Abstract
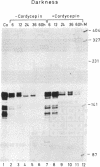
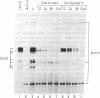
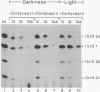
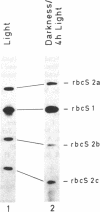
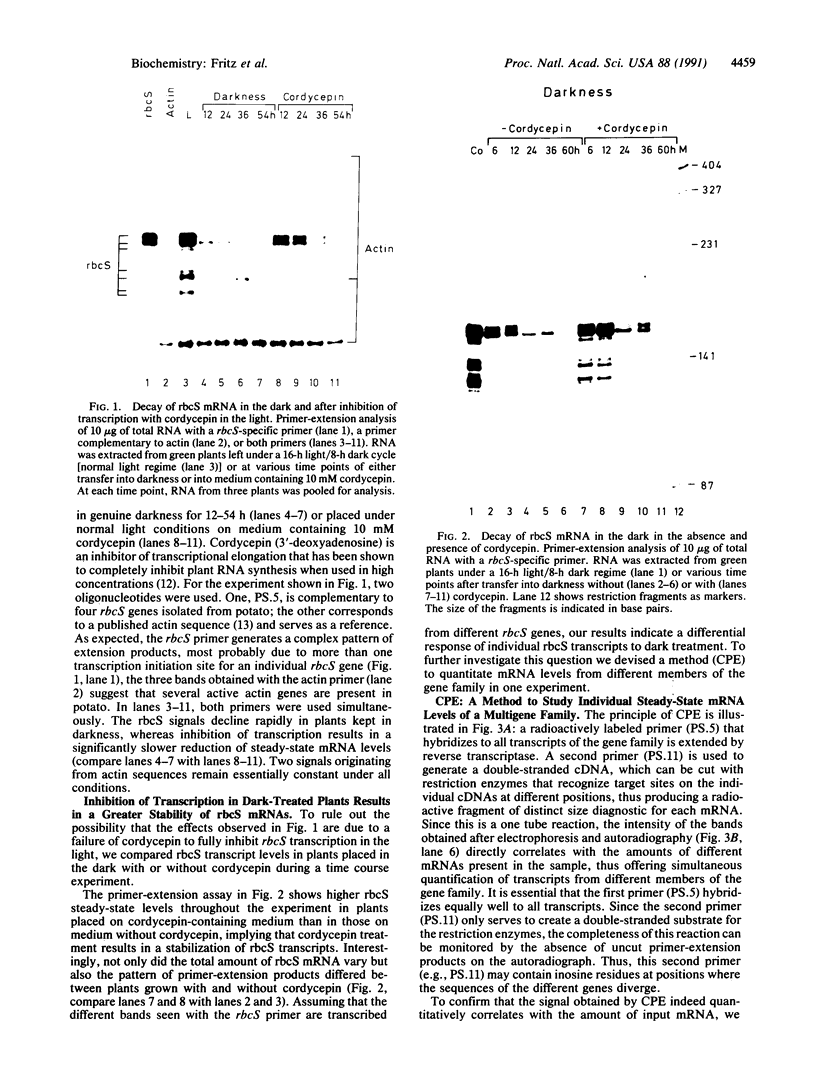
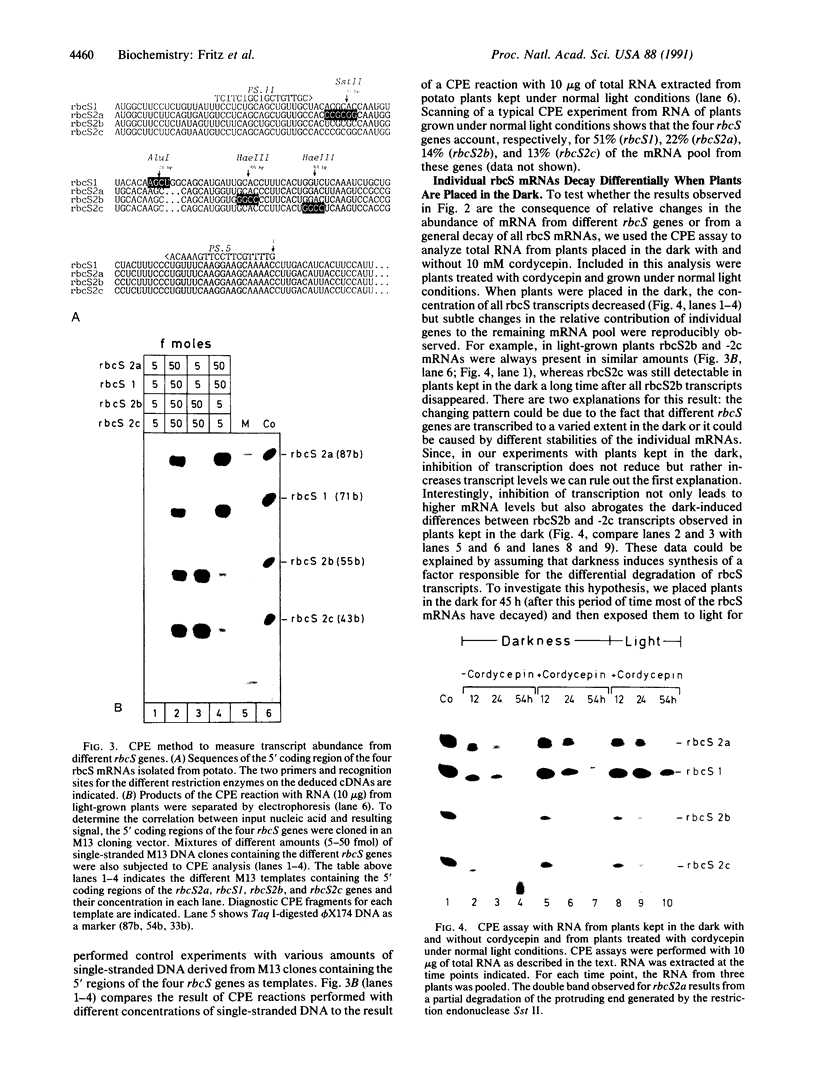
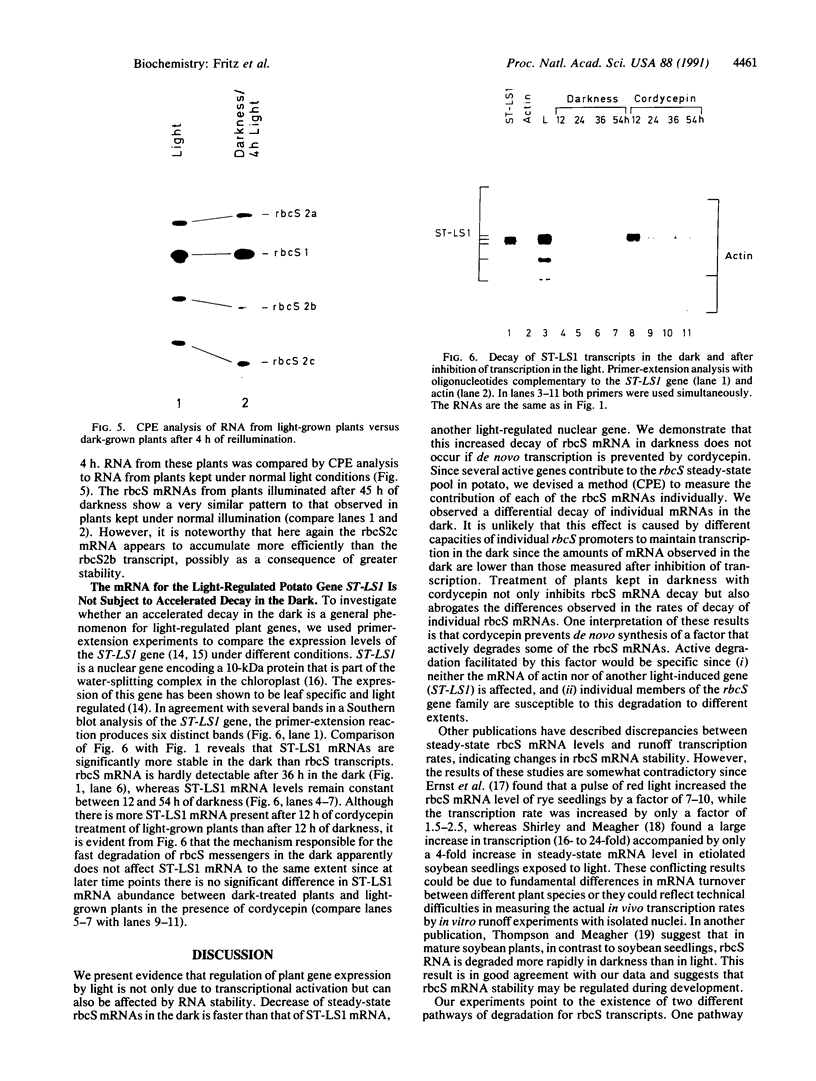
When plants are placed in the dark, the level of the abundant mRNA encoding the small subunit of ribulose-1,5-bisphosphate carboxylase (rbcS) declines rapidly. We present evidence demonstrating an active degradation of rbcS mRNA in the dark. Detailed analysis shows that transcripts originating from different members of the rbcS gene family are differentially affected by this degradation. This phenomenon is not common to all light-regulated plant genes since the mRNA for ST-LS1, another leaf-specific and light-induced gene, is not degraded in the dark within the same time scale.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry J. O., Carr J. P., Klessig D. F. mRNAs encoding ribulose-1,5-bisphosphate carboxylase remain bound to polysomes but are not translated in amaranth seedlings transferred to darkness. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4190–4194. doi: 10.1073/pnas.85.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. mRNA decay: finding the right targets. Cell. 1989 Apr 7;57(1):9–10. doi: 10.1016/0092-8674(89)90166-9. