It is known that parkinsonism is associated with abnormalities in basal ganglia activity and that deep brain stimulation of these structures, a common treatment for Parkinson's disease, strongly alters basal ganglia output. However, parkinsonism- and stimulation-related activity changes in the ventral thalamus, a major recipient of basal ganglia output, remain controversial. These primate experiments demonstrate such changes, emphasizing emerging oscillatory activity patterns, and changes of the coupling between local field potentials and neuronal spiking.
Keywords: parkinsonism, monkey, deep brain stimulation
Abstract
Deep brain stimulation of the internal globus pallidus (GPi) is a major treatment for advanced Parkinson's disease. The effects of this intervention on electrical activity patterns in targets of GPi output, specifically in the thalamus, are poorly understood. The experiments described here examined these effects using electrophysiological recordings in two Rhesus monkeys rendered moderately parkinsonian through treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), after sampling control data in the same animals. Analysis of spontaneous spiking activity of neurons in the basal ganglia-receiving areas of the ventral thalamus showed that MPTP-induced parkinsonism is associated with a reduction of firing rates of segments of the data that contained neither bursts nor decelerations, and with increased burst firing. Spectral analyses revealed an increase of power in the 3- to 13-Hz band and a reduction in the γ-range in the spiking activity of these neurons. Electrical stimulation of the ventrolateral motor territory of GPi with macroelectrodes, mimicking deep brain stimulation in parkinsonian patients (bipolar electrodes, 0.5 mm intercontact distance, biphasic stimuli, 120 Hz, 100 μs/phase, 200 μA), had antiparkinsonian effects. The stimulation markedly reduced oscillations in thalamic firing in the 13- to 30-Hz range and uncoupled the spiking activity of recorded neurons from simultaneously recorded local field potential (LFP) activity. These results confirm that oscillatory and nonoscillatory characteristics of spontaneous activity in the basal ganglia receiving ventral thalamus are altered in MPTP-induced parkinsonism. Electrical stimulation of GPi did not entrain thalamic activity but changed oscillatory activity in the ventral thalamus and altered the relationship between spikes and simultaneously recorded LFPs.
NEW & NOTEWORTHY
It is known that parkinsonism is associated with abnormalities in basal ganglia activity and that deep brain stimulation of these structures, a common treatment for Parkinson's disease, strongly alters basal ganglia output. However, parkinsonism- and stimulation-related activity changes in the ventral thalamus, a major recipient of basal ganglia output, remain controversial. These primate experiments demonstrate such changes, emphasizing emerging oscillatory activity patterns, and changes of the coupling between local field potentials and neuronal spiking.
the signs and symptoms of idiopathic Parkinson's disease (PD) are accompanied by altered firing of neurons in the movement-related portions of the internal pallidal segment (GPi) and other basal ganglia nuclei (as reviewed in Galvan et al. 2015). GPi activity has been described to be increased and abnormally patterned, with increased oscillatory and bursting activities of individual neurons, and increased synchrony between neighboring cells (Galvan et al. 2015). One of the principal recipients of GPi output is the ventral thalamus. The specific thalamic areas that receive the movement-related basal ganglia output are known under a variety of names in different nomenclature systems. To avoid confusion, we will refer to them here as the “basal ganglia motor thalamus” (BGMT).
Studies of neuronal activity in the BGMT in animal models of parkinsonism have provided conflicting evidence with reports of either decreased neuronal firing (Ni et al. 2000; Schneider and Rothblat 1996; Vitek et al. 1994), no change in firing rates (Pessiglione et al. 2005), or even an increase (Bosch-Bouju et al. 2014). In patients with idiopathic PD, neurons in the BGMT, recorded during deep brain stimulation (DBS) surgery, have a reduced mean firing rate compared with similar recordings from non-PD patients (Chen et al. 2010; Molnar et al. 2005). As in the basal ganglia, thalamic firing patterns also appear to be altered in parkinsonism, with descriptions of increased burst discharges (Guehl et al. 2003; Magnin et al. 2000; Molnar et al. 2005; Pessiglione et al. 2005; Zirh et al. 1998) and a greater-than-normal correlation of firing of neighboring neurons (Pessiglione et al. 2005). Few studies have evaluated local field potential (LFP) recordings in parkinsonism in the BGMT. Studies of neuronal activity in the pallidal receiving area of PD patients with drug-resistant tremor showed prominent oscillations in the tremor frequency range (4–9 Hz) in BGMT, correlated with frontal cortical oscillations (Sarnthein and Jeanmonod 2007).
Deep brain stimulation of the GPi motor territory is a highly effective treatment for patients with advanced PD (Moro et al. 2010; Rodrigues et al. 2007; Weaver et al. 2005; Zahodne et al. 2009) and is thought to reduce the impact of altered firing rates and patterns in GPi (Johnson et al. 2008; Kringelbach et al. 2007; Lempka and McIntyre 2013; Rubin et al. 2012). Experimental evidence shows that high-frequency GPi microstimulation leads (at least in the short term) to entrainment of pallidal activity to the stimulation (Bar-Gad et al. 2004; Chiken and Nambu 2013; Erez et al. 2009), and macrostimulation in parkinsonian nonhuman primates induces modest changes in pallidal firing rates, more substantial changes in oscillatory activity in the β-band of frequencies and the synchrony of firing, as well as entrainment of pallidal spiking activity to the stimulation (McCairn and Turner 2009). Recent studies have shown that pallidal DBS reduces cortical synchronous β-band oscillations (Johnson et al. 2009; McCairn and Turner 2015). While it is speculated that DBS of the subthalamic nucleus (STN) may exert its antiparkinsonian effects via antidromic activation of the cerebral cortex (Dejean et al. 2009; Devergnas and Wichmann 2011; Gradinaru et al. 2009; Li et al. 2012; Li et al. 2007), this is probably not the case for pallidal DBS (Devergnas and Wichmann 2011; McCairn and Turner 2015). It is more likely that the effects of pallidal stimulation on cortical activity are orthodromically mediated via the BGMT.
The effects of electrical pallidal stimulation on thalamic activity as its immediate downstream target in the parkinsonian state remain uncertain. The impact of high-frequency stimulation of GPi on BGMT activity has previously been examined in normal animals (Anderson et al. 2003), demonstrating predominately inhibitory effects, as well as in dystonic patients (Montgomery 2006; Montgomery and Gale 2008), showing stimulation-induced alterations in the patterning of neuronal firing. Pallidal stimulation in parkinsonian monkeys was recently also shown to entrain the activity of thalamic neurons (Agnesi et al. 2015), with strong inhibitory effects, although the stimulation in these experiments appears to have involved mostly the external pallidal segment. The present study examined in detail the effects of both parkinsonism and of GPi stimulation on BGMT activity, using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated Rhesus monkeys as a model of parkinsonism. We used stimulation parameters that mimicked those of DBS parameters in widespread clinical use.
METHODS
Animals, Surgery
The experiments involved two juvenile male rhesus monkeys (monkeys A and B; Macaca mulatta; 4–6 kg). The animals were pair-housed and had ad libitum access to food and water. All experiments were performed in accordance with the United States Public Health Service Policy on the humane care and use of laboratory animals, including the provisions of the Guide for the Care and Use of Laboratory Animals (Garber et al. 2010). All study protocols were submitted to, and approved by, the Institutional Biosafety Committee and the Animal Care and Use Committee of Emory University.
The animals were first conditioned to accept handling by the experimenters and to sit in a primate chair. They then underwent aseptic surgery under isoflurane anesthesia (1–3%) for placement of two stainless steel recording chambers (inner chamber diameter 19 mm; Crist Instruments, Hagerstown, MD), positioned stereotactically and embedded, along with a stainless steel head holder and bone screws, in an acrylic “cap.” The chambers were directed at the pallidum and the thalamus on the animal's left side. The pallidal chamber was placed at a 36° angle from the vertical in the coronal plane, and the thalamic chamber was positioned at a 30° angle anterior to the vertical in the sagittal plane. The animals were allowed to recover for 1 wk after the surgery before recording and other procedures begun. Some of the bone screws were stereotaxically positioned over the occipital lobe, motor cortex, and premotor cortex of the targeted hemisphere and were connected with wires to a 9-pin connector that was also embedded in the acrylic. These screws were used to monitor EEG signals to examine wakefulness.
MPTP Treatment and Behavioral Evaluation
After the surgical procedure, the animals were rendered progressively parkinsonian by weekly administration of small doses of MPTP (0.4–0.6 mg/kg im; total 2.8 mg/kg in one animal, divided into 6 weekly injections, and 8.2 mg/kg in the other animal, divided into 19 injections). To assess the degree and stability of the MPTP-induced motor disability, the severity of parkinsonism was assessed weekly for 15 min while the monkey was in an observation cage equipped with 8 infrared beams for activity monitoring. The sessions were also recorded with a video camera. In these sessions we scored the motor impairment in terms of 10 aspects of motor function (bradykinesia, freezing, extremity posture, trunk posture, action tremor, the frequency of arm and leg movements, finger dexterity, home cage activity, and balance). Each item was rated on a scale of zero to three (from normal to severely parkinsonian; maximal score: 30). During the time of recording, the animal scores were 19 and 20, respectively, corresponding to moderately severe parkinsonism, with prominent akinesia, bradykinesia, and balance problems. No supplemental MPTP injections were given during the time of the recording sessions.
The animals were also behaviorally scored during stimulation. For this purpose, we used a rating scale that was adapted for scoring of parkinsonism in head-restrained animals. We scored the severity of tremor and rigidity (tested through passive movements of the animal's extremities), bradykinesia, freezing, as well as body hypokinesia, each scored on a scale of zero and three (from normal to severely parkinsonian; maximal score: 15). These scores were obtained by an investigator who was unaware of the stimulation condition (no stimulation, 10-Hz stimulation, or DBS-like 120-Hz stimulation; see below for details). The stimulation lasted for 10 min, and assessments of the behavioral effects of stimulation were obtained in the time period between the 5th and 10th min of stimulation.
