Abstract
Aggregation of the pre-synaptic protein, α-synuclein (α-syn), is the key etiological factor in Parkinson's disease (PD) and other alpha-synucleinopathies, such as multiple system atrophy (MSA) and Dementia with Lewy bodies (DLB). Various triggers for pathological α-syn aggregation have been elucidated, including post-translational modifications, oxidative stress, and binding of metal ions, such as calcium. Raised neuronal calcium levels in PD may occur due to mitochondrial dysfunction and/or may relate to calcium channel dysregulation or the reduced expression of the neuronal calcium buffering protein, calbindin-D28k. Recent results on human tissue and a mouse oxidative stress model show that neuronal calbindin-D28k expression excludes α-syn inclusion bodies. Previously, cell culture model studies have shown that transient increases of intracellular free Ca(II), such as by opening of the voltage-gated plasma calcium channels, could induce cytoplasmic aggregates of α-syn. Raised intracellular free calcium and oxidative stress also act cooperatively to promote α-syn aggregation. The association between raised neuronal calcium, α-syn aggregation, oxidative stress, and neurotoxicity is reviewed in the context of neurodegenerative α-syn disease and potential mechanism-based therapies.
Keywords: Parkinson's disease, α-Synuclein, calcium, Multiple system atrophy, Dementia with Lewy bodies
Introduction: α-synuclein in neurodegeneration
α-Synuclein (α-syn) is a small (14 kDa), acidic protein that is highly conserved in vertebrate species. It is primarily expressed in the presynaptic terminals of dopaminergic neurons in the olfactory bulb, frontal cortex, striatum, and the hippocampus. α-Syn has also been observed in hypothalamus, thalamus, midbrain, cerebellum, and the pons/medulla oblongata with lower expression (Maroteaux et al., 1988; Iwai et al., 1995; Clayton and George, 1999). The normal function of α-syn remains unknown, however, it has been found that α-syn can interact with the lipid bilayer of neurons to prevent SNARE-mediated fusion of vesicles (DeWitt and Rhoades, 2013). Cytoplasmic and nuclear inclusion bodies containing α-syn have been found throughout the central nervous system (CNS) in a number of neurodegenerative disorders. These are collectively termed α-synucleinopathies and include Parkinson's disease (PD) and atypical PD, such as multiple system atrophy (MSA) and Dementia with Lewy bodies (DLB; Goedert et al., 2013; Eschbach and Danzer, 2014; Radford et al., 2014). Aggregation of α-syn results from dynamic instability of the native structure and may be promoted by gene mutations or by environmental stressors, such as oxidative stress or metal ion concentration changes.
PD can be classified into two main groups: idiopathic PD, accounting for 85–90% of all PD cases; and familial PD, accounting for 10–15%. Currently, six PD-linked mutations in PARK1/4, the α-syn gene, have been identified that may provide important clues to the pathways that may give rise to idiopathic PD. They include the A30P (Kruger et al., 1998), A53T (Polymeropoulos et al., 1997), E46K (Zarranz et al., 2004), H50Q (Proukakis et al., 2013), A53E (Pasanen et al., 2014), and G51D (Lesage et al., 2013) amino acid substitutions that disrupt the membrane binding domain (Figure 1A). The A53T and A30P mutations also alter neuronal cytotoxicity in response to hydrogen peroxide and 1-methyl-4-phenylpyridinium (MPP+) treatment. Expression of these mutant isoforms significantly increased cytotoxicity in comparison to cells expressing wild-type (WT) α-syn, which was similar to control cells (Kanda et al., 2000). Furthermore, both mutations showed increased fibril formation compared to WT (Conway et al., 1998, 2000; Narhi et al., 1999). E57K: mThy-1 human α-syn transgenic mice with increased oligomerisation of α-syn had decreased vesicles, synapse, and neuron degeneration in comparison to wildtype controls and E57K mice expressing lower levels of α-syn (Rockenstein et al., 2014). Gene duplication (Chartier-Harlin et al., 2004; Ibanez et al., 2004) and triplication of α-syn (Singleton et al., 2003) has also been found in familial PD, implicating increased expression of the wild-type protein. Recasens et al. (2014) have demonstrated that α-syn species found in PD-derived Lewy bodies (LBs) were pathogenic and had the capacity to initiate a PD-like pathological process. This included intracellular and presynaptic aggregation of pathological α-syn species in various parts of the brain and progressive cell loss of dopaminergic neurons. Several metals have been implicated in the aggregation of α-syn, including, iron, manganese, copper, and zinc (Santner and Uversky, 2010; McAllum and Finkelstein, 2016). However, this review will focus on calcium in alpha-synucleinopathies and its interaction with α-syn.
Figure 1.
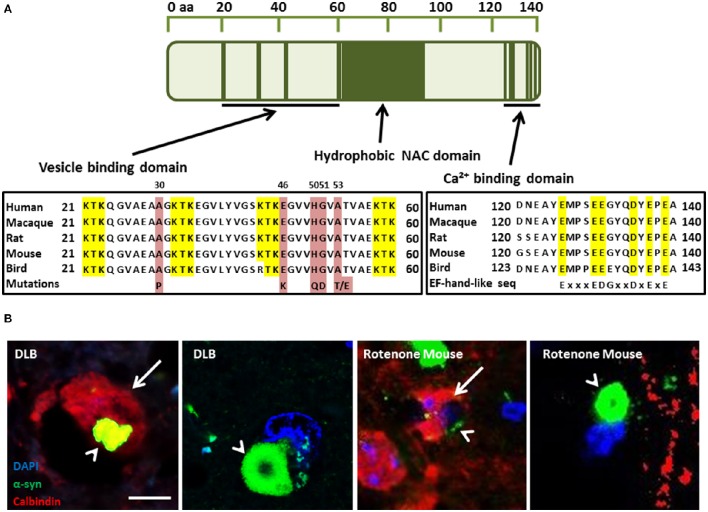
(A) α-Synuclein (α-syn) protein domain structure. KTK repeats in the N-terminus are involved in lipid interaction, the hydrophobic NAC domain is important for aggregation and the C-terminal Ca(II) binding site can increase the rate of oligomerization (Nielsen et al., 2001; Lowe et al., 2004). Parkinson's disease linked point mutations are indicated within the neurotransmitter vesicle binding domain. (B) Neuronal Calbindin-D28K (CB), inhibits α-syn inclusion bodies in DLB and mouse model tissue (Rcom-H'cheo-Gauthier et al., 2016). (B1-2) Dementia with Lewy bodies immunofluorescence for α-syn (green) and CB (red, arrow) showing rare, small cytoplasmic α-syn aggregates (arrowhead) in a CB+ neuron and a typical, large perinuclear α-syn inclusion body in a CB- neuron. (B3-4) Rotenone lesioned mouse (oxidative stress) model shows a similar pattern of large perinuclear α-syn inclusion bodies that are absent from CB expressing neurons, with only small α-syn puncta rarely detected in CB positive cells; scale bar 5 μm.
