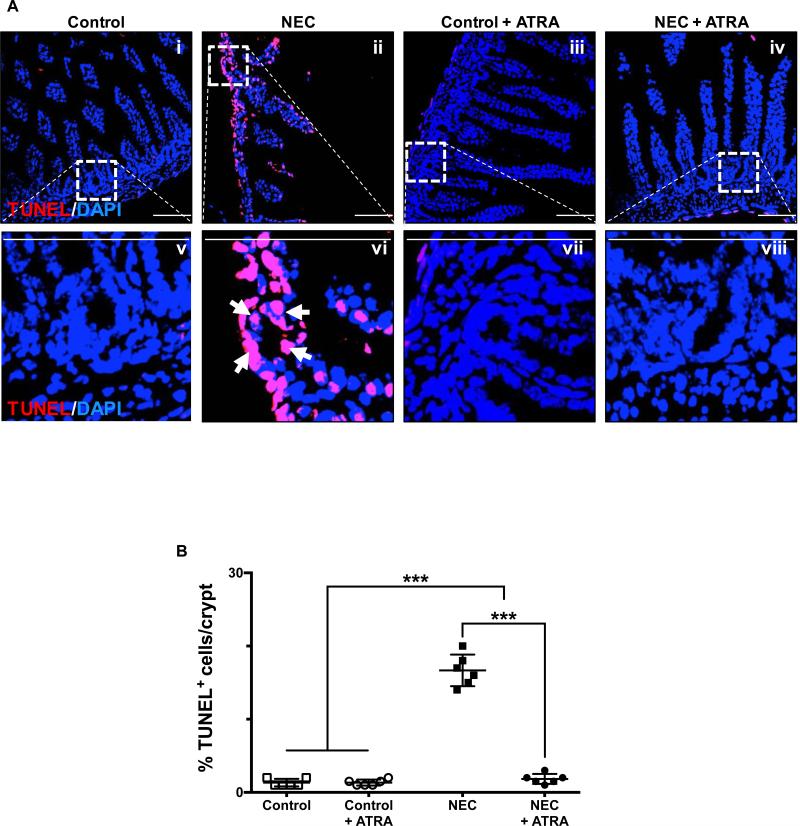
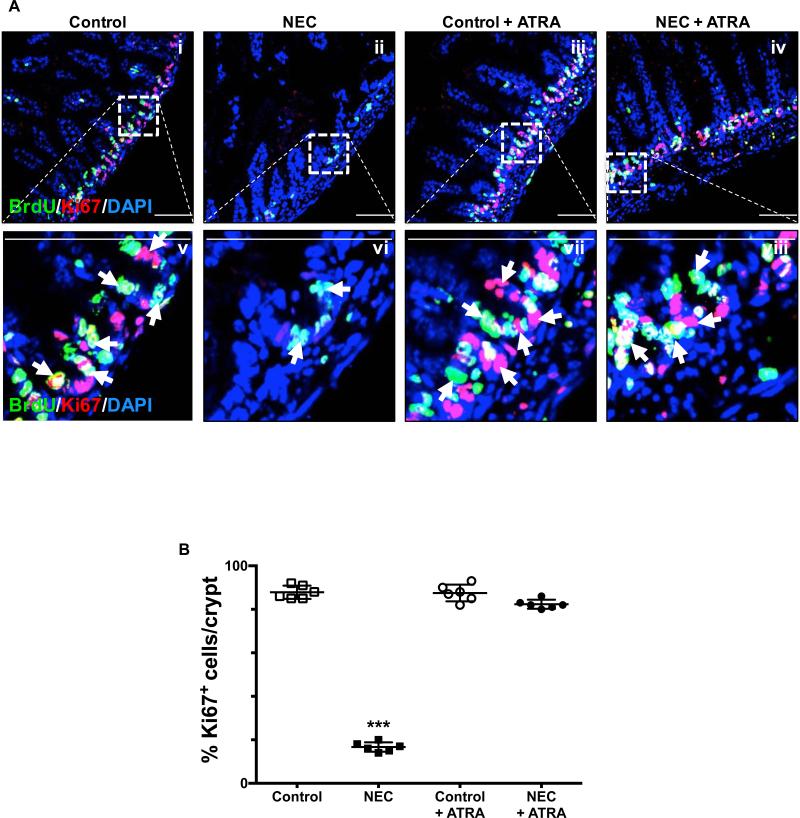
In seeking to understand the potential implications of our findings with ATRA on the premature intestinal mucosa, we assessed the level of apoptosis and proliferative capacity of the crypt-based Lgr5+ intestinal stem cells (ISCs) pool, which have been shown to give rise to all the major subtypes of cells present within the intestinal mucosa (20-22). We found that administration of ATRA during induction of experimental NEC led to decreased levels of crypt apoptosis as evidence by TUNEL staining of terminal ileum sections (Fig. 2A-B). Furthermore, the proliferative capacity of the crypt-based ISCs was assessed by BrdU label incorporation and Ki67 staining and found to be preserved in those mice that receive ATRA despite being submitted to the experimental model of the disease (Fig. 3A-B).