Electrophysiological Stimulation and Recording Methods
Recordings were made while the animals sat in a primate chair with their head immobilized but their body and limbs free to move. The locations of the GPi and ventral thalamus were mapped by extracellular electrophysiological recording with tungsten microelectrodes (Z = 0.5–1.0 MΩ at 1 kHz; FHC, Bowdoinham, ME). The dura was pierced with a guide tube, and the electrode was lowered into the brain with a microdrive (MO-95B; Narishige, Tokyo, Japan). The single unit signals were amplified (DAM-80 amplifier; WPI), filtered (300−6,000 Hz, model 3364 filter; Krohn-Hite, Brockton, MA), displayed on a digital oscilloscope (DPO2024; Tektronix, Beaverton, OR), and made audible via an audio amplifier and commercial speakers (Bose, Framingham, MA).
GPi neurons were identified by their location ventromedial to the external pallidal segment and by their continuous high-frequency discharge (DeLong 1971). The sensorimotor portion of GPi was then targeted for electrical stimulation, based on its ventral and lateral position within the borders of the nucleus and the results of sensory examination of the animals during electrophysiological mapping experiments. The stimulation was carried out with bipolar concentric electrodes (SNEX-100 × 120 mm; outer diameter, 250 μm; intercontact separation, 500 μm; impedance, 25–35 kΩ; Rhodes Medical Instruments, Tujunga, CA). On each recording day, this electrode was placed into GPi for stimulation. In each animal, we positioned the electrodes to the same chamber coordinates and depth (presumably, thus, the same GPi location) on each experimental day for a series of experimental sessions. While this method of tissue stimulation is different from the use of permanently implanted DBS electrodes in patients, it had the benefit of allowing us to verify (and, if needed, to correct) the location of the tip of the electrode by electrophysiological mapping of the boundaries of the stimulated nucleus after a few weeks of usage, and did not require the permanent implantation of the electrode. We repeated the electrophysiological mapping of GPi boundaries several times during the series of recordings, and adjusted the position of the stimulation electrode (resulting in slightly separate stimulation positions in each animal, as shown in Fig. 2, A and B). The size of the electrode and spacing of the contacts are scaled to approximate the electrode dimensions used in human DBS therapy.
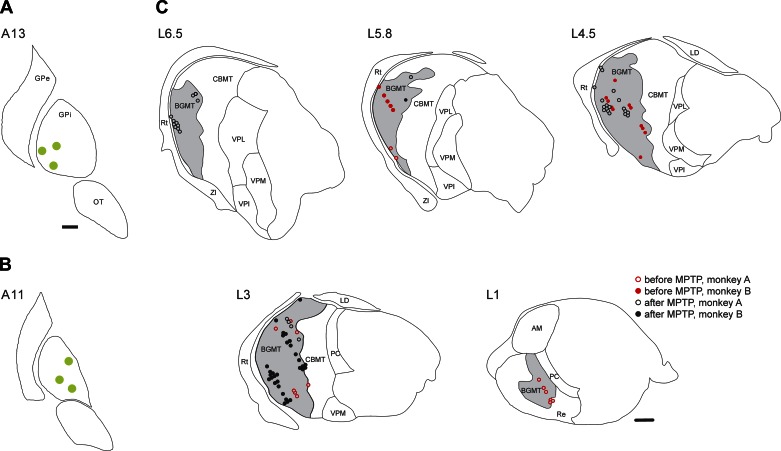
Fig. 2.
Stimulation sites in the internal globus pallidus (GPi, A and B) and microelectrode recording sites in the BGMT (C). The drawings in A and B are in coronal planes, showing the stimulation electrode tip sites (green circles) in monkeys A and B, respectively. The drawing of recording sites in C is based on histological reconstructions of parasagittal Nissl-, calbindin-, and MAP2-stained slides. Data from both monkeys are combined. The nuclear boundaries in A and B are based on the nuclear outlines in the atlas by Paxinos et al. (Paxinos et al. 2000), and those in C are drawn using the macaque brain atlas by Lanciego et al. (Lanciego and Vazquez 2012). In C, neurons recorded before 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment are shown as red symbols, neurons recorded after MPTP treatment are shown in black. Cells that were very closely spaced are shown next to each other. Open symbols represent recordings in monkey A, filled symbols are from monkey B. AM, anterior medial nucleus of the thalamus; CBMT, cerebellar receiving territory of the motor thalamus; GPe, external pallidal segment; LD, laterodorsal nucleus of the thalamus; OT, optic tract; PC, paracentral nucleus of the thalamus; Re, reuniens nucleus of the thalamus; Rt, reticular nucleus of the thalamus; VPI, ventral posterior inferior nucleus of the thalamus; VPL, ventral posterior lateral nucleus of the thalamus; VPM, ventral posterior medial nucleus of the thalamus; ZI, zona incerta. Scale bars, 1 mm. The scale bar in A also applies to B.
Subsequent to the insertion of the stimulation electrode, recording electrodes were introduced into the thalamus through the anterior recording chamber. The recording positions were chosen relative to the anterior edge of the thalamus found in preliminary mapping experiments, which also identified the characteristic neuronal activity patterns of surrounding structures, including the STN, the zona incerta, the internal capsule, and the globus pallidus. Stimulation and recording electrodes were removed at the end of each experimental day.
The electrical signals recorded in the thalamus were stored to computer disk (sampling rate 50 kHz, Power1401 with Spike2 interface; CED, Cambridge, UK). For most neurons, the raw electrical signal potentials were also filtered between 1 Hz and 1 kHz to extract LFP signals and stored along with the neuronal spiking data.
The animals were continuously video-monitored, and all recordings were done during periods when the monkey was awake (observed in a live camera feed and by corecorded occipital EEG) and sitting still with eye movements, orofacial movements, and occasional arm movements. Each thalamic cell was first recorded at baseline, for 3–15 min. After these baseline recordings, we recorded from the same neurons during GPi stimulation with biphasic square wave pulses (120 Hz, 100 μs/phase, 200 μA). The stimuli were delivered via a constant-current stimulus isolation device (A395R; WPI, Sarasota, Florida), and timed with the D/A interface of the Power1401 unit, along with digital time stamps, and voltage signals reflecting the stimulation currents. Depending on the stability of recording, we were able to use stimulation runs of up to 948.8 s (see results for details). We here report on observations made during the 1st and 10th min of stimulation.
Termination of the Experiment and Histology
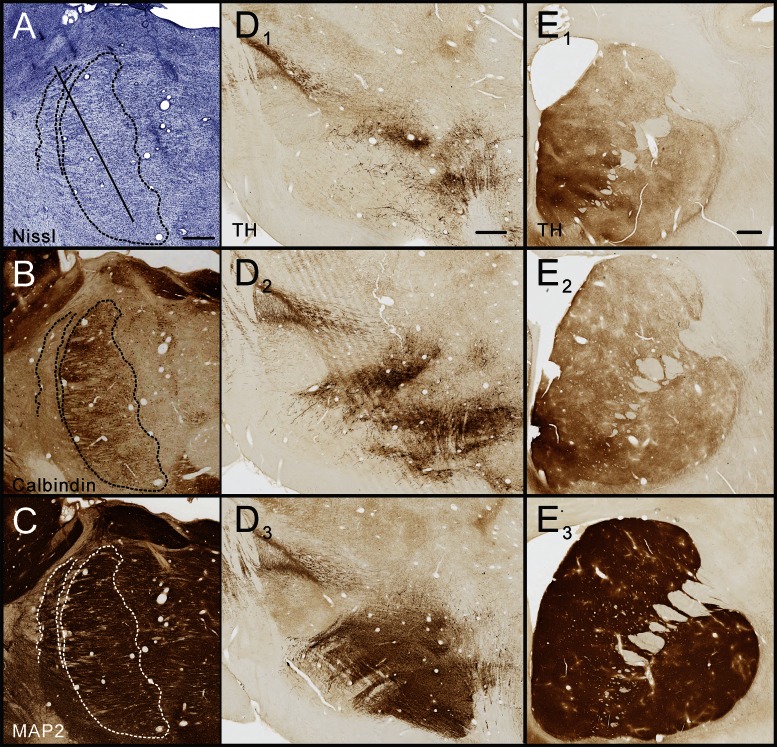
After completion of the experiments, the animals were killed with an overdose of pentobarbital sodium (100 mg/kg iv), followed by transcardial perfusion with oxygenated Ringer's solution, followed by 2 liters of fixative [4% paraformaldehyde, 0.1% glutaraldehyde in phosphate buffer (0.1 M, pH 7.2)]. The brain was blocked, cut further into 60-μm-thick sections with a vibratome, stained with cresyl violet, and immunostained for the neuronal marker microtubule-associated protein 2 and for calbindin to verify electrode positions, delineate the outlines of the BGMT, and to examine tissue damage produced by electrode insertions (Fig. 1, A–C). Sections through the striatum and substantia nigra pars compacta were also stained for tyrosine hydroxylase, to confirm the MPTP-induced loss of dopaminergic neurons and their striatal terminals. These results are shown in Fig. 1, D and E, compared with sections at the same levels from a normal animal. The sections were mounted on slides and analyzed by light microscopy.
Fig. 1.
Histological identification of recording sites and documentation of dopamine depletion. The images in A–C show examples of adjacent sagittal sections through the anterior thalamus stained for Nissl substance (A), calbindin (B), and microtubule-associated protein 2 (MAP2, C), documenting how the basal ganglia motor thalamus (BGMT) was delineated and how tissue damage from repeated electrode penetrations was assessed. A representative electrode penetration is shown by the diagnonal line in A to show the stereotaxic approach to the thalamus. D and E show tyrosine hydroxylase (TH) stains of the midbrain (including the substantia nigra pars compacta, D) and coronal sections of the striatum (E). D1, D2, E1, and E2 are sections from the monkeys used in this study while D3 and E3 are sections from a normal control monkey, shown here for comparison. All scale bars are 1 mm in length. The scale bar in A applies to images A–C, the scale bar in D1 applies to D1–D3, and the scale bar in E1 applies to E1–E3.
