Abstract
Tumor-selective oncolytic vesicular stomatitis viruses (VSVs) are being evaluated in clinical trials. Here, we report that the MPC-11 murine plasmacytoma model is so extraordinarily susceptible to oncolytic VSVs that a low dose of virus leads to extensive intratumoral viral replication, sustained viremia, intravascular coagulation, and a rapidly fatal tumor lysis syndrome (TLS). Rapid softening, shrinkage and hemorrhagic necrosis of flank tumors was noted within 1–2 days after virus administration, leading to hyperkalemia, hyperphosphatemia, hypocalcemia, hyperuricemia, increase in plasma cell free DNA, lymphopenia, consumptive coagulopathy, increase in fibrinogen degradation products, decreased liver function tests, dehydration, weight loss, and euthanasia or death after 5–8 days. Secondary viremia was observed but viral replication in normal host tissues was not detected. Toxicity could be mitigated by using VSVs with slowed replication kinetics, and was less marked in animals with smaller flank tumors. The MPC-11 tumor represents an interesting model to further study the complex interplay of robust intratumoral viral replication, tumor lysis, and associated toxicities in cases where tumors are highly responsive to oncolytic virotherapy.
Introduction
Tumor-selective replication-competent viruses represent a new class of anticancer drugs (oncolytic virotherapy) showing promising activity in clinical trials.1,2 The US FDA recently granted drug approval for an oncolytic herpes simplex virus encoding GM-CSF (Talimogene Laherparepvec) for melanoma.3,4 In contrast to traditional chemotherapy agents, oncolytic viruses are well tolerated by patients; fever, myalgia, chills, fatigue, and nausea are the most common reported adverse effects.5
Oncolytic virotherapy works initially by harnessing the replicative potential of the virus, to infect and spread preferentially in the tumor nodules, while sparing normal tissues, thereby causing selective tumor destruction and debulking.6 The second phase of virotherapy works by amplifying the host immune response to potentially increase tumor specific immunity through induction of tumor-specific T cells.7,8 We and others have been developing the attenuated laboratory adapted Indiana strain of vesicular stomatitis virus (VSV) as an oncolytic virus for a variety of cancers.9,10,11 A recombinant VSV encoding human interferon-β (VSV-hIFNβ) is in clinical testing after intratumoral injection into tumor nodules in the liver (Principal Investigator: Dr. Mitesh Borad, NCT#01628640 on clinicaltrials.gov). In our studies, the VSV-IFNβ-NIS virus was genetically modified to encode IFNβ which has several biological functions of relevance for virotherapy, such as the induction of primary innate immunity to restrict viral spread in type I interferon responsive normal tissues, direct inhibition of tumor cell proliferation, inhibition of intratumoral angiogenesis and modulation of the antitumor immune response to synergize with immune checkpoint inhibitors.12 VSV-IFNβ-NIS also encodes a reporter gene, the thyroidal sodium iodide symporter (NIS) that enables noninvasive imaging of the in vivo spread of the virus using Tc-99m pertechnetate and SPECT/CT.13,14 This virus has demonstrated antitumor activity against a variety of murine tumors and human xenografts in preclinical models. Additionally, the virus has been administered intravenously to purpose bred research hounds where the maximal tolerated dose was determined to be 1010 TCID50 (dose-limiting toxicity was mildly elevated transaminases).15
VSV-IFNβ-NIS is active against a broad spectrum of tumors of differing tissue origins and tumor response is typically not associated with significant toxicities. For example, in immunocompetent mice with large subcutaneous 5TGM1 myeloma tumors, an intravenous dose of 3 × 108 TCID50 VSV-murine IFNβ-NIS can result in sustained complete remission and induction of tumor-specific memory T cells that protect the mice from new tumor cell challenge.16 Formal toxicity testing of VSV-mIFNβ-NIS in tumor-free mice revealed an excellent safety profile even at doses up to 1,000-fold higher than the minimum effective dose. In contrast, as reported herein, VSV-mIFNβ-NIS administration to syngeneic Balb/c mice bearing subcutaneous MPC-11 plasmacytomas tumors caused a very rapid tumor response that is associated with extensive intratumoral viral replication, secondary viremia, high serum virally encoded interferon-β levels, tumor lysis, and intravascular coagulation, often requiring euthanasia of the animal due to poor body condition. We therefore propose that MPC-11 plasmacytoma tumors could serve as a useful model for the study of multifactorial toxicities associated with tumors that are highly responsive to oncolytic virotherapy.
Results
VSV-mIFNβ-NIS is active against MPC-11 plasmacytomas but causes lethal toxicity
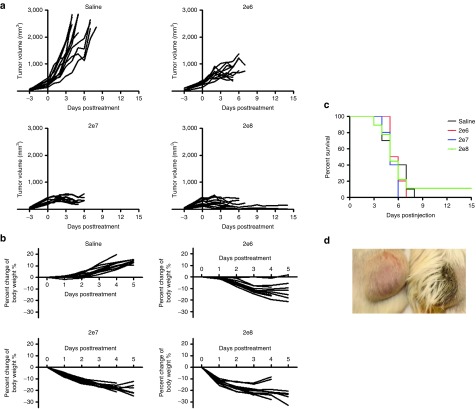
Balb/c mice with subcutaneous flank MPC-11 plasmacytomas were given one intravenous (IV) dose of VSV-mIFNβ-NIS (2 × 106, 2 × 107, or 2 × 108 TCID50). VSV treatment rapidly resulted in dose-dependent growth inhibition or regression of established tumors (Figure 1a). VSV at the highest dose of 2 × 108 TCID50 induced the most rapid tumor growth arrest and regression. However, by day 3 to 5, VSV-treated mice had lost 15–30% of pretreatment body weight, likely due to inadequate hydration and loss of appetite (Figure 1b). Despite the rapid tumor response to VSV treatment, the majority of VSV treated mice did not survive beyond day 9 and despite the impressive tumor responses, their survival curves were not different compared to controls (Figure 1c). Virus-treated moribund mice were inactive, shivering, cold to touch, had scruffy coats, and some were found dead. No signs of neurotoxicity or hindlimb paralysis were observed at any time. Responding tumors rapidly softened within 24 hours of virus administration and became hemorrhagic (Figure 1d).
