Abstract
Introduction
Muscle wasting (MW) in catabolic conditions (e.g. burn injury, BI) is a major risk factor affecting prognosis. Activation of IL-1β/NF-κB, IL-6/STAT3 and/or FoxO-mediated gene transcription pathways is the pivotal trigger of inflammatory response-induced protein catabolic processes in muscle. The α7 acetylcholine receptors (α7AChRs) are up-regulated in macrophages and peripheral tissues including skeletal muscle during MW conditions. Stimulation of α7AChRs mitigates inflammatory responses. Hypothesis tested is that attenuation of inflammation by α7AChRs stimulation with specific α7AChRs agonist, GTS-21, will reverse BI-induced body mass and MW by modulating inflammatory and proteolytic signals.
Methods
Body surface area (30%) BI or Sham BI mice were treated with GTS-21 or saline. Tibialis anterior (TA) muscle was harvested at 6 hours (6h), day 1 or 3 to examine inflammatory and proteolytic signals.
Results
GTS-21 significantly ameliorated the BI-induced increased expression of inflammatory cytokines IL-6, IL-1β, CXCL2 (6h), phosphorylated STAT3 and NF-κB (Day1) in TA muscle. GTS-21 also significantly inhibited BI-induced increase of MuRF1 and FOXO1 (Day1). Consistent with the cytokine and inflammatory mediator changes, BI-induced body weight and TA muscle mass loss at Day3 were mitigated by GTS-21 treatment. The beneficial effect of GTS-21 on BI changes were absent in methyllycaconitine (α7AChR antagonist) treated WT and α7AChR knock out mice.
Conclusion
GTS-21 stimulation of α7AChRs, by modulating multiple molecular signals related to inflammation and proteolysis, attenuates protein wasting, evidenced by maintenance of body weight and attenuation of distant muscle mass loss after BI. GTS-21 can be a novel, potent therapeutic option for reversal of BI-induced MW.
Introduction
Muscle wasting (MW) is seen in various muscle catabolic states including burn injury (BI), sepsis, cancer cachexia, immobilization and denervation (1-5). BI is a paradigm of critical illness-induced catabolic state. In severely burned patients, muscles distant from burned site are often affected because of systemic inflammatory responses and associated metabolic derangements (6). The muscle mass loss and consequent decreased tension generating capacity (muscle weakness) leads to hypoventilation with dependence on mechanical ventilator. The increased dependence on ventilators leads to ventilator-associated pneumonia and thromboembolic complications, and/or nosocomial sepsis, worsened prognosis and even death. The muscle weakness also prolongs hospitalization and after-hospital rehabilitation.
Skeletal muscle protein content is maintained by a dynamic balance between protein synthesis and degradation (7). Muscle protein degradation is characterized by increased expression of the muscle-specific ubiquitin ligases, “atrogenes”, namely, MuRF1 and atrogin-1 which cause muscle proteolysis. Disease-induced increased glucocorticoids, oxidative stress, dysfunction of anabolic (insulin/insulin-like growth factor-I) signals and cytokines are known effectors of atrogenes upregulation, mediated by multiple upstream signal cascades with cross-talk between these cascades. (1, 7-9). For example, Forkhead box O transcriptional factors (FOXOs), including FOXO1 are well known regulators of muscle ubiquitin ligases (1). We previously documented that activation of Akt, a negative regulator of FOXO1 is impaired by BI (10). BI also induces activation of macrophages with release of inflammatory cytokines that lead to systemic inflammatory responses mediated via multiple downstream signaling pathways (6). Inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and other ligands lead to activation of the canonical nuclear factor-kappa B (NF-κB) pathway. Nuclear translocation of activated NF-κB in turn results in transcription of its target genes associated with acute stress responses, inflammation and muscle atrophy (11). In addition, the Janus kinase and signal transducer and activator of transcription (JAK/STAT) pathway are other recognized alternative pathway of inflammatory responses. Specifically, STAT3, one of seven STAT family members activated by interleukin-6 (IL-6), is associated with muscle atrophy (5, 12, 13). Thus, suppression of systemic inflammatory signal pathways has been suggested as an important strategy against inflammation-related muscle mass loss. However, recently, clinical trials with use of specific TNFα antibody or IL-6 inhibitor conducted in patients with cancer proved ineffective in mitigating cancer-related MW or cachectic condition (14, 15). These results indicate that inhibition of single cytokine will show limited effect, suggesting that multiple inflammatory cytokines are involved in muscle proteolysis during systemic catabolic states.
Over the last decade, it has been documented that stimulation of vagus nerve can mediate anti-inflammatory effects. For example, Wang et al. discovered that stimulation of the alpha7 acetylcholine receptors (α7AChRs) constitutively expressed in macrophages suppressed lipopolysaccharide (LPS)-induced TNF production (16). Inflammatory leucocytes including macrophages are known to infiltrate muscle tissue in MW conditions of systemic inflammatory origin or even in simple disuse atrophy in the absence of systemic inflammation (17, 18). Although the pluripotent pathways of anti-inflammatory actions of α7AChRs remains to be established, stimulation of α7AChRs mediates some of its critical anti-inflammatory signals by inhibiting two important signaling proteins, namely NF-κB and IL-6/STAT3; the downstream effects of these two proteins cause muscle proteolysis (19-23). Whether the cholinergic anti-inflammatory actions mediated via α7AChRs can modulate the augmented proteolysis observed in inflammatory catabolic condition through suppression of activated NF-κB and IL-6/STAT3 is unknown. Using a well-established specific α7AChRs agonist, GTS-21 (23-26), the current study tested the hypothesis that the stimulation of α7AChRs with GTS-21 attenuates inflammation-induced distant MW of BI.
Materials & Methods
Materials
GTS-21 was kindly provided by Dr. William R. Kem (University of Florida, FL). Wild type and α7AChR knock out (Chrna7−/−) mice on C57BL6/J background were purchased from The Jackson Laboratory (Bar Harbor, ME). RNeasy Plus Universal kit was from Qiagen (Germantown, MD). High Capacity RNA-to-cDNA Kit, Platinum SYBR Green qPCR SuperMix-UDG, ViiA7 Real-Time PCR System, Enzyme Linked Immunosorbent Assay (ELISA) kit for Mouse IL-6, Pierce 660nm Protein Assay kit, NuPAGE LDS Sample Buffer, and NuPAGE 4-12% Bis-Tris Gel were from ThermoFisher Life Technologies (Grand Island, NY). Primary antibodies against FOXO1, p-Stat3-Tyr705, p-NF-κB p65-Ser536 and NF-κB p65 were obtained from Cell Signaling Technologies (Danvers, MA), p-Stat3-Ser727, Stat3, MuRF1 and atrogin-1 were from Santa Cruz Biotechnology (Santa Cruz, CA), GAPDH was from Trevigen (Gaithersurg, MD). HRP-conjugated anti-rabbit IgG antibody was from EMD Millipore (Danvers, MA). Methyllycaconitine and Protease inhibitor Cocktail were obtained from Sigma (St. Louis, MO). Chemiluminescent detection kit was from Denville Scientific Inc. (Metuchen, NJ).
Burn Mice Model
The study protocol was approved by the Institutional Animal Care Committee at Massachusetts General Hospital (Protocol # 2013N000193). Adult male C57BL6/J mice of 22-27g body weight were housed individually for acclimation with free access to food and water for 5-7 days prior to burn or sham injury. Prior to each perturbation, the mice received 0.1 mg/kg of buprenorphine subcutaneously as analgesia and 50 mg/kg of pentobarbital administered intraperitoneally as the anesthetic. A 30% total body surface area full thickness BI was produced under anesthesia by immersing the abdomen for 6 second and both sides of flank for 4 second in 80°C water as described previously (27). Sham burned mice were immersed in lukewarm water after anesthesia. As fluid resuscitation, 1ml of normal saline was injected immediately after burn or sham burn procedure regardless of the treatment. Burned mice were permitted to consume food ad libitum and sham burned mice were pair fed.
Experimental Design
The mice received intraperitoneal injection of 10mg/kg of GTS-21 or equal volume of vehicle (normal saline) 1hour after burn or sham burn procedure and every 12 hours thereafter until the termination of experiment. Based on the drug regimen, the mice were divided into 4 groups: Sham-Vehicle, Sham-GTS, Burn-Vehicle and Burn-GTS. The same protocol was applied for all the following experiment. The dose of GTS-21 was based on previous studies in rodents and our own preliminary studies. To examine systemic effects of BI or Sham procedure, the body weight changes were noted, and the tibialis anterior (TA) muscle, a site distant from burned area, was harvested for assessment of muscle mass and biochemical changes. The TA was harvested because the effect of BI has been previously noted more in fast-twitch, glycolytic muscle such as TA compared to that seen on slow-twitch, oxidative muscle such as soleus, where the muscle loss was limited (28).