Voltage-gated Ca(II) channels in neurodegeneration
Homeostatic Ca(II) regulation is essential to the health and functioning of cells. Although N-type voltage-gated Ca(II) channels (Cav) are the primary regulators of Ca(II) influx and corresponding neurotransmitter release in neurons (Simms and Zamponi, 2014), various studies highlight the broader range of Ca(II) channels found throughout the CNS. There are three types of Cav expressed in the brain, Cav1, Cav2, and Cav3 (Catterall et al., 2005), comprising four Cav1 subtypes (L-type configuration), three types of Cav2 channels (P/Q, N, and R configuration), with Cav2.1 and Cav2.2 responsible for neurotransmitter release, and the family of low-voltage activated T-type channels, Cav3.1, Cav3.2, and Cav3.3 (Talley et al., 1999; Heady et al., 2001; Catterall et al., 2005). Inhibition of Cav1, 2.1, and 2.2 channels have each been shown to decrease neuronal damage upon brain injury (Perez-Pinzon et al., 1997; Babu and Ramanathan, 2011; Kim et al., 2016), which also leads to increased α-syn expression, α-syn aggregation, and Parkinson's-like pathology (Acosta et al., 2015). Cav3 contribute to the pathogenesis of epilepsy (Chemin et al., 2001), and have also been reported in glial cells (Yunker et al., 2003). Bassoon, a neuronal zinc finger pre-synaptic scaffold protein shows increased expression in MSA cerebellum (Hashida et al., 1998). Interestingly, Bassoon increased Cav2.1 expression, associated with a longer calcium transient, when over-expressed and decreased Cav2.1 when knocked out in an epilepsy model (Davydova et al., 2014; Ivanova et al., 2015). Moreover, pathological α-syn species may mediate calcium entry and thus both further promote calcium-dependent α-syn aggregation and directly lead to calcium-dependent cytotoxicity (Angelova and Abramov, 2016). Wildburger et al. (2009) investigated the neuroprotective properties of the Ca(II) channel blocker, trimethadione (TMO), in cultured hippocampal and cortical neurons from C57BL/6 and α1H−/− mice, showing a significant decrease in cell death following TMO treatment (Wildburger et al., 2009). Moreover, Follett et al. (2013) showed that SH-SY5Y cells subjected to membrane depolarization by potassium resulted in Ca(II) influx and α-syn-positive cytoplasmic aggregates that could be blocked by TMO pre-treatment. Furthermore, Danish subjects taking dihydropyridine L-type calcium channel blockers between 1995 and 2006 were 27% less likely to develop PD (Ritz et al., 2010; Pasternak et al., 2012). Following this, a Phase II tolerability trial in early stage PD patients was conducted and found that isradipine was well-tolerated (Parkinson Study Group, 2013).
Ca(II) regulation in ageing and neurodegeneration
Regulation of calcium homeostasis requires a complex interplay between the cytoplasmic channels/pumps and intracellular Ca(II) stores, such as the mitochondria and endoplasmic reticulum (ER), and may be affected by age (Toescu and Verkhratsky, 2007). It has been established that resting intracellular Ca(II) levels remain the same despite the age of the neuron. However, the return time to resting levels is significantly increased in aged neurons after a stimulus. Aged neurons also exhibit a decreased capacity to recover from Ca(II) stimulus through uptake into the intracellular Ca(II) stores with a decline in sarco/endoplasmic reticulum Ca(II)-ATPase (SERCA) function (Pottorf et al., 2000). Moreover, Ca(II) pumping into the extracellular space via the plasma membrane Ca(II) ATPase (PMCA) has been shown to be impaired in aged neurons (Michaelis et al., 1996). The calcium buffering capacity of neurons was also shown to be decreased in aged neurons and some studies have shown that after stimulation, the concentration of Ca(II) may be decreased, but the recovery time back to resting levels is dramatically increased (Duckles et al., 1996). Calbindin D28K (CB), calretinin (CR), and parvalbumin (PV) are three cytosolic Ca(II) buffering proteins (CBP) that are capable of Ca(II) buffering in neurons and belong to the EF-hand protein family. The other CBP family is the annexin family and is characterized by proteins that bind Ca(II) in the presence of phospholipids-containing membranes (Andressen et al., 1993). The EF-hand proteins may function either as Ca(II) buffers, decreasing the free cytoplasmic concentration, or as triggers starting a cascade of responses (Dalgarno et al., 1984). The ubiquitous trigger protein calmodulin activates at least 20 different enzymes, while PV, CB, and CR, are more passive buffers decreasing the amplitude of Ca(II) signals. In a human study conducted by Bu et al. (2003), a decrease was found in both CR and CB in aged compared to young cortical neurons, but no difference in PV positive neurons. Yamada et al. (1990) found that dopaminergic neurons of the Substantia nigra pars compacta (SnPc) that were high in CB were preferentially spared in control brain sections compared with PD patients. The importance of these proteins has also been implicated by German et al. (1992) who studied the brains of human patients with PD, MPTP monkey or in C57BL/6. They found that in both idiopathic PD and in the above models, neurons that contained CB were spared while neurons in CB-negative (CB-) regions were lost. Gaspar et al. (1994) found that dopaminergic neurons expressing CB were spared from loss in a mouse model with Parkinsonian-like pathological features. Tsuboi et al. (2000) and Kim et al. (2000) found that CR expression in dopaminergic neurons of the SnPc were more protected against 6-hydroxydopamine (Kim et al., 2000; Tsuboi et al., 2000). Moreover, a recent study has described the over-representation of CB-positive (CB+) neurons in DLB, and the almost complete exclusion of α-syn aggregates in CB+ neurons. This study draws the same conclusions in a rotenone (oxidative stress) mouse model of α-synucleopathy (Rcom-H'cheo-Gauthier et al., 2016). In both DLB and the mouse model, occasional neurons with low CB expression showed small α-syn aggregates, but large perinuclear inclusion bodies were only detected in CB- cells (Figure 1B; Rcom-H'cheo-Gauthier et al., 2016).
