Abstract
Background
We investigated an association between baseline heart rate-corrected QT (QTc) interval before severe hypoglycemia (SH) and prolongation of QTc interval during SH in patients with type 2 diabetes mellitus (T2DM).
Methods
Between January 2004 and June 2014, 208 patients with T2DM, who visited the emergency department because of SH and underwent standard 12-lead electrocardiography within the 6-month period before SH were consecutively enrolled. The QTc interval was analyzed during the incidence of SH, and 6 months before and after SH. QTc intervals of 450 ms or longer in men and 460 ms or longer in women were considered abnormally prolonged.
Results
The mean age and diabetes duration were 68.1±12.1 and 14.1±10.1 years, respectively. The mean QTc intervals at baseline and SH episodes were 433±33 and 460±33 ms, respectively (P<0.001). One hundred and fourteen patients (54.8%) had a prolonged QTc interval during SH. There was a significant decrease in the prolonged QTc interval within 6 months after SH (QTc interval prolongation during SH vs. after recovery, 54.8% vs. 33.8%, P<0.001). The prolonged QTc interval was significantly associated with baseline QTc interval prolongation (odds ratio, 2.92; 95% confidence interval, 1.22 to 6.96; P=0.016) after adjusting for multiple confounders.
Conclusion
A prolonged QTc interval at baseline was significantly associated with prolongation of the QTc interval during SH in patients with T2DM, suggesting the necessity of QTc interval monitoring and attention to those with a prolonged QTc interval to prevent SH.
Keywords: Diabetes mellitus, type 2; Severe hypoglycemia; QT interval
INTRODUCTION
Because large epidemiologic studies have reported that a chronic uncontrolled glycemic status was related to the development of macrovascular and microvascular complications [1,2,3,4], intensive glycemic control has been emphasized for preventing long-term vascular complications in patients with type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM) [5]. Previously reported large randomized controlled studies, such as Action to Control Cardiovascular Risk in Diabetes (ACCORD), Action in Diabetes and Vascular Disease (ADVANCE), and Veterans Affairs Diabetes Trial (VADT), showed that intensive glycemic control reduces microvascular complications in patients with T2DM [6,7,8]. However, in spite of achieving a glycemic target, the trials did not demonstrate beneficial effects on cardiovascular events [6,7,8]. Weight gain and significantly increased hypoglycemia episodes have been thought to contribute to this unfavorable outcome in the intensive treatment group. There were 1.86- to 3-fold more severe hypoglycemia (SH) events in the intensive control group than in the standard control group in the ACCORD and ADVANCE studies [6,7].
The incidence rate of SH events has been reported as 0.4 to 16.6 events per 100 patients per year in patients with T2DM [6,7]. In Korea, the incidence of SH events has also recently increased to 2.11 to 2.71 events per 100 patients per year in patients with T2DM in 2009, which is higher than in 2004 (0.46 to 2.19 events per 100 patients per year) [9]. Therefore, to achieve a glycemic target for preventing chronic diabetic complications, we should consider and manage the accompanying risk of hypoglycemia in patients with T2DM.
SH is associated with cognitive dysfunction, seizure, coma, and major cardiovascular events, such as myocardial infarction, stroke, and arrhythmia, as well as all-cause mortality in T2DM [5,10]. Nocturnal hypoglycemia or SH is considered to be associated with sudden unexplained death in T1DM, named "dead in bed syndrome," and cardiovascular autonomic neuropathy with abnormal cardiac repolarization, as evidenced by corrected QT (QTc) interval prolongation, is suggested to play a role in cardiac arrhythmia [11]. In addition, the QTc interval has been reported to predict cardiac death in several diseases, including T1DM, advanced heart failure, systemic hypertension, and peripheral vascular disease in patients with diabetes [12,13]. Recent studies have found an association between SH and prolonged QTc in T2DM [14]. However, few studies have evaluated the relationship between QTc prolongation at baseline and during an SH episode in subjects with T2DM [14,15,16,17].
The aim of this study was to evaluate the relationship among baseline QTc, QTc during SH, and the changes in the QTc interval after SH recovery. In addition, we also sought to identify the risk factors that could predict QTc interval prolongation during SH.
METHODS
By retrospective medical record review, we screened patients with T2DM who were 25 years of age or older and visited the emergency department because of SH between January 2004 and June 2014. Among them, the subjects who had both undergone electrocardiography (ECG) that was conducted within 3 to 6 months of experiencing SH (baseline ECG) and had no evidence of cardiovascular events during that period were consecutively enrolled.