Fig. 2. Enteral administration of retinoic acid attenuates the effect of necrotizing enterocolitis by preventing the induction of apoptosis of crypt-based intestinal stem cells.
A, confocal images of TUNEL stained sections of the terminal ileum obtained from control mice that did not (i and v) or did (iii and vii) receive ATRA (50 μg/mouse orally per day) and mice submitted to experimental NEC in the absence (ii and vi) or presence (iv and viii) of ATRA. The outlined areas in i – iv are shown at a higher magnification in v – viii. Apoptotic cells within the crypts are identified by arrows and demonstrate the significant effect that ATRA has on the induction of NEC by preventing cell death within the ISC population. B, TUNEL staining intensity was quantified as described in the methods section and expressed as a percentage of the total number of cells identified within the crypts per high-magnification field. Scale bar = 50μm. Data depicted is presented as mean ± SD and represents 3 independent experiments with at least 10 mice per group. ***P ≤ 0.0001 determined by ANOVA followed by Tukey's multiple comparisons.
Fig. 3. Enteral administration of retinoic acid preserves the proliferative capacity of crypt-based intestinal stem cells.
A, the proliferative capacity of crypt-based ISCs was assessed by BrdU label incorporation and Ki67 staining of terminal ileum sections obtained from control mice that did not (i and v) or did (iii and vii) receive ATRA (50 μg/mouse orally per day) and mice submitted to experimental NEC in the absence (ii and vi) or presence (iv and viii) of ATRA. The outlined areas in i – iv are shown at a higher magnification in v – viii. Proliferating cells are indicated by arrows and demonstrate a conserved stable pool of dividing cells within the crypts. B, Ki67 staining was quantified as described in the methods section and is expressed as a percentage of the total number of cells identified within the crypts per high-magnification field. Scale bar = 50μm. Data depicted is presented as mean ± SD and represents 3 independent experiments with at least 10 mice per group. ***P ≤ 0.0001 determined by ANOVA followed by Tukey's multiple comparisons.
IL-17 impairs intestinal stem cell proliferation and differentiation and induces intestinal stem cell death
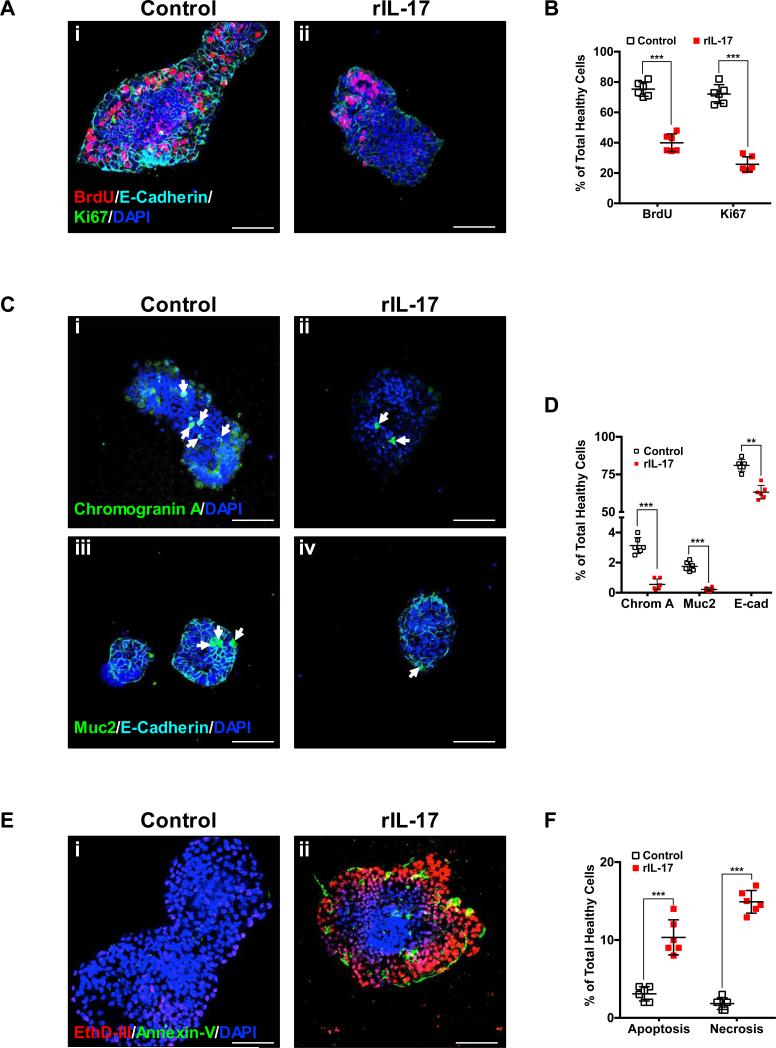
Having shown that experimental and human NEC is characterized by increased infiltration of Th17 cells and depletion of Tregs in the lamina propria(10), we next sought to determine the effect of the main cytokine released by Th17 cells (i.e. IL-17) as on the biology of isolated ISCs. For this purpose we generated enteroids from the small intestines of neonatal C57Bl/6 mice, and exposed these enteroids in culture to recombinant IL-17 (rIL-17, 100 ng/mL for 6 h) or vehicle. Our studies demonstrated that cell proliferation (Fig. 4, A and B) and the differentiation towards chromogranin or muc2-positive progeny (Fig. 4, C and D) were both significantly reduced when enteroids were exposed to rIL-17 (Fig. 4, Aii, C ii and Civ) as compared to saline. Furthermore, exposure of enteroids to rIL-17 significantly increased apoptosis and necrosis as demonstrated by the increased binding of annexin V and increased permeability to the DNA probe EthD-III, respectively (Fig. 4Eii).