Four sets of analyses were performed: In a first set of experiments, cytokines mRNA expression in skeletal muscle and IL-6 protein levels in serum were assessed 6 hours after BI. According to previous studies with burns and LPS-induced septic model, cytokine levels are elevated only in the very early phase from 4 to 12 hours after the perturbation (29, 30). Therefore, we chose time point of 6 hours after BI to assess cytokine levels. In a second set of experiment, molecular signals of inflammatory and proteolytic pathways were examined at post burn day1 (PBD1). Our previous work documented that proteolytic and inflammatory signals begin to change significantly at day1 and peak at day 2 after burn (31). Meanwhile, our preliminary study also indicated that atrogenes expression were significantly elevated at PBD1 and returned to basal levels at post burn day 3 (PBD3, Supplemental Figure 1). Therefore, we focused on examining muscle signaling changes at PBD1 in the second experiment. In a third experiment, body weight and muscle mass change were assessed at PBD3. In contrast to molecular signal changes, based on previous studies, muscle mass loss is most significantly observed at or after 2 days compared to control. This is as a consequence of cumulative effects of daily negative net protein balance (9). Thus, in this study, body weight and muscle mass changes were observed at day 3.
For the first experiment where cytokine levels were measured, the mice were anesthetized by 50mg/kg of pentobarbital and cardiac puncture was performed to obtain blood samples, followed by collection of TA muscle samples (N=6 for Sham-Vehicle, 4 for Sham-GTS, 10 for Burn-Vehicle, 11 for Burn-GTS group). Blood samples were centrifuged at 3000g for 10 min at 4 °C, and the supernatant (serum) was stored at −80 °C for subsequent analyses. TA samples were immediately frozen in liquid nitrogen and stored at −80°C for analyses of mRNA expression of cytokines later.
In the second experiment, TA samples were harvested at PBD1 in the same manner as the first experiment to examine molecular signals of inflammatory and proteolytic pathways. The number of mice in each group for the second experiment was as follows: N=8 for Sham-Vehicle, 4 for Sham-GTS, 11 for Burn-Vehicle, 12 for Burn-GTS group.
The third experiment consisted of measurement of body weight and muscle mass weight loss at PBD3. BI or Sham BI was produced in the same manner and treated daily for 3 days with GTS-21 or saline. After measurement of body weights, TA muscle samples were harvested at PBD3 to examine muscle mass changes (N=4 for Sham-Vehicle, 4 for Sham-GTS, 8 for Burn-Vehicle, 7 for Burn-GTS group). TA muscle weights were normalized to initial (pre-burn) body weight as the body weight on day of harvest can be inaccurate because the BI-induced organomegaly can artifactually increase total body weight (6, 32).
In the fourth experiment, specificity of GTS-21 for α7AChRs was assessed by using methyllycaconitine (MLA), an antagonist for α7AChR or by the use of α7AChR knock out (Chrna7−/−) mice on C57BL6/J background (α7KO mice). A new set of burned mice were treated with or without 3mg/kg of MLA administered intraperitoneally 30min before GTS-21 treatment. The dose of MLA was based on a previous study. TA muscle was harvested 6 hours after BI and cytokines mRNA expression were measured in the same manner as the first experiment (N=8 for Burn-GTS, 7 for Burn-GTS-MLA group). In the second part of the fourth experiment, burned or sham-burned α7KO mice with or without 10mg/kg of GTS-21 treatment served to test the specificity of GTS-21 actions via α7AChRs. Measurement of TA muscle mass at PBD3 was used as the marker for the physiologic effects of GTS-21. The number of animals used for this component of the experiment was as follows: N=3 for Sham-Vehicle, 4 for Burn-Vehicle, 4 for Burn-GTS. The absence of Chrna7 mRNA in α7KO mice was confirmed by real-time polymerase chain reaction.
Real-time Polymerase Chain Reaction (RT-PCR)
Total RNA from TA was purified by using RNeasy Plus Universal kit and purity was confirmed using the Nanodrop ND-1000 Spectrophotometer. The first strand cDNA was synthesized from 1 μg of total RNA using High Capacity RNA-to-cDNA Kit. RT-PCR reactions were performed with ViiA7 Real-Time PCR System by using Platinum SYBR Green qPCR SuperMix-UDG with the following amplification parameters: 50°C 2min, 95°C 2min, followed by 40 cycles of 95°C for 15sec and 60°C for 30 sec. Primers used in this study are shown in Table 1. The mRNA levels were normalized to GAPDH levels and were expressed as arbitrary units (AU).
Table 1.
Primer sequences used for real-time PCR
| Target | Primer sequences (5’-3’) | Product size (bp) |
|---|---|---|
| IL-6 | Forward 5’-AGCCAGAGTCCTTCAGAGA-3’ Reverse 5’-TCCTTAGCCACTCCTTCTGT-3’ |
146 |
| IL-1β | Forward 5’-GCC CAT CCT CTG TGA CTC AT-3’ Reverse 5’- AGG CCA CAG GTA TTT TGT CG-3’ |
230 |
| CXCL2 | Forward 5′-CAG AAGTCA TAG CCA CTC TCA AG-3′ Reverse 5′-CTT TCC AGGTCA GTT AGC CTT-3′ |
119 |
| IL-10 | Forward 5′-GGT TGC CAA GCC TTA TCG GA-3’ Reverse 5′-ACC TGC TCC ACT GCC TTG CT-3’ |
191 |
| MuRF1 | Forward 5’-GCTACCTTCCTCTCAAGTGC-3’ Reverse 5’-CCTCTGCTATGTGTTCTAAGTCC-3’ |
136 |
| atrogin-1 | Forward 5’-TGAATAGCATCCAGATCAGCA-3’ Reverse 5’-GATGTTCAGTTGTAAGCACACAG-3’ |
80 |
| FOXO1 | Forward 5’-CTT CAA GGA TAA GGG CGA CAG-3’ Reverse 5’-AGT TCC TTC ATT CTG CAC TCG-3’ |
106 |
| GAPDH | Forward 5’-AATGGTGAAGGTCGGTGTG-3’ Reverse 5’-GTGGAGTCATACTGGAACATGTAG-3’ |
150 |
ELISA
Serum IL-6 levels were determined at 6h after BI or Sham BI by using Mouse IL-6 ELISA Kit according to the manufacturer's instructions. Briefly, appropriately diluted serum samples were applied to ELISA plate along with IL-6 standards. After binding of the peroxidase-conjugated antibody, the bound quantity was measured by the reaction of chromogenic substrate at 450nm.
Immunoblotting
Muscle samples were homogenized as described previously (2). Briefly, TA samples were homogenized in ice-cold homogenization buffer (20mM Tris HCl, pH 7.4, 150mM NaCl, 1mM EDTA, 2% Triton X-100, 1mM PMSF, 10mM sodium fluoride, 2mM sodium vanadate, 10mM sodium pyrophosphate, 1mM DTT, Protease inhibitor cocktail). Protein concentration of the total homogenate after centrifugation at 800g for 10 min was assessed and the homogenate was boiled with NuPAGE LDS Sample Buffer. Equal amounts of total protein were loaded onto NuPAGE 4-12% Bis-Tris Gel, electrophoresed and transferred to nitrocellulose membranes. The membranes were blocked and incubated overnight at 4°C with antibodies against Foxo1, p-Stat3-Ser727, p-Stat3-Tyr705, Stat3, p-NF-κB p65-Ser536, NF-κB p65, MuRF1, atrogin-1, GAPDH followed by incubation with HRP-conjugated anti-rabbit IgG antibody for 1 hour at room temperature. Immunoreactive proteins were visualized by using a chemiluminescent detection kit and the detected bands were quantified by NIH Image J software and normalized to those of GAPDH.
Statistics
Results are reported as means +/− SEM. Initially, using Kolmogorov-Smirnov test, the data were tested for normality. For all data that was normally distributed, comparison among groups was performed by one-way ANOVA followed by Tukey's post hoc test (Figs. 2-4). When the data were not normally distributed (non-parametric), the Kruskal-Wallis test was used followed by two-tailed two independent sample Mann-Whitney-Wilcoxon test (Fig. 1). Comparison between two groups was performed by Welch's t test (Fig. 5). Data were analyzed using GraphPad Prism version 6.07 and R Ver. 3.3.0. A value of p≤0.05 was considered statistically significant.
Figure 2. Phosphorylation of STAT3 induced in skeletal muscle with burn injury and its attenuation by GTS-21 treatment.