The patients with previously diagnosed coronary artery disease, atrial fibrillation, T1DM, or any critical illness, such as liver cirrhosis, sepsis, malignancy, end stage renal disease, and those fitted with a pacemaker were excluded. In addition, patients with T1DM were excluded, which was defined as participants who were 25 years old and younger or had a history of diabetic ketoacidosis or had fasting serum C-peptide levels lower than 0.6 ng/mL (0.20 nmol/L) [18].
Patients taking drugs that influence the QT interval, such as β-blockers or antiarrhythmic, anti-psychiatric or antihistamine agents, and who were given glucose solution before arriving at the emergency department were also excluded from the study. The patients were defined as alcohol drinkers if their average consumption was 1 to 2 drinks per day [19]. The patients were also classified as to their current smoking status at the time of enrollment. The Catholic Medical Center Ethics Committee approved this study (IRB No. VC12EISI0103). Written informed consent was obtained from all participants.
Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or the use of antihypertensive medications. The plasma glucose levels were assayed using an enzymatic method, and the glycosylated hemoglobin (HbA1c) level was assayed by high-performance liquid chromatography (Bio-Rad, Montreal, QC, Canada). The total cholesterol, triglyceride level, and high density lipoprotein cholesterol were enzymatically assayed using an automatic analyzer (model 736-40; Hitachi, Tokyo, Japan) after at least 8 hours of fasting. To determine chronic kidney disease (CKD), the estimated glomerular filtration rate (eGFR) using the four-component Modification of Diet in Renal Disease equation was calculated during SH [20]. CKD was defined as a status with a decreased eGFR less than 60 mL/min/1.73 m2, or presence of microalbuminuria (30 to 300 mg/day) or macroalbuminuria (≥300 mg/day) for more than 3 months [21,22]. The urinary albumin excretion rate was assayed from a 24-hour urine collection using immunoturbidimetry (Eiken, Tokyo, Japan).
Evaluation of QTc interval prolongation
SH was defined as a hypoglycemic event requiring the assistance of another person to administer carbohydrates and other resuscitative actions, or to provide hospitalization or medical care in an emergency department [20]. The QTc interval was measured using a standard resting 12-lead ECG during the SH event, immediately on arrival using an ECG device (PI-19E; Fukuda Denshi, Tokyo, Japan). The ECG device recorded all leads simultaneously, which facilitated accurate assessment of the QT interval, which was defined as the interval from the onset of the QRS complex to the end of the T-wave in the cardiac electric cycle. The QT and RR intervals were measured by two experienced physicians, blinded to diagnosis, using five consecutive beats on the lead II. The QT interval was corrected for heart rate using Bazett's formula (QTc interval=QT/RR1/2) [23]. QTc measurements ≥450 ms in men and ≥460 ms in women were considered abnormally prolonged [24]. The interobserver variability of QT interval assessed by coefficient of variation was 3.1%. In addition to the baseline ECG, we also collected the standard 12-lead ECG between 3 and 6 months after the SH episode to evaluate the change in the QTc interval after SH recovery.
Statistical analyses
Clinical characteristics were described as the mean±standard deviation for continuous variables and frequency (percentage) for categorical variables. The clinical characteristics were compared between the patients with prolonged QTc and those without prolonged QTc interval stratified by sex. Chi-square tests or Fisher exact tests were used for the categorical variables, and independent Student t-test was used for continuous variables. Analyses of changes in the QTc interval from the baseline QTc to QTc interval after recovery were estimated using a repeated measures analysis of variance. Non-normally distributed data were log transformed. We used Pearson correlation to estimate the relationship between clinical variables and the QTc interval during SH. In addition, we used logistic regression analysis to test the associations between the prolongation of the QT interval at baseline which was conducted within 3 to 6 months before SH without any cardiovascular events, and during the SH episode. The relationships were explored after adjusting for the following factors: age, sex, duration of diabetes, body mass index (BMI), the habit of smoking, presence of hypertension, CKD, history of SH, and the use of diabetic medication such as insulin or sulfonylurea. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). P<0.05 was considered significant. The results are reported as the odds ratios (ORs) with 95% confidence intervals (CIs).
RESULTS
Clinical characteristics
During the study period, 208 patients were enrolled. The mean age and diabetes duration of participants were 68.1±12.1 and 14.1±10.1 years, respectively. One hundred and twenty-seven patients (61.1%) were women. On arrival at the emergency room following an episode of SH, the mean plasma glucose and HbA1c level were 35.9±14.6 mg/dL (1.9±0.8 mmol/L) and 7.4%±1.6%, respectively. Seventy-four subjects (35.6%) had CKD (Table 1). Female subjects had a higher BMI (24.5±4.0 kg/m2 vs. 22.3±3.5 kg/m2, P<0.001), had hypertension (67.2% vs. 51.9%, P=0.031), drank less alcohol (6.3% vs. 35.8%, P<0.001) and had a lower proportion of current smokers (3.9% vs. 34.6%, P<0.001), compared to those of male subjects (Table 1).