Fig. 4. IL-17 is detrimental to ISC homeostasis by inhibiting cell proliferation and differentiation and leading to apoptosis/necrosis.
The effects of the main cytokine released by Th17 cells (i.e. IL-17) on ISC biology was evaluated using enteroids generated from mouse ileum ISCs and exposed to recombinant IL-17 (rIL-17, 100 ng/mL for 6 h). Cell proliferation (A and B), cell differentiation (C and D) and apoptosis/necrosis (E and F) were assessed as described in the methods section. A, cell proliferation was evaluated in control and rIL-17 exposed enteroids using incorporation of BrdU label as well as expression of the proliferation marker Ki67. Representative confocal microscopy images of control (i) and rIL-17 treated (ii) enteroids are shown. C, cell differentiation was assessed by immunostaining for the enteroendocrine marker chromogranin A, the goblet cell marker muc-2 and the enterocyte marker E-cadherin. Representative confocal microscopy images of control (i and iii) and rIL-17 treated (ii and iv) enteroids are shown. Chromogranin A (i and ii) and muc-2 (iii and iv) positive cells are indicated by arrows. E, apoptosis and necrosis were assessed using the apoptotic cell marker Annexin V and necrotic cells were identified by their permeability to the nucleic acid probe ethidium homodimer III (EthD-III). Representative confocal microscopy images of control (i) and rIL-17 treated (ii) enteroids are shown. B, D and F, quantification of fluorescence intensity and cell number was performed using FIJI software and depicted as the average percentage of the total number of healthy cells. The overall effect of IL-17 on enteroids derived from ISCs is characterized by a significant decrease in cellular proliferation and differentiation as well as increased apoptosis/necrosis. All images depicted are representative of three separate experiments with at least five enteroids analyzed/group. Scale bar = 100μm. Data depicted is presented as mean ± SD. **P ≤ 0.01, ***P ≤ 0.001 determined by Student's t test.
Depletion of Foxp3+ regulatory T cells leads to intestinal crypt apoptosis and exacerbates the development of experimental necrotizing enterocolitis
We next sought to determine the role of Tregs on the degree of apoptosis in the intestinal crypts, and on the development and severity of NEC. To do so, we depleted Foxp3+ regulatory T cells (24, 25) using a technique shown in Figure 5A by breeding mice that express the human diphtheria toxin receptor (DTR) under the control of the promoter for the Treg dependent transcription factor forkhead box P3 (Foxp3) (23)(25, 26). The subsequent administration of diphtheria toxin (DT) leads to ablation of the Treg population (24, 26). As depicted in Fig. 5B, the administration of diphtheria toxin led to a significant decrease in the number of CD4+Foxp3+ T cells within the lamina propria as compared to control mice that were injected with saline. The effectiveness of Foxp3+ cellular depletion was further confirmed by real time qRT-PCR analysis of Foxp3 gene expression in CD4+ T cells (Fig. 5C), which were isolated by positive selection using magnetic anti-CD4 beads, and performed as we have previously shown (10).