Phosphorylation status STAT3 at tyrosine 705 (A), serine 727 (B) was analyzed by immunoblotting and quantified after normalization to total STAT3, and phosphorylated NF-κB by normalized to total NF-κB (C) at post burn day 1. Phosphorylated STAT3 at tyrosine 705, serine 727 and phosphorylated NF-κB were increased in burn injury to 8.1, 2.2 and 1.8 fold compared to Sham-burn. These increases were significantly ameliorated by GTS-21 treatment (from 8.1 to 4.1, 2.2 to 1.4 and 1.8 to 1.0 fold, respectively. *: p≤0.05 vs Sham-Vehicle, #: p≤0.05 vs Burn-Vehicle N=8 for Sham-Vehicle, 4 for Sham-GTS, 11 for Burn-Vehicle, 12 for Burn-GTS. The fold increase was calculated by comparing to the value of Sham-Vehicle group.
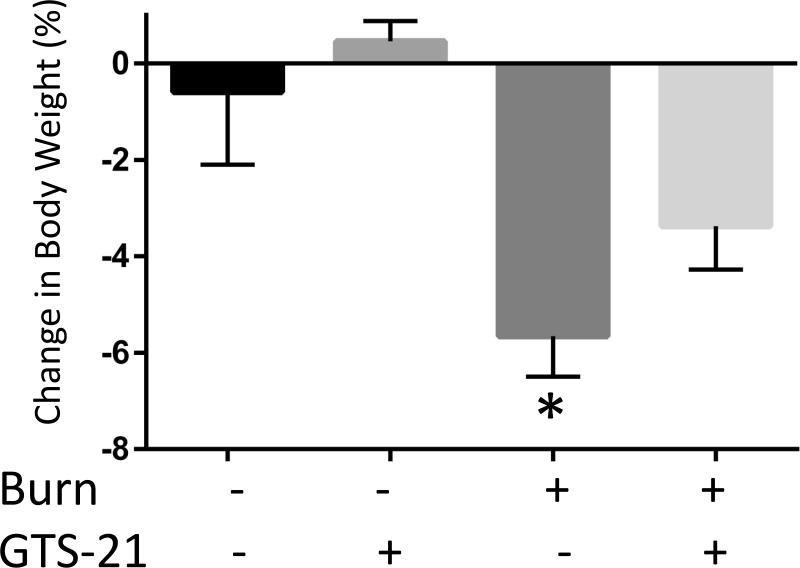
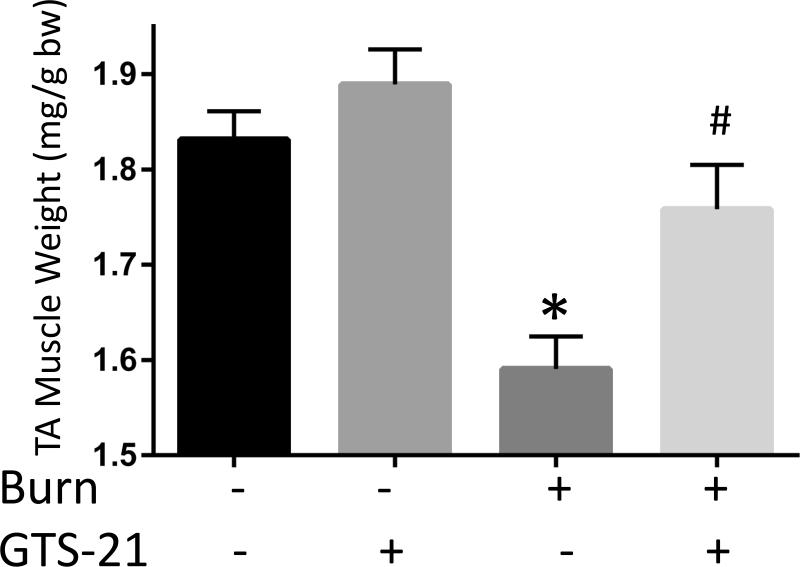
Figure 4. Reversal of burn injury-induced total body weight and muscle mass loss by GTS-21 treatment.
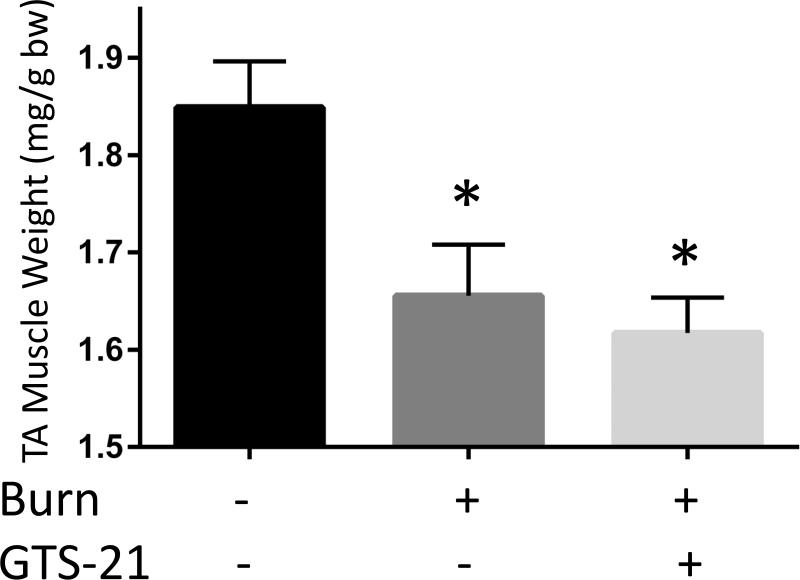
Total body weight in WT mice (A) and TA muscle mass in WT (B) at post burn day 3. TA muscle weight was normalized to initial body weight. Body weight decreased at day 3 after BI. The body weight of BI mice treated with GTS-21 was not different from Sham-burn mice (p=0.32, Sham-Vehicle vs. Burn-GTS). TA muscle mass significantly decreased at post burn day 3. GTS-21 mitigated the muscle mass loss (Sham-Vehicle vs. Burn-Vehicle vs. Burn-GTS = 1.83 (100%) vs. 1.59 (86.8%) vs. 1.76 mg/g (96.0%) b.w.). *: p≤0.05 vs Sham-Vehicle, #: p≤0.05 vs Burn-Vehicle N=4 for Sham-Vehicle, 4 for Sham-GTS, 8 for Burn-Vehicle, 7 for Burn-GTS.
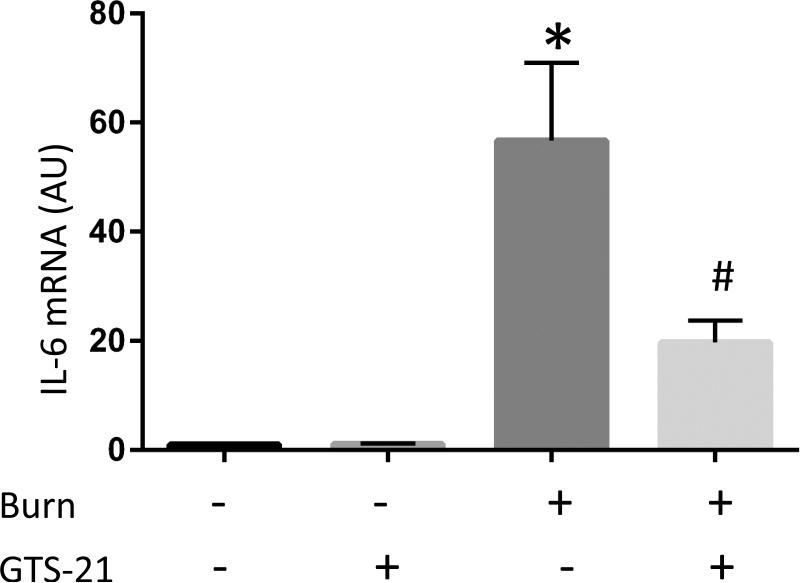
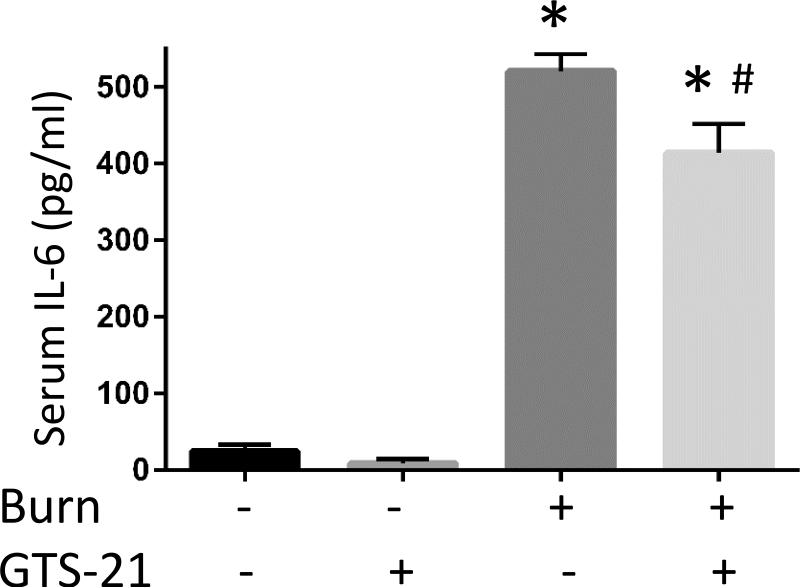
Figure 1. Cytokine mRNA expressions in burned mice and their amelioration by GTS-21 treatment.
