Abstract
Phosphodiesterase 4 (PDE4) inhibitors are approved for the treatment of some moderate to severe inflammatory conditions. However, dose-limiting side effects in the central nervous system and gastrointestinal tract, including nausea, emesis, headache, and diarrhea, have impeded the broader therapeutic application of PDE4 inhibitors. We sought to exploit the wealth of validation surrounding PDE4 inhibition by improving the therapeutic index through generation of an antibody–drug conjugate (ADC) that selectively targets immune cells through the CD11a antigen. The resulting ADC consisted of a human αCD11a antibody (based on efalizumab clone hu1124) conjugated to an analog of the highly potent PDE4 inhibitor GSK256066. Both the human αCD11a ADC and a mouse surrogate αCD11a ADC (based on the M17 clone) rapidly internalized into immune cells and suppressed lipololysaccharide (LPS)-induced TNFα secretion in primary human monocytes and mouse peritoneal cells, respectively. In a carrageenan-induced air pouch inflammation mouse model, treatment with the ADC significantly reduced inflammatory cytokine production in the air pouch exudate. Overall, these results provide compelling evidence for the feasibility of delivering drugs with anti-inflammatory activity selectively to the immune compartment via CD11a and the development of tissue-targeted PDE4 inhibitors as a promising therapeutic modality for treating inflammatory diseases.
Significance
PDE4 inhibitors are clinically validated molecules with considerable efficacy but relatively low safety profile in treating chronic inflammatory diseases. Therefore, the potential expansion of clinical indications of these molecules is relatively unexplored. Bringing to bear medicinal chemistry and bio-conjugation methods, we generated αhuCD11a-PDE4 and its mouse equivalent αmuCD11a-PDE4, which target the pan-immune cell surface antigen CD11a and demonstrated potent in vitro suppression of inflammation that is explicitly receptor-dependent. Pharmacokinetic and pharmacodynamic analysis of αmuCD11a in vivo revealed translation of these effects. With antibody-based therapies becoming a mainstay in the treatment of inflammation, this study provides critical validation for a new paradigm which could lead to second generation PDE4 inhibitors with an improved safety and efficacy.
Introduction
Antibody–drug conjugates (ADCs) are an attractive platform for highly potent drugs whose therapeutic potential can be improved by selective delivery to target tissues while avoiding nontarget tissues that drive dose-limiting toxicity. ADCs comprise an antibody that selectively targets a cell surface antigen and has been modified by a cleavable or noncleavable chemical linker that supports stable attachment and intracellular release of a small molecule “payload”, which is most often a cytotoxin1,2 and in limited examples can be a potent bioactive molecule.3,4,5 In this regard, targeted delivery with ADCs potentially represents a modular therapeutic platform to capture the efficacy of validated small molecules while eliminating their unwanted nontarget tissue side effects to yield a drug with improved safety profile. To date, significant efforts have been made to generate ADCs for oncology, with three ADCs receiving market approval. Comparatively, less emphasis has been placed on the application of ADCs in other disease areas such as inflammation and autoimmunity to deliver noncytotoxic, therapeutic drugs in a cell-specific manner in order to decrease potential side effects due to activity in off-target tissues.3,4,5
Immune responses are fine-tuned processes initiated by various cellular signals and mediated by complex intracellular cascades. Phosphodiesterases (PDEs) are a class of enzymes that control the amplitude and duration of the signal of cAMP, a key second messenger of inflammatory responses. Increases of cAMP dramatically decrease inflammatory responses of leukocytes to stimuli.6 PDE4 is a cAMP phosphodiesterase widely expressed in hematopoietic cells (e.g., myeloid, lymphoid), nonhematopoietic cells (e.g., smooth muscle, keratinocyte, endothelial), and sensory/memory neurons.7 To date, some small molecule PDE4 inhibitors had demonstrated wide-ranging preclinical efficacy in a variety of models of chronic inflammatory diseases, such as inflammatory bowel disease,8 rheumatoid arthritis,9 and psoriasis10 and several have progressed to clinical trials. However, dose-limiting side effects, including nausea, emesis, diarrhea, and headache, which primarily result from inhibiting PDE4 in the central nervous system and gastrointestinal tract, have impeded the clinical development of this class of drugs for a broad range of inflammatory disease indications.6 Several groups have sought to exploit tissue-restricted delivery of PDE4 inhibitors as a means of improving therapeutic index through inhaled11,12 and topical13 routes of administration. We hypothesized that improving the selectivity of PDE4 inhibitors through an ADC that targeted proinflammatory immune cells would enhance the safety profile of this molecule by reducing exposure of the molecule to the central nervous system and gastrointestinal tract; thus enabling the molecule to be used in the treatment of systemic inflammatory diseases. Based on this rationale, we generated and characterized a novel anti-inflammatory ADC that targets a potent PDE4 inhibitor to immune cells via a pan-immune cell targeting antibody against CD11a.
We selected the PDE4 inhibitor GSK256066 as the payload drug based on its high potency and correlated functional outputs (e.g., cytokine secretion) as well as its ability to be chemically derivatized to generate an ADC. An inhaled form of GSK256066 has demonstrated clinical efficacy in treating chronic obstructive pulmonary disease and asthma, but it appears unsuitable as a systemic drug.11,14,15,16 To deliver GSK256066 to the immune cell compartment we selected CD11a, a cell surface antigen expressed with high copy numbers by leukocytes, including monocytes, macrophages, lymphocytes, and granulocytes. CD11a is the alpha chain component of the integrin lymphocyte function-associated antigen-1 (LFA-1), which is involved in leukocyte adhesion and trafficking. Importantly, CD11a is rapidly internalized by cells upon binding to CD11a antibodies such as efalizumab, and surface expression of CD11a is refreshed with nonsaturating kinetics.17,18 Herein, we report a GSK256066 analog-based ADC and its delivery into primary human and mouse leukocytes, providing support for the use of ADCs to enhance the safety and efficacy of PDE4 inhibitors as treatments for inflammatory diseases.