The identification of stimulation and recording positions was based on postmortem reconstructions of electrode tracks on histological sections, comparison with stereotaxic atlas images (Lanciego and Vazquez 2012; Paxinos et al. 2000), and the written records of the anterior-posterior, mediolateral, and depth microdrive settings during the experimental sessions.
Data Analysis
Data preparation.
Neuronal data were considered for analysis if the neuron could be identified as belonging to the BGMT (see above) and had been recorded with sufficient quality (see below). An obvious possibility of error in these studies was that remnants of stimulation artifacts were misidentified as spikes. To remove stimulation artifacts from neuronal recordings during the electrical stimulation, we used a modified version of a previously described digital averaging method (Wichmann 2000), implemented in Spike2. For each cell, artifact templates were generated by averaging the waveforms of all stimulation artifacts. The artifacts were then scaled and subtracted from the record at the appropriate times relative to the timing of the stimuli. In recordings of eight of the thalamic cells that were studied during GPi stimulation, artifact components remained in the record even after the artifact subtraction procedure. These remaining artifact portions were replaced by brief zero-value segments, lasting an average of 0.60 ± 0.09 ms (range: 0.33–1.01 ms). For analysis of stimulation effects, similar brief zero-value segments were inserted in the baseline recordings of the neuronal spiking signals of the affected cells, so that the likelihood of detecting spikes was similarly affected during stimulation and during baseline portions of the record.
After artifact removal, the data segments were subjected to spike detection and spike sorting, using a waveform matching algorithm, followed by principal component analysis in Spike2. For each cell, distribution histograms of interspike intervals (ISIs) were constructed for an analysis of the recording quality. Spike trains containing >1% of ISIs shorter than 2 ms were excluded. We then extracted information regarding the timing of spikes and stimuli from the remaining files. This information was subsequently used for extensive analyses with custom-designed algorithms in Matlab.
Assessment of basic firing characteristics.
The possible association of changes in firing rates and patterns with parkinsonism was evaluated by comparing recordings from the pre-MPTP state with those from the MPTP-treated state. We assessed whether a cell responded to the stimulation, using data from the 1st and 10th min of stimulation, and comparing them with data from the control period immediately preceding the stimulation. We calculated firing rates (expressed as spikes/s), the coefficient of variation of ISI, as well as burst- and deceleration indexes. Calculation of the latter utilized the “surprise” method, which identifies bursts or decelerations as unexpected sequences of spiking events (Elias et al. 2007; Legendy and Salcman 1985; Wichmann and Soares 2006). We determined the incidence of such bursts (consisting of at least 3 spikes) or decelerations, using a surprise value of 3 (Aldridge and Gilman 1991; Wichmann and Soares 2006). Bursts were designated as “rebound bursts” if the immediate preburst ISI was at least two times as long as the average ISI of the respective neuron. Indexes were calculated as the proportion of spikes involved in the bursts or decelerations (relative to the total number of spikes), or the proportion of time spent by the cell in either bursts or decelerations.
We also generated “nonmodulated” data streams, i.e., ISI data from which bursts and decelerations were removed. Nonmodulated firing rates were calculated to determine whether firing rate changes were the product of, or related to, changes in bursting or decelerations, or whether the background activity of the cell changed independent of the presence of bursts or decelerations. The proportion of nonmodulated spikes and the proportion of time the cell spent in the nonmodulated state were then calculated.
A power spectral analysis to examine oscillatory properties of the neuronal discharge was carried out with the Neurospec 2.0 Matlab routines (written by Dr. David Halliday, http://www.neurospec.org, Halliday et al. 1995; Nielsen et al. 2005). For each cell, the raw spectra were generated, and integrated spectra, covering the 1- to 3-, 3- to 8-, 8- to 13-, 13- to 30-, and 30- to 100-Hz ranges were calculated. Spectra were normalized to the total power in the 1- to 100-Hz band (Soares et al. 2004).
Analysis of interstimulus time intervals.
We examined the temporal relationship between each neuronal spike recorded in the BGMT and the GPi stimulus that preceded it by constructing interstimulus histograms (binned in 0.2 ms intervals) and raster diagrams for each cell, plotting the period between consecutive stimuli during the 120-Hz stimulation trains. Entrainment of neuronal firing to the stimuli (if present) is easily visible in the peristimulus histograms as patterning with distinct maxima and minima, corresponding to maximal and minimal probabilities of spike occurrences during the interstimulus intervals. For each cell, the resulting interstimulus histogram values were z-scored by applying the same analysis to 250 series of shuffled ISIs (while maintaining the timing of stimuli) whose mean and SD values were then used to calculate z-scores. Histogram values outside of the mean ± 2 SD boundaries were considered to indicate inhibition or excitation. We examined separate interstimulus histograms for the 1st and 10th min of stimulation.
LFP analysis.
LFP data were analyzed to study the effects of parkinsonism on LFP oscillations in the thalamus. To this end, we determined the power spectral density of the LFP data from the pre-MPTP recordings, as well as the prestimulation (control) and stimulation periods from the post-MPTP recordings. The LFP traces were first band-pass-filtered using fourth-order Butterworth zero-phase-shift filtering (1–100 Hz). The power spectral density was computed using the Welch technique, with Hamming windowing, and a fast-Fourier transform segment length of 1,024 samples with no overlap, resulting in a final spectral resolution of 0.98 Hz across the 1.95- to 99.61-Hz range. The data were normalized to the total power of the respective recording session.
We also examined whether spiking occurred at a preferred phase of the LFP oscillations. This analysis used a circular analysis as described by Lobb and colleagues (Lobb and Jaeger 2015; Lobb et al. 2013). This analysis tests the ranked distribution of the Hilbert-transformed instantaneous phase of the LFP at each spike time for statistical significance using the omnibus test. For this analysis, the raw LFP data were filtered in 2-Hz-wide bands, allowing us to assess phase relationships between neuronal spiking and LFP signals with each of these bands. The data are presented as the proportion of neurons that showed a significant (P < 0.05) phase relationship for each of the frequency bands.
Statistics and data presentation.
The initial data preparation was carried out with Spike2 routines. All subsequent data extraction and analysis steps were done in Matlab. Data were plotted with Matlab and SigmaPlot. Unless indicated differently, statistical comparisons were carried out with SPSS using the Mann-Whitney test with correction for multiple comparisons for independent samples, and the Wilcoxon signed-rank test or paired t-tests, as appropriate, for related samples. As indicated above, the analysis of the spike/LFP phase relationship is based on the results of the Omnibus test. An α-error level of P < 0.05 was accepted as an indicator of significant differences.
RESULTS
Database
For the purpose of this analysis, data from the BGMT from both monkeys were pooled for most analyses. We included 31 neurons in the normal state and 66 neurons from the parkinsonian state. Both animals contributed similar numbers of cells to the analysis (Table 1).
Table 1.
MPTP-induced changes in descriptors of spontaneous spiking activity in the BGMT
|
Monkey A |
Monkey B |
Both Animals Combined |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Normal (n = 15) | MPTP (n = 33) | P | Normal (n = 16) | MPTP (n = 33) | P | Normal (n = 31) | MPTP (n = 66) | P |
| Firing rate, 1/s | 13.8 ± 5.9 | 6.4 ± 1.0 | 0.850 | 15.6 ± 2.5 | 12.5 ± 2.9 | 0.031 | 14.7 ± 3.1 | 9.5 ± 1.6 | 0.058 |
| ISI CV | 1.7 ± 0.1 | 1.8 ± 0.1 | 0.938 | 1.8 ± 0.3 | 2.1 ± 0.2 | 0.136 | 1.8 ± 0.2 | 1.9 ± 0.1 | 0.252 |
| Nonmodulation firing rate, 1/s | 13.9 ± 6.0 | 6.1 ± 1.0 | 0.734 | 15.0 ± 2.4 | 12.9 ± 2.9 | 0.037 | 14.5 ± 3.0 | 9.6 ± 1.6 | 0.030* |
| Proportion of nonmodulated spikes, % | 47.5 ± 5.6 | 45.4 ± 2.8 | 0.924 | 53.6 ± 3.5 | 48.1 ± 3.6 | 0.080 | 50.8 ± 3.1 | 46.8 ± 2.3 | 0.123 |
| Proportion of nonmodulation time, % | 54.3 ± 5.8 | 54.9 ± 3.0 | 0.903 | 57.7 ± 4.1 | 52.8 ± 3.9 | 0.115 | 56.2 ± 3.4 | 53.8 ± 2.5 | 0.226 |
| PS, % | |||||||||
| 1–3 Hz | 2.8 ± 0.4 | 3.3 ± 0.2 | 0.226 | 2.3 ± 0.2 | 2.7 ± 0.2 | 0.268 | 2.5 ± 0.2 | 3.0 ± 0.2 | 0.093 |
| 3–8 Hz | 6.6 ± 1.2 | 7.6 ± 0.6 | 0.196 | 4.0 ± 0.3 | 5.9 ± 0.4 | 0.006 | 5.2 ± 0.6 | 6.7 ± 0.3 | 0.002* |
| 8–13 Hz | 5.8 ± 0.9 | 6.4 ± 0.3 | 0.248 | 3.7 ± 0.2 | 5.3 ± 0.3 | 0.003 | 4.7 ± 0.5 | 5.8 ± 0.2 | 0.003* |
| 13–30 Hz | 18.7 ± 2.0 | 19.0 ± 0.7 | 0.596 | 15.0 ± 0.5 | 17.0 ± 0.7 | 0.306 | 16.7 ± 1.0 | 18.0 ± 0.5 | 0.150 |
| 30–100 Hz | 66.1 ± 4.3 | 63.7 ± 1.7 | 0.321 | 74.9 ± 1.1 | 69.2 ± 1.5 | 0.003 | 71.0 ± 2.1 | 66.5 ± 1.2 | 0.002* |
| Proportion of spikes in bursts, % | 45.6 ± 4.8 | 47.7 ± 2.4 | 0.775 | 40.8 ± 3.2 | 45.7 ± 3.3 | 0.348 | 43.0 ± 2.8 | 46.6 ± 2.0 | 0.257 |
| Spikes/burst | 5.2 ± 0.4 | 5.1 ± 0.2 | 0.989 | 6.0 ± 0.4 | 5.9 ± 0.3 | 0.241 | 5.7 ± 0.3 | 5.5 ± 0.2 | 0.336 |
| Maximal fold rate increase in bursts | 45.4 ± 15.6 | 50.6 ± 10.8 | 0.226 | 10.4 ± 1.3 | 37.1 ± 10.5 | 0.017 | 26.1 ± 7.6 | 43.6 ± 7.5 | 0.008* |
| Intraburst frequency, 1/s | 114.8 ± 31.4 | 80.0 ± 10.8 | 0.734 | 56.8 ± 7.2 | 70.7 ± 10.1 | 0.966 | 82.8 ± 15.3 | 75.2 ± 7.4 | 0.973 |
| Proportion of rebound bursts, % | 81.6 ± 10.1 | 93.2 ± 3.0 | 0.111 | 68.6 ± 9.5 | 78.4 ± 6.5 | 0.329 | 74.5 ± 6.9 | 85.5 ± 3.8 | 0.056 |
| Proportion of time in decelerations, % | 36.4 ± 5.2 | 38.0 ± 2.4 | 0.829 | 30.2 ± 3.8 | 38.4 ± 3.5 | 0.316 | 33.0 ± 3.1 | 38.2 ± 2.1 | 0.278 |
| Mean frequency in decelerations, 1/s | 4.1 ± 1.8 | 1.8 ± 0.3 | 0.874 | 3.9 ± 0.7 | 3.3 ± 0.8 | 0.021 | 4.0 ± 0.9 | 2.6 ± 0.5 | 0.038* |
Values are means ± SD; n, no. of experiments. MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; BGMT, basal ganglia motor thalamus; ISI, interspike intervals; CV, coefficient of variation of interspike intervals; PS, power spectrum.