Tibialis anterior muscles and serum samples were harvested at 6 hour after body trunk burn injury with or without 10mg/kg of GTS-21 or saline treatment b.i.d. Quantitative RT-PCR was performed on the total RNA purified from the harvested muscles. Relative quantification of pro-inflammatory IL-6 (A), IL-1β (B), CXCL2 (C) and anti-inflammatory IL-10 (D) transcripts normalized to that of GAPDH is shown as bar graph with the standard error. The levels of IL-6, IL-1β and CXCL2 transcripts were increased in burn injury to 56.7, 3.1 and 20.0 fold, respectively. GTS-21 treatment significantly ameliorated these cytokine levels from 56.7 to 19.7, 3.1 to 1.5 and 20.0 to 4.3 fold, respectively. The level of IL-10 was also increased in burn and reduced by GTS-21(Burn-Vehicle vs. Burn-GTS = 11.2 vs. 6.5 fold) Serum IL-6 levels (E) were also significantly increased in BI compared to Sham-burn group and significantly ameliorated by GTS-21 treatment (Sham-Vehicle vs. Burn-Vehicle vs. Burn-GTS = 24.5 +/− 7.9 vs. 520.3 +/− 21.2 vs 414.0 +/− 36.0 pg/ml). *: p≤0.05 vs Sham-Vehicle, #: p≤0.05 vs Burn-Vehicle N=6 for Sham-Vehicle, 4 for Sham-GTS, 10 for Burn-Vehicle, 11 for Burn-GTS. The fold increase was calculated by comparing to the value of Sham-Vehicle group.
Figure 5. Inhibition of GTS-21 effect by methyllycaconitine or genetic knock out of α7AChR.
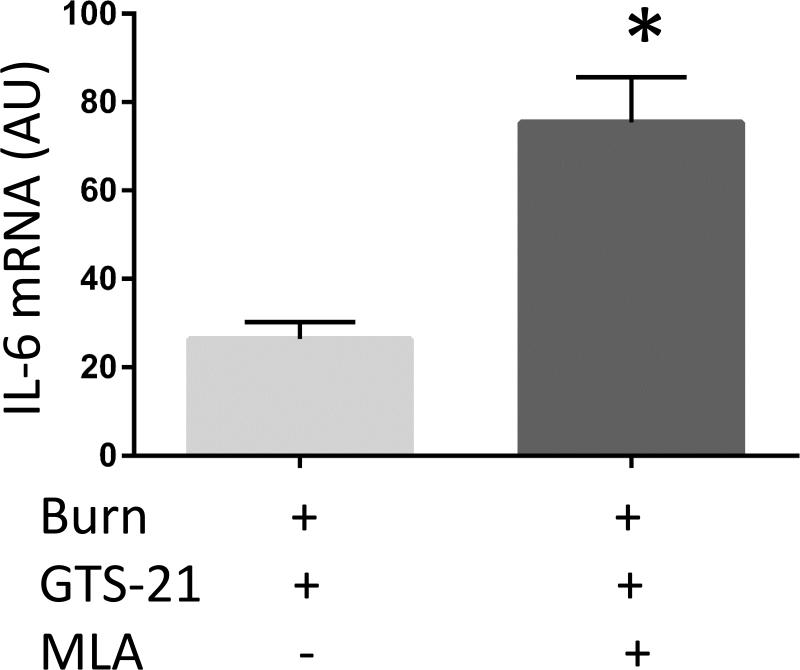
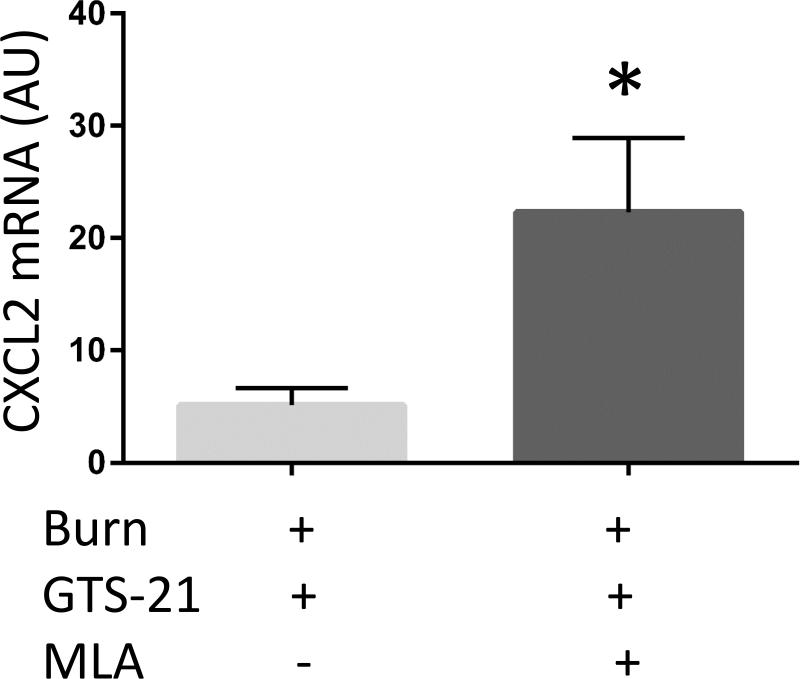
Burned mice were treated with GTS-21 alone or with combination of GTS-21 and methyllycaconitine (MLA). IL-6 (A), IL-1β (B) and CXCL2 (C) mRNA expression in skeletal muscle, quantified by RT-PCR, were increased in Burn-GTS-MLA group compared to Burn-GTS group (IL-6: 2.9 fold and CXCL2: 4.8 fold). (D) GTS-21 treatment did not improve TA muscle mass loss in α7KO mice. (A-C) *: p≤0.05 vs Burn-GTS, N=8 for Burn-GTS, 7 for Burn-GTS-MLA. (D) *: p≤0.05 vs Sham-Vehicle, N=3 for Sham-Vehicle, 4 for Burn-Vehicle, 4 for Burn-GTS.
Results
BI-induced inflammatory responses are reduced by GTS-21 treatment
In the first and second experiment, we examined whether BI activates inflammatory signals in the skeletal muscle distant from burned site. TA muscle samples were collected at 6 hour post burn for analyses of cytokine (IL-1β, IL-6 and IL-10) mRNA expression by RT-PCR (Fig. 1). One of the downstream signaling proteins of IL-6 is inflammatory protein, STAT3, which activates many genes including C-X-C motif chemokine ligand 2 (CXCL2) (a.k.a., STAT3 target gene). The downstream effects of cytokine expression on STAT3, and NF-κB were assessed by immunoblotting at PBD1
The mRNA levels of pro-inflammatory IL-6, IL-1β and CXCL2 in BI group compared to Sham-burn were upregulated to 56.7, 3.1 and 20.0 fold, respectively. GTS-21 treatment significantly ameliorated IL-6, IL-1β and CXCL2 mRNA expression from 56.7 to 19.7, 3.1 to 1.5 and 20.0 to 4.3 fold, respectively (p=0.01, 0.05 and <0.01, Fig. 1A-C). Meanwhile, mRNA level of anti-inflammatory IL-10 also presented significant upregulation in BI. This increase was ameliorated by GTS-21 treatment though still significant higher level compared to Sham-Vehicle group (Burn-Vehicle vs. Burn-GTS = 11.2 vs. 6.5 fold compared to Sham-Vehicle, p<0.01 for Sham-Vehicle vs. Burn-Vehicle, p=0.01 for Burn-Vehicle vs Burn-GTS and p<0.01 for Sham-Vehicle vs. Burn-GTS, Fig. 1D). Serum IL-6 protein levels were also markedly increased in BI compared to Sham-burn group and significantly ameliorated by GTS-21 treatment (Sham-Vehicle vs. Burn-Vehicle vs. Burn-GTS = 24.5 +/− 7.9 vs. 520.3 +/− 21.2 vs. 414.0 +/− 36.0 pg/ml, p<0.01 for Sham-Vehicle vs. Burn-Vehicle, and p=0.04 for Burn-Vehicle vs Burn-GTS, Fig. 1E). Consistent with increase of cytokines mRNA expression, protein expression of phosphorylated NF-κB at serine 536 and STAT3 at tyrosine 705 at PBD1 were significantly increased by BI compared to Sham-burn group (1.8 and 8.1 fold, respectively). GTS-21 treatment significantly attenuated BI-induced phosphorylation of NF-κB and STAT3 from 1.8 to 1.0 and 8.1 to 4.1 fold, respectively (Fig. 2).