Results
Human anti-CD11a-PDE4 inhibitor ADC generation
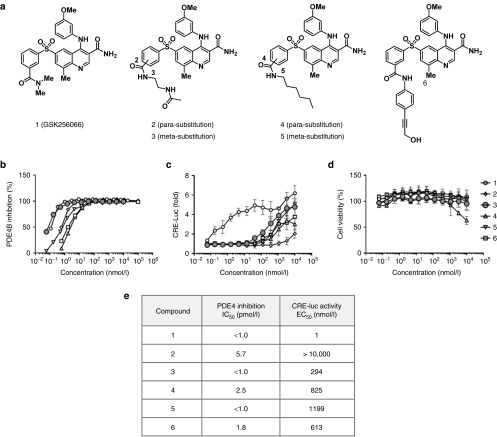
To develop an analog of GSK256066 with a linker for covalent attachment to the anti-CD11a antibody, we used structure-based design guided by the cocrystal structure of the GSK256066 with PDE4.19 This identified the dimethylcarboxamide as a solvent exposed moiety that we hypothesized could support the attachment of a chemical linker. We reasoned the use of a noncleavable linker would provide in vivo stability of the conjugate and correspondingly improve the specificity of drug delivery. Noncleavable ADC linkers have been shown to effectively deliver drugs that upon internalization and degradation of the antibody also have minimal extracellular release and reuptake into other tissues.20 Towards this end, we designed and synthesized model compounds containing different linker chemistries with varying hydrophilicity and flexibility at the meta- and para- positions of the arylsulfone moiety (Figure 1a). The in vitro activity of these derivatives was evaluated in a biochemical assay for inhibition of PDE4 enzymatic activity (Figure 1b) and a cell-based assay for cAMP accumulation and subsequent cAMP-response element (CRE)-dependent expression of luciferase in THP-1 (CRE-luc) cells (Figure 1c). GSK256066 potently inhibited PDE4B enzymatic activity at sub-picomolar EC50 as previously reported. Among the GSK256066 derivatives, compound 3 with substitution at the meta position was the most potent in both the enzyme and cell-based assays (Figure 1e). While the inhibitory effect of compound 3 against the isolated PDE4B enzyme is comparable to GSK256066 it exhibited a ~300-fold drop in cellular CRE-Luc activity. The attenuated cellular activity of compound 3 was hypothesized to be due to decreased cellular permeability, an attribute that should not adversely affect the efficiency of antibody-mediated uptake. This may favorably contribute to specificity by retention of the molecule inside the target cell, and decrease of recirculation of free drug payload following delivery by ADC. Importantly, compound 3 did not exhibit any cytotoxicity in THP1 cells up to 10 μmol/l (Figure 1d). Based on these results, we chose compound 3 as the basis for a linker conjugate to generate a PDE4 inhibitor ADC.
Figure 1.
Generation and characterization of PDE4 inhibitor payload drug. (a) GSK256066 and its derivative linker analogs. (b) PDE4B enzymatic activity inhibition by a range of concentrations of compound 1 to 6 was assessed. (c) THP-1 cells stably transfected with CRE-Luc (THP-1 CRE-Luc cells) were treated with a range of concentrations of compound 1 to 6 for 6 hours, CRE-Luc activity by a range of concentrations of compound 1 to 6 was assessed. (d) THP-1 CRE-Luc cells were treated with a range of concentrations of compound 1 to 6 for 24 hours, and the cell viability was assessed. (e) PDE4B inhibition IC50 of assay (b) and CRE-Luc activity EC50 of assay (c) of compound 1 to 6 was calculated. Results in (b–d) are presented as means ± SD.
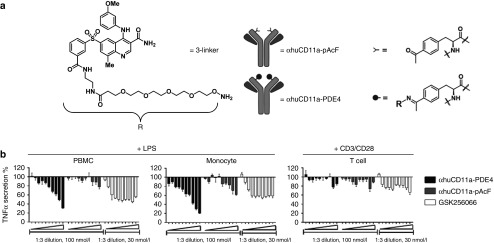
To enable site-specific conjugation of compound 3 to the anti-human CD11a antibody, we employed unnatural amino acid methodology. The unnatural residue p-acetylphenylalanine (pAcF) was incorporated at site A122 of the heavy chain of the anti-human CD11a antibody (clone hu1124, Efalizumab), as described previously.4 To eliminate nonspecific Fc binding and potential complications due to effector function, such as antibody-dependent cell-mediated cytotoxicity, we introduced 7 point mutations into the Fc region of anti-human CD11a to generate αhuCD11a-pAcF (Figure 2a). To enable conjugation of GSK256066, we generated a derivative containing a tetra-ethyleneglycol spacer terminated with an aminoxy group (3-linker, Figure 2a), which reacts under slightly acidic conditions to form stable covalent conjugates with the ketone of pAcF at site A122 of the heavy chain. Conjugation of 3-linker to αhuCD11a-pAcF yielded αhuCD11a-PDE4 with a drug to antibody ratio of exactly 2.0 (1 drug conjugated to each heavy chain) as confirmed by high resolution mass spectrometry (Supplementary Figure S1A and S1B).
Figure 2.
Characterization of the anti-inflammatory effect of αhuCD11a-PDE4 ADC in vitro. (a) 3-linker was synthesized based on compound 3 for antibody conjugation. This PDE4 inhibitor linker derivative was then conjugated to αhuCD11a-pAcF to generate αhuCD11a-PDE4 ADC. (b) Freshly isolated human PBMCs, monocytes and T cells were treated with various concentrations of αhuCD11a-PDE4, αhuCD11a-pAcF, and GSK256066. The highest concentration on right side for αhuCD11a-PDE4 and αhuCD11a-pAcF was 100 nmol/l, and for GSK256066 was 30 nmol/l. All treatments from right to left are in 1:3 serial dilution. After 7-hour incubation, PBMCs and monocytes were treated with 100 ng/ml LPS and T cells were treated with CD3/CD28 Dynabeads in 1:1 ratio. After 20-hour stimulation, TNFα levels in the cell culture supernatants were assessed by an HTRF assay. TNFα levels were normalized to LPS or CD3/CD28 treatment alone. Results are presented as means ± SD.
Human anti-CD11a-PDE4 inhibitor ADC characterization
To test the efficacy of the ADC, primary human peripheral blood mononuclear cells (PBMCs), monocytes, and T cells were treated with αhuCD11a-PDE4, αhuCD11a-pAcF, or GSK256066 for 7 hours followed by target cell stimulation. PBMCs and monocytes were stimulated with lipololysaccharide (LPS) whereas the T cells were stimulated with CD3/CD28 Dynabeads. After 20 hours of stimulation, TNFα released into the culture medium was measured. The αhuCD11a-PDE4 ADC significantly reduced LPS-induced TNFα secretion in both PBMCs and monocytes, and had a milder effect in reducing TNFα secretion from T cells (Figure 2b). This could be explained by the observation that GSK256066 did not have a dramatic effect on reducing CD3/CD28-induced T cell cytokine release (Supplementary Figure S1F). Unconjugated αhuCD11a-pAcF also reduced TNFα level in PBMCs and monocytes, albeit to a lesser degree, an effect we attribute to CD11a functional antagonism21; the additive effect of the αCD11a antibody itself likely explains the larger effect size observed with αhuCD11a-PDE4 compared with the parent small molecule GSK256066 (Figure 2b).
Mouse αCD11a-PDE4 inhibitor ADC Generation and characterization
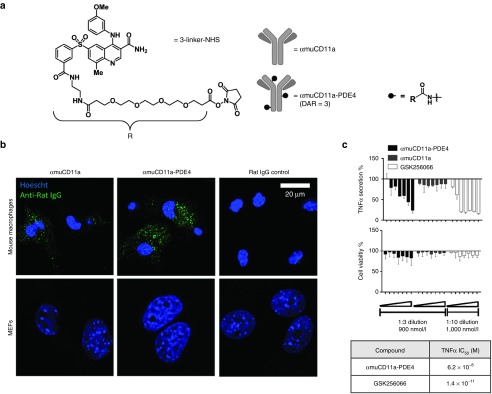
Because αhuCD11a clone hu1124 does not cross react murine CD11a, the rat anti-mouse antibody M17 (αmuCD11a) has been widely used as a surrogate.22 Thus, to assess our ADC in mouse models of inflammation, we generated an M17-based PDE4 conjugate (αmuCD11a-PDE4). The M17 IgG was purified from the M17/4.4.11.9 rat hybridoma. To enable conjugation of GSK256066 to the rat hybridoma-derived antibody, we synthesized a variant of molecule 3 in which the noncleavable linker terminated in an N-hydroxysuccinamide (NHS) ester (3-linker-NHS, Figure 3a) to conjugate nonspecifically to lysines of the αmuCD11a protein. After conjugation and removal of excess PDE4 inhibitor, the drug to antibody ratio was determined to be approximately 3 by high-resolution mass spectrometry (Figure 3a and Supplementary Figure S2A–C).