Figure 1.
Syngeneic MPC-11 tumors in Balb/c mice are highly responsive to systemic VSV-mIFNβ-NIS therapy. Mice were given saline or one intravenous dose of VSV at 2 × 106, 2 × 107, or 2 × 108 TCID50. (a) Tumor volume, (b) percent change in body weight, and (c) survival curves after virus treatment. (d) Photograph showing hemorrhagic VSV-treated tumor.
Intratumoral spread of VSV-mIFNβ-NIS results in viremia and systemic IFN-β
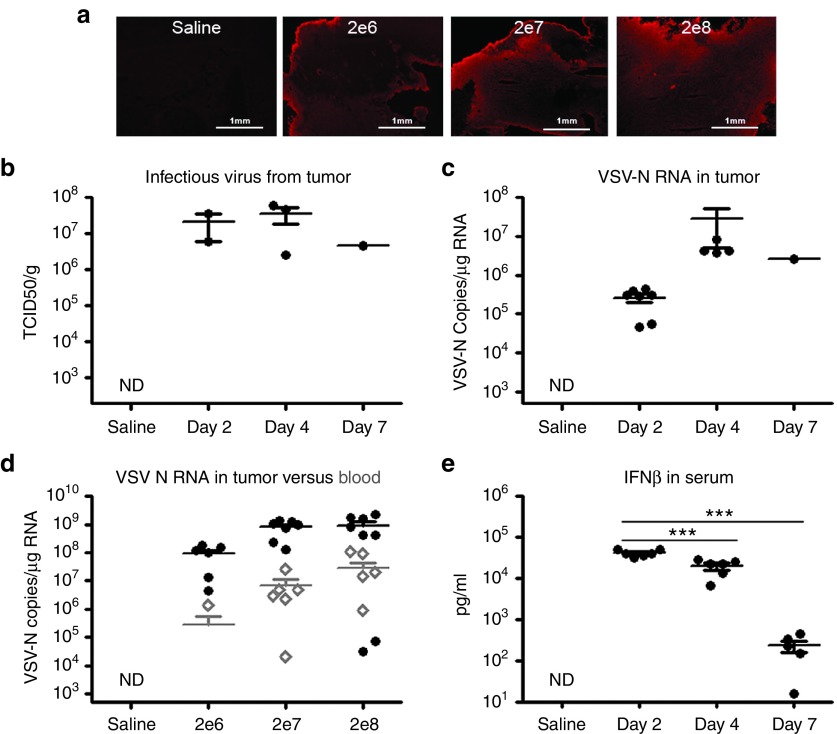
Immunohistochemical staining for VSV antigens showed extensive virus propagation in tumors from VSV-treated mice (Figure 2a). High levels of infectious virus (107 TCID50/g) were also recovered from the tumors (Figure 2b). Quantitative RT-PCR of RNA extracted from tumors showed a substantial increase in VSV nucleocapsid (N) RNA copy number from day 2 to day 4 (107 copies/µg RNA), confirming robust virus amplification in the tumors (Figure 2c). Secondary viremia was evident by day 3 at all dose levels tested, presumably due to release of progeny viruses and/or cell debris from infected tumor tissue into the bloodstream (Figure 2d). Viral RNA abundance was approximately 100-fold less in blood compared to tumor tissue. Serum IFNβ concentration, presumed to be a reflection of the total number of viable virus-infected tumor cells at a given time, was very high at 104 pg/ml on day 2 post-virus administration, significantly reduced to 20% of its peak level by day 4 (P < 0.001) and to only 1% of its peak level (P < 0.001) by day 7 (Figure 2e). Together, these findings suggested that the virus was rapidly and destructively spreading in the tumor and that progeny viruses were being released into the bloodstream.
Figure 2.
Robust vesicular stomatitis viruses (VSV) replication in MPC-11 tumor results in secondary viremia. (a) Immunohistochemical staining for VSV antigens (red) in tumor sections harvested at necropsy (day 3). (b) Titers of infectious virus recovered from explanted tumors of mice treated IV with 107 TCID50 VSV-mIFNβ-NIS. (c) Quantitative RT-PCR for VSV-N RNA indicate VSV replication in the tumors at days 2 and 4 (P = 0.17 between days 2 and 4), (d) Secondary viremia as a result of intratumoral replication of VSV, (e) Increase and decline in serum levels of IFNβ in mice, reflecting the numbers of VSV infected cells between day 2 to 7. ***P ≤ 0.001, Student's t-test. Mean ± SEM values are shown.
Biochemical evidence for rapid tumor lysis with increase in transaminases
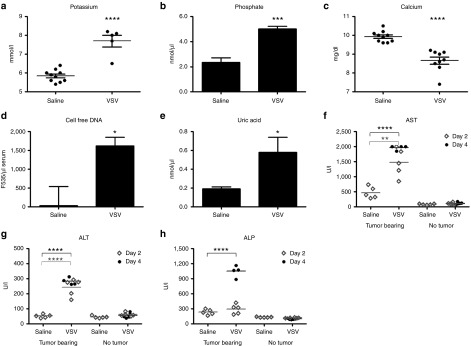
A comprehensive clinical chemistry panel was performed on the blood of mice at day 4 post-VSV. Plasma potassium and serum phosphate concentration were increased in virus-treated animals (Figure 3a,b) and this was accompanied by a substantial decline in calcium (Figure 3c). These are classical biochemical features of tumor lysis syndrome, due to release of intracellular fluid which is rich in potassium and phosphate, and subsequent precipitation of calcium phosphate. Cell-free DNA in the serum, presumably from lysis of tumor cells, was significantly higher in MPC-11 VSV-treated mice than the saline controls (Figure 3d). Serum uric acid levels also increased in tumor bearing mice after VSV treatment (Figure 3e), another classic feature of tumor lysis arising because of the rapid release and metabolism of intracellular nucleic acids. Liver function tests rapidly became abnormal in VSV-treated MPC-11 animals. Aspartate aminotransferase and alanine aminotransferase levels rose significantly compared to saline-treated MPC-11 animals (Figure 3f,g). Alkaline phosphatase levels were also elevated (fourfold) in VSV-treated MPC-11 bearing mice (Figure 3h). In contrast, no elevations in liver enzyme were seen in nontumor-bearing mice given the same dose of VSV (Figure 3f–h).