Table 1. Sex-stratified clinical characteristics of the participants during SH episode.
| Characteristic | All participants | Men | Women | P value |
|---|---|---|---|---|
| Number | 208 (100) | 81 (38.9) | 127 (61.1) | |
| QTc interval at baseline, ms | 433±33 | 419±29 | 443±33 | <0.001 |
| QTc prolongation at baseline, % | 24.8 | 21.3 | 27.0 | 0.353 |
| QTc interval during SH, ms | 460±33 | 450±31 | 467±32 | 0.002 |
| QTc prolongation at SH, % | 54.8 | 54.3 | 55.1 | 0.957 |
| Age, yr | 68.1±12.1 | 67.2±12.1 | 68.8±12.2 | 0.370 |
| Diabetes duration, yr | 14.1±10.1 | 13.8±10.1 | 14.3±10.1 | 0.730 |
| BMI, kg/m2 | 23.6±4.0 | 22.3±3.5 | 24.5±4.0 | <0.001 |
| Alcohol, % | 17.8 | 35.8 | 6.3 | <0.001 |
| Smoking, % | 15.9 | 34.6 | 3.9 | <0.001 |
| Hypertension, % | 61.4 | 51.9 | 67.2 | 0.031 |
| CKD, % | 35.6 | 42.0 | 31.5 | 0.124 |
| Diabetic retinopathy, % | 61.5 | 58.7 | 63.8 | 0.596 |
| SBP, mm Hg | 137±27 | 138±30 | 136±26 | 0.717 |
| DBP, mm Hg | 81±14 | 82±15 | 81±13 | 0.559 |
| Pulse rate, beats/min | 83±13 | 84±13 | 82±13 | 0.200 |
| Plasma glucose, mg/dL | 35.9±14.6 | 33.8±15.1 | 37.3±14.2 | 0.094 |
| eGFR, mL/min/1.73 m2 | 78.8±37.7 | 81.2±36.9 | 77.2±38.2 | 0.739 |
| HbA1c, % | 7.4±1.6 | 7.6±1.9 | 7.2±1.3 | 0.175 |
| Microalbuminuria, mg/day | 142.3±329.4 | 145.7±229.2 | 139.9±387.8 | 0.938 |
| Potassium, mEq/L | 3.9±0.6 | 3.9±0.7 | 3.9±0.6 | 0.881 |
| Calcium, mg/dL | 8.7±0.8 | 8.5±0.7 | 8.8±0.8 | 0.135 |
| Magnesium, mg/dL | 2.3±0.6 | 2.3±0.6 | 2.3±0.5 | 0.896 |
| TC, mg/dL | 165.9±45.5 | 156.7±38.4 | 172.3±49.1 | 0.071 |
| TG, mg/dL | 116.5±72.7 | 113.4±72.3 | 118.5±73.5 | 0.721 |
| HDL-C, mg/dL | 45.6±21.1 | 40.5±13.6 | 48.7±24.3 | 0.051 |
| LDL-C, mg/dL | 88.6±43.8 | 83.4±29.2 | 92.2±51.3 | 0.360 |
| Recurrent SH, % | 0.790 | |||
| First episode | 60.7 | 60.5 | 60.8 | |
| Second episode | 29.6 | 32.1 | 28.0 | |
| ≥Third episode | 9.7 | 7.4 | 11.2 | |
| Glucose-lowering treatment, % | ||||
| Insulin | 39.4 | 44.4 | 36.2 | 0.237 |
| Sulfonylure | 45.7 | 45.7 | 45.7 | 0.999 |
Values are presented as number (%) or mean±standard deviation. P value for difference between men and women, <0.05 was considered significant.
QTc, heart rate-corrected QT; SH, severe hypoglycemia; BMI, body mass index; CKD, chronic kidney disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
QTc interval prolongation during SH episodes
The mean QTc interval during the SH episode was 460±33 ms (males, 450±31 ms; females, 467±32 ms), and 114 patients (54.8%; males, 54.3%; females, 55.1%) had a prolonged QT interval during SH. As shown in Table 2, male patients with a prolonged QTc interval were more likely to have a faster pulse rate (87±13 beats per minute vs. 81±12 beats per minute, P=0.026), higher microalbuminuria level (241.9±287.4 mg/day vs. 43.8±49.0 mg/day, P=0.010), and higher low density lipoprotein cholesterol level (95.6±27.4 mg/dL vs. 73.1±27.2 mg/dL, P=0.020). There were no significant differences in the electrolyte disturbance during SH, including hypokalemia (potassium <3.5 mEq/L, P=0.523), hypocalcemia (calcium <8.2 mg/dL, P=0.131), and hypomagnesemia (magnesium <1.8 mg/dL, P=0.322) and the use of diabetic medications such as insulin (males, P=0.842; females, P=0.629) or sulfonylurea (males, P=0.165; females, P=0.744) (Table 2).