Figure 3.
Characterization of the anti-inflammatory effect of αmuCD11a-PDE4 ADC in vitro. (a) PDE4 inhibitor linker derivative (3-linker-NHS) basing on compound 3 was conjugated to αmuCD11a to generate αmuCD11a-PDE4 ADC. (b) Mouse primary peritoneal cells and mouse embryonic fibroblasts (MEFs) were incubated with αmuCD11a-PDE4, αmuCD11a or Rat IgG control for 7 hours before being fixed and stained with an anti-rat IgG secondary antibody conjugated with Alexa 488 (green). The nuclei was stained with Hoescht (blue). The cells were imaged with confocal microscopy. (c) Mouse primary peritoneal cells were treated with various concentrations of αmuCD11a-PDE4, αmuCD11a, or GSK256066. The highest concentration on the right side for αmuCD11a-PDE4 and αmuCD11a was 900 nmol/l with 1:3 serial dilution from right to left, and for GSK256066 was 1,000 nmol/l with 1:10 serial dilution from right to left. TNFα IC50 values for αmuCD11a-PDE4 and GSK256066 in this assay were calculated and shown in a table. Results are presented as means ± SD.
To confirm that αmuCD11a antibody is capable of delivering small molecule into leukocytes, internalization assays were carried out that demonstrated that αmuCD11a antibody effectively internalized into both normal peritoneal macrophages and thioglycollate-elicited peritoneal macrophages (Supplementary Figure S2E). We further confirmed that αmuCD11a and αmuCD11a-PDE4 were internalized into mouse peritoneal macrophages but not into CD11a-negative mouse embryonic fibroblasts (MEFs) by confocal microscopy analysis (Figure 3b).
To assess the anti-inflammatory effect of the mouse surrogate ADC upon internalization, we treated primary mouse peritoneal macrophages with various concentrations of αmuCD11a, αmuCD11a-PDE4, or GSK256066. After a 7-hour pretreatment, cells were stimulated with LPS for 20 hours before assessing TNFα release into the culture medium. Encouragingly, αmuCD11a-PDE4 inhibited LPS induced-TNFα secretion (Figure 3c). Based on these results, we further characterized αmuCD11a-PDE4.
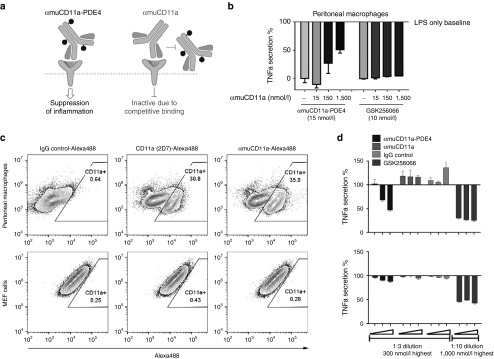
Anti-inflammatory effect of αmuCD11a-PDE4 inhibitor ADC is mediated through CD11a receptor
To confirm that the anti-inflammatory effect of αmuCD11a-PDE4 ADC is mediated through the CD11a receptor, we carried out two experiments. First, we used a competition assay whereby primary peritoneal macrophages were pretreated with an excess of αmuCD11a antibody ranging up to 1,500 nmol/l before cotreating the cells with either 15 nmol/l αmuCD11a-PDE4 ADC or 10 nmol/l GSK256066 (Figure 4a). After a 7-hour incubation, cells were stimulated with LPS as described above. αmuCD11a was found to compete with the effects of αmuCD11a-PDE4 in a dose-dependent manner, while as expected GSK256066 was unaffected by excess amount of αmuCD11a, indicating the effect of αmuCD11a-PDE4 was dependent on muCD11a internalization (Figure 4b). To further confirm the dependence of αmuCD11a-PDE4 activity on CD11a expression, we examined the effects of αmuCD11a-PDE4 and GSK256066 on MEFs, which do not express CD11a (Figure 4c), but are sensitive to PDE4 inhibition-mediated attenuation of LPS-induced TNFα secretion. In this experiment, MEFs only responded to GSK256066 (Figure 4d). Together, these two experiments provide strong evidence that CD11a internalization is required for αmuCD11a-PDE4's activity.
Figure 4.
Anti-inflammatory effect of αmuCD11a-PDE4 ADC is mediated through CD11a receptor. (a) A schematic graph showing that αmuCD11a-PDE4 binds CD11a receptor and exerts its anti-inflammatory effect, while occupancy of CD11a receptor by αmuCD11a blocks αmuCD11a-PDE4 from accessing to CD11a receptor, thus abrogating αmuCD11a-PDE4's anti-inflammatory effect. (b) Mouse peritoneal cells were treated with different concentrations of αmuCD11a as indicated for 30 minutes before αmuCD11a-PDE4 or GSK256066 was added. After 7-hour incubation, cells were stimulated with 100 ng/ml LPS for 20 hours. The TNFα content in the supernatant was assessed by an HTRF assay. The results are normalized to LPS treatment alone and are presented as means ± SD. (c) Peritoneal macrophages or MEFs were stained with IgG control, CD11a (2D7) or αmuCD11a antibodies conjugated with Alexa 488 and were analyzed by flow cytometry. (d) Mouse primary peritoneal cells (upper panel) or MEFs (lower panel) were treated with various concentrations of αmuCD11a-PDE4, αmuCD11a, rat IgG control or GSK256066. The highest concentration from right side for αmuCD11a-PDE4, αmuCD11a and IgG control was 300 nmol/l with 1:3 serial dilution from right to left, and for GSK256066 was 1,000 nmol/l with 1:10 serial dilution from right to left. The results are normalized to LPS treatment alone, and are presented as means ± SD.