Increased intracellular Ca(II) induces α-synuclein oligomerisation
Nielsen et al. (2001) first demonstrated that Ca(II) binding to α-syn regulates ligand binding and oligomerization. Subsequently, Lowe et al. (2004) found that calcium promoted α-syn annular oligomer formation that required the C-terminal calcium binding site (Figure 1A). The α-syn C terminus contains a sequence of six negatively charged acidic amino acids similar to an EF-hand-like motif (Zhou et al., 2006), although more studies are required to precisely delineate the calcium binding residues. More recent studies have shown that by increasing free intracellular Ca(II) concentration thapsigargin or Ca(II) ionophore chemical treatments caused a significant increase in the proportion of cells bearing microscopically-visible α-syn aggregates. It was also demonstrated that co-treatment with intracellular Ca(II) chelator, suppressed the aggregation, indicating that α-syn aggregation was induced by raised intracellular free Ca(II) directly, although a possible role for calpain protease activation could not be excluded. Moreover, supporting studies with recombinant α-syn indicated that direct binding of Ca(II) ion to α-syn promoted rapid oligomer formation in vitro (Nath et al., 2011). More recently, Follett et al. (2013) demonstrated that depolarization of the plasma membrane resulted in raised intracellular free Ca(II) and α-syn aggregation triggering the formation of large LB-like perinuclear α-syn inclusion bodies after 48 h that could be inhibited both by Ca(II) chelation and calcium channel blockade (Follett et al., 2013). Moreover, Chan et al. (2007) assessing Ca(II) blockade using brain slices from a MPTP mouse model of PD determined that the L-type Cav1.3 Ca(II) channel inhibitor, Isradipine, a drug used to treat high blood pressure, could recover dopaminergic neural activity. Furthermore, Singh et al. (2016) also used an L-type calcium channel blocker, nimodipine, in the same model and observed reduced loss of TH positive neurons and reduced reactive oxygen species (ROS) production in the striatal mitochondria.
Oxidative stress and α-synuclein oligomerization
Another potential target for α-synucleinopathy therapeutics could be to target oxidative stress in the brain. There is evidence that oxidative stress is increased in the normal aged brain, however the level of oxidative stress is greatly increased in patients with neurodegenerative diseases (Sims-Robinson et al., 2013). Thus, WT α-syn has been shown to induce mitochondrial NO when it is associated with mitochondria (Parihar et al., 2008). This indicates that not only will normal increases in oxidative stress cause aggregation but that aggregation of α-syn also induces more oxidative stress within the cell forming a positive feedback loop. Moreover, other research shows that α-syn protects cells from oxidative stress by deactivating the c-jun N-terminal kinase (JNK) pathway, although this data was from cells challenged with exogenous H2O2 (Hashimoto et al., 2002). The combination of oxidative stress and α-syn expression has been used to generate a model of MSA in mice, whereby the over-expression of α-syn in glial cells is combined with 3-nitropropionic acid. Treatment to induce mitochondrial oxidative stress was sufficient to induce MSA like pathology (Ubhi et al., 2009). Moreover, Kume et al. (2012) found urinary 8-OHdG levels were significantly higher in DLB cases compared to controls, indicating systemically increased oxidative stress. Oxidative stress in aging primates originates primarily from mitochondrial complexes I and III of the electron transport chain which leads to greater mitochondrial DNA damage compared to nuclear DNA (Castro Mdel et al., 2012). Indeed, widespread mitochondrial DNA damage occurs at early stages of DLB (Lin et al., 2012). Moreover, α-syn oligomers promoted Ca(II)-induced mitochondrial dysfunction (Luth et al., 2014) and oxidative stress characterized by α-syn lipoxidation precedes the formation of α-syn aggregates and the development of neocortical LB pathology in DLB (Dalfo and Ferrer, 2008). Furthermore, Quilty et al. (2006) showed that when mouse primary neocortical cells were incubated in the absence of antioxidants, mild oxidative stress caused α-syn accumulation in a subset of neurons. Indeed, recent studies have found the oxidized form of the endogenous oxidative stress sensor, DJ-1, progressively increased in the later stages of PD and more highly oxidized forms were likely present in DLB (Saito et al., 2014). Furthermore, it has been demonstrated that Ca(II) influx can interact with α-syn to mediate increased oxidative stress (Dryanovski et al., 2013; Surmeier et al., 2016). Indeed, the combination of oxidative stress and raised intracellular free Ca(II) showed a synergistic effect on α-syn aggregation (Goodwin et al., 2013). More recently, α-syn aggregation was studied using an oxidative stress mouse model where unilateral lesion was performed using the mitochondrial complex I inhibitor, rotenone. The results showed an increase in α-syn aggregates in the lesioned hemisphere, compared to sham or unlesioned, especially in aged animals, combined with an exclusion of α-syn aggregates from CB-expressing neurons, further implicating a cooperative interaction between calcium buffering and oxidative stress (Rcom-H'cheo-Gauthier et al., 2016).
α-synuclein aggregation, raised Ca(II), and oxidative stress
Hettiarachchi et al. (2009) demonstrated that elevated levels of intracellular α-syn elevated levels of intracellular Ca(II), although reduced Ca(II) was observed in primary neurons overexpressing α-syn when treated with manganese (Dučić et al., 2015). Furthermore, secreted α-syn induced an increase in capacitive Ca(II) entry in differentiated SH-SY5Y cells (Melachroinou et al., 2013). Transmembrane entry of Ca(II) or other cations may be via α-syn pore-like oligomers (Pountney et al., 2005; Mironov, 2015) and can mediate cytotoxicity (Angelova et al., 2016). Transgenic mice over-expressing human WT α-syn showed that α-syn transgenic mice exhibited augmented, long-lasting Ca(II) transients characterized by considerable deviation from the exponential decay. Furthermore control and α-syn knock out groups demonstrated low percentages of neurons with Ca(II) abnormalities whereas the α-syn transgenic group showed Ca(II) response alteration, suggesting these alterations are related to α-syn expression (Reznichenko et al., 2012; Mironov, 2015). Furthermore, Navarria et al. (2015) showed that overexpression of α-syn reduced NMDAR-mediated Ca(II) influx.