Increased muscle proteolytic signals of burn are ameliorated by GTS-21
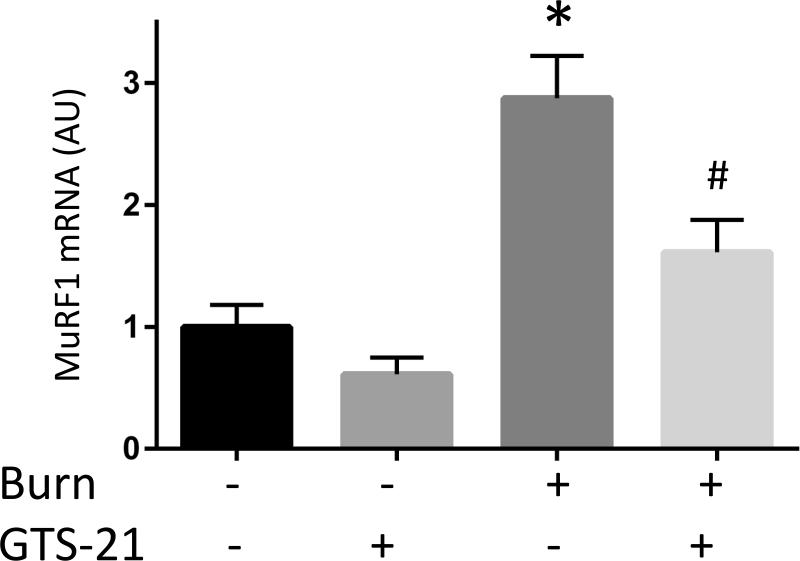
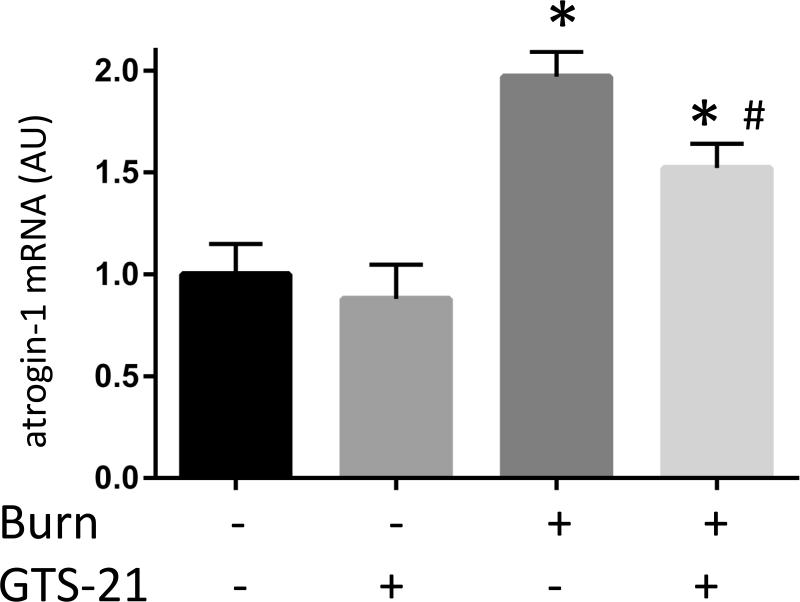
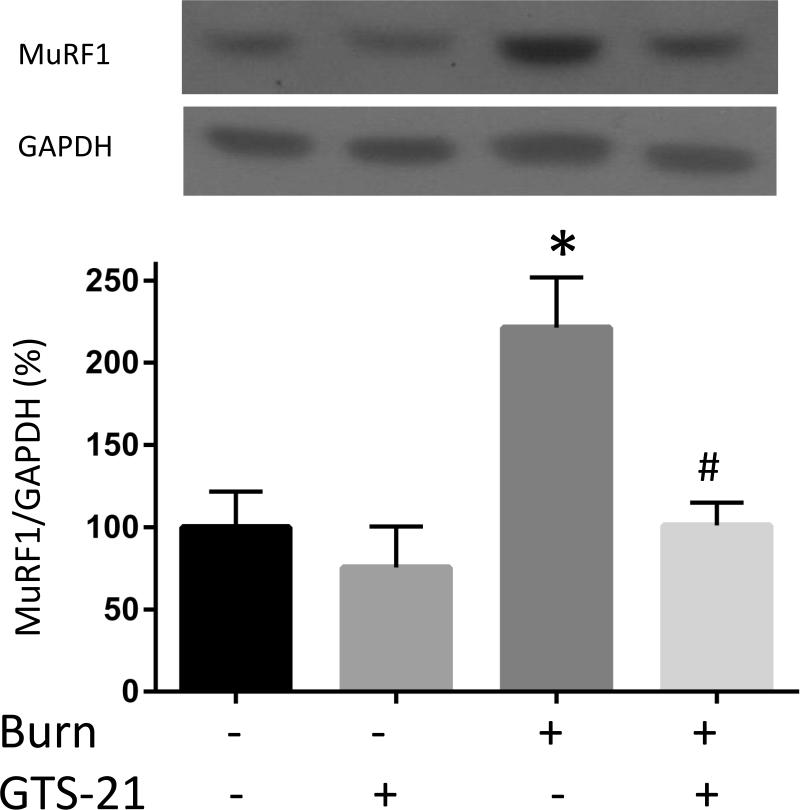
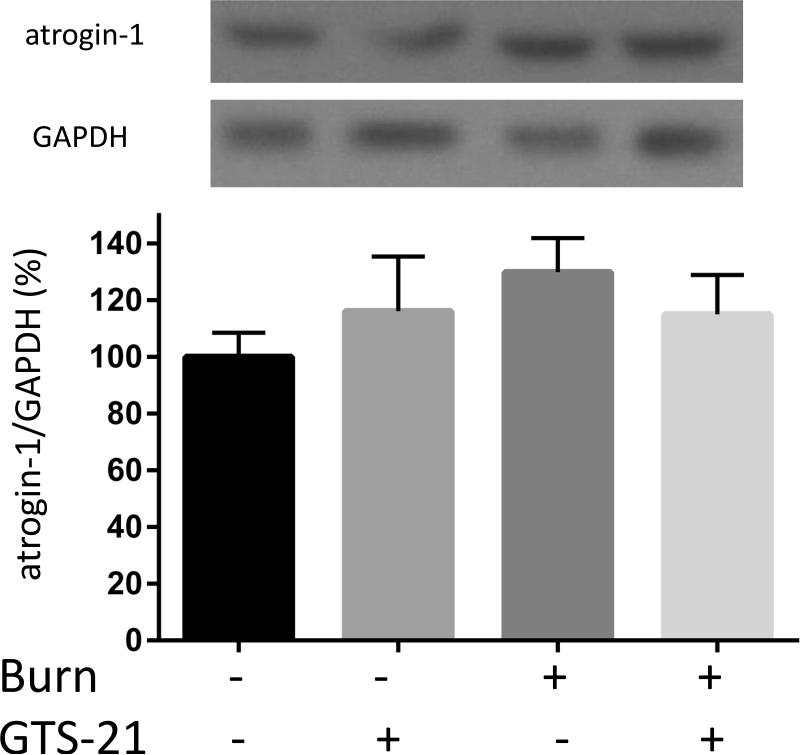
Although previous studies have reported increased expression of muscle specific ubiquitin ligases (MuRF1 and atrogin-1) in rats with BI (9), this study in mice examined the effect of GTS-21 on these atrogenes in the distant tibialis muscle at PBD1. The mRNA expression of MuRF1 in BI group increased 3.0 fold compared to Sham-burn group and this increase was prevented by GTS-21 treatment (from 3.0 to 1.6 fold, Fig. 3A). Consistent with mRNA levels, the protein expression of MuRF1 increased after BI and was reversed by GTS-21 treatment (Fig. 3D). Meanwhile, the mRNA expression of atrogein-1 in BI group increased 2.0 folds compared to Sham-burn group and GTS-21 treatment ameliorated the increase (from 2.0 to 1.5 fold, Fig. 3B). Intriguingly, neither BI nor GTS-21 treatment significantly altered atrogin-1 protein levels (Fig. 3E).
Figure 3. Burn-induced proteolytic signals and their amelioration by GTS-21 treatment.