Mouse surrogate αmuCD11a-PDE4 inhibitor ADC reducing inflammation in a mouse carrageenan air pouch model
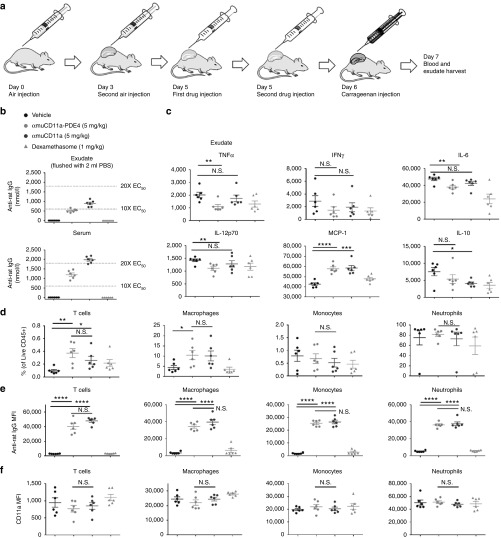
To demonstrate the anti-inflammatory effect of αmuCD11a-PDE4 in vivo, we used a mouse air pouch model of carrageenan-induced sterile-inflammation. Carrageenan induces inflammation in an air pouch created on the back of the mouse, which serves as a reservoir for cells and soluble mediators that can be easily measured in the fluid that accumulates locally.23 In our model, an air pouch was created by subcutaneously injecting filtered air into the backs of individual mice on Day 0 and Day 3. Test compounds (5 mg/kg) or dexamethasone (1 mg/kg) were injected directly into the air pouch (i.e., intra-pouch) 24 and 3 hours before challenging the mice with carrageenan (Figure 5a). Whole blood and air pouch exudate samples (flushed out with 2 ml phosphate-buffered saline (PBS)) were collected 24 hours after carrageenan injection to assess both local and systemic inflammation, pharmacokinetics, and pharmacodynamics. The exposure of both αmuCD11a-PDE4 and αmuCD11a in the PBS-diluted exudate and serum exceeded the EC50 of the cell-based TNF secretion assay by almost more than 10-fold (Figure 5b). Encouragingly, αmuCD11a-PDE4 significantly attenuated carrageenan-induced inflammatory cytokines in the exudate, including TNFα, IL-6, and IL-12p70, while αmuCD11a antibody treatment alone did not. Both αmuCD11a-PDE4 and αmuCD11a elevated MCP-1 levels in the exudate (Figure 5c). As a key chemokine that regulates migration and infiltration of monocytes and macrophages, elevated MCP-1 corresponds to the increased local macrophage and T cell infiltration in the air pouch (Figure 5d), which might contribute to the anti-inflammatory responses to carrageenan stimulus. Consistent with a localized inflammation inside the air pouch, serum levels of these cytokines are much lower compared to exudate, although αmuCD11a-PDE4 exhibited a modest attenuation of TNFα, IL-6, and IL-12p70 even with this small dynamic range (Supplementary Figure S3).
Figure 5.
Mouse surrogate αmuCD11a-PDE4 inhibitor ADC reduces inflammation in a mouse carrageenan air pouch model. (a) A schematic graph showing timelines for air pouch generation and drug administration. (b) αmuCD11a (M17 clone) levels in the diluted air pouch exudate and serum samples were assessed using an anti-rat IgG ELISA. (c) Exudate cytokines including TNFα, IFNγ, IL-6, IL-12p70, MCP-1, and IL-10 were assessed by a mouse inflammatory CBA assay kit. The results are presented as means ± SEM. n = 6 per group. (d) Exudate cells were stained with appropriate antibodies and subjected to flow cytometry analysis. The live cells that were CD45-positive and live/dead stain negative were designated as live CD45+ cells, within which the percentage of different cell populations were analyzed, including CD3+ T cells, CD11b+ Ly6C+ F4/80- monocytes, CD11b+ Ly6C+ F4/80+ macrophages, CD11b+ Ly6G+ Ly6Cint neutrophils. (e) Binding of αmuCD11a (M17 clone) on different exudate immune cell population was assessed by anti-rat IgG stain. (f) CD11a expression on different immune cell populations in the exudate was assessed by flow cytometry.
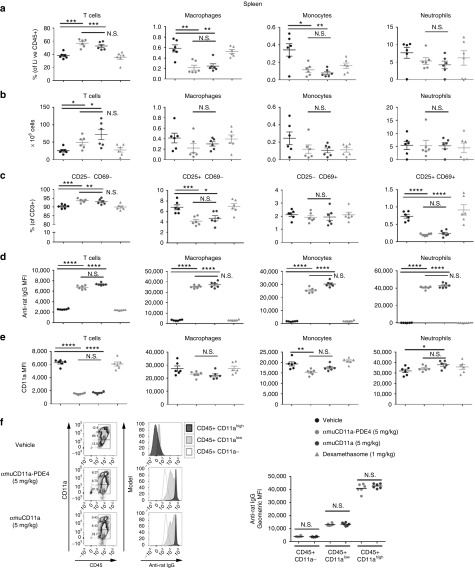
To examine how αmuCD11a-PDE4 regulates immune cell homeostasis and activation, we analyzed the composition of T cells, monocytes, macrophages, and neutrophils in the exudate (Figure 5d,e), spleen (Figure 6a,b), lymph node (Supplementary Figure S4A,B), and blood (Supplementary Figure S5A,B). T cell numbers were significantly increased in the spleen while reduced in the lymph node of mice that were treated with αmuCD11a-PDE4 or αmuCD11a (Figure 6a,b, and Supplementary Figure S4A), suggesting both αmuCD11a-PDE4 and αmuCD11a resulted in T cell retention in the spleen. We further examined the activation status of these splenic T cells, and we found treatment with αmuCD11a-PDE4 or αmuCD11a promotes naive T cell population as they lack one or both of CD25 and CD69 (Figure 6c), suggesting that T cell activity might be generally suppressed by αmuCD11a. This result is consistent with previous reports (http://www.accessdata.fda.gov/ drugsatfda_docs/nda/2003/125075_0000_Raptiva_Pharmr_P1.pdf). Myeloid cell composition analysis shows αmuCD11a-PDE4 treatment significantly reduced splenic macrophage and monocyte populations while increased macrophage population in the exudate, suggesting in response to local carrageenan-induced inflammation, splenic monocytes and macrophages exited the spleen en masse, accumulated in inflammatory site, and participated in immune responses and wound healing (Figure 5d and Figure 6a,b).
Figure 6.
αmuCD11a-PDE4 and αmuCD11a modulate the composition and activation of immune cell population in the spleen. (a) Spleens of mice in the experiment as shown in Figure 5 were collected. The single cell suspension was stained with appropriate antibodies and subjected to flow cytometry analysis. The cells that were CD45-positive and live/dead stain negative were designated as live CD45+ cells, within which the percentage of different cell populations were analyzed, including CD3+ T cells, CD11b+ Ly6C+ F4/80- monocytes, CD11b+ Ly6C+ F4/80+ macrophages, CD11b+ Ly6G+ Ly6Cint neutrophils. (b) Actual numbers of different immune cells in the spleen as calculated basing on the total numbers of cells of each spleen sample. (c) Percentage of different subsets of CD3+ T cell population with differential CD25 and CD69 expression. (d) Binding of αmuCD11a (M17 clone) on different exudate immune cell population was assessed by anti-rat IgG stain. (e) CD11a expression on different immune cell populations in the exudate was assessed by flow cytometry. (f) Binding of αmuCD11a (M17 clone) on CD45+ splenic cells that express different levels of CD11a from mice that were treated with αmuCD11a-PDE4 and αmuCD11a.
To assess which cell types αmuCD11a-PDE4 was predominantly targeting in the in vivo model, we used an anti-rat IgG secondary antibody to probe αmuCD11a antibody bound to the cell surface. αmuCD11a-PDE4 was found predominantly on T cells, monocytes, macrophages, and neutrophils in the exudate (Figure 5e), spleen (Figure 6d), lymph node (Supplementary Figure S4C), and blood (Supplementary Figure S5C). In particular, more αmuCD11a-PDE4 and αmuCD11a were found on CD45+ CD11ahigh cells than CD45+ CD11alow or CD45+ CD11a- cells, and there was no significant difference in αmuCD11a-PDE4 and αmuCD11a binding to CD11a expressing cells (Figure 6f, Supplementary Figures S4E and S5E). This provides further evidence that the antibody may deliver the PDE4 inhibitor into CD11a expressing cells in vivo resulting in an anti-inflammatory phenotype (Figure 5b).