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Delseny M., Teresa M., Guitton Y. Effects of cordycepin on RNA metabolism in germinating seedlings. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1278–1285. doi: 10.1016/0006-291x(75)90831-1. [DOI] [PubMed] [Google Scholar]
- Herget T., Schell J., Schreier P. H. Elicitor-specific induction of one member of the chitinase gene family in Arachis hypogaea. Mol Gen Genet. 1990 Dec;224(3):469–476. doi: 10.1007/BF00262442. [DOI] [PubMed] [Google Scholar]
- Lautner A., Klein R., Ljungberg U., Reiländer H., Bartling D., Andersson B., Reinke H., Beyreuther K., Herrmann R. G. Nucleotide sequence of cDNA clones encoding the complete precursor for the "10-kDa" polypeptide of photosystem II from spinach. J Biol Chem. 1988 Jul 25;263(21):10077–10081. [PubMed] [Google Scholar]
- Mösinger E., Batschauer A., Schäfer E., Apel K. Phytochrome control of in vitro transcription of specific genes in isolated nuclei from barley (Hordeum vulgare). Eur J Biochem. 1985 Feb 15;147(1):137–142. doi: 10.1111/j.1432-1033.1985.tb08729.x. [DOI] [PubMed] [Google Scholar]
- Piechulla B., Gruissem W. Diurnal mRNA fluctuations of nuclear and plastid genes in developing tomato fruits. EMBO J. 1987 Dec 1;6(12):3593–3599. doi: 10.1002/j.1460-2075.1987.tb02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Shirley B. W., Meagher R. B. A potential role for RNA turnover in the light regulation of plant gene expression: ribulose-1,5-bisphosphate carboxylase small subunit in soybean. Nucleic Acids Res. 1990 Jun 11;18(11):3377–3385. doi: 10.1093/nar/18.11.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu A. B., Greenberg M. E., Belasco J. G. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989 Jan;3(1):60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- Silverthorne J., Tobin E. M. Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1112–1116. doi: 10.1073/pnas.81.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhaus J., Eckes P., Rocha-Sosa M., Schell J., Willmitzer L. Analysis of cis-active sequences involved in the leaf-specific expression of a potato gene in transgenic plants. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7943–7947. doi: 10.1073/pnas.84.22.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. M., Meagher R. B. Transcriptional and post-transcriptional processes regulate expression of RNA encoding the small subunit of ribulose-1,5-biphosphate carboxylase differently in petunia and in soybean. Nucleic Acids Res. 1990 Jun 25;18(12):3621–3629. doi: 10.1093/nar/18.12.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter F. P., Fritz C. C., Willmitzer L., Schell J., Schreier P. H. rbcS genes in Solanum tuberosum: conservation of transit peptide and exon shuffling during evolution. Proc Natl Acad Sci U S A. 1988 Feb;85(3):846–850. doi: 10.1073/pnas.85.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]