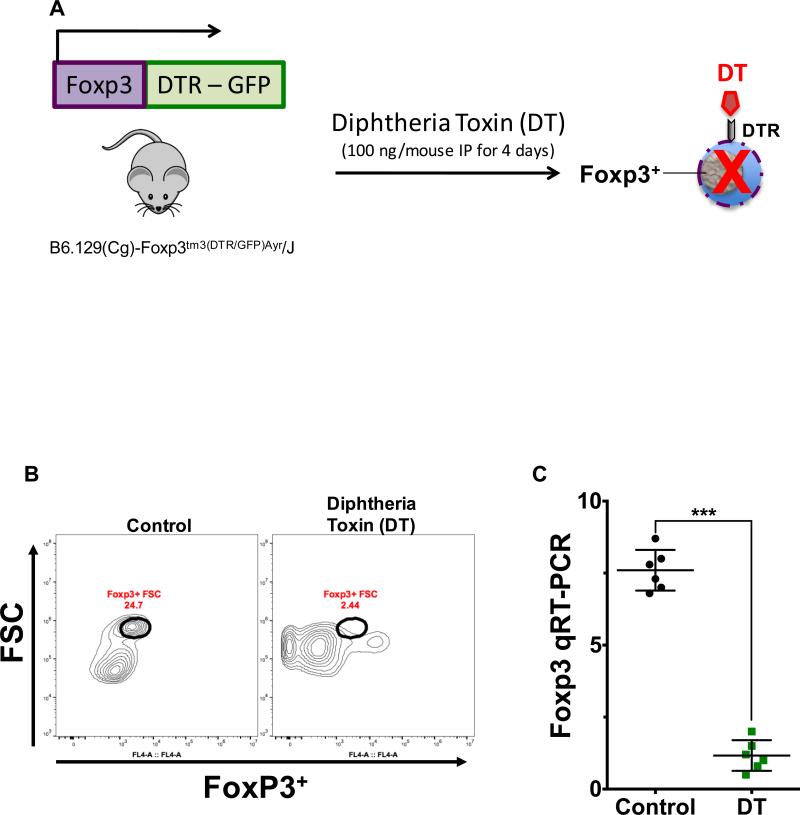
Fig. 5. Diphtheria toxin-induced depletion of Foxp3+ regulatory T cells.
A, schematic diagram depicts the experimental strategy as described in the methods section utilized for the depletion of Foxp3+ regulatory T cells (Tregs) in Foxp3DTR mice. Depletion of this cell population was confirmed by flow cytometry (B) and qRT-PCR (C). B, representative plot demonstrates the depletion of Tregs within the lamina propria of the terminal ileum obtained from Foxp3DTR mice that received diphtheria toxin (DT 100 ng/mouse IP, daily for 4 days). Flow cytometric analysis was performed on CD4+ T cells, which were magnetically isolated by positive selection using anti-CD4 beads. Control samples were obtained from mice that received saline. C, depletion of Tregs was further confirmed by qRT-PCR analysis of Foxp3 expression in the purified cell population obtained after magnetic isolation as described above. Data depicted in (C) is presented as mean ± SD and represents 3 independent experiments with at least 6 mice per group. ***P ≤ 0.0001 determined by Student's t test.
Having confirmed the level of depletion of Tregs within the lamina propria of the terminal ileum after DT injection (Fig. 5, B and C), we proceeded to evaluate the role of Tregs in the development and severity of NEC (Fig. 6 and Fig. 7). Foxp3+DTR mice were submitted to our previously described experimental model to induce NEC (7, 10) while receiving diphtheria toxin for the duration of the model as described in the methods section. Depletion of Tregs leads to increased NEC severity as evidenced by H&E histological assessment (Fig. 6A), as well as increased level of crypt apoptosis (Fig. 6, Biv, Bviii and C) and increased injury severity score (Fig. 6D). While cell proliferation was significantly decreased in both wild-type and Foxp3+DTR mice exposed to the NEC model (Fig. 7, A and B), no significant difference in the number of Ki67 positive crypt-cells was found between the two mouse strains.