To determine whether CD11a surface expression was altered due to treatment with αmuCD11a-PDE4 or αmuCD11a, CD11a levels on the cell surface were quantified with an anti-mouse CD11a antibody (clone 2D7) recognizing a different epitope as M17 binds.24 The results show CD11a expression was dramatically decreased on T cells of the spleen, lymph node, and blood of mice that were treated with αmuCD11a-PDE4 or αmuCD11a, but only modestly changed on monocytes, macrophages, and neutrophils (Figure 6e and Supplementary Figures S4D and S5D). As CD11a is an adhesion molecule regulating leukocyte trafficking, αmuCD11a binding and internalization-induced dramatic reduction of CD11a result in an immune suppressive phenotype of T cells, but not macrophages, monocytes, or neutrophils. Interestingly, unlike the spleen, lymph node and blood, CD11a expression on T cells treated with αmuCD11a-PDE4 and αmuCD11a in the exudate was not significantly reduced, suggesting these effector T cells engaging the inflammatory response were not suppressed. There was no significant difference in either αmuCD11a binding or CD11a expression in all analyzed immune cells in the spleen, lymph node, blood and exudate between αmuCD11a-PDE4 and αmuCD11a treated groups (Figure 5e,f and Figure 6d,e, Supplementary Figures S4C, D and S5C, D), indicating the anti-inflammatory effect of αmuCD11a-PDE4 in reducing inflammatory cytokines was attributable to the delivery of PDE4 inhibitors. Overall, these results show αmuCD11a-PDE4 effectively targeted immune cell populations and delivered PDE4 inhibitor into CD11a expressing cells in the in vivo mouse model.
Discussion
This work provides important support for extending the ADC paradigm beyond oncology to improve the efficacy and safety of PDE4 inhibitors in treating inflammatory diseases. To avoid the central nervous system- and gastrointestinal tract-mediated side effects of PDE4 inhibition, others have limited the exposure of PDE4 inhibitors to the tissue of interest using intranasal or topical routes of administration. While effective for targeting certain organ systems, these approaches are not extendable to systemic inflammatory conditions and require complex formulation. In recent years, two oral small molecule PDE4 inhibitors, including Daliresp (Roflumilast) from Takeda and Otezla (Apremilast) from Celgene, were approved for treating chronic obstructive pulmonary disease and psoriasis, respectively. However, both oral drugs were limited to treating moderate to severe conditions, because of their suboptimal therapeutic indices. We sought to target PDE4 inhibitors to cell types of interest—rather than tissues of interest—in order to create a more general solution to safely and effectively treating inflammatory diseases through targeted pharmacology. Here, we demonstrated that both human and mouse αCD11a-PDE4 inhibitor ADCs robustly inhibit TNFα secretion in immune cells in vitro and in vivo.
We showed here that the treatment of αmuCD11a-PDE4 significantly reduced local inflammation in a carrageenan-induced air pouch mouse model, and this reduction of inflammation was due to the effect of the PDE4 inhibitor moiety of the ADC, but not the αmuCD11a, because αmuCD11a treatment did not significantly reduce inflammatory cytokines in either the air pouch or the serum (Figure 5c). However, treatment of αmuCD11a-PDE4 and αmuCD11a resulted in similar changes in immune cell composition and activation status in the major lymphoid organs, blood and air pouch (Figures 5 and 6, Supplementary Figures S4 and S5), suggesting these changes of immune responses were attributed to αmuCD11a instead of the PDE4 inhibitor moiety of the ADC. The air pouch model, an acute local inflammation model, has been widely employed for studying pharmacology and genetics in regulating immune cell trafficking and inflammatory responses.23,25,26 While the nature of the air pouch model prevents a strict PK/PD correlation, due to imprecise exposure measurements in the exudate, precise levels in the serum and approximate levels in the exudate support an in vivo potency that is in line with predictions based on in vitro experiments.
PDE4 inhibitor-induced emesis is known to be caused by the elevation of cAMP in the central noradrenergic terminals.27 We expect αCD11a-PDE4 inhibitor ADCs will lack such effects on neuronal cells, which are negative for CD11a (Supplementary Figure S6), since the ADC did not have an effect on cells lacking CD11a surface expression (Figure 4d) and the anti-inflammatory effect of the ADC was specifically mediated by CD11a receptor (Figure 4b). Future studies will directly explore this connection by evaluating the αCD11a-PDE4 inhibitor ADC in a mouse pica feeding model.28
Efalizumab, a humanized αCD11a antibody, was developed and approved by FDA for treating psoriasis, but was withdrawn from the market due to severe side effects, including bacterial sepsis, viral meningitis, and progressive multifocal leukoencephalopathy.29,30 As integrins play important roles in leukocyte trafficking, reduction of surface expression of CD11a due to antibody-mediated receptor internalization may significantly impair the immune cell function and attribute to the immunosuppressing phenotype. Similar to the literature report,22 we also observed that upon binding and internalization of the αmuCD11a antibody, the surface expression of CD11a on T cells, but not monocytes, macrophages, or neutrophils, were dramatically impaired in the spleen, lymph node and blood (Figure 6d,e, Supplementary Figures S4C, D and S5C, D). As the antibody internalization rate is an important factor in determining the efficacy of the ADC and resurfacing of the receptor is important for proper immune function, the balance between both rates is essential to determine whether the antibody is an appropriate vector in delivering payload drug. While we were encouraged to see a lack of saturation of CD11a binding and internalization on macrophages, the saturation presumed by the reduction of surface CD11a expression on T cells suggests CD11a is not an ideal antigen to be safely targeted for delivering PDE4 inhibitors.
Following this set of encouraging proof-of-principle experiments, our current work is focused on identification of other targeting strategies to further restrict the effects of PDE4 inhibitor ADCs to subsets of activated immune cells that drive inflammation, while sparing effects on leukocyte trafficking that make the αCD11a-PDE4 inhibitor ADC challenging to develop. Key considerations in selecting a surface antigen to target include: restricted expression on target cell types (ideally activation state-dependent); high copy number and ability to internalize and recycle surface expression; and non-saturated surface engagement, especially important for surface molecules with physiological functions. The work presented herein has laid important groundwork to continue these studies and provided evidence that such methods are feasible and attractive approaches to next-generation therapies for inflammatory disease with a best-in-class balance of safety and efficacy.
Materials and Methods
Animal studies. All procedure were carried out in accordance with protocols approved by the California Institute for Biomedical Research (Calibr) Animal Care and Use Committee. 8–10-week-old C57BL/6 mice were purchased from the Jackson Laboratory and housed in Calibr's animal facility. Air pouches were generated as previously described31 by subcutaneous injection of 3 ml of sterile air given through intra-pouch route on Day 0 and Day 3. Drugs were administered through intra-pouch route on Day 5—24 hours and 3 hours before inflammation being induced on Day 6 by injection of 1 ml of 2 % carrageenan suspension. Twenty-four hours after carrageenan injection, mice were killed by CO2 narcosis, and the spleen, lymph node, and blood samples were collected, and the exudates were harvested with 2 ml PBS. Lymph node and spleen were collected and homogenized through a 70 µm mesh in FACS buffer and single cell suspension was prepared for flow cytometry analysis. Blood was centrifuged at 400 × g for 5 minutes at 4 °C to separate plasma and blood cells. Plasma was collected into a fresh tube, and blood cells were prepared for flow cytometry analysis. Concentrations of cytokines in the serum and exudate were measured by a Cytometric Bead Assay Mouse Inflammatory Kit (Becton Dickinson, Franklin Lakes, NJ). αmuCD11a-PDE4 and αmuCD11a concentrations were determined by a Rat IgG total ELISA kit (eBioscience, San Diego, CA).