Figure 3.
Biochemical characterization of the blood or serum from nontumor or MPC-11 tumor bearing mice treated IV with 107 TCID50 VSV-mIFNβ-NIS. (a) Potassium, (b) phosphate, (c) calcium, (d) cell-free DNA, (e) uric acid, (f) aspartate aminotransferase, (g) alanine aminotransferase, (h) alkaline phosphatase. *P ≤ 0.05, ***P ≤ 0.001, ****P ≤ 0.0001, Student's t-test. Mean ± SEM values are shown.
Evidence for intravascular coagulation and consumptive coagulopathy
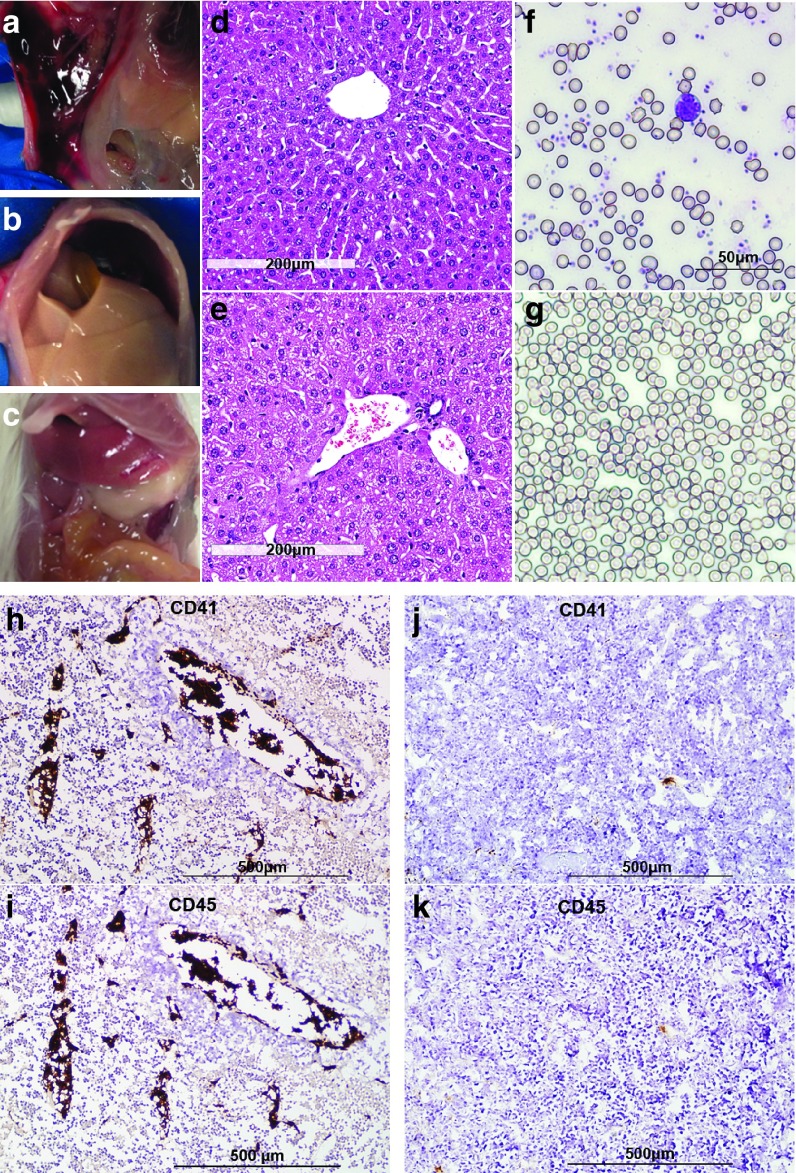
Besides intratumoral hemorrhaging seen in VSV-treated animals, we noted extensive subcutaneous and abdominal wall bruising at necropsy (Figure 4a), particularly in those animals showing the most severe clinical signs of inactivity and weight loss. Necropsy of these animals also revealed that their livers were strikingly pale in color compared to normal livers (Figure 4b,c). H&E-stained liver sections from these VSV-treated mice showed presence of blood clots in the blood vessels and ballooning of hepatocytes (Figure 4e). Because of the extensive bruising seen at necropsy and the intravascular blood clots seen on liver histology, we measured the platelet counts and prothrombin coagulation times (PT) in some virus-treated mice bearing MPC-11 tumors on day 3 post-virus administration. Saline-treated control mice had platelet counts of 561.3 ± 43.98 × 109/l (n = 3) and PT of 18s (n = 1). In contrast, the average platelet count of VSV-mIFNβ-NIS-treated mice was 73 × 109/l (34, 122, n = 2) and PT of 22.5s (22, 23, n = 2) at day 3 post-VSV. By day 6 post-therapy, the platelet counts had dropped below 10 × 109/l (n = 3) and the animals were moribund. May-Grünwald-Giemsa–stained blood smears confirmed the depletion of platelets in VSV-treated mice. In contrast to blood smears from saline-treated mice (Figure 4f), platelets were noticeably reduced in blood smears from VSV-treated mice at day 4 post-treatment (Figure 4g). Immunostaining for platelet glycoprotein IIb (CD41) in tumor sections showed striking evidence for intravascular coagulation with large amounts of CD41 platelets within the blood vessels of tumors (Figure 4h). Abundant lymphocytes (strong CD45 staining) were found to colocalize with the activated platelets in these VSV-treated tumors (Figure 4i), and is probably the cause of the significant lymphopenia seen in these animals. Measurement of fibrinogen degradation product (FDP) in serum showed a significant increase (P = 0.005) in VSV-mIFNβ-NIS-treated MPC-11 mice (day 4, 948.91 ± 93.68 ng/ml, n = 4) compared to saline-treated mice (527.90 ± 29.61 ng/ml, n = 4) further supporting intravascular coagulation as a source of toxicity in this model.