Cali et al. (2012) observed significantly greater levels of mitochondrial Ca(II) in α-syn treated cells compared to control cells. In SH-SY5Y and HeLa cells, ER-mitochondria interactions and increased mitochondrial Ca(II) were enhanced by overexpressed α-syn and increased mitochondrial Ca(II) transient. Moreover, treatment with native α-syn increased Ca(II) entry in primary rat cortical neurons and induced mitochondrial Ca(II) uptake. Dryanovski et al. (2013) found that in dopaminergic neurons bearing inclusions, ROS levels in the mitochondria were greater in the soma and proximal dendrites than in neurons not bearing any inclusions. Treatment with isradipine significantly reduced the ROS levels in CB− dopaminergic neurons when compared to CB+ dopaminergic neurons, suggesting ROS production in the cytosol is triggered by the formation of α-syn inclusions. Buttner et al. (2013) established that α-syn leads to elevated cytosolic Ca(II) in yeast that coincides with an increase in oxidative stress. This suggests that the formation of α-syn aggregates triggers an increase in mitochondrial Ca(II) transient and then leads to oxidative stress. WT α-syn also displayed the capacity to induce mitochondrial NO when associated with mitochondria (Parihar et al., 2008) and oxidative stress induced by Ca(II) influx was worsened in a DJ-1 mutant mouse PD model (Goldberg et al., 2012). Thus, an increase in oxidative stress can cause α-syn aggregation and that aggregation can also induce further oxidative stress within the cell forming a positive feedback loop. Though, this contradicts other research by Hashimoto et al. (2002) which shows that α-syn protects cells from oxidative stress by inactivating the JNK pathway. More recently, the antioxidant capacity of SH-SY5Y cells was shown to be decreased by changes in SOD1 and SOD2 enzyme activity and decreased gluthathione levels following overexpression of α-syn (Perfeito et al., 2016). Koch et al. (2016) also found that α-syn fibrils increase SOD1 aggregation in vitro and in vivo.
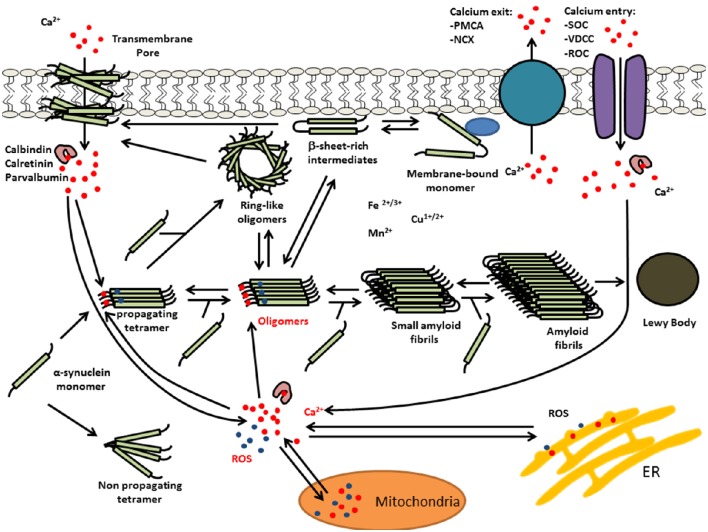
Recent findings indicate that oxidative stress and increased intracellular free Ca(II) synergistically increased the number of α-syn-positive aggregates (Goodwin et al., 2013). Moreover, in vitro experiments demonstrated that Ca(II) binding alone was able to induce non-stable α-syn aggregates, whereas, co-treatments with Ca(II) and oxidant resulted in the formation of larger, stable aggregates. Furthermore, kinetic experiments of α-syn aggregate formation by Nath et al. (2010) were consistent with an auto-catalytic mechanism. Thus, Ca(II)/oxidation stabilized α-syn aggregates could in turn serve to increase nucleation centers in disease. This is supported by work performed by Krishnan et al. (2003) that show that oxidatively di-tyrosine cross linked α-syn dimers were the rate limiting step in the fibrillation process in forming nucleation centers. Moreover, Ca(II) influx into neurons can induce oxidative stress that may in turn result in further α-syn aggregation. Figure 2 summarizes the potential synergies between raised calcium levels, oxidative stress, and α-syn aggregation.
Figure 2.
Multiple interactions of Ca(II) and oxidative stress on α-syn aggregation. Elevated levels of intracellular Ca(II) may cause α-syn aggregation and the formation of LBs. This calcium dysregulation could be mediated by elevated reactive oxygen species (ROS) and/or calcium mobilization from mitochondria, α-syn oligomer pores or dysregulation of voltage-dependent calcium channel (VDCC), plasma membrane Ca(II) ATPase (PMCA), or Na(I)-Ca(II) exchanger (NCX).
Conclusion: challenges for future therapeutics
Calcium channel antagonists are currently undergoing clinical trials for PD therapy, however many of these drugs may display undesirable features when used chronically. Modulating neuronal calcium homoeostasis via endogenous CBP or through differential calcium channel recruitment could offer efficient alternatives.
Author contributions
DP: Research group leader coordinated writing, editing of text and figures. AR: lead author contributed majority of text. SO: contributed some text. AM: reviewing co-author.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Supported by Griffith University, Australian Research Council and Menzies Health Institute Queensland and Parkinson's Queensland.
Glossary
Abbreviations
- α-syn
α-synuclein
- CB
Calbindin D28K
- CBP
Calcium buffering protein
- CNS
Central nervous system
- CR
Calretinin
- DLB
Dementia with Lewy bodies
- ER
Endoplasmic reticulum
- GC
Glial cytoplasmic inclusion
- JNK
c-jun N-terminal kinase
- LBs
Lewy bodies
- MSA
Multiple system atrophy
- NCX
Na(I)-Ca(II) exchanger
- PD
Parkinson's disease
- PMCA
Plasma membrane Ca(II) ATPase
- PV
Parvalbumin
- SNpc
Substantia nigra pars compacta
- ROS
Reactive oxygen species
- TMO
Trimethadione
- VDCC
Voltage-dependent calcium channel
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase.