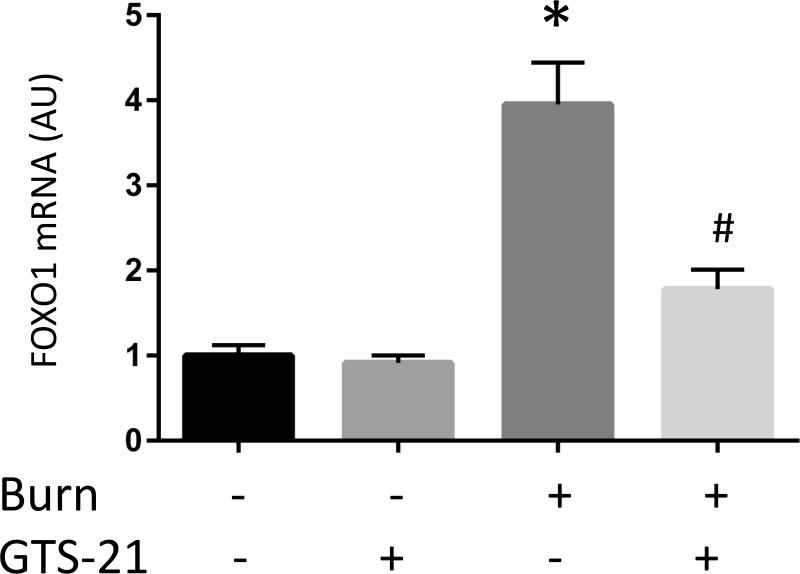
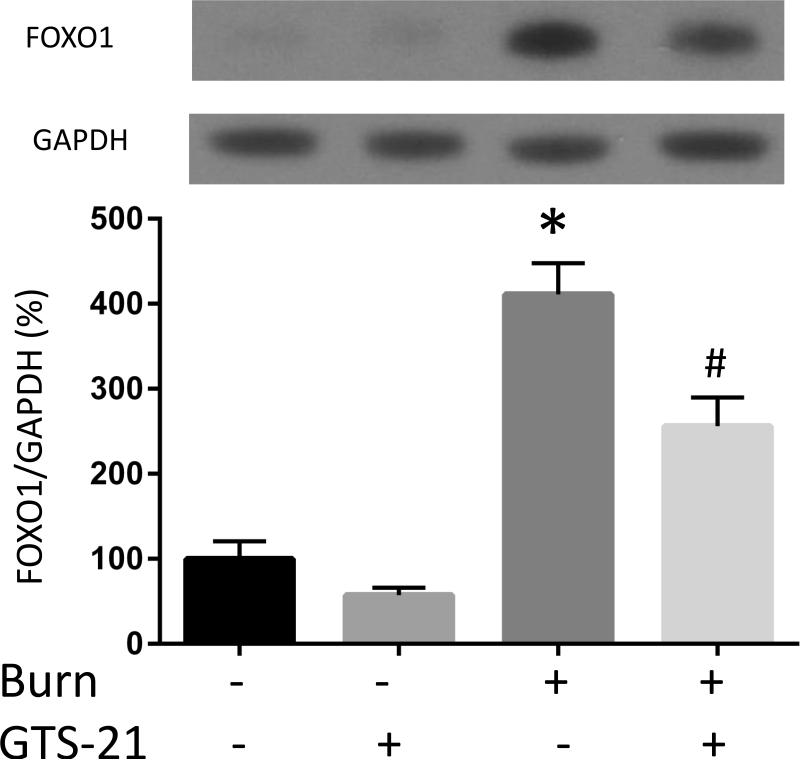
The mRNA (A-C) and protein (D-F) levels of MuRF1, atrogin-1 and FOXO1 were quantified at post burn day 1. MuRF1 and FOXO1 protein levels were increased in burn injury (3.0 and 4.1 fold compared to Sham-burn). These increases were significantly ameliorated by GTS-21 treatment (from 3.0 to 1.6 and 4.1 to 2.0 fold). Neither burn injury nor GTS-21 treatment altered atrogin-1 protein level although atrogin-1 mRNA expression levels presented similar trend as MuRF1. *: p≤0.05 vs Sham-Vehicle, #: p≤0.05 vs Burn-Vehicle N=8 for Sham-Vehicle, 4 for Sham-GTS, 11 for Burn-Vehicle, 12 for Burn-GTS. The fold increase was calculated by comparing to the value of Sham-Vehicle group.
GTS-21 treatment ameliorated induction of FOXO1 after BI
It has been reported that IL-6/STAT3 pathway is an upstream signaling partner involved in regulation of MuRF1 and atrogin-1. However, these same genes are also regulated by an alternative pathway, namely FOXO1. We, therefore, next examined whether BI and GTS-21 treatment affect FOXO1 expression. FOXO1 mRNA expression in BI mice was increased 4.0 fold compared to Sham-burn mice. GTS-21 treatment reduced the expression (from 4.0 to 1.8 fold, Fig. 3C). Consistent with the mRNA data, BI significantly increased FOXO1 protein expression and the increase was ameliorated by GTS-21 (from 4.1 to 2.6 fold, Fig. 3F).
Total body weight and distant skeletal muscle mass loss of BI are mitigated by GTS-21
The third experiment examined the body weight and muscle mass changes induced by BI and GTS-21 therapy. The total body weight decreased 5.6% in Burn-Vehicle compared to Sham-Vehicle group at day 3 after BI (p=0.02). GTS-21 treatment after BI mitigated the weight loss and maintained the body weight similar to that of Sham BI (p=0.32, Sham-Vehicle vs. Burn-GTS, Fig. 4A). Furthermore, TA muscle weight in Burn-GTS group improved significantly compared to Burn-Vehicle group (1.76 vs 1.59mg/g b.w., p=0.02, Fig. 4B). In other words, BI decreased TA muscle mass 13.2 % at day 3 and this loss was significantly decreased to 4.0 % by GTS-21.
MLA treatment of WT or genetic deletion of α 7KO mice abrogates the GTS-21 effects
The previous experiments presented the beneficial effects of GTS-21 on skeletal muscle mass loss as well as abrogation of the inflammatory and proteolytic signaling pathways after BI. The following study examined whether GTS-21 specifically exerts its effect through α7AChRs. A new set of BI mice were treated with GTS-21 alone or in combination with MLA, a specific antagonist of α7AChRs. The addition of MLA to GTS-21 treated mice increased mRNA expression of IL-6 2.9 fold (p<0.01) and CXCL2 4.4 fold (p<0.01) Fig. 5A, C). IL-1β mRNA expression presented similar trend but was not statistically significantly different (1.4 fold increase, p=0.20, Fig. 5B). Consistent with the absence of GTS-21 effects on BI injured mice receiving α7AChRs antagonist, MLA, GTS-21 treatment did not improve TA muscle mass loss in α7KO mice (Burn-Vehicle vs Burn GTS = 1.66 vs 1.62 mg/g b.w., p=0.81, Fig. 5D).
Discussion
The current study documents for the first time that stimulation of α7AChRs by specific agonist, GTS-21 ameliorated: (1) BI-induced mRNA expression of inflammatory cytokines in skeletal muscle and serum IL-6 protein expression; (2) increased phosphorylation (activation) of NF-κB and STAT3 signals; (3) increased expression of proteolytic signals (MuRF1 and FOXO1); and (4) BI-induced body weight loss and skeletal muscle mass loss in wild type mice. Importantly, as opposed to beneficial responses to GTS-21 treatment in BI wild type mice, the treatment of BI in α7KO mice with GTS- 21 or concomitant treatment of WT mice with GTS-21 and MLA failed to exert the protective effect on muscle mass loss or cytokine expression. These data indicate that the beneficial effects of GTS-21 were mediated specifically via α7AChRs.
Most acquired MW conditions are associated with some form of systemic or local inflammation (4, 6, 8, 11, 12). The infiltration of circulating leucocytes into muscle during MW is also now well established (17). Even in simple disuse atrophy, despite the absence of systemic inflammatory response, muscle inflammation and infiltration of macrophages have been evidenced (18). There is also a priori evidence that there is de novo expression of α7AChRs in muscle during muscle cachexia even in the absence of systemic inflammation. The upregulation of α7AChRs has been observed in skeletal muscle under certain catabolic condition including simple disuse (3) and motor nerve denervation (33). In view of the fact that these leucocytes and muscle express α7AChRs, we exploited the anti-inflammatory properties of α7AChRs to decrease inflammation in skeletal muscle. This was achieved by the use of a specific α7AChRs agonist GTS-21. Our studies provide evidence that stimulation of α7AChRs does indeed decrease systemic inflammation evidenced by decrease of IL-6 protein levels in association with maintenance of body weight and attenuation of muscle mass loss. Our studies, however, identified neither if the effects of GTS-21 were more prominent systemically or locally, nor if the effects of GTS-21 were predominantly on muscle or leucocyte α7AChRs.