Figure 4.
Histological analysis of blood and tissues from vesicular stomatitis viruses (VSV)-treated MPC-11 bearing mice suggest intravascular coagulation and consumptive coagulopathy. Photographs of VSV treated mice taken at necropsy showed cases of (a) abdominal wall bleeding, and (b) pale ischemic liver. (c) A normal liver is shown for comparison. H&E stained liver sections from (d) saline-treated or (e) VSV-treated MPC-11 mice showing fibrin deposits and trapped erythrocytes in vessels. (f) Erythrocytes and platelets were seen in May-Grünwald-Giemsa stained blood smears from saline-treated mice. (g) Platelets were noticeably reduced in blood smears of VSV-treated mice at day 4 post-treatment. Immunohistochemical staining for platelets (CD41) and lymphocytes (CD45) in tumors harvested from (h, i) VSV-treated and (j, k) saline-treated mice. Aggregates of platelets and lymphocytes, as a result of intravascular coagulation, can be seen in the blood vessels of VSV-treated animals at day 4.
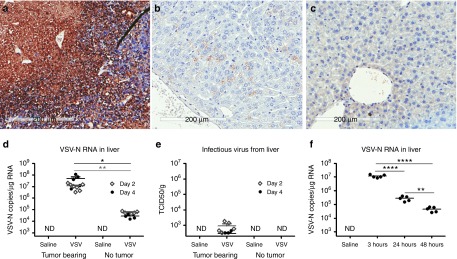
Limited VSV replication in liver tissue
In contrast to the strong positive VSV antigen staining in tumors, there was minimal VSV staining in their livers of VSV-treated animals (Figure 5). Quantitative RT-PCR (Figure 5d) revealed a higher amount of VSV-N RNA (107 copy numbers/µg RNA) in livers of VSV-treated MPC-11 mice than in nontumor-bearing mice (104–105 copy number/µg RNA). There could be some VSV infection of hepatocytes due to the sustained viremia due to release of progeny viruses from infected tumor cells. However, the level of VSV-RNA in the liver and blood were similar at 107 copies/µg RNA, suggesting that VSV replication in the liver was limited. Indeed, only a low level of infectious virus (103 TCID50/g) was recovered from the livers of MPC-11 mice (Figure 5e) compared to 106–109 TCID50/g tumor tissue. To confirm that VSV-mIFNβ-NIS cannot propagate efficiently in normal liver tissue, we administered the virus intravenously to nontumor-bearing mice and performed Quantitative RT-PCR analysis of their livers 3, 24, and 48 hours post-virus administration. Abundant viral RNA (107 copies/µg RNA) was detected in liver tissues 3 hours after virus administration but by 24 hours the level had fallen approximately 50-fold with a further 10-fold reduction by 48 hours (Figure 5f). No infectious virus was recovered from liver tissue at any of these time points.
Figure 5.
Systemically administered vesicular stomatitis viruses (VSV) replication is highly tumor selective with minimal infection of the liver. Immunohistochemical staining for VSV antigen (brown DAB (3,3′-Diaminobenzidine) stain) reflect robust VSV expression in (a) tumors at day 4 post-virus, and (b) minimal VSV expression in the liver of these VSV-treated mouse. (c) VSV staining of a liver section from a saline-treated animal. (d) QRT-PCR for VSV-N RNA and (e) infectious virus recovered from the liver of VSV-treated nontumor or MPC-11 bearing animals at day 2 and 4 post-virotherapy. ND, not detectable. (f) QRT-PCR showed minimal VSV replication in liver as indicated by declining VSV-N RNA in nontumor bearing Balb/c mice. Limit of detectable of VSV-N RNA by qRT-PCR is ≥1,000 copies/µg RNA, and infectious virus is >190 TCID50/ml. *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001, Student's t-test.
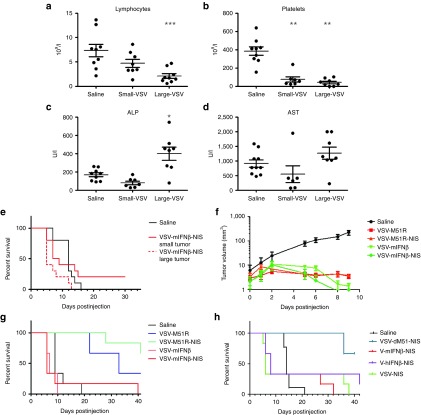
Larger tumor burden is associated with earlier TLS-induced death
Since tumor lysis syndrome (TLS) (Figure 3) and associated intravascular consumptive coagulation contribute to toxicity in tumor-bearing mice, we hypothesized that mice with smaller MPC-11 tumors might experience less severe clinical effects. Mice with small tumors (15.9 ± 3.7 mm3, n = 10) or larger tumors (55.6 ± 15 mm3, n = 10) were treated IV with 107 TCID50 VSV-mIFNβ-NIS. Mice with larger tumor volumes experienced more significant lymphopenia and thrombocytopenia (Figure 6a,b). The biochemical abnormalities detected after virus therapy of the MPC-11 tumors were typically less severe in mice with smaller tumors (Figure 6c, Alkaline phosphatase and Figure 6d, Aspartate aminotransferase). Median survival was 12 days for saline-treated mice, 8.5 days for virus-treated mice with small tumors, and 5 days for virus-treated mice with larger tumors (Figure 6e). Survival curves of VSV-treated mice with small versus larger tumors were significantly different (P = 0.035).
Figure 6.