Significant difference, P < 0.05.
The cells in the normal state were recorded for 152.6 ± 16.6 s (range: 47.7–471.0 s). Post-MPTP, baseline recordings lasted 229.5 ± 12.5 s (range: 74.7–629.4). Of the post-MPTP neurons, data for the 1st min of stimulation were available from 61 cells, and data for the 10th min of stimulation were available for 52 cells. On average, the stimulation lasted 662.6 ± 20 s (range: 143.0–948.8 s). The locations of the stimulation and recording sites are shown in Fig. 2.
MPTP-Induced Changes
MPTP-induced changes in spontaneous activity of neurons in the BGMT are shown in Table 1. While the variability of the results was greater in monkey A than in monkey B, the polarity of changes between the two animals was the same. The induction of parkinsonism was not accompanied by significant changes in firing rates in either of the animals, or in the pooled analysis. The firing rate was significantly reduced in segments of the data stream that did not show bursts or decelerations of discharge (nonmodulated spikes). Oscillatory firing in the 3- to 8- and 8- to 13-Hz ranges was increased, whereas oscillations in the 30- to 100-Hz (γ-) range of frequencies were decreased. There was also an overall increase of the maximal fold rate increase in bursts. Almost 75% of all bursts satisfied criteria for “rebound bursting” in the normal state, and 86% in the parkinsonian state. The decelerations reached lower frequencies in the parkinsonian state.
Stimulation-Induced Changes
Behavior.
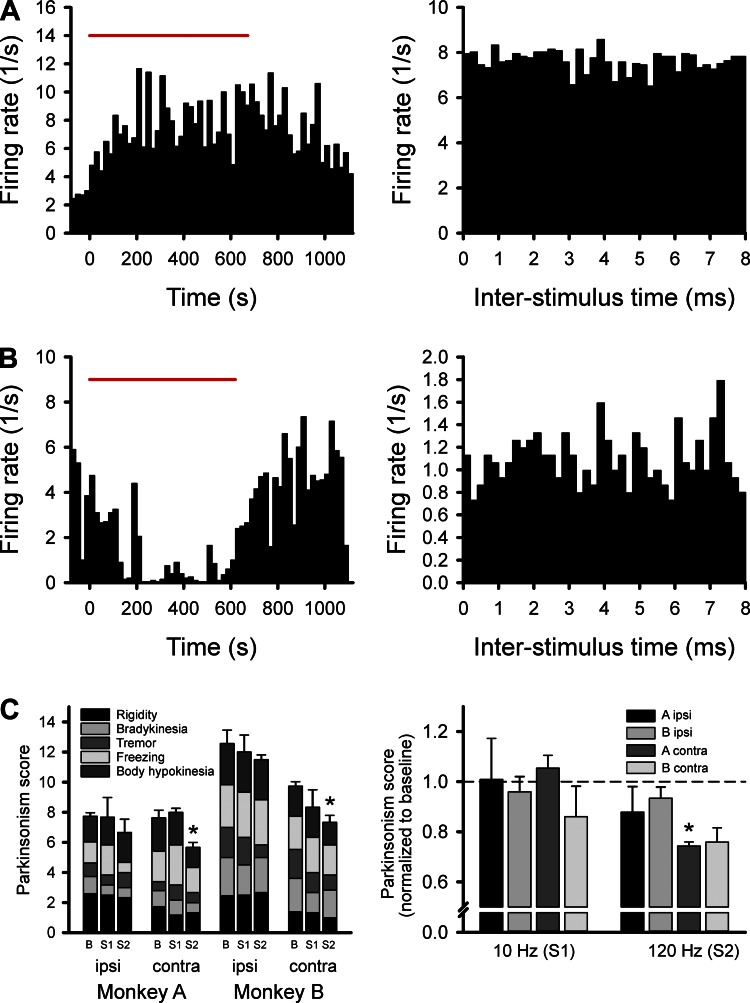
We examined the effects of 10- and 120-Hz stimulation. For the behavioral testing, the stimulation (or baseline) was applied for 10 min, and the effects shown were scored within the period between the 5th and 10th min. The behavioral effects were scored by an observer unaware of the stimulation condition with the rating scale mentioned in methods. As shown in Fig. 3C, we found that 10-Hz stimulation (“S1” in the plot on Fig. 3C, left) had no effect ipsi- or contralateral to the stimulation, whereas the 120-Hz stimulation (“S2”) lowered the parkinsonism scores in both monkeys contralateral to the stimulation (P = 0.037 in monkey A and P = 0.015 in monkey B). In the analysis of raw parkinsonism scores (shown in Fig. 3C, left), there was no consistent effect on any one of the five subscores (rigidity, tremor, bradykinesia, freezing, and body hypokinesia). A similar result was obtained when the effects of stimulation were evaluated with paired tests, comparing the overall parkinsonism scores with the baseline from the specific days of testing. This result is shown in Fig. 3C, right (contralateral stimulation: P = 0.012 for monkey A and P = 0.057 for monkey B).
Fig. 3.
Response to stimulation. The plots in A and B show results from two different BGMT cells, showing an increase (A) and a reduction (B), respectively, in firing in response to deep brain stimulation (DBS)-like 120-Hz stimulation in the GPi. The plots on the left show firing rate readouts, binned in 20-s intervals before, during, or after GPi stimulation (applied during the time indicated by the red bar). The plots on the right show poststimulus histograms for the same cells. The bar graphs in C show the behavioral responses to GPi stimulation at 10 or 120 Hz. The graph on the left shows the parkinsonism scores from each monkey, with gray scale depiction of the average subscores for rigidity, bradykinesia, tremor, freezing, and hypokinesia. Error bars and asterisks refer to the average overall score. The behavioral data were generated in three separate experimental days in each monkey. The graph on the right shows baseline-normalized overall parkinsonism scores from the sides ipsi- or contralateral to the stimulation, in monkeys A and B. *P < 0.05, difference from baseline. B, baseline; S1, 10-Hz stimulation; S2, 120-Hz stimulation.
Effects on spiking activity of thalamic neurons.
The responses of BGMT cells to the stimulation were variable. Figure 3, A and B, shows the responses of two example cells in the BGMT, responding to 120-Hz GPi stimulation. The cell in Fig. 3A increased its firing during the stimulation, and gradually returned back toward its prestimulation baseline after the stimulation was stopped. The cell in Fig. 3B showed the opposite response, a reduction in firing during the time of stimulation. Neither of the two cells showed substantial stimulus-related changes in firing, as is evident from the poststimulus histograms on the right side of Fig. 3, A and B.
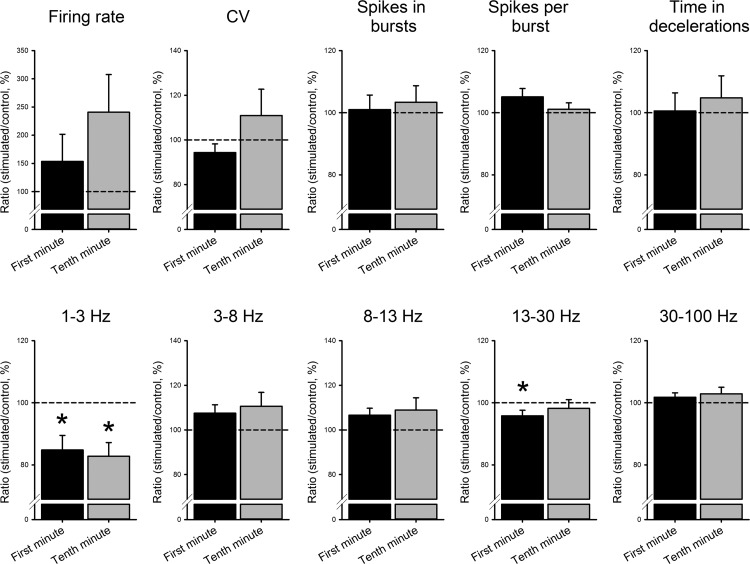
Summary analyses of stimulation-induced changes in firing rates, the coefficient of variation, as well as descriptors of burst and deceleration behavior in thalamic neurons, and oscillatory firing patterns recorded before and during the stimulation are presented in Table 2 and Fig. 4. The data shown in Table 2 demonstrate that there were no significant changes in any of the parameters tested in the analysis of pooled data from all thalamic cells (as assessed with Mann-Whitney tests). Note that the stimulation results from the 1st and 10th min of stimulation are presented separately, because each has its own set of baseline data (not all cells that were recorded for 1 min of stimulation could be recorded for the full 10-min period). The lack of changes in the group analysis is likely the result of the substantial variability of the individual baseline data and stimulation responses (see examples in Fig. 3). The data in Fig. 4 were analyzed with paired tests to examine changes within the 1st and 10th min of recording (as a proportion of the respective cell's baseline). We found that GPi stimulation increased the average firing rate (but not significantly, P = 0.877 in the 1st min and P = 0.964 in the 10th min of stimulation), and decreased 1- to 3-Hz oscillatory spiking (P = 0.000 for the 1st and 10th min of stimulation), as well as 13- to 30-Hz oscillations (P = 0.007 for the 1st min of stimulation). There were no significant overall changes in the parameters describing bursting, or in other oscillatory frequency ranges (compared with the respective recordings from unstimulated controls). The comparison between the 1st and 10th min of stimulation did not indicate significant changes in these parameters during the stimulation.