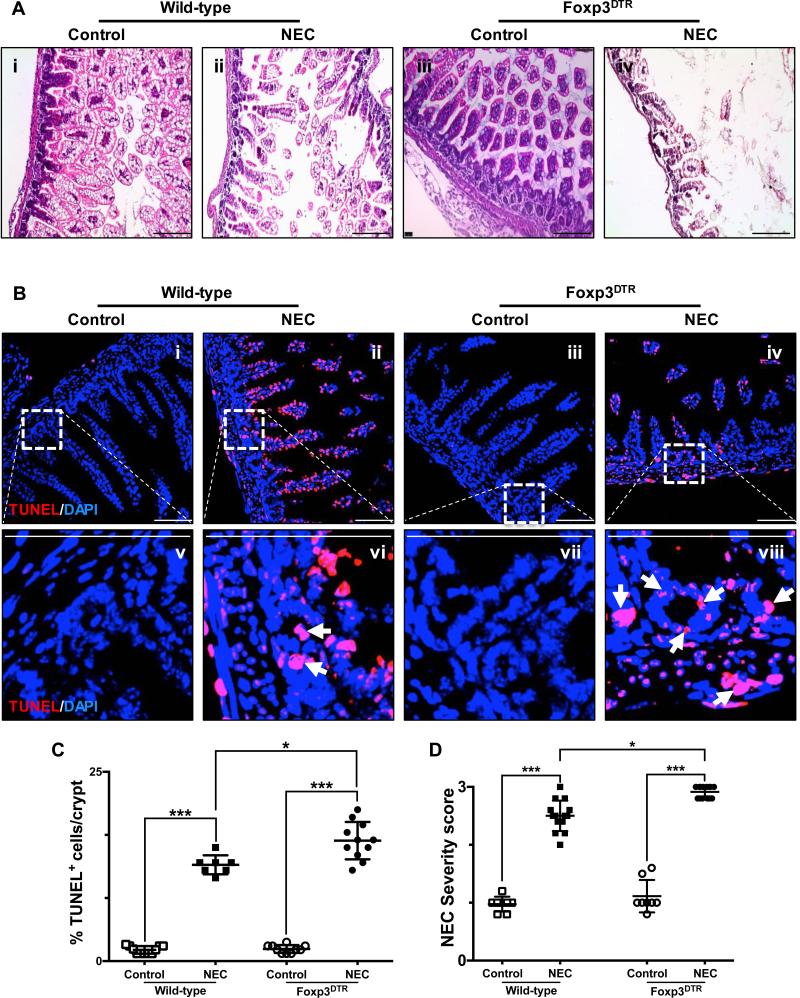
Fig. 6. Depletion of regulatory T cells exacerbates the development of experimental necrotizing enterocolitis.
A, H&E sections of the terminal ileum obtained from wild-type C57Bl/6 and Foxp3+DTR mice submitted to experimental NEC (ii and iv, respectively) or breast-fed controls (i and iii, respectively). B, confocal images of TUNEL stained sections of the terminal ileum obtained from wild-type C57Bl/6 and Foxp3+DTR mice submitted to experimental NEC (ii, vi and iv, viii respectively) or breast-fed controls (i, v and iii, vii respectively). The outlined areas in i – iv are shown at a higher magnification in v – viii. Apoptotic cells within the crypts are identified by arrows and demonstrate the significant effect that depletion of Foxp3+ T cells has on the induction and severity of NEC, exacerbating the damage to the crypt-based ISC population. TUNEL staining intensity (C) was quantified as described in the methods section and is expressed as a percentage of the total number of cells identified within the crypts per high-magnification field. D, injury severity score as determined by a pathologist blinded to the experimental conditions, demonstrates a significant increase in the incidence and severity of experimental NEC among Foxp3+DTR mice. Scale bar = 50μm. Data depicted is presented as mean ± SD and represents 3 independent experiments with at least 10 mice per group. *P ≤ 0.05, ***P ≤ 0.0001 determined by ANOVA followed by Tukey's multiple comparisons.
