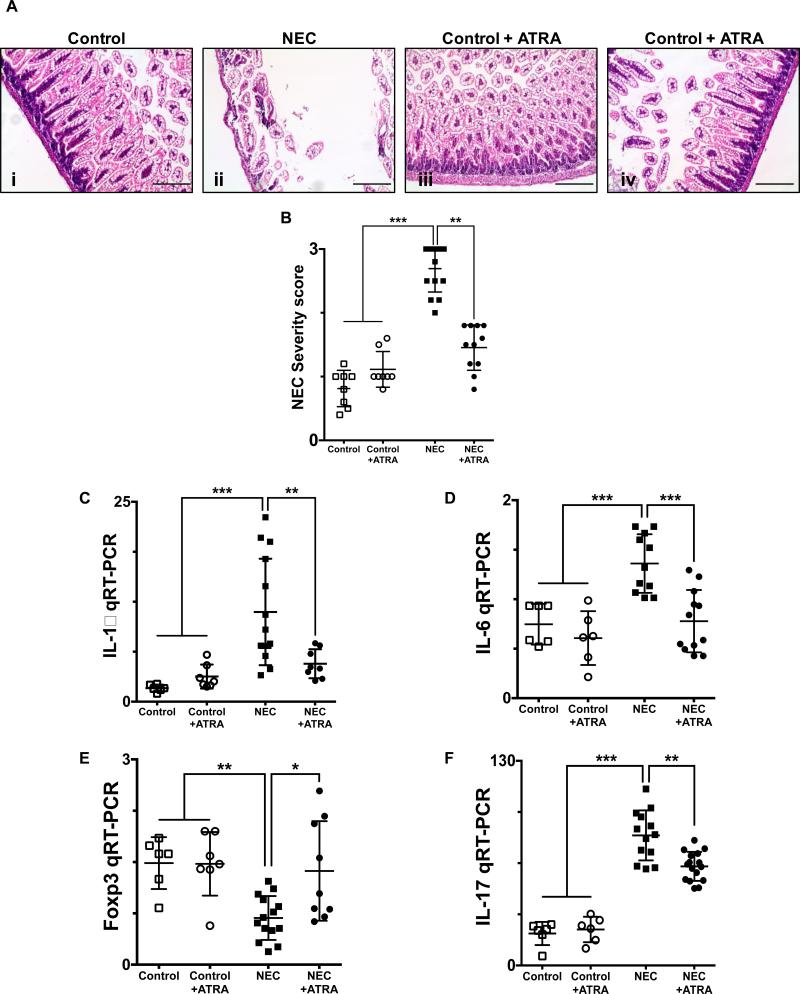
Fig. 1. Enteral administration of retinoic acid prevents the development of necrotizing enterocolitis.
A, H&E sections of the terminal ileum obtained from control mice that did not (i) or did (iii) receive ATRA (50 μg/mouse orally per day) and mice submitted to experimental NEC in the absence (ii) or presence (iv) of ATRA. Histological evaluation demonstrates a preserved intestinal mucosa in mice that received ATRA during the course of induction of experimental NEC. B, Injury severity score as determined by a pathologist blinded to the experimental conditions, demonstrates a significant decrease in the incidence and severity of experimental NEC in the presence of ATRA intervention. Gene expression analysis of the terminal ileum (C – D) or lamina propria isolated CD4+ T cells (E – F), obtained from control mice and mice submitted to experimental NEC in the absence or presence of ATRA. Inflammatory mediators IL-1β (C) and IL-6 (D) demonstrate a significant decrease in the inflammatory response associated with NEC in animals that received ATRA. Furthermore, CD4+ T cell gene expression profile was evaluated using Foxp3 (E) and IL-17 (F) expression; demonstrating preserved levels of Tregs (E) and absence of Th17 induction (F) among animals that were exposed to NEC and received oral ATRA. Scale bar = 50μm. Data depicted is presented as mean ± SD and represents 3 independent experiments with at least 10 mice per group. *P ≤ 0.05, **P ≤ 0.001, ***P ≤ 0.0001 determined by ANOVA followed by Tukey's multiple comparisons test.