Table 2.
Stimulation-induced changes in descriptors of spontaneous spiking activity in the BGMT
| Parameter | Baseline | Stimulation, 1st min | P | Baseline | Stimulation, 10th min | P |
|---|---|---|---|---|---|---|
| Firing rate, 1/s | 9.9 ± 1.7 | 9.5 ± 1.6 | 0.914 | 10.7 ± 2.0 | 10.5 ± 2.1 | 0.944 |
| ISI CV | 2.0 ± 0.1 | 1.8 ± 0.1 | 0.116 | 2.0 ± 0.1 | 2.3 ± 0.4 | 0.480 |
| Nonmodulation firing rate, 1/s | 11.0 ± 1.9 | 10.5 ± 1.9 | 0.605 | 12.6 ± 2.5 | 12.4 ± 2.7 | 0.791 |
| Proportion of nonmodulated spikes, % | 46.8 ± 2.2 | 46.0 ± 2.7 | 0.989 | 46.2 ± 2.5 | 46.7 ± 2.5 | 0.857 |
| Proportion of nonmodulation time, % | 54.2 ± 2.5 | 54.0 ± 3.1 | 0.769 | 53 ± 2.9 | 54.2 ± 3.3 | 0.711 |
| PS, % | ||||||
| 1–3 Hz | 3.0 ± 0.1 | 3.2 ± 0.3 | 0.972 | 3.0 ± 0.2 | 2.9 ± 0.2 | 0.679 |
| 3–8 Hz | 6.6 ± 0.4 | 6.5 ± 0.4 | 0.732 | 6.3 ± 0.4 | 5.9 ± 0.3 | 0.465 |
| 8–13 Hz | 5.9 ± 0.3 | 5.5 ± 0.3 | 0.497 | 5.7 ± 0.3 | 5.3 ± 0.2 | 0.421 |
| 13–30 Hz | 18.0 ± 0.6 | 17.2 ± 0.5 | 0.409 | 17.4 ± 0.6 | 16.9 ± 0.4 | 0.775 |
| 30–100 Hz | 66.5 ± 1.3 | 67.6 ± 1.3 | 0.600 | 67.6 ± 1.4 | 68.9 ± 1.1 | 0.511 |
| Proportion of spikes in bursts, % | 47.6 ± 1.8 | 48.4 ± 2.2 | 0.983 | 46.9 ± 2.1 | 47.2 ± 2.2 | 0.951 |
| Spikes/burst | 5.5 ± 0.2 | 5.8 ± 0.3 | 0.829 | 5.6 ± 0.3 | 5.6 ± 0.3 | 0.846 |
| Maximal fold rate increase in bursts | 35.5 ± 7.0 | 33.7 ± 5.1 | 0.880 | 34.8 ± 8.9 | 28.5 ± 5.3 | 0.743 |
| Intraburst frequency, 1/s | 83.9 ± 8.3 | 68.8 ± 6.8 | 0.220 | 79.8 ± 9.6 | 75.4 ± 10.1 | 0.647 |
| Proportion of rebound bursts, % | 82.9 ± 4.4 | 79.5 ± 4.8 | 0.215 | 78.3 ± 5.6 | 81.1 ± 5.3 | 0.322 |
| Proportion of time in decelerations, % | 37.1 ± 2.1 | 37.0 ± 2.8 | 0.565 | 37.5 ± 2.7 | 35.8 ± 2.9 | 0.574 |
| Mean frequency in decelerations, 1/s | 2.9 ± 0.5 | 2.7 ± 0.4 | 0.722 | 3.3 ± 0.7 | 3.0 ± 0.6 | 0.956 |
Values are means ± SD.
Fig. 4.
Effects of GPi stimulation on parameters describing nonoscillatory and oscillatory properties of neuronal firing in the BGMT neurons. For each cell, data from the stimulated portion of the cell's record were normalized to the recording of the cell's baseline activity before the stimulation. *P < 0.05.
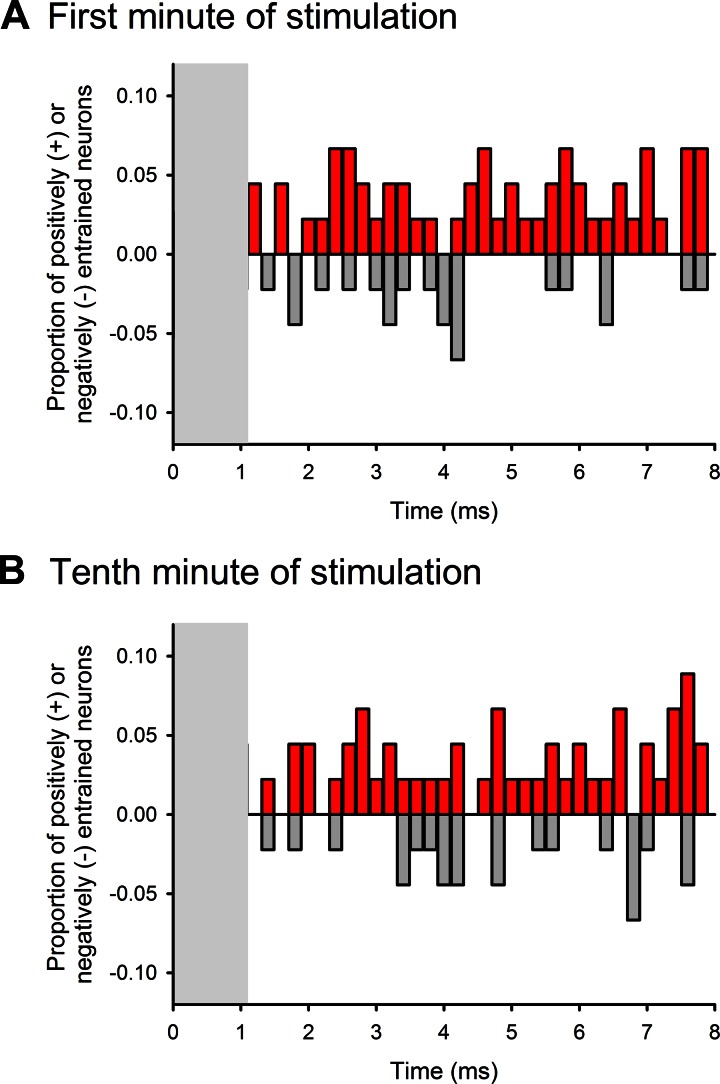
Entrainment of thalamic activity to GPi stimulation.
To assess the temporal coupling of increases or decreases in spiking activity to stimuli, we constructed interstimulus time histograms for all available cells. Examples of such interstimulus histograms are shown in Fig. 3, A and B, right. Figure 5 shows the results from the 1st and 10th min of stimulation from the subset of cells that were recorded for at least 10 min of stimulation. We calculated for each 0.2-ms bin of the 8-ms interstimulus interval the proportion of cells in which the likelihood of firing was outside of the mean ± 2 SD of data for that bin, generated based on analyses of shuffled ISI data streams. Both in the 1st and 10th min of stimulation, the firing of <10% of all tested neurons was found to be “entrained” to the stimulation at any specific time during the stimulation cycle. Note that the first millisecond of the interstimulus histograms was not considered, because it contained remnants of stimulation artifacts in a few cases (see methods).
Fig. 5.
Analysis of GPi stimulation-related activity changes of BGMT cells at specific interstimulus times. Response profiles are shown for the 1st and 10th min of stimulation, summarized across all cells (see methods and results for details of analysis). Increases in the probability of firing are shown as red (upward), whereas decreases are shown in gray (downward). In some cells, the first ms of the interstimulus interval was affected by the stimulation artifact. These intervals are therefore not shown (gray rectangles).
Relationship between thalamic spiking and LFPs.