Flow cytometry analysis of animal studies. One milliliter ACK lysing buffer (Cat No. A1049201, ThermoFisher, Waltham, MA) was added to blood cells and spleen cells to get rid of red blood cells according to the manufacturer's protocol. Cells were washed with 5 ml FACS buffer, and were resuspended in FACS buffer. Lymph node single cell suspension and exudate cells were washed with FACS buffer once to prepare for antibody staining. All cells were counted and 2 × 106 cells/sample were used for antibody staining. Cells were first stained with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Cat No: L34957, ThermoFisher) at 4 °C in dark according to manufacturer's protocol. After 30 minutes, cells were washed with FACS buffer and stained with APC-Anti-rat IgG (clone poly4054) to probe αmuCD11a (clone M17) bound on the cell surface. After 30 minutes, cells were then washed with FACS buffer. Fc block (Cat No: 101320, Biolegend, San Diego, CA) was added to cells to block nonspecific binding. After incubation for 10 minutes on ice, an antibody cocktail, including PerCP Cy5.5-CD3 (clone 17A2, Biolegend), APC Cy7-Ly6C (clone HK1.4, Biolegend), BV650 Ly6G (clone 1A8, Biolegend), BV711-CD11c (clone HL3, BD biosciences, San Diego, CA), Alexa 488-CD11b (clone M1/70, Biolegend), BV785-CD25 (clone PC61, Biolegend), BV421-CD69 (clone H1.2F3, Biolegend), PE-Cy7-F4/80 (clone BM8, Biolegend), A700-CD45 (clone 30-F11, Biolegend), and PE-CD11a (clone 2D7, Biolegend) was added to all samples. Single color stains for all antibodies and Fluorescence Minus One for αmuCD11a (clone M17) and CD11a were prepared in parallel. After incubation at 4 °C in dark for 30 minutes, the cells were washed three times with FACS buffer and analyzed by flow cytometry with BD LSRFortessa. All the results were processed with FlowJo software.
In vitro PDE4B enzymatic assay. Enzymatic PDE4B assays were performed at Nanosyn (Santa Clara, CA). Recombinant hPDE4B1 (full length with N-terminal GST-tag) was from BPS biosciences (Cat#60041, San Diego, CA). The reactions were assembled in 384-well plates in a total volume of 25 µl. Test compounds were serially prediluted in DMSO and added to the reaction buffer comprising: 100 mmol/l HEPES, pH7.5, 10 mmol/l MgCl2, 0.1% bovine serum albumin, 0.01% Triton X-100, 0.06 nmol/l PDE4B enzyme, and 1% DMSO from compounds (all concentrations are final). The reactions were initiated by addition of the fluorescently labeled FAM-cAMP substrate to a final concentration of 0.5 μmol/l and incubated for 1 hour at room temperature. Following incubation, reactions were quenched by addition of 45 µl of termination buffer (100 mmol/l HEPES, pH7.5, 0.01% Triton X-100, 50 mmol/l EDTA). The terminated plates were analyzed using Caliper LabChip 3000 microfluidic electrophoresis instrument (Caliper Life Sciences/Perkin Elmer). The enzymatic hydrolysis of the 3' cyclic phosphate bond in cAMP results in a change of net charge enabling electrophoretic separation of product from substrate. As substrate and product are separated by electrophoresis, two peaks of fluorescence were observed. Change in the relative fluorescence intensity of the substrate and product peaks was the parameter measured, reflecting enzyme activity. Activity in each test sample was determined as the product to sum ratio (PSR): P/(S+P), where P is the peak height of the product and S is the peak height of the FAM-cAMP substrate. Percent inhibition (Pinh) was determined using the following equation: Pinh = (PSR0% - PSRinh%)/(PSR0% - PSR100%)*100, where PSRinh is the product sum ratio in the presence of inhibitor, PSR0% is the product sum ratio in the absence of inhibitor and PSR100% is the product sum ratio in 100%-inhibition control samples.
In vitro CRE-luciferase assay. THP-1 cells were purchased from ATCC (Manassas, VA) and were cultured in RPMI (1 mmol/l glucose) with 10% fetal bovine serum (FBS), 0.1% 2-Mercaptoethanol, 1 mmol/l sodium pyruvate, and 100 U/ml penicillin-streptomycin. Cignal lenti CRE luciferase reporter (cat no: CCS-002L) was purchased from Qiagen (Valencia, CA). Cells were transfected with lenti CRE luciferase reporter according to manufacturer's instruction, and positively transfected cells were selected by adding 1 µg/ml puromycin. For PDE4 inhibitor activity assay, 10,000 cells/well were plated in 384-well plate with different compounds at concentrations as suggested in a total volume of 25 µl. After incubation for 24 hours, 5 µl ONE-Glo luciferase assay reagent (Promega, Madison, WI) was added into the cell culture according to manufacturer's instruction, and the reporter gene activity signal was read by a PerkinElmer EnVision Multilabel Reader.
Cytotoxicity assay. After cells were incubated for certain time period as indicated for each experiment, CellTiter-Glo Luminescent cell viability reagent (Promega) was added at a ratio of 1:5 in volume to cell culture directly. The reagent was mixed with cell culture on a plate shaker. The signal was read on a PerkinElmer EnVision Multilabel Reader after 10 minutes incubation.
Preparation of human PBMCs, monocytes, and T cells. PBMCs were isolated by carefully overlaying 35 ml 1:1 PBS diluted whole blood on 15 ml Ficoll reagent, followed by centrifugation at room temperature for 30 minutes at 400×g with the brake turned off. After centrifugation, cells present at the white cell interface were carefully collected, washed with PBS three times, and resuspended in complete PBMCs culture media consisting of RPMI-1640 plus 10% FBS, 10 mmol/l penicillin-streptomycin and 10 mmol/l L-glutamine. If monocytes and T cells were prepared, PBMCs were resuspended in PBS containing 2% FBS and 1 mmol/l EDTA followed by procedures described in StemCell EasySep negative selection kits (Cat No: 19359 for monocytes and 19051 for T cells, STEMCELL Technologies, Vancouver, Canada). Monocytes and T cells were also cultured in complete RPMI1640 culture media supplemented with 10% FBS, 1% L-glutamine, and 1% Penn/Strep.
Preparation of mouse peritoneal cells. Resident peritoneal macrophages were extracted from 8- to 10-week-old C57BL/6 mice as previously described32 and incubated at 37 °C for 2 hours prior to stimulation. Thioglycolate-elicited macrophages were prepared by injecting 2-month-old C57BL/6 mice with 2 ml 3% aged thioglycolate media (Difco, Sparks, MD). On day 4, cells were isolated by lavage and prepared as above for resident macrophages. The cells were maintained in complete DMEM media supplemented with 10% FBS, 1% sodium pyruvate, 1% Penn/Strep, 1% L-glutamine, and 1% nonessential amino acid.