References
- Acosta S. A., Tajiri N., de la Pena I., Bastawrous M., Sanberg P. R., Kaneko Y., et al. (2015). Alpha-synuclein as a pathological link between chronic traumatic brain injury and Parkinson's disease. J. Cell. Physiol. 230, 1024–1032. 10.1002/jcp.24830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andressen C., Blümcke I., Celio M. R. (1993). Calcium-binding proteins: selective markers of nerve cells. Cell Tissue Res. 271, 181–208. 10.1007/BF00318606 [DOI] [PubMed] [Google Scholar]
- Angelova P. R., Abramov A. Y. (2016). Alpha-synuclein and beta-amyloid - different targets, same players: calcium, free radicals and mitochondria in the mechanism of neurodegeneration. Biochem. Biophys. Res. Commun. [Epub ahead of print]. 10.1016/j.bbrc.2016.07.103 [DOI] [PubMed] [Google Scholar]
- Angelova P. R., Ludtmann M. H., Horrocks M. H., Negoda A., Cremades N., Klenerman D., et al. (2016). Ca2+ is a key factor in α-synuclein-induced neurotoxicity. J. Cell Sci. 129, 1792–1801. 10.1242/jcs.180737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu C. S., Ramanathan M. (2011). Post-ischemic administration of nimodipine following focal cerebral ischemic-reperfusion injury in rats alleviated excitotoxicity, neurobehavioural alterations and partially the bioenergetics. Int. J. Dev. Neurosci. 29, 93–105. 10.1016/j.ijdevneu.2010.08.001 [DOI] [PubMed] [Google Scholar]
- Bu J., Sathyendra V., Nagykery N., Geula C. (2003). Age-related changes in calbindin-D28k, calretinin, and parvalbumin-immunoreactive neurons in the human cerebral cortex. Exp. Neurol. 182, 220–231. 10.1016/S0014-4886(03)00094-3 [DOI] [PubMed] [Google Scholar]
- Buttner S., Faes L., Reichelt W. N., Broeskamp F., Habernig L., Benke S., et al. (2013). The Ca2+/Mn2+ ion-pump PMR1 links elevation of cytosolic Ca2+ levels to alpha-synuclein toxicity in Parkinson's disease models. Cell Death Differ. 20, 465–477. 10.1038/cdd.2012.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali T., Ottolini D., Negro A., Brini M. (2012). alpha-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J. Biol. Chem. 287, 17914–17929. 10.1074/jbc.M111.302794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro Mdel R., Suarez E., Kraiselburd E., Isidro A., Paz J., Ferder L., et al. (2012). Aging increases mitochondrial DNA damage and oxidative stress in liver of rhesus monkeys. Exp. Gerontol. 47, 29–37. 10.1016/j.exger.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Perez-Reyes E., Snutch T. P., Striessnig J. (2005). International union of pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 57, 411–425. 10.1124/pr.57.4.5 [DOI] [PubMed] [Google Scholar]
- Chan C. S., Guzman J. N., Ilijic E., Mercer J. N., Rick C., Tkatch T., et al. (2007). ‘Rejuvenation’ protects neurons in mouse models of Parkinson's disease. Nature 447, 1081–1086. 10.1038/nature05865 [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin M. C., Kachergus J., Roumier C., Mouroux V., Douay X., Lincoln S., et al. (2004). alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364, 1167–1169. 10.1016/S0140-6736(04)17103-1 [DOI] [PubMed] [Google Scholar]
- Chemin J., Monteil A., Perez-Reyes E., Nargeot J., Lory P. (2001). Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. EMBO J. 20, 7033–7040. 10.1093/emboj/20.24.7033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., George J. M. (1999). Synucleins in synaptic plasticity and neurodegenerative disorders. J. Neurosci. Res. 58, 120–129. [DOI] [PubMed] [Google Scholar]
- Conway K. A., Harper J. D., Lansbury P. T. (1998). Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat. Med. 4, 1318–1320. 10.1038/3311 [DOI] [PubMed] [Google Scholar]
- Conway K. A., Lee S. J., Rochet J. C., Ding T. T., Williamson R. E., Lansbury P. T., Jr. (2000). Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. U.S.A. 97, 571–576. 10.1073/pnas.97.2.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalfo E., Ferrer I. (2008). Early alpha-synuclein lipoxidation in neocortex in Lewy body diseases. Neurobiol. Aging 29, 408–417. 10.1016/j.neurobiolaging.2006.10.022 [DOI] [PubMed] [Google Scholar]
- Dalgarno D., Klevit R. E., Levine B. A., Williams R. J. P. (1984). The calcium receptor and trigger. Trends Pharmacol. Sci. 5, 266–271. 10.1016/0165-6147(84)90442-5 [DOI] [Google Scholar]
- Davydova D., Marini C., King C., Klueva J., Bischof F., Romorini S., et al. (2014). Bassoon specifically controls presynaptic P/Q-type Ca(2+) channels via RIM-binding protein. Neuron 82, 181–194. 10.1016/j.neuron.2014.02.012 [DOI] [PubMed] [Google Scholar]
- DeWitt D. C., Rhoades E. (2013). α-Synuclein can inhibit SNARE-mediated vesicle fusion through direct interactions with lipid bilayers. Biochemistry 52, 2385–2387. 10.1021/bi400236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryanovski D. I., Guzman J. N., Xie Z., Galteri D. J., Volpicelli-Daley L. A., Lee V. M., et al. (2013). Calcium entry and alpha-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J. Neurosci. 33, 10154–10164. 10.1523/JNEUROSCI.5311-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dučić T., Carboni E., Lai B., Chen S., Michalke B., Lázaro D. F., et al. (2015). Alpha-synuclein regulates neuronal levels of manganese and calcium. ACS Chem. Neurosci. 6, 1769–1779. 10.1021/acschemneuro.5b00093 [DOI] [PubMed] [Google Scholar]
- Duckles S. P., Tsai H., Buchholz J. N. (1996). Evidence for decline in intracellular calcium buffering in adrenergic nerves of aged rats. Life Sci. 58, 2029–2035. 10.1016/0024-3205(96)00194-4 [DOI] [PubMed] [Google Scholar]
- Eschbach J., Danzer K. M. (2014). alpha-Synuclein in Parkinson's disease: pathogenic function and translation into animal models. Neurodegener. Dis. 14, 1–17. 10.1159/000354615 [DOI] [PubMed] [Google Scholar]