Table 2. Comparison of clinical characteristics between patients with prolonged QTc and patients without prolonged QTc during the SH episode.
| Characteristic | Men | Women | ||||
|---|---|---|---|---|---|---|
| QTc <460 ms | QTc ≥460 ms | P value | QTc <460 ms | QTc ≥460 ms | P value | |
| Number | 44 (54.3) | 37 (38.3) | 57 (44.9) | 70 (55.1) | ||
| QTc interval during SH, ms | 425±18 | 475±20 | <0.001 | 438±17 | 486±25 | <0.001 |
| Age, yr | 65.1±12.0 | 69.0±12.0 | 0.149 | 68.0±12.2 | 69.3±12.2 | 0.553 |
| Diabetes duration, yr | 12.4±9.5 | 14.9±10.6 | 0.296 | 13.0±8.8 | 15.3±11.1 | 0.196 |
| BMI, kg/m2 | 22.3±3.1 | 22.4±3.9 | 0.854 | 24.7±4.4 | 24.4±3.8 | 0.762 |
| Alcohol, % | 35.1 | 36.4 | 0.909 | 3.5 | 8.6 | 0.245 |
| Smoking, % | 43.2 | 27.3 | 0.132 | 3.5 | 4.3 | 0.823 |
| Hypertension, % | 45.7 | 50.0 | 0.708 | 66.1 | 68.1 | 0.809 |
| CKD, % | 43.2 | 40.9 | 0.832 | 28.1 | 34.3 | 0.453 |
| Diabetic retinopathy, % | 52.2 | 65.2 | 0.369 | 53.8 | 71.9 | 0.155 |
| SBP, mm Hg | 135±24 | 140±34 | 0.494 | 138±28 | 135±25 | 0.549 |
| DBP, mm Hg | 81±14 | 82±16 | 0.705 | 82±15 | 80±11 | 0.334 |
| Pulse rate, beats/min | 81±12 | 87±13 | 0.026 | 84±12 | 80±14 | 0.058 |
| Plasma glucose, mg/dL | 33.2±14.3 | 34.3±15.8 | 0.738 | 36.3±14.4 | 38.1±14.1 | 0.465 |
| eGFR, mL/min/1.73 m2 | 73.4±37.5 | 87.8±35.5 | 0.080 | 82.8±36.6 | 72.8±39.1 | 0.146 |
| HbA1c, % | 7.6±1.9 | 7.7±2.0 | 0.882 | 7.2±1.2 | 7.3±1.4 | 0.736 |
| Microalbuminuria, mg/day | 43.8±49.0 | 241.9±287.4 | 0.010 | 220.8±545.8 | 74.0±164.3 | 0.235 |
| Potassium, mEq/L | 3.9±0.8 | 3.8±0.6 | 0.365 | 3.9±0.6 | 3.9±0.7 | 0.713 |
| Calcium, mg/dL | 8.7±0.8 | 8.2±0.4 | 0.077 | 9.0±0.9 | 8.6±0.7 | 0.142 |
| Magnesium, mg/dL | 2.4±0.8 | 2.2±0.3 | 0.563 | 2.5±0.5 | 2.2±0.5 | 0.117 |
| TC, mg/dL | 149.7±36.3 | 163.3±39.9 | 0.228 | 165.7±50.8 | 180.6±46.5 | 0.215 |
| TG, mg/dL | 115.4±73.9 | 111.2±72.3 | 0.849 | 112.7±46.5 | 122.9±73.3 | 0.577 |
| HDL-C, mg/dL | 38.3±12.8 | 43.1±14.4 | 0.267 | 54.3±32.4 | 44.6±14.8 | 0.112 |
| LDL-C, mg/dL | 73.1±27.2 | 95.6±27.4 | 0.020 | 101.9±63.4 | 85.6±40.1 | 0.267 |
| Recurrent SH, % | 0.476 | 0.608 | ||||
| First episode | 51.4 | 68.2 | 56.4 | 64.3 | ||
| Second episode | 40.5 | 25.0 | 32.7 | 24.3 | ||
| ≥Third episode | 8.1 | 6.8 | 10.9 | 11.4 | ||
| Glucose-lowering treatment, % | ||||||
| Insulin | 43.2 | 45.5 | 0.842 | 36.5 | 40.9 | 0.629 |
| Sulfonylurea | 54.1 | 38.6 | 0.165 | 50.0 | 53.0 | 0.744 |
Values are presented as number (%) or mean±standard deviation.