TNFα secretion HTRF assays. Human PBMCs, monocytes, T cells, or mouse peritoneal cells were seeded in a white opaque 384-well plate (Greiner, Monroe, NC). The cells were incubated with or without Fc block for 10 minutes before adding ADC, antibody or small molecules. After incubation for 7 hours, cells were stimulated with 100 ng/ml LPS for 16 hours. For the homogeneous time resolved fluorescence (HTRF) assay, cell culture supernatant was then transferred into a low-volume round-bottom black-opaque 384 well plate (Greiner, Monroe, NC). The TNFa level in the supernatant was then determined by either human or mouse TNFα secretion assay (Cisbio, Bedford, MA) according to manufacturer's protocol. The signal was read by a PerkinElmer EnVision Multilabel Reader.
Confocal microscopy. For human primary T cells and monocytes, cells were plated in 24 well-plate and treated with 50 nmol/l antibodies or ADC as indicated for 7 hours at 37 °C. The cells were then washed once before being concentrated onto a glass slide using cytofunnel (Cat No. 10–354, ThermoFisher) by spinning at 400 rpm for 5 minutes. The cells were fixed with 4% PFA for 10 minutes at room temperature. The cells were washed three times with PBST and permeabilized with 0.5% saponin in PBS for 10 minutes. Cells were washed three times with PBST and incubated with 2 μg/ml Alexa 488 anti-human secondary antibody (Cat No. A-11013, Life Technologies, Carlsbad, CA) for 1 hour at room temperature in dark. Cells were then washed three times with PBST and incubated with 2 µg/ml Hoescht dye for 5 minutes before being washed and mounted with ProLong Diamond Antifade Mountant (Cat No: p36961, ThermoFisher). For mouse peritoneal macrophages and embryonic fibroblasts, cells were plated onto the poly-lysine-coated coverslip in 24-well plate. Cells were allowed to attach overnight before treatment with 50 nmol/l antibodies or ADC as indicated for 7 hours at 37 °C. The cells were then fixed with 4% paraformaldehyde for 10 minutes at room temperature. The cells were washed three times with PBST and permeabilized with 0.5% saponin in PBS for 10 minutes. Cells were washed three times with PBST and incubated with Alexa 488 anti-rat secondary antibody for 1 hour at room temperature. Cells were then washed three times with PBST and incubated with 2 µg/ml Hoescht dye for 5 minutes before being washed and mounted with ProLong Diamond Antifade Mountant (Cat No: p36961, ThermoFisher). Cells were imaged using a Zeiss confocal microscope with a 63× oil objective.
Flow cytometry-based binding and internalization assay. For the binding assay, cells were plated in 96-well plate and treated with or without Fc block in 100 μl FACS buffer (2% FBS in 1× PBS) for 10 minutes at room temperature, followed by incubation with each of the IgG-Alexa 488 conjugated (except for the control cells) at concentration as indicated in FACS buffer at 4 °C in the dark. After 1 hour, cells were washed three times with FACS buffer. Finally, cells were resuspended in 100 μl FACS buffer and analyzed with BD Accuri. For the internalization assay, cells were plated in 96-well plate and treated with or without Fc block in cell culture media for 10 minutes, followed by incubation with each of the IgG-Alexa 488 conjugated (except for the control cells) at 50 nmol/l at 37 °C for various period of time as indicated. In the end of the experiment, the fluorescence of uninternalized antibody was quenched by an Alexa 488 antibody (ThermoFisher). The cells were washed three times with FACS buffer and analyzed by flow cytometry with BD Accuri. All the results were processed with FlowJo software.
Mouse primary brain cells isolation and flow cytometry analysis. Mouse primary brain cells were separated into CD11b+ population (microglial cell) and CD11b- population using MACS Miltenyi Biotec MicroBeads (Cat No. 130-093-634, Miltenyi Biotec, San Diego, CA) as described by the manufacturer's instruction. Briefly, the brain of a C57BL/6 mouse was dissociated into single cell suspension using the Neural Tissue Dissociation Kit (P) (# 130-092-628, Miltenyi Biotec). The cell suspension was then washed and resuspended and labelled with CD11b MicroBeads under 4 °C for 15 minutes. The cells were then washed and subjected to magnetic separation to obtain CD11b+ (microglial cells) and CD11b- (nonmicroglial cells) fractions. These two populations were then incubated with Alexa 488-αmuCD11a or Alexa 488-IgG control together with PE-CD11a (clone 2D7, Biolegend) for 30 minutes on ice protected from light. The cells were washed with FACS buffer and analyzed by flow cytometry on a BD Accuri. The data was analyzed by FlowJo software.
Statistics. The number of animals used in each study was indicated. Statistics analysis was conducted using unpaired student T-test or one-way analysis of variance with post-Tukey's multiple comparisons test on Prism 5 software with significance of *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, and N.S. standing for not significant. Results are presented as means ± SD or means ± SEM, which is specified in the figure descriptions.
SUPPLEMENTARY MATERIAL Figure S1. Validation and characterization of αhuCD11a-PDE4 ADC. Figure S2. Validation and characterization of αmuCD11a-PDE4 ADC. Figure S3. Serum cytokines levels of the experiment of carrageenan-induced inflammation in the air pouch. Figure S4. αmuCD11a-PDE4 and αmuCD11a modulate the composition and activation of immune cell population in the lymph node. Figure S5. αmuCD11a-PDE4 and αmuCD11a modulate the composition and activation of immune cell population in the blood. Figure S6. Binding of αmuCD11a on mouse primary brain cells. Supplementary Materials and Methods.
Author Contributions
S.Y. and M.S.T. drafted the manuscript; S.Y., M.S.T., T.S.Y., D.T.R., D.W., and S.A.K. designed the biology studies; S.Y., R.K.V.L., D.T.R., S.L., H.B.P., M.W., E.N.H., M.J.B., J.S., A.K.W., and S.A.K. performed biology studies; A.D.P. synthesized PDE4 inhibitor analogs; P.G.S., T.M.W., T.S.Y., and M.S.T. supervised the work; S.Y. D.T.R., S.A.K., P.G.S., T.S.Y., and M.S.T. contributed to experimental design and interpretation.
Acknowledgments
We would like to thank Magdalena Mazagova and Lance Sherwood for helping with the in vivo study, Jennifer Ma for providing suggestions on the antibody selection and flow cytometry experiment, and Warren Plaisted for providing primary mouse brain cells. We would like to acknowledge the hybridoma contributor and the Developmental Studies Hybridoma Bank for providing the M17/4.4.11.9 rat hybridoma. S.Y. is supported by Crohn's and Colitis Foundation of America Research Fellowship Award #368561. Competing interests: None.