Early death of vesicular stomatitis viruses (VSV)-treated MPC-11 mice can be mitigated by modulating tumor size or using VSV with different replication profiles. (a) Lymphocyte, (b) platelet count, (c) Alkaline phosphatase, and (d) Aspartate aminotransferase levels from mice with small or larger tumors, that were treated IV with 107 TCID50 VSV-mIFNβ-NIS. (e) Survival of VSV-treated mice with smaller tumors is significantly prolonged (P = 0.035) compared to those with larger (but responding) tumors. (f) Tumor volumes in mice treated with the respective viruses. (g) Recombinant VSV-M51R viruses with slower tumor regression resulted in prolonged survival of mice compared to VSV-mIFNβ viruses (P < 0.05). (h) VSV-NIS, a noninterferon inducing virus with wild-type M protein, is equally lethal to MPC-11 mice as the interferon expressing viruses. VSV-M51-NIS is the only group that is significantly different from saline (P = 0.0002). Survival curves were compared using Mantel Cox Log rank test. Mean ± SEM values are shown. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, Student's t-test.
Virotherapy of MPC-11 tumors with other oncolytic viruses
To determine if speed of virus propagation could impact the likelihood of treatment-associated toxicity in the MPC-11 tumor model, we treated animals with a slower replicating strain of VSV that has an attenuating mutation in methionine residue 51 in its matrix protein (VSV-M51R).17 Both sets of VSV-M51R and VSV-mIFNβ viruses rapidly halted tumor growth within 2 days of virus administration (Figure 6f). Tumor volumes in VSV-M51R-treated mice remained small and mice survived significantly longer (P < 0.05) than mice treated with VSV-mIFN or VSV-mIFN-NIS viruses (Figure 6g). Survival curves between the two VSV-M51R viruses were not significantly different (P = 0.217). VSV-M51R-treated MPC-11 mice did experience lymphopenia and thrombocytopenia but the severity was greatly reduced in comparison to those animals treated with VSV-mIFNβ-NIS). In saline mice, the white cell count and platelet count were 12.5 ± 2.3 × 106/μl and 561.3 ± 23.2 × 106/μl (n = 3) compared to 5.6 ± 0.6 × 106/μl and 277.2 ± 39.5 × 106/μl for VSV-M51- and VSV-M51R-NIS-treated mice (n = 5), compared to 0.67 + 0.14 × 106/μl and 104.2 ± 18.1 × 106/μl for VSV-mIFNβ- and VSV-mIFNβ-NIS-treated mice (n = 4).
Serum IFN-β levels were very high (104 pg/ml) in the VSV-miFN-NIS-treated MPC-11 tumor-bearing mice. To determine whether the virally encoded IFNβ was a major factor determining the worse toxicity profile of VSV-mIFNβ-NIS versus VSV-M51R-NIS, we compared the toxicities of VSV-m/h-IFNβ-NIS viruses versus VSV-NIS which has a wild-type matrix protein and replicates with high efficiency in tumor cells. Median survival of animals treated with VSV-mIFNβ-NIS and VSV-human IFNβ-NIS were similar at 8 days, compared to 6 days for the faster-replicating VSV-NIS virus (Figure 6h). Importantly, human IFNβ is not biologically active in mice. Hence, toxicity of VSV therapy in the MPC-11 plasmacytoma model appears to correlate more closely to speed of intratumoral virus propagation, tumor response and the cascade of downstream events.
Discussion
Here, we describe a highly reproducible tumor model of virotherapy-induced multifactorial toxicity arising from extensive intratumoral viral replication and tumor lysis. In this MPC-11 tumor model, a relatively small dose of an oncolytic VSV administered intravenously can lead to a rapidly fatal scenario. In addition to the classical biochemical signs of TLS, hyperkalemia, hyperphosphatemia, hypocalcemia, and hyperuricemia, we also observed rapid softening, shrinkage and hemorrhagic necrosis of flank tumors within 24 to 48 hours of virus administration, lymphopenia, consumptive coagulopathy, hepatic toxicity, secondary VSV viremia, dehydration, weight loss, and euthanasia/death after as little as 5 days following virus administration. The virus does not appear to spread to normal tissues and toxicity is mitigated by using VSVs with slower replication kinetics, or by treating animals with lower tumor burden.
Tumor lysis syndrome is an oncologic emergency characterized by spillage of intracellular material into the blood caused by disruption of a massive load of tumor cells.18 It can have fatal consequences due to renal and multi-organ failure and arrhythmias due to electrolyte imbalance.19 The classic electrolyte and metabolic disturbances occurring in TLS are (i) hyperkalemia and hyperphosphatemia due to release of potassium- and phosphate-rich intracellular fluid from dying tumor cells; (ii) hypocalcemia secondary to complexation of extracellular fluid calcium by released intracellular phosphate to form insoluble calcium phosphate crystals; and (iii) hyperuricemia due to incomplete metabolism of nucleic acids released by dying tumor cells. TLS occurs most commonly after initiation of therapy in patients with rapidly growing tumors and/or large disease burden whose tumors are particularly responsive to the administered treatment. Beyond these classical early features of TLS, the subsequent evolution of the syndrome can differ significantly from case to case, presumably a reflection of several key variables such as tumor histology, tumor burden, pre-existing organ damage, as well as size, quantity, and thrombogenicity of circulating cellular components derived from lysed tumor cells.20
MPC-11 is highly unique in its exquisite permissiveness to viral infection and spread. In vitro, MPC-11 cells support high levels of VSV replication, yielding 1010 TCID50/ml of VSV-GFP virus within 24 hours, is not interferon responsive and does not produce interferon after VSV infection.21 As such, MPC-11 is the “ideal” tumor in which oncolytic virotherapy is most potent due defective IFN antiviral response pathways in the tumor. However, as shown in this report, severe adverse events could occur as a result of rapid and extensive destruction of tumor cells, and TLS is a potential toxicity that should be considered in a virotherapy clinical trial. In a nontumor-bearing Balb/c mouse, no toxicity was observed with IV administration of VSV-mIFNβ-NIS and the maximal tolerated dose is more than 1.5e1010 per mouse with transient elevation in transaminases as the dose-limiting toxicity. In the MPC-11 model described here, the intravenously administered VSV extravasates at sites of tumor growth and propagates very rapidly in the tumor leading to tumor cell killing with release of cell debris, cell-free DNA and virus progeny into the bloodstream. While untreated MPC-11 tumors are highly vascular and hemorrhagic, treated tumors softened rapidly to the touch, and became very intensely hemorrhagic. In this model, intravascular coagulation is an important contributor to toxicity to the mice. Large aggregates of leukocytes and platelets, potentially arrested due to intravascular coagulation, were found in the blood vessels of treated tumors at day 4 post-virus administration. These aggregates were not found in untreated tumors. We have not yet defined the trigger for intravascular coagulation within the tumor blood vessels. It is therefore possible that the administered virus also infected the endothelial cells lining tumor neovessels, leading to activation of the coagulation cascade, and rapid vascular occlusion. Indeed, Breitbach et al. reported that VSV can directly infect and destroy activated tumor blood vessels but not normal vasculature.22