QTc, heart rate-corrected QT; SH, severe hypoglycemia; BMI, body mass index; CKD, chronic kidney disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
As shown in Table 3, the QTc interval was significantly prolonged during SH and improved after SH recovery (baseline vs. during SH vs. after SH recovery, 433±33 ms vs. 460±33 ms vs. 442±29 ms, P<0.001). Compared with the group without a prolonged QTc at baseline, male and female patients with a prolonged QTc at baseline had significant QTc interval prolongation during SH (P<0.001) and showed recovery after SH (P<0.001).
Table 3. Prolongation of QTc interval during SH compared with baseline QTc interval and recovery after SH.
| Variable | Baseline | During SH event | After SH | P value |
|---|---|---|---|---|
| QTc interval, ms | ||||
| Total | 433±33 | 460±33 | 442±29 | <0.001 |
| Men | 419±29 | 450±31 | 434±28 | <0.001 |
| Women | 443±33 | 467±32 | 447±28 | <0.001 |
| QTc interval prolongation, % | ||||
| Total | 24.8 | 54.8 | 33.8 | <0.001 |
| Men | 21.3 | 54.3 | 18.2 | <0.001 |
| Women | 27.0 | 55.1 | 28.3 | <0.001 |
| Group without prolonged QTc interval, ms | ||||
| Total | 424±26 | 432±18 | 432±22 | 0.019 |
| Men | 413±24 | 425±18 | 428±21 | 0.003 |
| Women | 434±24 | 438±17 | 435±23 | 0.509 |
| Group with prolonged QTc interval, ms | ||||
| Total | 441±37 | 482±24 | 450±31 | <0.001 |
| Men | 425±33 | 475±20 | 441±34 | <0.001 |
| Women | 450±37 | 486±25 | 456±29 | <0.001 |
Values are presented as mean±standard deviation.
QTc, heart rate-corrected QT; SH, severe hypoglycemia.
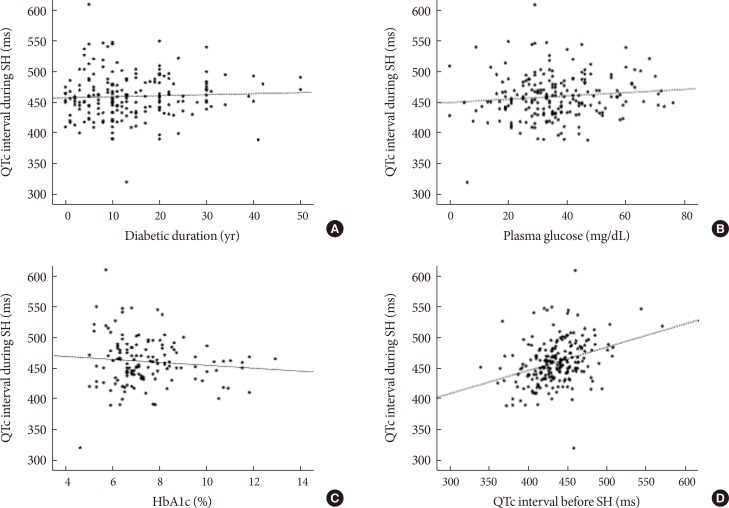
We assessed the correlations between clinical variables and QTc interval during SH. There were no significant correlations between QTc interval (ms) and diabetic duration (r=0.036, P=0.621), plasma glucose level (r=0.108, P=0.120), or HbA1c level (r=−0.098, P=0.251) during SH (Fig. 1A-C). However, baseline QTc interval before SH was significantly correlated with QTc interval during SH (r=0.333, P<0.001) (Fig. 1D).
Fig. 1. Correlations between clinical variables and heart rate-corrected QT (QTc) interval during severe hypoglycemia (SH) episode. (A) Duration of diabetes (years; r=0.036, P=0.621). (B) Plasma glucose (mg/dL; r=0.108, P=0.120). (C) Glycosylated hemoglobin (HbA1c) level (%; r=−0.098, P=0.251). (D) QTc interval before SH (ms; r=0.333, P<0.001).
Logistic regression analysis showed that QTc prolongation at baseline was significantly associated with QTc prolongation during SH in univariable analysis. In multivariable analysis, independent of the patient's age, sex, diabetes duration, BMI, habit of smoking, presence of hypertension and CKD, previous SH, and use of insulin or sulfonylurea, the prolongation of the QTc interval at baseline was strongly associated with a prolonged QTc interval during SH (OR, 2.92; 95% CI, 1.22 to 6.96; P=0.016) (Table 4).