- Follett J., Darlow B., Wong M. B., Goodwin J., Pountney D. L. (2013). Potassium depolarization and raised calcium induces alpha-synuclein aggregates. Neurotox. Res. 23, 378–392. 10.1007/s12640-012-9366-z [DOI] [PubMed] [Google Scholar]
- Gaspar P., Ben Jelloun N., Febvret A. (1994). Sparing of the dopaminergic neurons containing calbindin-D28k and of the dopaminergic mesocortical projections in weaver mutant mice. Neuroscience 61, 293–305. 10.1016/0306-4522(94)90232-1 [DOI] [PubMed] [Google Scholar]
- German D. C., Manaye K. F., Sonsalla P. K., Brooks B. A. (1992). Midbrain dopaminergic cell loss in Parkinson's disease and MPTP-induced parkinsonism: sparing of calbindin-D28k-containing cells. Ann. N. Y. Acad. Sci. 648, 42–62. 10.1111/j.1749-6632.1992.tb24523.x [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Del Tredici K., Braak H. (2013). 100 years of Lewy pathology. Nat. Rev. Neurol. 9, 13–24. 10.1038/nrneurol.2012.242 [DOI] [PubMed] [Google Scholar]
- Goldberg J. A., Guzman J. N., Estep C. M., Ilijic E., Kondapalli J., Sanchez-Padilla J., et al. (2012). Calcium entry induces mitochondrial oxidant stress in vagal neurons at risk in Parkinson's disease. Nat. Neurosci. 15, 1414–1421. 10.1038/nn.3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J., Nath S., Engelborghs Y., Pountney D. L. (2013). Raised calcium and oxidative stress cooperatively promote alpha-synuclein aggregate formation. Neurochem. Int. 62, 703–711. 10.1016/j.neuint.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Hashida H., Goto J., Zhao N., Takahashi N., Hirai M., Kanazawa I., et al. (1998). Cloning and mapping of ZNF231, a novel brain-specific gene encoding neuronal double zinc finger protein whose expression is enhanced in a neurodegenerative disorder, multiple system atrophy (MSA). Genomics 54, 50–58. 10.1006/geno.1998.5516 [DOI] [PubMed] [Google Scholar]
- Hashimoto M., Hsu L. J., Rockenstein E., Takenouchi T., Mallory M., Masliah E. (2002). alpha-Synuclein protects against oxidative stress via inactivation of the c-Jun N-terminal kinase stress-signaling pathway in neuronal cells. J. Biol. Chem. 277, 11465–11472. 10.1074/jbc.M111428200 [DOI] [PubMed] [Google Scholar]
- Heady T. N., Gomora J. C., Macdonald T. L., Perez-Reyes E. (2001). Molecular pharmacology of T-type Ca2+ channels. Jpn. J. Pharmacol. 85, 339–350. 10.1254/jjp.85.339 [DOI] [PubMed] [Google Scholar]
- Hettiarachchi N. T., Parker A., Dallas M. L., Pennington K., Hung C. C., Pearson H. A., et al. (2009). alpha-Synuclein modulation of Ca2+ signaling in human neuroblastoma (SH-SY5Y) cells. J. Neurochem. 111, 1192–1201. 10.1111/j.1471-4159.2009.06411.x [DOI] [PubMed] [Google Scholar]
- Ibanez P., Bonnet A. M., Debarges B., Lohmann E., Tison F., Pollak P., et al. (2004). Causal relation between alpha-synuclein gene duplication and familial Parkinson's disease. Lancet 364, 1169–1171. 10.1016/S0140-6736(04)17104-3 [DOI] [PubMed] [Google Scholar]
- Ivanova D., Dirks A., Montenegro-Venegas C., Schone C., Altrock W. D., Marini C., et al. (2015). Synaptic activity controls localization and function of CtBP1 via binding to Bassoon and Piccolo. EMBO J. 34, 1056–1077. 10.15252/embj.201488796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai A., Masliah E., Yoshimoto M., Ge N., Flanagan L., de Silva H. A., et al. (1995). The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron 14, 467–475. 10.1016/0896-6273(95)90302-X [DOI] [PubMed] [Google Scholar]
- Kanda S., Bishop J. F., Eglitis M. A., Yang Y., Mouradian M. M. (2000). Enhanced vulnerability to oxidative stress by alpha-synuclein mutations and C-terminal truncation. Neuroscience 97, 279–284. 10.1016/S0306-4522(00)00077-4 [DOI] [PubMed] [Google Scholar]
- Kim B. G., Shin D. H., Jeon G. S., Seo J. H., Kim Y. W., Jeon B. S., et al. (2000). Relative sparing of calretinin containing neurons in the substantia nigra of 6-OHDA treated rat parkinsonian model. Brain Res. 855, 162–165. 10.1016/S0006-8993(99)02374-4 [DOI] [PubMed] [Google Scholar]
- Kim T. Y., Yoshimoto T., Aoyama Y., Niimi K., Takahashi E. (2016). Analysis of the protective effects of a neuronal Cav2.1 calcium channel in brain injury. Neuroscience 313, 110–121. 10.1016/j.neuroscience.2015.11.035 [DOI] [PubMed] [Google Scholar]
- Koch Y., Helferich A. M., Steinacker P., Oeckl P., Walther P., Weishaupt J. H., et al. (2016). Aggregated α-synuclein increases SOD1 oligomerization in a mouse model of amyotrophic lateral sclerosis. Am. J. Pathol. 186, 2152–2161. 10.1016/j.ajpath.2016.04.008 [DOI] [PubMed] [Google Scholar]
- Krishnan S., Chi E. Y., Wood S. J., Kendrick B. S., Li C., Garzon-Rodriguez W., et al. (2003). Oxidative dimer formation is the critical rate-limiting step for Parkinson's disease alpha-synuclein fibrillogenesis. Biochemistry 42, 829–837. 10.1021/bi026528t [DOI] [PubMed] [Google Scholar]
- Kruger R., Kuhn W., Muller T., Woitalla D., Graeber M., Kosel S., et al. (1998). Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat. Genet. 18, 106–108. 10.1038/ng0298-106 [DOI] [PubMed] [Google Scholar]
- Kume K., Kikukawa M., Hanyu H., Takata Y., Umahara T., Sakurai H., et al. (2012). Telomere length shortening in patients with dementia with Lewy bodies. Eur. J. Neurol. 19, 905–910. 10.1111/j.1468-1331.2011.03655.x [DOI] [PubMed] [Google Scholar]
- Lesage S., Anheim M., Letournel F., Bousset L., Honore A., Rozas N., et al. (2013). G51D alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann. Neurol. 73, 459–471. 10.1002/ana.23894 [DOI] [PubMed] [Google Scholar]