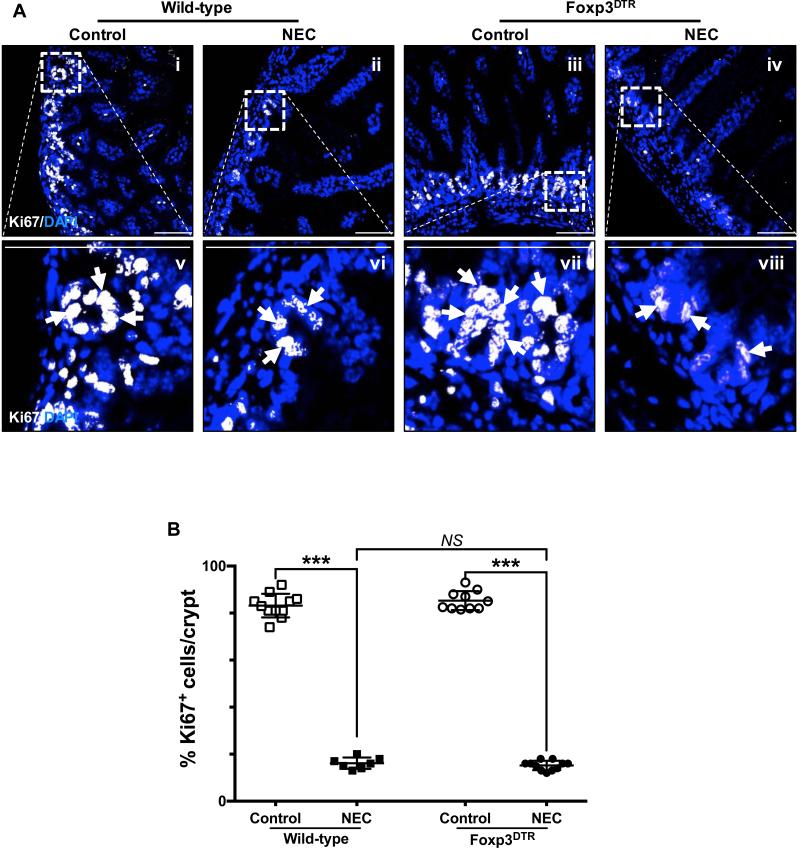
Fig. 7. Depletion of regulatory T cells exacerbates the development of experimental necrotizing enterocolitis by decreasing the proliferative capacity of crypt-based intestinal stem cells.
A, the proliferative capacity of crypt-based ISCs was assessed by Ki67 staining of terminal ileum sections obtained from wild-type C57Bl/6 and Foxp3+DTR mice submitted to experimental NEC (ii, vi and iv, viii respectively) or breast-fed controls (i, v and iii, vii respectively). The outlined areas in i – iv are shown at a higher magnification in v – viii. Proliferating cells are indicated by arrows and demonstrate a significant decrease of dividing cells within the crypts of mice submitted to NEC. B, Ki67 staining was quantified as described in the methods section and expressed as a percentage of the total number of cells identified within the crypts per high-magnification field. Scale bar = 50μm. Data depicted is presented as mean ± SD and represents 3 independent experiments with at least 10 mice per group. ***P ≤ 0.0001 determined by ANOVA followed by Tukey's multiple comparisons.
DISCUSSION
Necrotizing enterocolitis is the most lethal gastrointestinal disease that affects premature infants (1, 2). Despite promising advancements in the field, effective treatment and preventive strategies remain elusive, primarily due to the lack of a clear understanding of the cellular and molecular pathogenesis of the disease, which has hampered the identification of specifically targeted interventions. Our lab has recently identified the critical role that the innate immune receptor toll-like receptor 4 (TLR4) plays in modulating the influx of T lymphocytes into the newborn intestinal mucosa, which characterizes the development of the disease (10). The study presented here provides further evidence of the role of T lymphocyte balance within the newborn intestinal mucosa in the development and severity of NEC. In particular, our previous report (10) had demonstrated that NEC leads to induction of highly inflammatory Th17 cells with concomitant depletion of Tregs. These results are consistent with recent reports from human studies demonstrating decreased levels of Tregs within the lamina propria of the intestine of premature infants afflicted with NEC (23). Interestingly, the balance between these two opposing cell populations has been shown to be of critical relevance in the development of autoimmunity (27) and inflammatory bowel disease (28) further demonstrating the relevance of these cell populations in health and disease. Strikingly, this imbalance can be modulated by enteral administration of the vitamin A metabolite, retinoic acid (Fig. 1), which as we had previously shown (10) prevents the induction of Th17 cells and promotes the expression of the transcription factor Foxp3 and the subsequent regulatory T cell phenotype, ultimately preventing the development of this devastating disease (Figs. 1 – 3).