Table 4. Univariable and multivariable logistic regression analysis for QTc prolongation during SH episode.
| Variable | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Age, yr | 1.02 (0.99–1.04) | 0.171 | 1.02 (0.99–1.06) | 0.215 |
| Diabetes duration, yr | 1.39 (0.98–1.97) | 0.067 | 1.45 (0.92–2.30) | 0.110 |
| Female sex | 1.03 (0.59–1.81) | 0.910 | 1.15 (0.51–2.62) | 0.736 |
| BMI, kg/m2 | 1.01 (0.93–1.09) | 0.885 | 1.01 (0.92–1.12) | 0.772 |
| Smoking, % | 0.64 (0.30–1.35) | 0.242 | 0.64 (0.22–1.88) | 0.415 |
| Hypertension | 0.99 (0.56–1.75) | 0.968 | 0.58 (0.26–1.30) | 0.185 |
| CKD | 1.13 (0.64–2.002) | 0.675 | 1.23 (0.59–2.56) | 0.587 |
| Previous SH | 0.82 (0.54–1.24) | 0.351 | 0.59 (0.29–1.21) | 0.152 |
| Insulin | 1.24 (0.71–2.17) | 0.457 | 0.55 (0.20–1.54) | 0.258 |
| Sulfonylurea | 0.75 (0.43–1.30) | 0.301 | 0.51 (0.20–1.32) | 0.165 |
| Baseline QTc prolongation | 2.22 (1.13–4.33) | 0.020 | 2.92 (1.22–6.96) | 0.016 |
QTc, heart rate-corrected QT; SH, severe hypoglycemia; CI, confidence interval; BMI, body mass index; CKD, chronic kidney disease.
Cardiovascular outcomes during follow-up period
During 6 months follow-up after SH, one episode of acute myocardial infarction developed within 3 months after SH, and five patients were diagnosed with atrial fibrillation during SH episodes. Among five patients with newly diagnosed atrial fibrillation, four patients had prolonged QTc interval during SH episodes. In addition, six patients have been diagnosed with a stroke within 3 months after SH, which was confirmed by radiological examination.
We also found that two patients died because of ventricular tachycardia or ventricular fibrillation 1 month after an episode of SH, and one patient died of sudden death 6 months after SH, showing pulseless electrical activity on arrival at the emergency department. In addition, one patient died because of acute myocardial infarction 6 months after SH.
DISCUSSION
We investigated the changes in the QTc interval at baseline, during SH, and recovery after SH and associated factors with prolonged QTc interval in patients with T2DM. We demonstrated that baseline prolongation in the QTc interval was an independent predictor of a prolonged QTc interval during SH in patients with T2DM after adjusting for age, sex, diabetes duration, BMI, the habit of smoking, presence of hypertension, CKD, previous SH, and the use of insulin or sulfonylurea.
Hypoglycemia is a clinically important obstacle for glycemic control in patients with T1DM and T2DM, especially with intensive glucose-lowering treatment [2,9,25,26]. Hypoglycemia causes acute problems in the brain, immediately affecting cognition, mood, and the level of consciousness [2,27]. Recent episodes of hypoglycemia cause a vicious cycle of recurrent hypoglycemia, which is known as hypoglycemia-associated autonomic failure [28]. Additionally, hypoglycemia can have fatal outcomes with an estimated mortality of 3.6% to 5.1% per year in patients with T2DM [5]. The link between hypoglycemia or SH and mortality has been studied in previous work. Tattersall and Gill [29] reported unexpected deaths in patients with T1DM who lacked a history of complications. This has become known as "dead-in-bed syndrome," and it was suggested that the deaths were due to hypoglycemia [29].
According to the ACCORD and ADVANCE trials, participants with T2DM during SH have a 2.28- to 4.86-fold higher incidence of mortality: ACCORD intensive treatment group (hazard ratio [HR], 1.28; 95% CI, 1.19 to 2.47), standard treatment group (HR, 2.87; 95% CI, 1.73 to 4.76); ADVANCE (HR, 4.86; 95% CI, 3.60 to 6.57; P<0.001) [5,30]. Zoungas et al. [5] reported that SH was associated with all-cause, cardiovascular, and non-cardiovascular mortality. Postulated mechanisms of cardiovascular mortality included sympathoadrenal activation, abnormal cardiac repolarization, leukocyte activation, increased thrombogenesis, inflammation, and vasoconstriction [15,31].