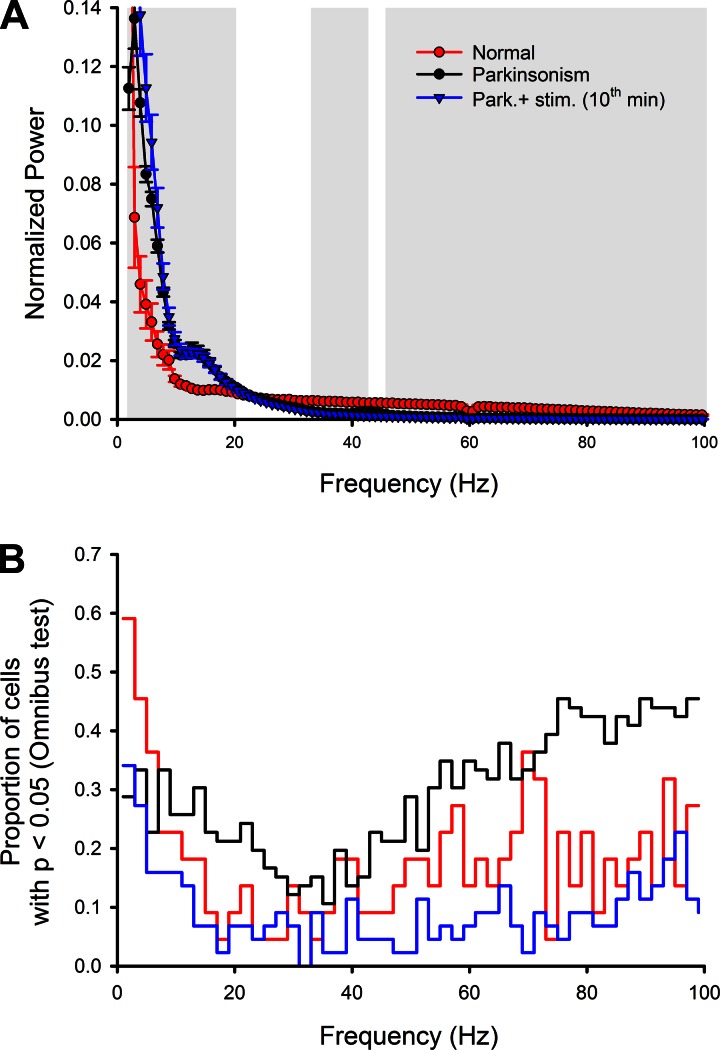
Figure 6A shows a comparison of LFP records from the normal state, from the MPTP-treated state, and from the MPTP-treated state during stimulation (10th min). MPTP treatment resulted in an increase of oscillatory LFP activity throughout the 2- to 20-Hz range, with a distinct peak in the 10- to 15-Hz region of the summary power spectrum. In the range from 30 to 100 Hz, MPTP treatment instead lowered the spectral LFP power. The GPi stimulation had no significant effect on the LFP power spectra. Note that the recordings from parkinsonian state without and with stimulation come from the same recording sessions, whereas those from the normal state are obviously from a different group of recordings.
Fig. 6.
Analysis of the relationship between local field potential (LFP) activity and spiking activity of thalamic neurons. A shows the average (normalized) power spectra of LFP signals in the normal (red) and parkinsonian (black/blue) states. The black and blue curves and symbols depict data from the same recording sites before (black) and during (blue) stimulation. Portions of the spectra that differed significantly between the normal and parkinsonian states (P < 0.05) are indicated by the gray background. The analysis in B shows the proportion of neurons in which a specific relationship between narrow-band filtered LFP activity (2-Hz bandwidth) and spiking was detected (Omnibus test, P < 0.05). Data from the parkinsonian state with ongoing GPi stimulation (10th min) are shown as blue lines.
We found that the spiking of few neurons in the normal state showed a phase relationship to simultaneously recorded LFPs (Fig. 6B). MPTP treatment strongly increased the proportion of neurons whose discharge appeared to be phase coupled to LFPs (Fig. 6B, P = 0.000, Wilcoxon test, pairing frequencies). This affected two frequency ranges in particular, i.e., the 10- to 30-Hz range and the range of frequencies over 40 Hz. Stimulation substantially lowered the proportion of neurons related to the simultaneously recorded LFPs (Fig. 6B, P = 0.000).
DISCUSSION
Our results show that MPTP treatment in monkeys results in significant changes in parameters describing spontaneous firing in the BGMT, and in the entrainment pattern between LFPs and the neuronal spiking of single cells in this area. Cells recorded after MPTP exposure fired at a lower nonmodulated rate and showed more intense rebound bursting. There were also changes in the oscillatory firing properties of BGMT neurons, with an increase in the 3- to 13-Hz range of oscillations and a reduction in the 30- to 100-Hz range. LFP signals, recorded at the same time as the spike discharges, showed an increase in low-frequency spectral power (with a peak in the 10- to 15-Hz range) and a reduction in the 30- to 100-Hz range. The coupling between LFP signals and neuronal spiking was strongly increased throughout most of the 1- to 100-Hz spectral range.
Stimulation at 120 Hz had antiparkinsonian effects along with variable changes in the firing rates of BGMT neurons (with increases or decreases in firing, as shown in Fig. 3). In the aggregate, these changes did not reach significance. We also saw a reduction of low-frequency neuronal oscillatory activity. The coupling between neuronal spikes and LFP activity was substantially reduced by the stimulation. In distinction from studies in the basal ganglia, we found little direct entrainment between the stimulation and thalamic spike patterns.
MPTP-Induced Changes in Firing
The literature on MPTP-induced changes in the firing of neurons in the basal-ganglia-receiving portion of the thalamus differs considerably, ranging from reports of reduced spontaneous firing (Ni et al. 2000; Schneider and Rothblat 1996; Vitek et al. 1994) to no change (Pessiglione et al. 2005) or even increased firing (Bosch-Bouju et al. 2014). Our results indicate that MPTP lowered the firing rates of thalamic neurons in these animals, consistent with the hypothesis that inhibitory basal ganglia output to the thalamus is generally increased in the parkinsonian state. The differences between this and some of the other studies may have been caused by the use of different animal species or may have occurred because of differences in the state of arousal of the animals, which is well known to strongly influence thalamic firing, specifically the generation of burst firing (see review by Crunelli et al. 2015). We paid careful attention to keep the animals awake during the recording sessions, using video and EEG observations to monitor the animal's state of wakefulness, and examined whether the remaining presence of bursts or decelerations in firing strongly altered firing rates in our sample, by removing th ose ISIs from the analysis that were engaged in bursts or decelerations. In our animals, firing rates were lower in these nonmodulated data streams in the parkinsonian state.
Burst discharges in the thalamus were more common in general, particularly with regard to rebound bursts (defined as bursts that were preceded by pauses in firing). In absolute terms, the average intraburst frequency, or the length of bursts, did not change, but the bursts occurred on the background of a reduced average firing rate, so that the relative increase in firing rates in bursts was much higher in the parkinsonian than in the normal state.
The spectral distribution of spikes changed in the parkinsonian state, with an increase in the 3- to 8- and 8- to 13-Hz spectral ranges, and a reduction in the 30- to 100-Hz (γ-) range. These differences reflect those seen previously in basal ganglia recordings (Galvan et al. 2015), as well as in those from subgroups of cortical neurons (Pasquereau and Turner 2013, 2011). Unlike what has been described for neurons in the basal ganglia (Bar-Gad et al. 2004; Hashimoto et al. 2003; McCairn and Turner 2009), the spectral power was increased in broad ranges of frequencies rather than distinct spectral peaks.
LFPs are strongly influenced by the size and synchrony of synaptic potentials, triggered by afferent inputs (see, e.g., Buzsaki et al. 2012). As shown in Fig. 6A, the spectral distribution, normalized to the total power in the 2- to 100-Hz range, changed significantly, with increased power at low frequencies (below 20 Hz) and reduced spectral power in the γ-range. These findings are, of course, not surprising, since they likely reflect similar network changes in the basal ganglia and at the cortical level and correspond to frequency band modulation in human parkinsonism and DBS (Brittain et al. 2014; Swann et al. 2015).
Stimulation-Induced Changes in the Basal Ganglia-Receiving Territory
In the first study of the effects of electrical stimulation of the GPi on neuronal discharge in the thalamus, Anderson and colleagues (Anderson et al. 2003) reported that thalamic firing rates were in many cells reduced upon GPi stimulation, easily explained by the possibility that pallidal output to the thalamus was stimulated. By contrast, we observed that high-frequency stimulation did not consistently change thalamic firing rates, but induced effects that differed substantially from cell to cell. The reasons for the differences between our study and the previously published results remain unclear, but could relate to experimental differences, perhaps most importantly the fact that our studies were done in the parkinsonian state while those by Anderson et al. were done in normal monkeys. The greater pallidal output in parkinsonism may hyperpolarize thalamic neurons, so that further increase in pallidal output through electrical stimulation may be sufficient to lower the membrane potential of thalamic neurons enough to render them prone to rebound burst discharges.
While the effects on firing rates reversed some of the abnormalities that were seen in the parkinsonian state, it is clear that the stimulation did not reverse all of the “parkinsonian” abnormalities, concomitant with a relevant but incomplete reduction of parkinsonian clinical signs. This was particularly obvious for the oscillatory discharge patterns, where the stimulation resulted in a strong reduction of 1- to 3-Hz oscillation, but much smaller (or no) changes in the 13- to 30- (β) and 30- to 100- (γ) Hz ranges. β- And γ-range oscillations in the basal ganglia have been widely implicated in the pathophysiology of parkinsonism (Brown et al. 2001; Galvan et al. 2015; Hammond et al. 2007), and were previously shown to respond to electrical basal ganglia stimulation (Eusebio et al. 2011; Kuhn et al. 2008; Quinn et al. 2015; Rosa et al. 2011; Wingeier et al. 2006). The observed differences between the oscillatory activity in neuronal spiking activity in the basal ganglia and in the thalamus suggest that oscillations at these frequencies are not simply “transmitted” from the basal ganglia to the thalamus, and from there on to the cerebral cortex.
Stimulation of the STN results in obvious entrainment of pallidal neurons in primates (Agnesi et al. 2015; Bar-Gad et al. 2004; Erez et al. 2009; Hashimoto et al. 2003; Johnson et al. 2009; Ma et al. 2007; McCairn and Turner 2009). It has therefore been widely expected that stimulation of the basal ganglia “motor” output nucleus GPi would similarly entrain thalamic neurons. However, while our study shows that some thalamic neurons are entrained to the GPi stimulation (see also Agnesi et al. 2015), we found that the level of entrainment is much smaller than that reported in the basal ganglia, adding further evidence to the notion that the effects of electrical basal ganglia stimulation are not a simple driving or frequency band propagation of thalamic cells (see, e.g., Neuenschwander et al. 2002). The difference between the entrainment in GPi after STN stimulation and the lack of entrainment of thalamic cells after GPi stimulation may be caused by the difference in the kinetics of synaptic interactions between the glutamatergic STN-GPi interaction and the GABAergic GPi-thalamic interaction (Alexander et al. 2006; Park et al. 2014). The electrical high-frequency stimulation of the pallidum may induce a tonic change of polarization of thalamic neurons, especially after episodes of extended stimulation.