- Lin M. T., Cantuti-Castelvetri I., Zheng K., Jackson K. E., Tan Y. B., Arzberger T., et al. (2012). Somatic mitochondrial DNA mutations in early Parkinson and incidental Lewy body disease. Ann. Neurol. 71, 850–854. 10.1002/ana.23568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe R., Pountney D. L., Jensen P. H., Gai W. P., Voelcker N. H. (2004). Calcium(II) selectively induces alpha-synuclein annular oligomers via interaction with the C-terminal domain. Protein Sci. 13, 3245–3252. 10.1110/ps.04879704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luth E. S., Stavrovskaya I. G., Bartels T., Kristal B. S., Selkoe D. J. (2014). Soluble, prefibrillar α-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J. Biol. Chem. 289, 21490–21507. 10.1074/jbc.m113.545749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L., Campanelli J. T., Scheller R. H. (1988). Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 8, 2804–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllum E. J., Finkelstein D. I. (2016). Metals in Alzheimer's and Parkinson's disease: relevance to dementia with lewy bodies. J. Mol. Neurosci. 60, 279–288. 10.1007/s12031-016-0809-5 [DOI] [PubMed] [Google Scholar]
- Melachroinou K., Xilouri M., Emmanouilidou E., Masgrau R., Papazafiri P., Stefanis L., et al. (2013). Deregulation of calcium homeostasis mediates secreted alpha-synuclein-induced neurotoxicity. Neurobiol. Aging 34, 2853–2865. 10.1016/j.neurobiolaging.2013.06.006 [DOI] [PubMed] [Google Scholar]
- Michaelis M. L., Bigelow D. J., Schoneich C., Williams T. D., Ramonda L., Yin D., et al. (1996). Decreased plasma membrane calcium transport activity in aging brain. Life Sci. 59, 405–412. 10.1016/0024-3205(96)00319-0 [DOI] [PubMed] [Google Scholar]
- Mironov S. L. (2015). α-Synuclein forms non-selective cation channels and stimulates ATP-sensitive potassium channels in hippocampal neurons. J. Physiol. 593(Pt 1), 145–159. 10.1113/jphysiol.2014.280974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narhi L., Wood S. J., Steavenson S., Jiang Y., Wu G. M., Anafi D., et al. (1999). Both familial Parkinson's disease mutations accelerate alpha-synuclein aggregation. J. Biol. Chem. 274, 9843–9846. 10.1074/jbc.274.14.9843 [DOI] [PubMed] [Google Scholar]
- Nath S., Goodwin J., Engelborghs Y., Pountney D. L. (2011). Raised calcium promotes alpha-synuclein aggregate formation. Mol. Cell. Neurosci. 46, 516–526. 10.1016/j.mcn.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Nath S., Meuvis J., Hendrix J., Carl S. A., Engelborghs Y. (2010). Early aggregation steps in α-synuclein as measured by FCS and FRET: evidence for a contagious conformational change. Biophys. J. 98, 1302–1311. 10.1016/j.bpj.2009.12.4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarria L., Zaltieri M., Longhena F., Spillantini M. G., Missale C., Spano P., et al. (2015). Alpha-synuclein modulates NR2B-containing NMDA receptors and decreases their levels after rotenone exposure. Neurochem. Int. 85–86, 14–23. 10.1016/j.neuint.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Nielsen M. S., Vorum H., Lindersson E., Jensen P. H. (2001). Ca2+ binding to alpha-synuclein regulates ligand binding and oligomerization. J. Biol. Chem. 276, 22680–22684. 10.1074/jbc.M101181200 [DOI] [PubMed] [Google Scholar]
- Parihar M. S., Parihar A., Fujita M., Hashimoto M., Ghafourifar P. (2008). Mitochondrial association of alpha-synuclein causes oxidative stress. Cell. Mol. Life Sci. 65, 1272–1284. 10.1007/s00018-008-7589-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson Study Group (2013). Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson's disease (STEADY-PD). Mov. Disord. 28, 1823–1831. 10.1002/mds.25639 [DOI] [PubMed] [Google Scholar]
- Pasanen P., Myllykangas L., Siitonen M., Raunio A., Kaakkola S., Lyytinen J., et al. (2014). A novel α-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson's disease-type pathology. Neurobiol. Aging 35, 2180.e1–e5. 10.1016/j.neurobiolaging.2014.03.024 [DOI] [PubMed] [Google Scholar]
- Pasternak B., Svanstrom H., Nielsen N. M., Fugger L., Melbye M., Hviid A. (2012). Use of calcium channel blockers and Parkinson's disease. Am. J. Epidemiol. 175, 627–635. 10.1093/aje/kwr362 [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon M. A., Yenari M. A., Sun G. H., Kunis D. M., Steinberg G. K. (1997). SNX-111, a novel, presynaptic N-type calcium channel antagonist, is neuroprotective against focal cerebral ischemia in rabbits. J. Neurol. Sci. 153, 25–31. 10.1016/S0022-510X(97)00196-2 [DOI] [PubMed] [Google Scholar]
- Perfeito R., Ribeiro M., Rego A. C. (2016). Alpha-synuclein-induced oxidative stress correlates with altered superoxide dismutase and glutathione synthesis in human neuroblastoma SH-SY5Y cells. Arch Toxicol. [Epub ahead of print]. 10.1007/s00204-016-1788-6 [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., et al. (1997). Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047. 10.1126/science.276.5321.2045 [DOI] [PubMed] [Google Scholar]
- Pottorf W. J., Duckles S. P., Buchholz J. N. (2000). SERCA function declines with age in adrenergic nerves from the superior cervical ganglion. J. Auton. Pharmacol. 20, 281–290. 10.1046/j.1365-2680.2000.00194.x [DOI] [PubMed] [Google Scholar]
- Pountney D. L., Voelcker N. H., Gai W. P. (2005). Annular alpha-synuclein oligomers are potentially toxic agents in alpha-synucleinopathy. Neurotox. Res. 7, 59–67. 10.1007/BF03033776 [DOI] [PubMed] [Google Scholar]
- Proukakis C., Dudzik C. G., Brier T., MacKay D. S., Cooper J. M., Millhauser G. L., et al. (2013). A novel α-synuclein missense mutation in Parkinson disease. Neurology 80, 1062–1064. 10.1212/WNL.0b013e31828727ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilty M. C., King A. E., Gai W. P., Pountney D. L., West A. K., Vickers J. C., et al. (2006). Alpha-synuclein is upregulated in neurones in response to chronic oxidative stress and is associated with neuroprotection. Exp. Neurol. 199, 249–256. 10.1016/j.expneurol.2005.10.018 [DOI] [PubMed] [Google Scholar]