Because the QT interval reflects the duration of myocardial depolarization and repolarization, a prolonged QTc interval is associated with an increased risk of ventricular arrhythmia, which could lead to sudden cardiac death [32]. Bazett's formula, which is most commonly used to correct heart rates, overcorrects at faster heart rates and undercorrects at slower heart rates [24,32]. The QT interval overcorrects heart rates; therefore, it is essential to correct the QT interval for heart rate. Tsujimoto et al. [14] reported that SH was associated with QTc prolongation, severe hypertension, and newly developed atrial fibrillation in patients with T1DM and T2DM. Robinson et al. [15] also reported that the QTc interval during experimental hypoglycemia, using a hyperinsulinemic clamp, increased (67 ms, P<0.001). Therefore, sudden death or ventricular arrhythmia that develops during an SH occurrence might be predicted by the QTc interval prolongation in T1DM and T2DM.
We also found that two patients died because of ventricular tachycardia or ventricular fibrillation 1 month after SH, and one patient died of sudden death 6 months after SH. Prolonged QTc interval after SH might affect depolarization and repolarization of the myocardium and cause death, but correlation and a causal relationship between the development of SH and death could not be investigated because of short-term follow-up and a small number of subjects. Further studies are needed to evaluate clinical outcomes of patients with SH and QTc prolongation.
In this study, we demonstrated that 54.8% of patients with T2DM experienced QTc interval prolongation during SH and that the prolongation was significantly associated with the baseline QTc interval. In addition to the baseline QTc interval, only the female sex was associated with QTc prolongation during SH. Many studies have reported that young and middle-aged females have a longer QT interval than males and diabetes patients [24,33]. The relationship between female sex and QTc interval in patients with T2DM was consistent with previous studies. For these reasons, we used 450 ms as the cut-off of QTc interval prolongation in men and 460 ms in women, respectively [24]. In addition, we could not identify any correlation between the QTc interval during an SH episode and diabetic duration or glycemic control status.
To the best of our knowledge, no previously published observational studies have analyzed the serial relationship of the QTc interval before and after an SH episode in T2DM. This study suggests that we should pay more scrupulous attention to the QTc interval and the presence of arrhythmia during SH as well as educating patients to avoid SH because of these prolonged QTc intervals, which are associated with an increased risk of a life-threatening cardiac arrhythmia. In addition, we suggest checking a 12-lead ECG to evaluate the QTc interval during the annual check-up of patients with diabetic complications.
The limitations of this study are as follows. First, this study was conducted retrospectively with a relatively small number of participants. Prospective studies are needed to estimate the causal relationship between fatality or higher mortality and QTc prolongation during SH. Second, we could not definitively explain the mechanism of QTc prolongation in SH. Third, we analyzed a single QT interval at the time of SH episodes and 3 to 6 months after SH; however, serial short-term follow-up of the QTc interval after SH would improve our understanding of the changes in the QTc prolongation and clinical outcomes.
In conclusion, the presence of a prolonged baseline QTc interval was an important predictive marker of QTc prolongation during an SH event in patients with T2DM. Therefore, we recommend that QTc interval monitoring be performed with screening for typical diabetic complications, especially in patients with a high risk of developing SH, such as patients with T2DM.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 2.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The DCCT Research Group. Diabetes Control and Complications Trial (DCCT): results of feasibility study. Diabetes Care. 1987;10:1–19. doi: 10.2337/diacare.10.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 5.Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S, Grobbee DE, Kengne AP, Marre M, Heller S ADVANCE Collaborative Group. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 6.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 8.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 9.Kim JT, Oh TJ, Lee YA, Bae JH, Kim HJ, Jung HS, Cho YM, Park KS, Lim S, Jang HC, Lee HK. Increasing trend in the number of severe hypoglycemia patients in Korea. Diabetes Metab J. 2011;35:166–172. doi: 10.4093/dmj.2011.35.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, Heller SR, Rodriguez H, Rosenzweig J, Vigersky R American Diabetes Association; Endocrine Society. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab. 2013;98:1845–1859. doi: 10.1210/jc.2012-4127. [DOI] [PubMed] [Google Scholar]