In addition to TLS and consumptive coagulopathy, there were high levels of infectious virus released into the bloodstream which could infect normal tissues including the liver and brain. No neurotoxic signs were observed at any time point. Only a low level of infectious virus was recovered from nonperfused liver of these animals and VSV staining was minimal in liver sections. Hence, viral replication in normal tissues is limited in the Balb/c mouse despite the sustained viremia. As to the cause of liver failure and death in this VSV-treated MPC-11 model, our working hypothesis is that the rapid lysis of VSV-infected tumor cells caused the release of procoagulants, or thrombogenic cell debris and progeny virus particles into the circulation. These particles were trapped preferentially in hepatic sinusoids where they initiated a process of intravascular coagulation with extensive clot propagation into the larger portal blood vessels, leading ultimately to consumptive coagulopathy as evidenced by profound thrombocytopenia, prolonged prothrombin time and extensive spontaneous subcutaneous bleeding. In parallel with this process of blood coagulation in the portal blood vessels, we hypothesize that the mice became significantly hypotensive due to TLS-associated metabolic disturbances, and that the drop in hepatic arterial pressure in the context of a compromised portal circulation was the proximate cause of ischemic liver damage which led to transaminase and bilirubin elevations, and a strikingly pale liver at postmortem. This model for the pathogenesis of liver damage is further supported by the presence of intravascular fibrin deposits in liver sections, and by the lack of evidence to suggest direct infection of hepatocytes by progeny viruses.
During the course of our studies, the question arose as to whether the virally encoded IFNβ may be contributing to the liver damage seen in this model. The levels of circulating IFNβ in mice that were treated with VSV-IFNβ-NIS were extremely high even at very early timepoints post-virus administration. IFNα, which is used extensively for the treatment of chronic hepatitis C virus infection, binds to the same cellular receptor as IFNβ, and is contraindicated in patients with decompensated liver disease (Child-Pugh class B and C) because of potentially fatal toxicity in this situation (Micromedix). At one time, it therefore seemed likely that the virally encoded IFNβ might in part be responsible for the fatal outcome in this model. It has been reported that IFNβ therapy could result in transient hepatic dysfunction in multiple sclerosis patients, and there was increase in platelet aggregation in vitro in one study of 10 carcinoma patients who received IFNβ.23,24 However, similar lethal toxicity was seen, with equivalent survival times, in MPC-11 tumor-bearing mice after treatment with VSVs encoding mouse or human IFNβ, and even after the administration of an IFNβ-null VSV with an unmutated M protein. Since human IFNβ is only minimally active on the mouse IFNα/β receptor25 and since the unmutated M protein in the IFNβ-null virus actively suppresses IFNβ production,17 we conclude that, while active IFNβ may be capable of contributing to the liver damage and lethal outcome in this model, it is not the primary driver.
Where it is anticipated, TLS can be prevented or ameliorated by prehydrating the patient to enhance renal clearance of potassium and phosphate, and by the administration of rasburicase to accelerate metabolism of uric acid.18 Prehydration did not ameliorate the severity of the syndrome in this oncolytic VSV-treated MPC-11 model, nor was it impacted by the administration of rasburicase, even though the serum level of uric acid was substantially reduced (Russell and Peng, unpublished data). However, the syndrome could be substantially ameliorated by treating the tumor-bearing animals with viruses VSV-M51R or VSV-M51R-NIS that propagate and lyse the MPC-11 tumors less rapidly than the interferon-encoding viruses. TLS toxicity was also less severe when treating animals with smaller MPC-11 tumors. While there are a few reports on spontaneous tumor lysis in transgenic mouse models,26,27 reproducible mouse models of treatment-associated TLS have not to our knowledge been previously reported in the literature. The MPC-11 model described in this manuscript may therefore prove extremely useful and informative for the future study of treatment-associated TLS, and to guide the development of novel approaches for its prevention and/or treatment. Furthermore, the MPC-11 model serves as an interesting model to study the multifactorial toxicities that could arise from robust viral replication and infection of tumor cells. Recently, it was reported that high doses of type I IFN can cause thrombotic microangiopathy. The role of IFNb induced toxicity is under further investigation in this model.32
Materials and Methods
Cell culture and viruses. MPC-11 cells (ATCC CCL-167) obtained from American Type Cell Culture were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin. The cell line tested negative for mycoplasma contamination. The VSV-M51R and VSV-M51R-NIS viruses were a kind gift from Vyriad (Rochester, MN) and viral stocks were generated and titered by Imanis Life Sciences on Vero cells (Rochester, MN). The VSV-mIFNβ and VSV-mIFNβ-NIS virus used for all in vivo experiments were previously published.16,28,29 These viruses were manufactured by the Mayo Clinic Viral Vector Production Laboratory using HEK293 suspension cells under serum-free conditions. The cell culture supernatant was clarified to remove intact cells and treated with Benzonase to digest contaminating nucleic acid. This processed cell culture supernatant was then concentrated and purified using tangential flow filtration and diafiltration into a final buffer of 5% Sucrose 50 mmol/l Tris HCl, 2 mmol/l MgCL2, pH 7.4. The purified virus was filtered through a final 1.5 μm filter prior to final vialing. The titer of the stock was 6.13 × 109 TCID50/ml on Vero cells.