We have now focused our attention on two critical aspects of the disease process, namely the specific contribution to the pathogenesis of NEC by the two cell types involved (i.e. Th17 cells – Fig. 4 and Tregs – Fig. 6 and Fig. 7) and the subsequent implication on the homeostasis of the intestinal stem cell population available for the healing process to occur. Our study demonstrates in the context of NEC the specific contribution of the increased activation of Th17 cells and depletion of Tregs on the viability of the crypt-based ISCs. Furthermore, we demonstrate that interventions, such as the administration of retinoic acid (Figs. 1 – 3), that modulate the fate of naïve T cells can be highly effective in the prevention and treatment of this devastating disease. In reaching these conclusions we have relied heavily on the use of intestinal enteroids derived from stem cells. The use of enteroids as a model system has been extensively validated in our lab (8, 10, 12) and several others (11, 29, 30) for the study of ISCs biology and physiology. Our findings clearly demonstrate the detrimental effect of IL-17 on ISC biology as enteroids exposed to this cytokine display a decreased rate of proliferation (Fig. 4, A and B), differentiation (Fig. 4, C and D) as well as an increased rate of apoptosis and necrosis (Fig. 4, E and F). These findings are supported by our previous observations using IEC-6 cells (10), which are immature crypt-derived cells. In these cells as well as we now demonstrate in enteroids, rIL-17 has a profound detrimental effect on viability and cellular proliferation and differentiation (10). These results were further validation of our previous observations in mice in which intraperitoneal injection of IL-17A led to increased crypt apoptosis and decreased enterocyte proliferation (10). Effects that were demonstrated by Egan et al. to be prevented by administration of an anti-IL-17 receptor (IL-17R) antibody (10). Taken together these data demonstrate the critical role of IL-17 in the development of the mucosal injury associated with NEC. Furthermore, our results are consistent with current literature demonstrating the detrimental effects of Th17 cells in other pathologies of the gut such as inflammatory bowel disease (31, 32).
We have now further established a critical role for T cells in the maintenance of normal ISCs during NEC, by showing that Treg depletion leads to large scale crypt destruction in this disease. Interestingly, no significant difference in cell proliferation, as evaluated by the number of Ki67 positive cells within the intestinal crypts, was found between wild-type and Foxp3+DTR mice exposed to the NEC model. Further studies are required to determine why Tregs play a role in preventing cell death but not maintaining the proliferative capacity of these cells, although one could speculate that those very factors responsible for ISC function, including Notch, Wnt, and beta catenin, may be influenced by IL-17 to some degree (7, 10, 33).
In summary, our studies provide evidence for the development of enteral administration strategies that could serve to overcome the pathogenesis of this devastating disease by modulating the phenotypic characteristics of the cellular mediators of NEC.
Acknowledgments
Funding sources: DJH is supported by R01GM078238 and R01DK083752 from the National Institutes of Health.
ABBREVIATIONS
- ATRA
all-trans retinoic acid
- Foxp3
forkhead box P3
- ISC
intestinal stem cell
- NEC
necrotizing enterocolitis
Footnotes
Conflict of Interest Statement: The authors have declared that no conflict of interest exists.
REFERENCES
- 1.Nino DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016 doi: 10.1038/nrgastro.2016.119. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Laptook AR, Sanchez PJ, Van Meurs KP, Wyckoff M, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015;314(10):1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stey A, Barnert ES, Tseng CH, Keeler E, Needleman J, Leng M, Kelley-Quon LI, Shew SB. Outcomes and costs of surgical treatments of necrotizing enterocolitis. Pediatrics. 2015;135(5):e1190–1197. doi: 10.1542/peds.2014-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand RJ, Leaphart CL, Mollen KP, Hackam DJ. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock. 2007;27(2):124–133. doi: 10.1097/01.shk.0000239774.02904.65. [DOI] [PubMed] [Google Scholar]
