Abstract
The multicopper oxidase CueO had previously been demonstrated to exhibit phenoloxidase activity and was implicated in intrinsic copper resistance in Escherichia coli. Catecholates can potentially reduce Cu(II) to the prooxidant Cu(I). In this report we provide evidence that CueO protects E. coli cells by oxidizing enterobactin, the catechol iron siderophore of E. coli, in the presence of copper. In vitro, a mixture of enterobactin and copper was toxic for E. coli cells, but the addition of purified CueO led to their survival. Deletion of fur resulted in copper hypersensitivity that was alleviated by additional deletion of entC, preventing synthesis of enterobactin. In addition, copper added together with 2,3-dihydroxybenzoic acid or enterobactin was able to induce a Φ(cueO-lacZ) operon fusion more efficiently than copper alone. The reaction product of the 2,3-dihydroxybenzoic acid oxidation by CueO that can complex Cu(II) ions was determined by gas chromatography-mass spectroscopy and identified as 2-carboxymuconate.
Recently, two copper efflux exporters were described in Escherichia coli, CopA, a copper/silver P-type ATPase (27), and CusCFBA, a protein complex encompassing both the cytoplasmic and outer membranes (10, 21). The multicopper oxidase CueO was identified as an additional component involved in copper homeostasis in E. coli (12, 13, 22), and the three-dimensional structure of this protein was solved at a resolution of 1.4 Å (29). It was shown that CueO is a laccase-like enzyme (EC 1.10.3.2), as proposed by Alexandre and Zhulin (2). CueO is a periplasmic protein and translocated into the periplasm via the TAT pathway (7). Four copper ions are bound in the CueO monomer, forming an active center typical of multicopper oxidases such as ascorbate oxidase, fungal laccases, Saccharomyces cerevisiae Fet3, and human ceruloplasmin (29). The enzyme is activated by binding of a labile Cu(II) at a novel copper-binding site (30).
The mechanism of protection from copper-mediated toxicity by CueO was postulated to be the oxidation of cuprous copper (12, 23). This hypothesis was recently strengthened by the demonstration that the related multicopper oxidases Fet3 and human ceruloplasmin are able to oxidize Cu(I) to Cu(II), maintaining the cuprous-cupric redox balance in aerobic organisms and preventing copper-mediated toxicity (33, 34).
CueO was responsible for the oxidation of cuprous copper and the potential Cu(II) reductant enterobactin. Enterobactin is the indigenous catecholate siderophore of E. coli secreted to sequester iron from the environment. Recently it was demonstrated that enterobactin is a substrate of CueO in vitro (16). Copper in combination with the catecholate siderophore is much more toxic than copper alone because enterobactin and other catecholates can act as a Cu(II) reductants (15, 18). We showed that CueO oxidized the siderophore enterobactin and its precursor 2,3-dihydroxybenzoic acid and thus protected E. coli cells against copper-induced killing. The product of 2,3-dihydroxybenzoic acid oxidation was able to bind copper. In addition, the global iron-dependent repressor Fur was identified as an important determinant of copper tolerance, since a fur mutant overproduced enterobactin. Reduction of Cu(II) by enterobactin facilitated Cu(I) uptake into cells. These results give insight into the complex mode of CueO-mediated protection from copper toxicity.
MATERIALS AND METHODS
Bacterial strains and growth media.
The strains used in this work are listed in Table 1. E. coli was grown in Luria-Bertani (LB) medium or Tris-buffered mineral salts medium (19) containing 2 ml of glycerol and 1 g of yeast extract or 3 g of Casamino Acids per liter. Antibiotics [chloramphenicol (15 to 20 μg/ml) pr kanamycin (25 μg/ml)] and CuCl2 were added where appropriate.
TABLE 1.
E. coli strains
Gene disruptions, deletions, and operon fusions.
Genes were disrupted by the insertion of a chloramphenicol resistance cassettes with a protocol developed in the laboratory of B. Wanner which is based on the λ Red recombinase system as described previously (6).
CAS liquid assay and plates.
E. coli strains GR1 (ΔcueO- cat) and W3110 were grown overnight in Luria-Bertani medium with shaking at 37°C, diluted 1:500 into Tris-buffered minimal medium (19) supplemented with 0.2% glycerol and 0.3% Casamino Acids. Cultures were grown overnight, diluted 1:500 into fresh minimal medium without iron but with 0.3% deferrated Casamino Acids (25) and different concentrations of CuCl2 and grown for 16 h to an optical density at 600 nm of 1.0. The cell density was adjusted, spent medium was centrifuged at 12,000 rpm for 2 min, and cleared spent medium was stored at 4°C.
The chrome azurol S (CAS) assay solution was prepared as described by Payne (25). In short, 0.0219 g of hexadecyltrimethylammonium bromide was dissolved in 50 ml of water; 1.5 ml of 1 mM FeCl3 · 6H2O in 10 mM HCl was mixed with 7.5 ml of 2 mM CAS solution. This Fe-CAS solution and also piperazine buffer (4.307 g of piperazine dissolved in 30 ml of water with 6.75 ml of concentrated HCl to bring the pH to 6.5) was added to the hexadecyltrimethylammonium bromide solution, and the volume was brought up to 100 ml. CAS agar plates were prepared as described by Schwyn and Neilands (33); 0.5 ml of cleared spent culture medium was added to 0.5 ml of CAS assay solution and mixed, and 10 μl of shuttle solution (0.2 M 5-sulfosalicylic acid, stored in the dark) was added to facilitate transfer of iron from the CAS complex to enterobactin, and the sample was mixed. After 5 min, the absorbance at 655 nm was measured.