GTS-21 suppressed muscle inflammatory responses
The current study demonstrated the beneficial effects of GTS-21 on inflammatory responses after BI. GTS-21 treatment suppressed pro-inflammatory cytokines mRNA expression including IL-6, IL-1β and CXCL2 in skeletal muscle. IL-10, an anti-inflammatory cytokine was also elevated after BI at this time pint of the current study, which most likely means simultaneously increased synthesis of anti-inflammatory markers counteracting the pro-inflammatory cytokines as similarly observed in a previous study (24). Unexpectedly, IL-10 level was reduced by GTS-21 but still significantly higher than Sham-Vehicle group. Though the precise reason about this result is unclear, this observation might be because of secondary effect through suppression of systemic and local inflammation. The effect of α7AChRs on anti-inflammatory cytokines is controversial (24, 26, 34). Further study is required to elucidate the involvement of α7AChRs in anti-inflammatory cytokines release and their effects on muscle mass loss and/or recovery.
Having demonstrated the beneficial effects of GTS-21 on inflammatory responses after BI, we next examined the biochemical and anti-inflammatory effects of GTS-21. Systemic administration of GTS-21 suppressed inflammatory responses in muscle as indicated in change of inflammatory cytokine mRNA expression and STAT3/NF-κB signals in skeletal muscle. It has been demonstrated that cholinergic agonists suppress inflammatory responses in macrophages (19) and endothelial cells (20) by inhibiting NF-κB activation in vitro. Similarly, STAT3 activity is also reported to be modulated by cholinergic agonists. The study by de Jonge et al. demonstrated that nicotine, α7AChRs agonist induced STAT3 activation in macrophages treated with LPS (21). In contrast, Chatterjee et al. reported that treatment of endothelial cells with nicotine or GTS-21 reduced STAT3 activation (22). Thus, whether stimulation of α7AChRs induces or reduces STAT3 activation depends on target organs/tissues and/or experimental condition. However, both reports are in agreement that stimulation of α7AChRs has anti-inflammatory effects eventually suppressing NF-κB activation and/or inflammatory cytokines. Thus, our studies are consistent with these studies that agonistic stimulation of α7AChRs leads to NF-κB inhibition in vivo.
Although phosphorylation of STAT3 on tyrosine 705 is the canonical JAK target site, serine 727 phosphorylation site was also phosphorylated by BI (Fig. 2). The significance of phosphorylation of serine 727 following BI in terms of inflammation is unclear. This latter observation is possibly due to multiple and complex inflammatory and immune responses induced by severe BI. Importantly, however, both tyrosine 705 and serine 727 phosphorylation of STAT3 were significantly reduced by GTS-21 treatment. Phosphorylation of STAT3 on serine 727 is mediated by signals including protein kinase C, Jun N-terminal kinase, extracellular signal-regulated kinase and the mitogen-activated protein kinase p38 (35). Thus, GTS-21 may affect these signaling molecules in addition to canonical NF-κB and IL-6/STAT3 pathways. These results indicate that stimulation of α7AChRs by GTS-21 has pluripotent beneficial effects.
GTS-21 suppressed BI-induced up-regulated proteolytic signals
Several in vitro and in vivo studies have already documented the important roles of cytokines in muscle protein loss (1, 13). These studies suggest that increased expression of inflammatory cytokines, at least in part, may contribute MW in catabolic conditions. MW of critical illness of BI results from an imbalance of the anabolic (synthetic) and catabolic (proteolytic) machinery (1). In BI there is evidence for increased protein degradation (9). In rodent studies, although Akt signaling is impaired in BI, the phosphorylation of p70S6, one of the major signals for protein synthesis, was increased compared to controls, suggesting higher than normal protein synthesis (10). Regardless of increased protein synthesis, MW persists in BI, suggesting that protein degradation is greater than synthesis. Our studies demonstrate that elevated atrogenes expression is mitigated by GTS-21 in association with maintenance of muscle mass after BI. This suggests that the protein degradation has been effectively inhibited by GTS-21.The effects of GTS-21 on synthesis is unclear and subject to future studies.
Previous studies identified FOXO1, NF-κB and STAT3 as effectors of MW (1, 4, 5, 7, 11-13). In the current study NF-κB, STAT3 and FOXO1 activation was reduced by GTS-21 treatment in BI animals. Both mRNA and protein expression of MuRF1, a major ubiquitin ligase related to muscle atrophy was reduced by GTS-21 treatment. Although mRNA expression of atrogin-1 presented the same trend as MuRF1, the level of atrogin-1 protein expression was not significantly altered by BI or GTS-21. This discrepancy between protein and mRNA has been previously observed ventilator-induced MW (12), possibly due to the difference in the kinetics between protein and mRNA regulation.
The current study also provides evidence that stimulation of α7AChRs suppresses BI-induced increased expression of FOXO1 in skeletal muscle. In this study, total FOXO1 mRNA and protein expression were examined because it had been reported that FOXO1 activation is caused by increased level of total FOXO1 protein in catabolic conditions such as starvation (36) or sepsis (37) although reduction of phosphorylated FOXO1 is also an important mechanism of activation of FOXO1. Although the mechanisms which regulate FOXO1 signal were not investigated in this study, Wang et al. reported that stimulation of α7AChRs ameliorates obesity-induced insulin resistance by enhancing insulin signals such as Akt which also works as a regulator of FOXO1 signal (38). It is conceivable that GTS-21 treatment affects Akt activity leading to suppression of FOXO1 signal. However, it does not exclude the possibility that GTS-21 reduces FOXO1 expression secondarily through suppression of systemic and local inflammation. Further studies are necessary to elucidate a link between α7AChRs and the FOXO1 pathway. Taken together, the current results suggest that stimulation of α7AChRs by GTS-21 treatment regulates not only inflammatory responses but also proteolytic signals such as MuRF1 and FOXO1 signal.
GTS-21 retarded BI-induced body weight and muscle mass loss
Next the physiological consequences of attenuation of BI-induced inflammatory responses and augmented proteolytic signals by GTS-21 were examined by observation of body weight and muscle mass changes since this is the most important goal from a clinical perspective. Our study documents that the increased expression of muscle specific ubiquitin ligases by BI caused skeletal muscle mass loss at PBD3 (Fig. 4). Stimulation of α7AChRs by GTS-21 treatment significantly ameliorated not only the TA muscle mass loss but also whole body weight loss induced by BI.
The beneficial effects of GTS-21 was blocked by specific inhibition of α 7AChRs
Finally, the specificity of GTS-21 for α7AChRs was examined. Pharmacological inhibition of α7AChRs by MLA treatment blocked the effect of GTS-21 which reduced pro-inflammatory cytokines mRNA expression after BI. Similarly, GTS-21 treatment did not prevent BI-induced muscle mass loss in α7KO mice. These observations confirm the specificity of GTS-21 actions being exerted via stimulation of α7AChRs.
In conclusion, the current study demonstrates that pharmacotherapeutic stimulation of α7AChRs by a specific agonist, GTS-21 ameliorates BI-induced induction of proteolytic signals including MuRF1 and FOXO1, as well as inflammatory responses mediated by NF-κB and IL-6/STAT3 pathways in skeletal muscle. These effects lead to amelioration of body weight and skeletal muscle mass loss after BI. Therefore, stimulation of α7AChRs can be a potent therapeutic target for MW under catabolic condition of BI.