- 6.Lu P, Sodhi CP, Hackam DJ. Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis. Pathophysiology. 2014;21(1):81–93. doi: 10.1016/j.pathophys.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179(7):4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 8.Neal MD, Sodhi CP, Jia H, Dyer M, Egan CE, Yazji I, Good M, Afrazi A, Marino R, Slagle D, et al. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J Biol Chem. 2012;287(44):37296–37308. doi: 10.1074/jbc.M112.375881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afrazi A, Branca MF, Sodhi CP, Good M, Yamaguchi Y, Egan CE, Lu P, Jia H, Shaffiey S, Lin J, et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J Biol Chem. 2014;289(14):9584–9599. doi: 10.1074/jbc.M113.526517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, Lu P, Ma C, Branca MF, Weyandt S, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest. 2016;126(2):495–508. doi: 10.1172/JCI83356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 12.Shaffiey SA, Jia H, Keane T, Costello C, Wasserman D, Quidgley M, Dziki J, Badylak S, Sodhi CP, March JC, et al. Intestinal stem cell growth and differentiation on a tubular scaffold with evaluation in small and large animals. Regen Med. 2016;11(1):45–61. doi: 10.2217/rme.15.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yazji I, Sodhi CP, Lee EK, Good M, Egan CE, Afrazi A, Neal MD, Jia H, Lin J, Ma C, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci U S A. 2013;110(23):9451–9456. doi: 10.1073/pnas.1219997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T, Jr., Branca M, Russo A, Gribar SC, Ma C, et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology. 2010;138(1):185–196. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer CT, Lahl K, Milanez-Almeida P, Watts D, Dittmer U, Fyhrquist N, Huehn J, Kopf M, Kretschmer K, Rouse B, et al. Advantages of Foxp3(+) regulatory T cell depletion using DEREG mice. Immun Inflamm Dis. 2014;2(3):162–165. doi: 10.1002/iid3.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheridan BS, Lefrancois L. Isolation of mouse lymphocytes from small intestine tissues. Curr Protoc Immunol. 2012;19 doi: 10.1002/0471142735.im0319s99. Chapter 3:Unit3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nino DF, Cauvi DM, De Maio A. Itraconazole, a commonly used antifungal, inhibits Fcgamma receptor-mediated phagocytosis: alteration of Fcgamma receptor glycosylation and gene expression. Shock. 2014;42(1):52–59. doi: 10.1097/SHK.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Good M, Sodhi CP, Egan CE, Afrazi A, Jia H, Yamaguchi Y, Lu P, Branca MF, Ma C, Prindle T, Jr., et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 2015;8(5):1166–1179. doi: 10.1038/mi.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346(6205):1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 21.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7(5):349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 22.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15(1):19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 23.Weitkamp JH, Koyama T, Rock MT, Correa H, Goettel JA, Matta P, Oswald-Richter K, Rosen MJ, Engelhardt BG, Moore DJ, et al. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut. 2013;62(1):73–82. doi: 10.1136/gutjnl-2011-301551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204(1):57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Lahl K, Hori S, Loddenkemper C, Chaudhry A, deRoos P, Rudensky A, Sparwasser T. Cutting edge: depletion of Foxp3+ cells leads to induction of autoimmunity by specific ablation of regulatory T cells in genetically targeted mice. J Immunol. 2009;183(12):7631–7634. doi: 10.4049/jimmunol.0804308. [DOI] [PubMed] [Google Scholar]
- 26.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 27.Eisenstein EM, Williams CB. The T(reg)/Th17 cell balance: a new paradigm for autoimmunity. Pediatr Res. 2009;65(5 Pt 2):26R–31R. doi: 10.1203/PDR.0b013e31819e76c7. [DOI] [PubMed] [Google Scholar]
- 28.Yamada A, Arakaki R, Saito M, Tsunematsu T, Kudo Y, Ishimaru N. Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2016;22(7):2195–2205. doi: 10.3748/wjg.v22.i7.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stelzner M, Helmrath M, Dunn JC, Henning SJ, Houchen CW, Kuo C, Lynch J, Li L, Magness ST, Martin MG, et al. A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol. 2012;302(12):G1359–1363. doi: 10.1152/ajpgi.00493.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foulke-Abel J, In J, Yin J, Zachos NC, Kovbasnjuk O, Estes MK, de Jonge H, Donowitz M. Human Enteroids as a Model of Upper Small Intestinal Ion Transport Physiology and Pathophysiology. Gastroenterology. 2016;150(3):638–649. e638. doi: 10.1053/j.gastro.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12(5):382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 32.Troncone E, Marafini I, Pallone F, Monteleone G. Th17 cytokines in inflammatory bowel diseases: discerning the good from the bad. Int Rev Immunol. 2013;32(5-6):526–533. doi: 10.3109/08830185.2013.823421. [DOI] [PubMed] [Google Scholar]
- 33.Markel TA, Crisostomo PR, Wairiuko GM, Pitcher J, Tsai BM, Meldrum DR. Cytokines in necrotizing enterocolitis. Shock. 2006;25(4):329–337. doi: 10.1097/01.shk.0000192126.33823.87. [DOI] [PubMed] [Google Scholar]