We found that the spectral power of LFPs recorded in the BGMT in the α- and β-ranges was increased, with a peak of oscillatory power in the β-band range in the parkinsonian state, and that the spectral power in the γ-band was reduced. We also found the spiking activity of more neurons to be coupled to the phase of concomitantly recorded LFPs in the parkinsonian than in the normal state across the entire range of frequencies studied. Figure 6 demonstrates that the stimulation reduced the coupling between spikes and LFP signals while not significantly altering the power spectra of LFPs. This apparent stimulation-induced uncoupling of thalamic spiking and LFPs would support the notion that the stimulation disrupts the synchrony between neurons. On the basis of neural modeling and other evidence, it has been previously suggested that desynchronization may, in fact, be a fundamental effect of DBS (Wilson et al. 2011), and experimental studies in primates have shown such decoupling effects to occur in the basal ganglia (e.g., Moran et al. 2012). Our results demonstrate that similar uncoupling may also affect BGMT activity.
Limitations
The current study is limited by the number of cells recorded in the different behavioral states. The number of cells that could be analyzed for this report was limited because we chose cells with very good to excellent signal-to-noise ratio that were clearly within the basal ganglia-related territory of the thalamus. It would also have been preferable to have more animals available for this analysis.
We acutely inserted high-impedance electrodes to record both single neuron potentials as well as LFPs. While this optimized the conditions for single-neuron recording, this approach limits LFP recording fidelity due to inherent hardware filter characteristics of the electrodes (Nelson et al. 2008). A specific potential problem is that the insertion and recording of potentials at different locations in the thalamus do not allow us to adequately compare absolute LFP amplitudes before and after the induction of parkinsonism.
Stimulation occurred for several minutes instead of the time scale one would use in human PD patients that measures in months and years. While this may account for differences between the MPTP stimulation model and PD patients, it should be noted that the antiparkinsonian effects of DBS become observable within seconds of activation in human patients with PD (Hristova et al. 2000), although gradual changes with much slower time constants also occur (Angeli et al. 2015). Most LFP recordings in human studies are based on perioperative recordings with similar short time scales as chosen for the present study. Therefore, the 10-min stimulation cycles used here may be considered sufficient indicators of thalamic activity during DBS on a short (ms to s)- and intermediate (min)-term scale.
This study was performed in the animals' resting state with explorative eye movements, orofacial movements, and occasional arm movements. While the corticothalamic information flow probably does not completely cease under such “resting state” conditions, it likely differs from that activated when the animal is purposefully moving. For instance, functional imaging studies in humans have discovered a cortical “default mode” network that is active during physical and mental inactivity in awake attentive individuals who are not engaged in a challenging task (Greicius and Menon 2004). This network differs from that seen during performance of challenging tasks (van den Heuvel and Hulshoff Pol 2010). Parkinsonian signs such as bradykinesia are mostly (or only) apparent during active movement. It is therefore possible that more and different abnormalities in thalamic processing in the parkinsonian state would have been apparent in more activated states. Likewise, stimulation effects on thalamic processing in the active state may differ from their effects in the resting state that was studied here. Specific questions about the relevance of disturbances of thalamic information transmission for active behavior in the parkinsonian state, or about the specific contribution of thalamic transmission to parkinsonian bradykinesia or tremor, remain to be examined in future studies involving recordings during active movements.
Finally, related to the previous point, it is worth mentioning that the effects of stimulation with a stimulation electrode in the GPi are not specific for any one basal ganglia output pathway. Stimulation with long trains of stimuli, as used here (and in DBS therapy), is likely to engage pathways other than the direct pallidothalamic connection. For instance, the stimulation may also have engaged the GPi projection to the caudal intralaminar nuclei of the thalamus [the centromedian and parafascicular nucleus (Smith et al. 2014; Smith et al. 2009; Smith et al. 2004)], affecting the animal's state of arousal, which, as mentioned before, may then have altered thalamic firing. While we limited our recordings to epochs of wakefulness, it is possible that small shifts in arousal contributed to the results shown here. Effects on other networks, for instance, involving cortico-cortical connections or connections between GPi and the pedunculopontine nucleus of the brain stem, can also not be excluded.
Most of the mentioned limitations are not specific to this study but apply to any study of electrophysiological effects of DBS-like stimulation.
Conclusions
In conclusion, antiparkinsonian high-frequency stimulation of the GPi ameliorated some of the thalamic firing abnormalities that were associated with parkinsonism, and resulted in an uncoupling of spiking activity from LFP activity in the thalamus, which may reflect a desynchronizing property of GPi stimulation. Future studies need to examine whether similar changes also apply during episodes of thalamic activation during learned motor tasks, and whether neighboring cells in the thalamus truly change their synchronization state.
GRANTS
This project was supported through National Institutes of Health Grants P50-NS-071669 (Udall Center grant) and P51-OD-11132 (Yerkes Center infrastructure grant). S. Kammermeier was partially funded through the Young Researcher Fellowship program of the Deutsche Gesellschaft für Klinische Neurophysiologie (German Clinical Neurophysiology Society).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.K., I.H., and T.W. conception and design of research; S.K., D.P., and I.H. performed experiments; S.K., I.H., and T.W. analyzed data; S.K., I.H., and T.W. interpreted results of experiments; S.K., D.P., I.H., and T.W. edited and revised manuscript; S.K., D.P., I.H., and T.W. approved final version of manuscript; I.H. and T.W. prepared figures; T.W. drafted manuscript.
ACKNOWLEDGMENTS
We acknowledge the help of Susan Jenkins with the histology and many helpful discussions with Drs. Adriana Galvan and Annaelle Devergnas.
Current address for S. Kammermeier: Dept. of Neurology, Ludwig-Maximilian-University of Munich, Klinikum Grosshadern, 81377 München, Germany.
REFERENCES
- Agnesi F, Muralidharan A, Baker KB, Vitek JL, Johnson MD. Fidelity of frequency and phase entrainment of circuit-level spike activity during DBS. J Neurophysiol 114: 825–834, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JW, Gilman S. The temporal structure of spike trains in the primate basal ganglia: afferent regulation of bursting demonstrated with precentral cerebral cortical ablation. Brain Res 543: 123–138, 1991. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Fisher TL, Godwin DW. Differential response dynamics of corticothalamic glutamatergic synapses in the lateral geniculate nucleus and thalamic reticular nucleus. Neuroscience 137: 367–372, 2006. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Postupna N, Ruffo M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophys 89: 1150–1160, 2003. [DOI] [PubMed] [Google Scholar]
- Angeli A, Akram H, Zacharia A, Limousin P, Hariz M, Zrinzo L, Foltynie T. Varying time-course of effects of high frequency stimulation of sub-regions of the globus pallidus in patients with parkinson's disease. Parkinsonism Relat Disord 21: 597–602, 2015. [DOI] [PubMed] [Google Scholar]
- Bar-Gad I, Elias S, Vaadia E, Bergman H. Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation. J Neurosci 24: 7410–7419, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch-Bouju C, Smither RA, Hyland BI, Parr-Brownlie LC. Reduced reach-related modulation of motor thalamus neural activity in a rat model of Parkinson's disease. J Neurosci 34: 15836–15850, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain JS, Sharott A, Brown P. The highs and lows of beta activity in cortico-basal ganglia loops. Eur J Neurosci 39: 1951–1959, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci 21: 1033–1038, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents: EEG, ECoG, LFP and spikes. Nat Rev Neurosci 13: 407–420, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhuang P, Miao SH, Yuan G, Zhang YQ, Li JY, Li YJ. Neuronal firing in the ventrolateral thalamus of patients with Parkinson's disease differs from that with essential tremor. Chin Med J (Engl) 123: 695–701, 2010. [PubMed] [Google Scholar]
- Chiken S, Nambu A. High-frequency pallidal stimulation disrupts information flow through the pallidum by GABAergic inhibition. J Neurosci 33: 2268–2280, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, David F, Lorincz ML, Hughes SW. The thalamocortical network as a single slow wave-generating unit. Curr Opin Neurobiol 31: 72–80, 2015. [DOI] [PubMed] [Google Scholar]
- Dejean C, Hyland B, Arbuthnott G. Cortical effects of subthalamic stimulation correlate with behavioral recovery from dopamine antagonist induced akinesia. Cereb Cortex 19: 1055–1063, 2009. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol 34: 414–427, 1971. [DOI] [PubMed] [Google Scholar]
- Devergnas A, Wichmann T. Cortical potentials evoked by deep brain stimulation in the subthalamic area. Front Syst Neurosci 5: 30, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias S, Joshua M, Goldberg JA, Heimer G, Arkadir D, Morris G, Bergman H. Statistical properties of pauses of the high-frequency discharge neurons in the external segment of the globus pallidus. J Neurosci 27: 2525–2538, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez Y, Czitron H, McCairn K, Belelovsky K, Bar-Gad I. Short-term depression of synaptic transmission during stimulation in the globus pallidus of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates. J Neurosci 29: 7797–7802, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebio A, Thevathasan W, Doyle Gaynor L, Pogosyan A, Bye E, Foltynie T, Zrinzo L, Ashkan K, Aziz T, Brown P. Deep brain stimulation can suppress pathological synchronisation in parkinsonian patients. J Neurol Neurosurg Psychiatry 82: 569–573, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Devergnas A, Wichmann T. Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Front Neuroanat 9: 5, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber JC, Barbee RW, Bielitzki JT, Clayton LA, Donovan JC, Hendriksen CFM, Kohn DF, Lipman NS, Locke PA, Melcher J, Quimby FW, Turner PV, Wood GA, Würbel H. Guide for the Care and Use of Laboratory Animals (8th ed). Washington, DC: Nat Acad Press, 2010. [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science 324: 354–359, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci 16: 1484–1492, 2004. [DOI] [PubMed] [Google Scholar]