Supplementary Material
References
- Kovtun, YV and Goldmacher, VS (2007). Cell killing by antibody-drug conjugates. Cancer Lett 255: 232–240. [DOI] [PubMed] [Google Scholar]
- Ducry, L and Stump, B (2010). Antibody-drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjug Chem 21: 5–13. [DOI] [PubMed] [Google Scholar]
- Graversen, JH, Svendsen, P, Dagnæs-Hansen, F, Dal, J, Anton, G, Etzerodt, A et al. (2012). Targeting the hemoglobin scavenger receptor CD163 in macrophages highly increases the anti-inflammatory potency of dexamethasone. Mol Ther 20: 1550–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, RK, Yu, S, Cheng, B, Li, S, Kim, NJ, Cao, Y et al. (2015). Targeted delivery of LXR agonist using a site-specific antibody-drug conjugate. Bioconjug Chem 26: 2216–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, RE, Liu, T, Wang, Y, Cao, Y, Du, J, Luo, X et al. (2015). An immunosuppressive antibody-drug conjugate. J Am Chem Soc 137: 3229–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice, DH, Ke, H, Ahmad, F, Wang, Y, Chung, J and Manganiello, VC (2014). Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discov 13: 290–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay, MD and Adams, DR (2003). PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J 370(Pt 1): 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari-Sharif, P and Abdollahi, M (2010). Phosphodiesterase 4 inhibitors in inflammatory bowel disease: a comprehensive review. Curr Pharm Des 16: 3661–3667. [DOI] [PubMed] [Google Scholar]
- McCann, FE, Palfreeman, AC, Andrews, M, Perocheau, DP, Inglis, JJ, Schafer, P et al. (2010). Apremilast, a novel PDE4 inhibitor, inhibits spontaneous production of tumour necrosis factor-alpha from human rheumatoid synovial cells and ameliorates experimental arthritis. Arthritis Res Ther 12: R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp, K, Reich, K, Leonardi, CL, Kircik, L, Chimenti, S, Langley, RG et al. (2015). Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol 73: 37–49. [DOI] [PubMed] [Google Scholar]
- Singh, D, Petavy, F, Macdonald, AJ, Lazaar, AL and O'Connor, BJ (2010). The inhaled phosphodiesterase 4 inhibitor GSK256066 reduces allergen challenge responses in asthma. Respir Res 11: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Savi, C, Cox, RJ, Warner, DJ, Cook, AR, Dickinson, MR, McDonough, A et al. (2014). Efficacious inhaled PDE4 inhibitors with low emetic potential and long duration of action for the treatment of COPD. J Med Chem 57: 4661–4676. [DOI] [PubMed] [Google Scholar]
- Nazarian, R and Weinberg, JM (2009). AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs 10: 1236–1242. [PubMed] [Google Scholar]
- Tralau-Stewart, CJ, Williamson, RA, Nials, AT, Gascoigne, M, Dawson, J, Hart, GJ et al. (2011). GSK256066, an exceptionally high-affinity and selective inhibitor of phosphodiesterase 4 suitable for administration by inhalation: in vitro, kinetic, and in vivo characterization. J Pharmacol Exp Ther 337: 145–154. [DOI] [PubMed] [Google Scholar]
- Nials, AT, Tralau-Stewart, CJ, Gascoigne, MH, Ball, DI, Ranshaw, LE and Knowles, RG (2011). In vivo characterization of GSK256066, a high-affinity inhaled phosphodiesterase 4 inhibitor. J Pharmacol Exp Ther 337: 137–144. [DOI] [PubMed] [Google Scholar]
- Watz, H, Mistry, SJ and Lazaar, AL; IPC101939 investigators (2013). Safety and tolerability of the inhaled phosphodiesterase 4 inhibitor GSK256066 in moderate COPD. Pulm Pharmacol Ther 26: 588–595. [DOI] [PubMed] [Google Scholar]
- Jullien, D, Prinz, JC, Langley, RG, Caro, I, Dummer, W, Joshi, A et al. (2004). T-cell modulation for the treatment of chronic plaque psoriasis with efalizumab (Raptiva): mechanisms of action. Dermatology 208: 297–306. [DOI] [PubMed] [Google Scholar]
- Boehncke, WH (2007). Efalizumab in the treatment of psoriasis. Biologics 1: 301–309. [PMC free article] [PubMed] [Google Scholar]
- Woodrow, MD, Ballantine, SP, Barker, MD, Clarke, BJ, Dawson, J, Dean, TW et al. (2009). Quinolines as a novel structural class of potent and selective PDE4 inhibitors. Optimisation for inhaled administration. Bioorg Med Chem Lett 19: 5261–5265. [DOI] [PubMed] [Google Scholar]
- Hughes, B (2010). Antibody-drug conjugates for cancer: poised to deliver? Nat Rev Drug Discov 9: 665–667. [DOI] [PubMed] [Google Scholar]
- Reisman, NM, Floyd, TL, Wagener, ME, Kirk, AD, Larsen, CP and Ford, ML (2011). LFA-1 blockade induces effector and regulatory T-cell enrichment in lymph nodes and synergizes with CTLA-4Ig to inhibit effector function. Blood 118: 5851–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey, GP, Fox, JA, Pippig, S, Palmieri, S, Reitz, B, Gonzales, M et al. (2005). Tissue distribution and receptor-mediated clearance of anti-CD11a antibody in mice. Drug Metab Dispos 33: 623–629. [DOI] [PubMed] [Google Scholar]
- Romano, M, Faggioni, R, Sironi, M, Sacco, S, Echtenacher, B, Di Santo, E et al. (1997). Carrageenan-induced acute inflammation in the mouse air pouch synovial model. Role of tumour necrosis factor. Mediators Inflamm 6: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, CL, Wilson, TJ, Strauch, P, Colonna, M, Pelanda, R and Torres, RM (2010). Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J Exp Med 207: 1485–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Boyle, G, Fox, CR, Walden, HR, Willet, JD, Mavin, ER, Hine, DW et al. (2012). Chemokine receptor CXCR3 agonist prevents human T-cell migration in a humanized model of arthritic inflammation. Proc Natl Acad Sci USA 109: 4598–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy, SR, Jala, VR, Bodduluri, SR, Krishnan, E, Hegde, B, Hoyle, GW et al. (2015). Crystalline silica-induced leukotriene B4-dependent inflammation promotes lung tumour growth. Nat Commun 6: 7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina, D (2008). PDE4 inhibitors: current status. Br J Pharmacol 155: 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, K, Matsunaga, S, Matsui, M, Takeda, N and Yamatodani, A (2002). Pica in mice as a new model for the study of emesis. Methods Find Exp Clin Pharmacol 24: 135–138. [DOI] [PubMed] [Google Scholar]
- Berger, JR, Houff, SA and Major, EO (2009). Monoclonal antibodies and progressive multifocal leukoencephalopathy. MAbs 1: 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major, EO (2010). Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med 61: 35–47. [DOI] [PubMed] [Google Scholar]
- Montesinos, MC, Takedachi, M, Thompson, LF, Wilder, TF, Fernández, P and Cronstein, BN (2007). The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5'-nucleotidase: findings in a study of ecto-5'-nucleotidase gene-deficient mice. Arthritis Rheum 56: 1440–1445. [DOI] [PubMed] [Google Scholar]
- Morrison, AC and Correll, PH (2002). Activation of the stem cell-derived tyrosine kinase/RON receptor tyrosine kinase by macrophage-stimulating protein results in the induction of arginase activity in murine peritoneal macrophages. J Immunol 168: 853–860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.