In vivo experiments in mice using VSV-mIFNβ-NIS. 5 × 106 MPC-11 murine myeloma cells were implanted subcutaneously on the right flank of 5-6 weeks-old female Balb/c mice (Harlan Laboratories, Indianapolis, IN). Mice were randomized by body weight to four groups (n = 13 per group). At day 0, mice were given saline or VSV-mIFNβ-NIS (2 × 106, 2 × 107, and 2 × 108/100 μl) intravenously by tail vein injection. Mice were observed daily for adverse clinical signs and were euthanized when tumor exceeded 10% of body weight, inactive, or moribund. A separate cohort of mice with subcutaneous flank MPC-11 tumors received 107 TCID50 VSV-mIFNβ-NIS IV. Mice were harvested at different time points post-virus administration to harvest samples for correlative analysis (e.g., CBC, clinical chemistry, tissue histology, and viral load). Tissues (tumor, liver) were collected and cut into four pieces at necropsy, into RNALater (Am7021, Thermo Scientific, Waltham, MA) for RT-PCR, into 10% neutral-buffered formalin for paraffin embedding and histology, embedded in optimal cut temperature (OCT) medium for immunohistochemistry and quick frozen in liquid nitrogen for storage at ≤ −80 °C.
Analysis for hematological, clinical chemistry, and biochemical parameters. Blood was collected by cardiac puncture prior to necropsy and collected for clinical chemistry (200 μl into lithium heparin tubes, BD BioSciences, 365971), for complete blood count (CBC, 100 µl in EDTA tubes, BD BioSciences, 365973), for RNA extraction (100 µl in blood protect tube, Qiagen, 76544), serum (600 µl in serum separator tube, BD BioSciences, 365967). Blood chemistry was analyzed using the ABAXIS Piccolo Xpress (Union City, CA) and CBC was done on the ABAXIS VetScan HM5 hematology machines. Clotting times were determined using the Coag Dx Analyzer (IDEXX VetLab Station, Westbrook, ME). Phosphate and uric acid were run on serum samples, respectively using a phosphate Assay Kit (ab65622, Abcam, Cambridge, MA) and uric acid assay kit (MAK07, Sigma, St. Louis, MO). Murine interferon beta was analyzed using VeriKine TM Mouse IFNβ ELISA kit (42400, PBL Assay Science, Piscataway, NJ).
Histology, immunofluorescence, and immunohistochemistry. Tissues embedded in OCT were sectioned and used for immunofluorescence staining. A rabbit antibody against VSV (M2168, Imanis Life Sciences, Rochester, MN) was used, coupled with Alexa Fluor 555-conjugated goat anti-rabbit secondary antibody (A21429, Invitrogen, Carlsbad, CA). For histology and immunohistochemistry, paraffin-embedded tissues were serially sectioned, and were stained with hematoxylin and eosin (Mayo Histology Core Laboratory, Arizona), or used for VSV immunohistochemistry using the rabbit anti-VSV antibody (M2168, 1:4,000 dilution, Imanis Life Sciences), anti-CD41 staining for platelets (ab33661, 1:1,000 dilution, BD BioSciences), and anti-CD45 staining for lymphocytes (553086, 1:1,000 dilution, BD BioSciences).
Infectious virus recovery from tissues. Frozen samples were weighed and homogenized in 4 volumes (w/v) of Opti-MEM buffer, the supernatant was clarified by centrifugation at 12,000×g for 10 minutes and 10-fold serial dilutions of samples were prepared in Opti-MEM. Aliquots (50 μl) of each dilution were placed in 96-well plates containing Vero cells. The cytopathic effects were recorded at day 3 and calculation for TCID50 titer was previously described.30
RNA extraction and q-PCR analysis. RNA from various sources were extracted using standard kits according to manufacturer's instructions; RNeasy Plus Universal Kit for tissues (73404, Qiagen, Hilden, Germany), Rneasy Protect animal blood kit (73224, Qiagen). Quantitative reverse transcription-PCR was performed according to the manufacturer's instructions (04991885001, Roche, Basel, Switzerland) on the Roche LightCycler 480 Real Time PCR Thermocycler as previously described.31
Statistical analysis. The GraphPad Prism 5.0 program (GraphPad Software, La Jolla, CA) was used for data handling, analysis, and graphic representation. All the data were shown as mean with standard error of the mean. Kaplan-Meier survival curves were plotted and survival data was analyzed using log rank test. Student's T-tests were used for comparison between two groups.
Acknowledgments
This work was funded by the NIH/NCI (R01CA175795), the Mayo Clinic NCI-designated Comprehensive Cancer Center, the Mayo Comprehensive Cancer Center Support Grant (P30 CA015083), Al and Mary Agnes McQuinn, Dorothea Berggren and the Mayo Foundation. We thank Vyriad for the kind gift of VSV-M51R and VSV-M51R-NIS viral stocks. We would like to acknowledge the work of the Mayo Clinic Viral Vector Production Laboratory in producing, purifying and characterizing the VSV-mIFNβ-NIS virus, in particular Kirsten Langfield, Sharon Stephan, Deborah Melder, Henry Walker and Gennett Pike. S.J.R., M.J.F., S.N., and K-W.P. and the Mayo Clinic have a financial conflict of interest related to this research. This conflict is being managed by the Mayo Clinic Conflict of Interest Review Board in compliance with Mayo Clinic Conflict of Interest policies.