- 11.Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Characterizing sudden death and dead-in-bed syndrome in type 1 diabetes: analysis from two childhood-onset type 1 diabetes registries. Diabet Med. 2011;28:293–300. doi: 10.1111/j.1464-5491.2010.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barr CS, Naas A, Freeman M, Lang CC, Struthers AD. QT dispersion and sudden unexpected death in chronic heart failure. Lancet. 1994;343:327–329. doi: 10.1016/s0140-6736(94)91164-9. [DOI] [PubMed] [Google Scholar]
- 13.Darbar D, Luck J, Davidson N, Pringle T, Main G, McNeill G, Struthers AD. Sensitivity and specificity of QTc dispersion for identification of risk of cardiac death in patients with peripheral vascular disease. BMJ. 1996;312:874–878. doi: 10.1136/bmj.312.7035.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsujimoto T, Yamamoto-Honda R, Kajio H, Kishimoto M, Noto H, Hachiya R, Kimura A, Kakei M, Noda M. Vital signs, QT prolongation, and newly diagnosed cardiovascular disease during severe hypoglycemia in type 1 and type 2 diabetic patients. Diabetes Care. 2014;37:217–225. doi: 10.2337/dc13-0701. [DOI] [PubMed] [Google Scholar]
- 15.Robinson RT, Harris ND, Ireland RH, Lee S, Newman C, Heller SR. Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycemia. Diabetes. 2003;52:1469–1474. doi: 10.2337/diabetes.52.6.1469. [DOI] [PubMed] [Google Scholar]
- 16.Gruden G, Giunti S, Barutta F, Chaturvedi N, Witte DR, Tricarico M, Fuller JH, Cavallo Perin P, Bruno G. QTc interval prolongation is independently associated with severe hypoglycemic attacks in type 1 diabetes from the EURODIAB IDDM complications study. Diabetes Care. 2012;35:125–127. doi: 10.2337/dc11-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen TF, Tarnow L, Randlov J, Kristensen LE, Struijk JJ, Eldrup E, Hejlesen OK. QT interval prolongation during spontaneous episodes of hypoglycaemia in type 1 diabetes: the impact of heart rate correction. Diabetologia. 2010;53:2036–2041. doi: 10.1007/s00125-010-1802-0. [DOI] [PubMed] [Google Scholar]
- 18.Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30:803–817. doi: 10.1111/dme.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal DP. Cardioprotective effects of light-moderate consumption of alcohol: a review of putative mechanisms. Alcohol Alcohol. 2002;37:409–415. doi: 10.1093/alcalc/37.5.409. [DOI] [PubMed] [Google Scholar]
- 20.Workgroup on Hypoglycemia, American Diabetes Association. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Bazett HC. An analysis of the time relation of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 24.Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, van Herpen G, Wagner GS, Wellens H American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV. The ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Circulation. 2009;119:e241–e250. doi: 10.1161/CIRCULATIONAHA.108.191096. [DOI] [PubMed] [Google Scholar]
- 25.Johnson ES, Koepsell TD, Reiber G, Stergachis A, Platt R. Increasing incidence of serious hypoglycemia in insulin users. J Clin Epidemiol. 2002;55:253–259. doi: 10.1016/s0895-4356(01)00479-6. [DOI] [PubMed] [Google Scholar]
- 26.Yun JS, Ko SH. Avoiding or coping with severe hypoglycemia in patients with type 2 diabetes. Korean J Intern Med. 2015;30:6–16. doi: 10.3904/kjim.2015.30.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren RE, Frier BM. Hypoglycaemia and cognitive function. Diabetes Obes Metab. 2005;7:493–503. doi: 10.1111/j.1463-1326.2004.00421.x. [DOI] [PubMed] [Google Scholar]
- 28.Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013;369:362–372. doi: 10.1056/NEJMra1215228. [DOI] [PubMed] [Google Scholar]
- 29.Tattersall RB, Gill GV. Unexplained deaths of type 1 diabetic patients. Diabet Med. 1991;8:49–58. doi: 10.1111/j.1464-5491.1991.tb01516.x. [DOI] [PubMed] [Google Scholar]
- 30.Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B, Horowitz KR, Savage PJ, Seaquist ER, Simmons DL, Sivitz WI, Speril-Hillen JM, Sweeney ME. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev. 2008;24:353–363. doi: 10.1002/dmrr.865. [DOI] [PubMed] [Google Scholar]
- 32.Stettler C, Bearth A, Allemann S, Zwahlen M, Zanchin L, Deplazes M, Christ ER, Teuscher A, Diem P. QTc interval and resting heart rate as long-term predictors of mortality in type 1 and type 2 diabetes mellitus: a 23-year follow-up. Diabetologia. 2007;50:186–194. doi: 10.1007/s00125-006-0483-1. [DOI] [PubMed] [Google Scholar]
- 33.Rana BS, Lim PO, Naas AA, Ogston SA, Newton RW, Jung RT, Morris AD, Struthers AD. QT interval abnormalities are often present at diagnosis in diabetes and are better predictors of cardiac death than ankle brachial pressure index and autonomic function tests. Heart. 2005;91:44–50. doi: 10.1136/hrt.2003.017632. [DOI] [PMC free article] [PubMed] [Google Scholar]