- Guehl D, Pessiglione M, Francois C, Yelnik J, Hirsch EC, Feger J, Tremblay L. Tremor-related activity of neurons in the “motor” thalamus: changes in firing rate and pattern in the MPTP vervet model of parkinsonism. Eur J Neurosci 17: 2388–2400, 2003. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data–Theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64: 237–278, 1995. [DOI] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci 30: 357–364, 2007. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci 23: 1916–1923, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristova A, Lyons K, Troster AI, Pahwa R, Wilkinson SB, Koller WC. Effect and time course of deep brain stimulation of the globus pallidus and subthalamus on motor features of Parkinson's disease. Clin Neuropharmacol 23: 208–211, 2000. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Miocinovic S, McIntyre CC, Vitek JL. Mechanisms and targets of deep brain stimulation in movement disorders. Neurotherapeutics 5: 294–308, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Vitek JL, McIntyre CC. Pallidal stimulation that improves parkinsonian motor symptoms also modulates neuronal firing patterns in primary motor cortex in the MPTP-treated monkey. Exp Neurol 219: 359–362, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ. Translational principles of deep brain stimulation. Nat Rev Neurosci 8: 623–635, 2007. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Kempf F, Brucke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider GH, Hariz MI, Vandenberghe W, Nuttin B, Brown P. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson's disease in parallel with improvement in motor performance. J Neurosci 28: 6165–6173, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Vazquez A. The basal ganglia and thalamus of the long-tailed macaque in stereotaxic coordinates. A template atlas based on coronal, sagittal and horizontal brain sections. Brain Struct Funct 217: 613–666, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophys 53: 926–939, 1985. [DOI] [PubMed] [Google Scholar]
- Lempka SF, McIntyre CC. Theoretical analysis of the local field potential in deep brain stimulation applications. PLoS One 8: e59839, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ke Y, Chan DC, Qian ZM, Yung KK, Ko H, Arbuthnott GW, Yung WH. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron 76: 1030–1041, 2012. [DOI] [PubMed] [Google Scholar]
- Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophys 98: 3525–3537, 2007. [DOI] [PubMed] [Google Scholar]
- Lobb CJ, Jaeger D. Bursting activity of substantia nigra pars reticulata neurons in mouse parkinsonism in awake and anesthetized states. Neurobiol Dis 75: 177–185, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb CJ, Zaheer AK, Smith Y, Jaeger D. In vivo electrophysiology of nigral and thalamic neurons in alpha-synuclein-overexpressing mice highlights differences from toxin-based models of parkinsonism. J Neurophysiol 110: 2792–2805, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Bevan M, Wichmann T. Effects of subthalamic nucleus stimulation on neuronal activity in the internal pallidal segment in monkeys. Soc Neurosci Abstracts 693.16: 2007. [Google Scholar]
- Magnin M, Morel A, Jeanmonod D. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience 96: 549–564, 2000. [DOI] [PubMed] [Google Scholar]
- McCairn KW, Turner RS. Deep brain stimulation of the globus pallidus internus in the parkinsonian primate: local entrainment and suppression of low-frequency oscillations. J Neurophys 101: 1941–1960, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairn KW, Turner RS. Pallidal stimulation suppresses pathological dysrhythmia in the parkinsonian motor cortex. J Neurophysiol 113: 2537–2548, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar GF, Pilliar A, Lozano AM, Dostrovsky JO. Differences in neuronal firing rates in pallidal and cerebellar receiving areas of thalamus in patients with Parkinson's disease, essential tremor, and pain. J Neurophys 93: 3094–3101, 2005. [DOI] [PubMed] [Google Scholar]
- Montgomery EB., Jr Effects of GPi stimulation on human thalamic neuronal activity. Clin Neurophysiol 117: 2691–2702, 2006. [DOI] [PubMed] [Google Scholar]
- Montgomery EB Jr, Gale JT. Mechanisms of action of deep brain stimulation(DBS). Neurosci Biobehav Rev 32: 388–407, 2008. [DOI] [PubMed] [Google Scholar]
- Moran A, Stein E, Tischler H, Bar-Gad I. Decoupling neuronal oscillations during subthalamic nucleus stimulation in the parkinsonian primate. Neurobiol Dis 45: 583–590, 2012. [DOI] [PubMed] [Google Scholar]
- Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S, Volkmann J, Kulisevsky J, Obeso JA, Albanese A, Hariz MI, Quinn NP, Speelman JD, Benabid AL, Fraix V, Mendes A, Welter ML, Houeto JL, Cornu P, Dormont D, Tornqvist AL, Ekberg R, Schnitzler A, Timmermann L, Wojtecki L, Gironell A, Rodriguez-Oroz MC, Guridi J, Bentivoglio AR, Contarino MF, Romito L, Scerrati M, Janssens M, Lang AE. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson's disease. Mov Disord 25: 578–586, 2010. [DOI] [PubMed] [Google Scholar]
- Nelson MJ, Pouget P, Nilsen EA, Patten CD, Schall JD. Review of signal distortion through metal microelectrode recording circuits and filters. J Neurosci Methods 169: 141–157, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenschwander S, Castelo-Branco M, Baron J, Singer W. Feed-forward synchronization: propagation of temporal patterns along the retinothalamocortical pathway. Philos Trans R Soc Lond B Biol Sci 357: 1869–1876, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni ZG, Gao DM, Benabid AL, Benazzouz A. Unilateral lesion of the nigrostriatal pathway induces a transient decrease of firing rate with no change in the firing pattern of neurons of the parafascicular nucleus in the rat. Neuroscience 101: 993–999, 2000. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Conway BA, Halliday DM, Perreault MC, Hultborn H. Organization of common synaptic drive to motoneurones during fictive locomotion in the spinal cat. J Physiol 569: 291–304, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A, Hoffman K, Keller A. Roles of GABAA and GABAB receptors in regulating thalamic activity by the zona incerta: a computational study. J Neurophysiol 112: 2580–2596, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquereau B, Turner RS. Primary motor cortex of the parkinsonian monkey: altered neuronal responses to muscle stretch. Front Syst Neurosci 7: 98, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquereau B, Turner RS. Primary motor cortex of the parkinsonian monkey: differential effects on the spontaneous activity of pyramidal tract-type neurons. Cereb Cortex 21: 1362–1378, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 2000. [Google Scholar]
- Pessiglione M, Guehl D, Rolland AS, Francois C, Hirsch EC, Feger J, Tremblay L. Thalamic neuronal activity in dopamine-depleted primates: evidence for a loss of functional segregation within basal ganglia circuits. J Neurosci 25: 1523–1531, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn EJ, Blumenfeld Z, Velisar A, Koop MM, Shreve LA, Trager MH, Hill BC, Kilbane C, Henderson JM, Bronte-Stewart H. Beta oscillations in freely moving Parkinson's subjects are attenuated during deep brain stimulation. Mov Disord 30: 1750–1758, 2015. [DOI] [PubMed] [Google Scholar]
- Rodrigues JP, Walters SE, Watson P, Stell R, Mastaglia FL. Globus pallidus stimulation in advanced Parkinson's disease. J Clin Neurosci 14: 208–215, 2007. [DOI] [PubMed] [Google Scholar]
- Rosa M, Giannicola G, Servello D, Marceglia S, Pacchetti C, Porta M, Sassi M, Scelzo E, Barbieri S, Priori A. Subthalamic local field beta oscillations during ongoing deep brain stimulation in Parkinson's disease in hyperacute and chronic phases. Neuro-Signals 19: 151–162, 2011. [DOI] [PubMed] [Google Scholar]
- Rubin JE, McIntyre CC, Turner RS, Wichmann T. Basal ganglia activity patterns in parkinsonism and computational modeling of their downstream effects. Eur J Neurosci 36: 2213–2228, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnthein J, Jeanmonod D. High thalamocortical theta coherence in patients with Parkinson's disease. J Neurosci 27: 124–131, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Rothblat DS. Alterations in intralaminar and motor thalamic physiology following nigrostriatal dopamine depletion. Brain Res 742: 25–33, 1996. [DOI] [PubMed] [Google Scholar]
- Smith Y, Galvan A, Ellender TJ, Doig N, Villalba RM, Huerta-Ocampo I, Wichmann T, Bolam JP. The thalamostriatal system in normal and diseased states. Front Syst Neurosci 8: 5, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Raju D, Nanda B, Pare JF, Galvan A, Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull 78: 60–68, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci 27: 520–527, 2004. [DOI] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate parkinsonism: comparison of the effects of MPTP-induced parkinsonism and lesions of the external pallidal segment. J Neurosci 24: 6417–6426, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, de Hemptinne C, Aron AR, Ostrem JL, Knight RT, Starr PA. Elevated synchrony in Parkinson disease detected with electroencephalography. Ann Neurol 78: 742–750, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 20: 519–534, 2010. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Ashe J, Kaneoke Y. Spontaneous neuronal activity in the motor thalamus: alteration in pattern and rate in parkinsonism. Soc Neurosci Abstr 20: 561, 1994. [Google Scholar]
- Weaver F, Follett K, Hur K, Ippolito D, Stern M. Deep brain stimulation in Parkinson disease: a metaanalysis of patient outcomes. J Neurosurg 103: 956–967, 2005. [DOI] [PubMed] [Google Scholar]
- Wichmann T. A digital averaging method for removal of stimulus artifacts in neurophysiologic experiments. J Neurosci Methods 98: 57–62, 2000. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophys 95: 2120–2133, 2006. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Beverlin B, Netoff T 2nd. Chaotic desynchronization as the therapeutic mechanism of deep brain stimulation. Front Syst Neurosci 5: 50, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingeier B, Tcheng T, Koop MM, Hill BC, Heit G, Bronte-Stewart HM. Intra-operative STN DBS attenuates the prominent beta rhythm in the STN in Parkinson's disease. Exp Neurol 197: 244–251, 2006. [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Okun MS, Foote KD, Fernandez HH, Rodriguez RL, Wu SS, Kirsch-Darrow L, Jacobson CE t Rosado C, Bowers D. Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidus as compared to the subthalamic nucleus. J Neurol 256: 1321–1329, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirh TA, Lenz FA, Reich SG, Dougherty PM. Patterns of bursting occurring in thalamic cells during parkinsonian tremor. Neurosci 83: 107–121, 1998. [DOI] [PubMed] [Google Scholar]