References
- Russell, SJ, Peng, KW and Bell, JC (2012). Oncolytic virotherapy. Nat Biotechnol 30: 658–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour, LW and Fisher, KD (2016). Oncolytic viruses: finally delivering. Br J Cancer 114: 357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andtbacka, RH, Kaufman, HL, Collichio, F, Amatruda, T, Senzer, N, Chesney, J et al. (2015). Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 33: 2780–2788. [DOI] [PubMed] [Google Scholar]
- Coffin, RS (2015). From virotherapy to oncolytic immunotherapy: where are we now? Curr Opin Virol 13: 93–100. [DOI] [PubMed] [Google Scholar]
- Pol, J, Bloy, N, Obrist, F, Eggermont, A, Galon, J, Cremer, I et al. (2014). Trial Watch:: Oncolytic viruses for cancer therapy. Oncoimmunology 3: e28694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, SJ, Federspiel, MJ, Peng, KW, Tong, C, Dingli, D, Morice, WG et al. (2014). Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc 89: 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichty, BD, Breitbach, CJ, Stojdl, DF and Bell, JC (2014). Going viral with cancer immunotherapy. Nat Rev Cancer 14: 559–567. [DOI] [PubMed] [Google Scholar]
- Harrington, KJ, Puzanov, I, Hecht, JR, Hodi, FS, Szabo, Z, Murugappan, S et al. (2015). Clinical development of talimogene laherparepvec (T-VEC): a modified herpes simplex virus type-1-derived oncolytic immunotherapy. Expert Rev Anticancer Ther 15: 1389–1403. [DOI] [PubMed] [Google Scholar]
- Hastie, E and Grdzelishvili, VZ (2012). Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J Gen Virol 93(Pt 12): 2529–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojdl, DF, Lichty, BD, tenOever, BR, Paterson, JM, Power, AT, Knowles, S et al. (2003). VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4: 263–275. [DOI] [PubMed] [Google Scholar]
- Ozduman, K, Wollmann, G, Piepmeier, JM and van den Pol, AN (2008). Systemic vesicular stomatitis virus selectively destroys multifocal glioma and metastatic carcinoma in brain. J Neurosci 28: 1882–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski, TF and Corrales, L (2015). New perspectives on type I IFNs in cancer. Cytokine Growth Factor Rev 26: 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A and Russell, SJ (2016). The use of the NIS reporter gene for optimizing oncolytic virotherapy. Expert Opin Biol Ther 16: 15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, K, Kirk, A, Naik, S, Nace, R, Steele, MB, Suksanpaisan, L et al. (2013). Mathematical model for radial expansion and conflation of intratumoral infectious centers predicts curative oncolytic virotherapy parameters. PLoS One 8: e73759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc, AK, Naik, S, Galyon, GD, Jenks, N, Steele, M, Peng, KW et al. (2013). Safety studies on intravenous administration of oncolytic recombinant vesicular stomatitis virus in purpose-bred beagle dogs. Hum Gene Ther Clin Dev 24: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik, S, Nace, R, Federspiel, MJ, Barber, GN, Peng, KW and Russell, SJ (2012). Curative one-shot systemic virotherapy in murine myeloma. Leukemia 26: 1870–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, M, McKenzie, MO, Puckett, S, Hojnacki, M, Poliquin, L and Lyles, DS (2003). Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J Virol 77: 4646–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride, A and Westervelt, P (2012). Recognizing and managing the expanded risk of tumor lysis syndrome in hematologic and solid malignancies. J Hematol Oncol 5: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, SC, Jones, DP and Pui, CH (2011). The tumor lysis syndrome. N Engl J Med 364: 1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi, M (2009). Disseminated intravascular coagulation in cancer patients. Best Pract Res Clin Haematol 22: 129–136. [DOI] [PubMed] [Google Scholar]
- Liu, YP, Suksanpaisan, L, Steele, MB, Russell, SJ and Peng, KW (2013). Induction of antiviral genes by the tumor microenvironment confers resistance to virotherapy. Sci Rep 3: 2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach, CJ, De Silva, NS, Falls, TJ, Aladl, U, Evgin, L, Paterson, J et al. (2011). Targeting tumor vasculature with an oncolytic virus. Mol Ther 19: 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferroni, P, Roselli, M, Diodati, A, Casciani, CU and Gazzaniga, PP (1994). Effects on platelet function by human interferon-beta in carcinoma patients. Anticancer Res 14(6B): 2779–2784. [PubMed] [Google Scholar]
- Francis, GS, Grumser, Y, Alteri, E, Micaleff, A, O'Brien, F, Alsop, J et al. (2003). Hepatic reactions during treatment of multiple sclerosis with interferon-beta-1a: incidence and clinical significance. Drug Saf 26: 815–827. [DOI] [PubMed] [Google Scholar]
- Mogensen, KE, Lewerenz, M, Reboul, J, Lutfalla, G and Uzé, G (1999). The type I interferon receptor: structure, function, and evolution of a family business. J Interferon Cytokine Res 19: 1069–1098. [DOI] [PubMed] [Google Scholar]
- Treuting, PM, Albertson, TM and Preston, BD (2010). Case series: acute tumor lysis syndrome in mutator mice with disseminated lymphoblastic lymphoma. Toxicol Pathol 38: 476–485. [DOI] [PubMed] [Google Scholar]
- Vogel, P, Pletcher, JM and Liang, Y (2010). Spontaneous acute tumor lysis syndrome as a cause of early deaths in short-term carcinogenicity studies using p53 +/- mice. Vet Pathol 47: 719–724. [DOI] [PubMed] [Google Scholar]
- Obuchi, M, Fernandez, M and Barber, GN (2003). Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J Virol 77: 8843–8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik, S, Nace, R, Barber, GN and Russell, SJ (2012). Potent systemic therapy of multiple myeloma utilizing oncolytic vesicular stomatitis virus coding for interferon-β. Cancer Gene Ther 19: 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadac, EM, Peng, KW, Nakamura, T and Russell, SJ (2004). Reengineering paramyxovirus tropism. Virology 329: 217–225. [DOI] [PubMed] [Google Scholar]
- Jenks, N, Myers, R, Greiner, SM, Thompson, J, Mader, EK, Greenslade, A et al. (2010). Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-beta in rodents and nonhuman primates. Hum Gene Ther 21: 451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh, D, McGlasson, S, Jury, A, Williams, J, Scolding, N, Bellamy, C et al. (2016). Type I interferon causes thrombotic microangiopathy by a dose-dependent toxic effect on the microvasculature. Blood, 05–715987.http://www.ncbi.nlm.nih.gov/pubmed/27663672. [DOI] [PMC free article] [PubMed]