- Radford R., Wong M. B., Pountney D. L. (2014). Neurodegenerative aspects of multiple system atrophy, in Handbook of Neurotoxicity, ed Kostrzewa R. M. (New York, NY: Springer Science + Business Media; ), 2157–2180. [Google Scholar]
- Rcom-H'cheo-Gauthier A., Davis A., Meedeniya A., Pountney D. L. (2016). Alpha-Synuclein aggregates are excluded from Calbindin-D28k-positive neurons in Dementia with Lewy bodies and a unilateral rotenone mouse model. Mol.Cell. Neurosci. 77, 65–75. 10.1016/j.mcn.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Recasens A., Dehay B., Bove J., Carballo-Carbajal I., Dovero S., Perez-Villalba A., et al. (2014). Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann. Neurol. 75, 351–362. 10.1002/ana.24066 [DOI] [PubMed] [Google Scholar]
- Reznichenko L., Cheng Q., Nizar K., Gratiy S. L., Saisan P. A., Rockenstein E. M., et al. (2012). In vivo alterations in calcium buffering capacity in transgenic mouse model of synucleinopathy. J. Neurosci. 32, 9992–9998. 10.1523/JNEUROSCI.1270-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B., Rhodes S. L., Qian L., Schernhammer E., Olsen J. H., Friis S. (2010). L-type calcium channel blockers and Parkinson disease in Denmark. Ann. Neurol. 67, 600–606. 10.1002/ana.21937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E., Nuber S., Overk C. R., Ubhi K., Mante M., Patrick C., et al. (2014). Accumulation of oligomer-prone alpha-synuclein exacerbates synaptic and neuronal degeneration in vivo. Brain 137(Pt 5), 1496–1513. 10.1093/brain/awu057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Miyasaka T., Hatsuta H., Takahashi-Niki K., Hayashi K., Mita Y., et al. (2014). Immunostaining of oxidized DJ-1 in human and mouse brains. J. Neuropathol. Exp. Neurol. 73, 714–728. 10.1097/NEN.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santner A., Uversky V. N. (2010). Metalloproteomics and metal toxicology of α-synuclein. Metallomics. 2, 378–392. 10.1039/b926659c [DOI] [PubMed] [Google Scholar]
- Simms B. A., Zamponi G. W. (2014). Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron 82, 24–45. 10.1016/j.neuron.2014.03.016 [DOI] [PubMed] [Google Scholar]
- Sims-Robinson C., Hur J., Hayes J. M., Dauch J. R., Keller P. J., Brooks S. V., et al. (2013). The role of oxidative stress in nervous system aging. PLoS ONE 8:e68011. 10.1371/journal.pone.0068011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Verma P., Balaji G., Samantaray S., Mohanakumar K. P. (2016). Nimodipine, an L-type calcium channel blocker attenuates mitochondrial dysfunctions to protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice. Neurochem. Int. 99, 221–232. 10.1016/j.neuint.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Singleton A. B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., et al. (2003). α-Synuclein locus triplication causes Parkinson's disease. Science 302:841. 10.1126/science.1090278 [DOI] [PubMed] [Google Scholar]
- Surmeier D. J., Schumacker P. T., Guzman J. D., Ilijic E., Yang B., Zampese E. (2016). Calcium and Parkinson's disease. Biochem. Biophys. Res. Commun. [Epub ahead of print]. 10.3760/cma.j.issn.0376-2491.2016.41.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley E. M., Cribbs L. L., Lee J. H., Daud A., Perez-Reyes E., Bayliss D. A. (1999). Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J. Neurosci. 19, 1895–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu E. C., Verkhratsky A. (2007). Role of calcium in normal aging and neurodegeneration. Aging Cell 6:265. 10.1111/j.1474-9726.2007.00299.x [DOI] [PubMed] [Google Scholar]
- Tsuboi K., Kimber T. A., Shults C. W. (2000). Calretinin-containing axons and neurons are resistant to an intrastriatal 6-hydroxydopamine lesion. Brain Res. 866, 55–64. 10.1016/S0006-8993(00)02219-8 [DOI] [PubMed] [Google Scholar]
- Ubhi K., Lee P. H., Adame A., Inglis C., Mante M., Rockenstein E., et al. (2009). Mitochondrial inhibitor 3-nitroproprionic acid enhances oxidative modification of alpha-synuclein in a transgenic mouse model of multiple system atrophy. J. Neurosci. Res. 87, 2728–2739. 10.1002/jnr.22089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildburger N. C., Lin-Ye A., Baird M. A., Lei D., Bao J. (2009). Neuroprotective effects of blockers for T-type calcium channels. Mol. Neurodegener. 4:44. 10.1186/1750-1326-4-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., McGeer P. L., Baimbridge K. G., McGeer E. G. (1990). Relative sparing in Parkinson's disease of substantia nigra dopamine neurons containing calbindin-D28K. Brain Res. 526, 303–307. [DOI] [PubMed] [Google Scholar]
- Yunker A. M., Sharp A. H., Sundarraj S., Ranganathan V., Copeland T. D., McEnery M. W. (2003). Immunological characterization of T-type voltage-dependent calcium channel CaV3.1 (alpha 1G) and CaV3.3 (alpha 1I) isoforms reveal differences in their localization, expression, and neural development. Neuroscience 117, 321–335. 10.1016/S0306-4522(02)00936-3 [DOI] [PubMed] [Google Scholar]
- Zarranz J. J., Alegre J., Gomez-Esteban J. C., Lezcano E., Ros R., Ampuero I., et al. (2004). The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55, 164–173. 10.1002/ana.10795 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Yang W., Kirberger M., Lee H. W., Ayalasomayajula G., Yang J. J. (2006). Prediction of EF-hand calcium binding proteins and analysis of bacterial EF hand proteins. Proteins 65, 643–655. 10.1002/prot.21139 [DOI] [PubMed] [Google Scholar]