Supplementary Material
Acknowledgements
Supported in part by grants from Shriners Hospital for Children (#86100), Tampa, FL and from the NIH, P-50 GM2500 Project 1 and RO1 GM118947(to JAJM)
Reference
- 1.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14(1):58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 2.Hosokawa S, Koseki H, Nagashima M, Maeyama Y, Yomogida K, Mehr C, Rutledge M, Greenfeld H, Kaneki M, Tompkins RG, Martyn JA, Yasuhara SE. Title efficacy of phosphodiesterase 5 inhibitor on distant burn-induced muscle autophagy, microcirculation, and survival rate. Am J Physiol Endocrinol Metab. 2013;304(9):E922–33. doi: 10.1152/ajpendo.00078.2013. [DOI] [PubMed] [Google Scholar]
- 3.Khan MA, Sahani N, Neville KA, Nagashima M, Lee S, Sasakawa T, Kaneki M, Martyn JA. Nonsurgically induced disuse muscle atrophy and neuromuscular dysfunction upregulates alpha7 acetylcholine receptors. Can J Physiol Pharmacol. 2014;92(1):1–8. doi: 10.1139/cjpp-2013-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castillero E, Alamdari N, Aversa Z, Gurav A, Hasselgren PO. PPARbeta/delta regulates glucocorticoid- and sepsis-induced FOXO1 activation and muscle wasting. PLoS One. 2013;8(3):e59726. doi: 10.1371/journal.pone.0059726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, Koniaris LG, Zimmers TA. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab. 2012;303(3):E410–21. doi: 10.1152/ajpendo.00039.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedroso FE, Spalding PB, Cheung MC, Yang R, Gutierrez JC, Bonetto A, Zhan R, Chan HL, Namias N, Koniaris LG, Zimmers TA. Inflammation, organomegaly, and muscle wasting despite hyperphagia in a mouse model of burn cachexia. J Cachexia Sarcopenia Muscle. 2012;3(3):199–211. doi: 10.1007/s13539-012-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117(3):399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai D, Frantz JD, Tawa NE, Jr., Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119(2):285–98. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Lang CH, Huber D, Frost RA. Burn-induced increase in atrogin-1 and MuRF-1 in skeletal muscle is glucocorticoid independent but downregulated by IGF-I. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R328–36. doi: 10.1152/ajpregu.00561.2006. [DOI] [PubMed] [Google Scholar]
- 10.Sugita H, Kaneki M, Sugita M, Yasukawa T, Yasuhara S, Martyn JA. Burn injury impairs insulin-stimulated Akt/PKB activation in skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288(3):E585–91. doi: 10.1152/ajpendo.00321.2004. [DOI] [PubMed] [Google Scholar]
- 11.Jackman RW, Cornwell EW, Wu CL, Kandarian SC. Nuclear factor-kappaB signalling and transcriptional regulation in skeletal muscle atrophy. Exp Physiol. 2013;98(1):19–24. doi: 10.1113/expphysiol.2011.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith IJ, Godinez GL, Singh BK, McCaughey KM, Alcantara RR, Gururaja T, Ho MS, Nguyen HN, Friera AM, White KA, McLaughlin JR, Hansen D, Romero JM, Baltgalvis KA, Claypool MD, Li W, Lang W, Yam GC, Gelman MS, Ding R, Yung SL, Creger DP, Chen Y, Singh R, Smuder AJ, Wiggs MP, Kwon OS, Sollanek KJ, Powers SK, Masuda ES, Taylor VC, Payan DG, Kinoshita T, Kinsella TM. Inhibition of Janus kinase signaling during controlled mechanical ventilation prevents ventilation-induced diaphragm dysfunction. FASEB J. 2014;28(7):2790–803. doi: 10.1096/fj.13-244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985) 98(3):911–7. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 14.Jatoi A, Ritter HL, Dueck A, Nguyen PL, Nikcevich DA, Luyun RF, Mattar BI, Loprinzi CL. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer. 2010;68(2):234–9. doi: 10.1016/j.lungcan.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayliss TJ, Smith JT, Schuster M, Dragnev KH, Rigas JR. A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin Biol Ther. 2011;11(12):1663–8. doi: 10.1517/14712598.2011.627850. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 17.Fink H, Helming M, Unterbuchner C, Lenz A, Neff F, Martyn JA, Blobner M. Systemic inflammatory response syndrome increases immobility-induced neuromuscular weakness. Crit Care Med. 2008;36(3):910–6. doi: 10.1097/CCM.0B013E3181659669. [DOI] [PubMed] [Google Scholar]
- 18.Zhu S, Nagashima M, Khan MA, Yasuhara S, Kaneki M, Martyn JA. Lack of caspase-3 attenuates immobilization-induced muscle atrophy and loss of tension generation along with mitigation of apoptosis and inflammation. Muscle Nerve. 2013;47(5):711–21. doi: 10.1002/mus.23642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugano N, Shimada K, Ito K, Murai S. Nicotine inhibits the production of inflammatory mediators in U937 cells through modulation of nuclear factor-kappaB activation. Biochem Biophys Res Commun. 1998;252(1):25–8. doi: 10.1006/bbrc.1998.9599. [DOI] [PubMed] [Google Scholar]
- 20.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201(7):1113–23. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6(8):844–51. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee PK, Al-Abed Y, Sherry B, Metz CN. Cholinergic agonists regulate JAK2/STAT3 signaling to suppress endothelial cell activation. Am J Physiol Cell Physiol. 2009;297(5):C1294–306. doi: 10.1152/ajpcell.00160.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giebelen IA, van Westerloo DJ, LaRosa GJ, de Vos AF, van der Poll T. Local stimulation of alpha7 cholinergic receptors inhibits LPS-induced TNF-alpha release in the mouse lung. Shock. 2007;28(6):700–3. doi: 10.1097/shk.0b013e318054dd89. [DOI] [PubMed] [Google Scholar]
- 24.Nullens S, Staessens M, Peleman C, Schrijvers DM, Malhotra-Kumar S, Francque S, Matteoli G, Boeckxstaens GE, De Man JG, De Winter BY. Effect of GTS-21, An Alpha7 Nicotinic Acetylcholine Receptor Agonist, on Clp-Induced Inflammatory, Gastrointestinal Motility and Colonic Permeability Changes in Mice. Shock. 2015 doi: 10.1097/SHK.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 25.Khan MA, Farkhondeh M, Crombie J, Jacobson L, Kaneki M, Martyn JA. Lipopolysaccharide upregulates alpha7 acetylcholine receptors: stimulation with GTS-21 mitigates growth arrest of macrophages and improves survival in burned mice. Shock. 2012;38(2):213–9. doi: 10.1097/SHK.0b013e31825d628c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kox M, Pompe JC, Gordinou de Gouberville MC, van der Hoeven JG, Hoedemaekers CW, Pickkers P. Effects of the alpha7 nicotinic acetylcholine receptor agonist GTS-21 on the innate immune response in humans. Shock. 2011;36(1):5–11. doi: 10.1097/SHK.0b013e3182168d56. [DOI] [PubMed] [Google Scholar]
- 27.Nakazawa H, Yamada M, Tanaka T, Kramer J, Yu YM, Fischman AJ, Martyn JA, Tompkins RG, Kaneki M. Role of protein farnesylation in burn-induced metabolic derangements and insulin resistance in mouse skeletal muscle. PLoS One. 2015;10(1):e0116633. doi: 10.1371/journal.pone.0116633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiao G, Lieberman M, Fischer JE, Hasselgren PO. Intracellular regulation of protein degradation during sepsis is different in fast- and slow-twitch muscle. Am J Physiol. 1997;272(3 Pt 2):R849–56. doi: 10.1152/ajpregu.1997.272.3.R849. [DOI] [PubMed] [Google Scholar]
- 29.Plackett TP, Colantoni A, Heinrich SA, Messingham KA, Gamelli RL, Kovacs EJ. The early acute phase response after burn injury in mice. J Burn Care Res. 2007;28(1):167–72. doi: 10.1097/BCR.0b013E31802CB84F. [DOI] [PubMed] [Google Scholar]
- 30.Paris D, Beaulieu-Abdelahad D, Abdullah L, Bachmeier C, Ait-Ghezala G, Reed J, Verma M, Crawford F, Mullan M. Anti-inflammatory activity of anatabine via inhibition of STAT3 phosphorylation. Eur J Pharmacol. 2013;698(1-3):145–53. doi: 10.1016/j.ejphar.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Kaneki M, Fukushima Y, Shinozaki S, Fukaya M, Habiro M, Shimizu N, Chang K, Yasuhara S, Martyn JA. iNOS inhibitor, L-NIL, reverses burn-induced glycogen synthase kinase-3beta activation in skeletal muscle of rats. Metabolism. 2013;62(3):341–6. doi: 10.1016/j.metabol.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrow RE, Hawkins HK, Aarsland A, Cox R, Rosenblatt J, Barrow LN, Jeschke MG, Herndon DN. Identification of factors contributing to hepatomegaly in severely burned children. Shock. 2005;24(6):523–8. doi: 10.1097/01.shk.0000187981.78901.ee. [DOI] [PubMed] [Google Scholar]
- 33.Tsuneki H, Salas R, Dani JA. Mouse muscle denervation increases expression of an alpha7 nicotinic receptor with unusual pharmacology. J Physiol. 2003;547(Pt 1):169–79. doi: 10.1113/jphysiol.2002.036368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez-Aleman E, Quintanar-Stephano A, Escobedo G, Campos-Esparza Mdel R, Campos-Rodriguez R, Ventura-Juarez J. Vagotomy induces deregulation of the inflammatory response during the development of amoebic liver abscess in hamsters. Neuroimmunomodulation. 2015;22(3):166–80. doi: 10.1159/000362240. [DOI] [PubMed] [Google Scholar]
- 35.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19(21):2628–37. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 36.Furuyama T, Kitayama K, Yamashita H, Mori N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem J. 2003;375(Pt 2):365–71. doi: 10.1042/BJ20030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith IJ, Alamdari N, O'Neal P, Gonnella P, Aversa Z, Hasselgren PO. Sepsis increases the expression and activity of the transcription factor Forkhead Box O 1 (FOXO1) in skeletal muscle by a glucocorticoid-dependent mechanism. Int J Biochem Cell Biol. 2010;42(5):701–11. doi: 10.1016/j.biocel.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Yang Z, Xue B, Shi H. Activation of the cholinergic antiinflammatory pathway ameliorates obesity-induced inflammation and insulin resistance. Endocrinology. 2011;152(3):836–46. doi: 10.1210/en.2010-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.