Enterobactin concentration determination.
The reaction between the CAS solution, Fe(III), and enterobactin proceeds as Fe(III)-CAS + enterobactin → Fe(III)-enterobactin + CAS. The decrease in absorbance at 655 nm can be used to calculate the concentration of enterobactin-bound iron with Fe(III)-CAS having a molar extinction coefficient of 105,000 M−1 cm−1. It is assumed that the Fe(III) and enterobactin form 1:1 complexes (14). Enterobactin concentrations were calculated from desferrioxamine units via a calibration curve.
Ferric enterobactin oxidation by CueO.
Purified CueO was suspended at 1 μg/ml in 0.05 M morpholinepropanesulfonic acid (MOPS) buffer, pH 6.5, containing 0.5 mM CuCl2. Enterobactin was purified from culture supernatants of E. coli K-12 (17), complexed with iron, and chromatographically purified (33). Various concentrations of ferric enterobactin were added to reaction mixtures at 25°C, and the oxidation of ferric enterobactin was monitored by the change in absorption at 393 nm. The data were plotted and analyzed to determine Km with the enzyme kinetics algorithm of Grafit (version 4.013; Erithacus Ltd.). Visible spectra of the product were collected on a Beckman DU7 spectrophotometer.
Copper-enterobactin toxicity measurement.
E. coli strains were grown overnight in LB medium with shaking at 37°C, diluted 1:400 into Tris-buffered minimal medium (19) with 0.2% glycerol and 0.3% Casamino Acids, grown overnight, and diluted 1:400 into fresh minimal medium without iron. Cultures were incubated for 16 h and centrifuged, and the spent medium was filter sterilized (20-μm pore size; Nalgene). Cells to be challenged were also subsequently grown in LB medium and regular minimal medium. Copper challenge was carried out as follows: 5 μl of stationary-phase culture was added to 1 ml of sterile spent medium containing 5 mM CuCl2, mixed, and incubated at room temperature for 15 min. The mixture was diluted 1:200 into LB medium, and 25 μl was plated onto LB agar plates.
Precipitate preparation, UV-visible spectrum, and EDX analysis.
Purified CueO protein (5 μg) was added to a solution of 100 mM sodium acetate (pH 6.5), 500 μM CuCl2 and 2 mM 2,3-dihydroxybenzoic acid. The reaction was incubated at 50°C for 2 h. For UV-visible analysis, the reaction product was scanned on a Uvikon 922 (Kontron Instruments). For energy-dispersive X-ray (EDX) analysis, the colored oxidation product was harvested by centrifugation, and the resulting pellet was redissolved in water and recentrifuged to remove excess copper. The washed precipitate was subjected to analysis with an EM 912 OMEGA electron transmission microscope (Leo, Oberkochen, Germany) equipped with an EDX system (energy-dispersible X-ray analysis, LINK eXIII; Oxford Instruments, High Wycombe, United Kingdom) in the spot mode (100-nm spot size at 80 keV and 20 μA emission current). For analysis, a computer program considering the net counts and excitation probability, determined with the in-column filter, was used.
2,3-Dihydroxybenzoic acid oxidation by CueO.
Purified CueO was incubated with 1 mM 2,3-dihydroxybenzoic acid in the presence of 0.5 mM CuCl2 and 100 mM Tris-HCl buffer, pH 8.0. The reaction was stopped after 1 min by rapid chilling and acidification with HCl to pH 2, and the reaction mixture was extracted twice with ethyl acetate. The combined organic phase was dried with sodium sulfate and filtered, and the solvent was removed by evaporation in a SpeedVac concentrator (Eppendorf). The sample was redissolved in 20 μl of methanol treated with 100 μl of ethereal diazomethane which was prepared from N-nitrosomethyl urea and transferred to an autosampler vial (Chromacol 05-CTV[A]116; Fisher Scientific, Schwerte, Germany). Excessive diazomethane and solvent were removed in a gentle stream of nitrogen, and the methylated compounds were taken up in 20 μl of chloroform.
An aliquot of 1 μl was applied to the gas chromatography-mass spectroscopy (GC-MS) system for mass fragment analysis of main signals. All spectra were recorded with a Finnigan Magnum ion-trap mass spectrometer connected to a Varian GC 3400 gas chromatograph (Varian, Walnut Creek, Calif.). Injections were made with a CTC A200S autoinjector. The following conditions were chosen for GC: splitless injector temperature 260°C, transfer line temperature 260°C, capillary column Zebron ZB-5 (Phenomenex, Aschaffenburg, Germany), film thickness 30 m by 0.25 mm by 0.25 μm, and helium as the carrier gas. The temperature program was constant for 1 min at 60°C and a linear increase was chosen (30°C per min) to 280°C. The mass spectrometer was operated in full scan mode (m/z 50 to 400) with chemical ionization and methanol as the reactant gas.
Miscellaneous.
Standard molecular genetic techniques were used (31). PCR was performed in the presence of Pwo or Taq DNA polymerase (Roche, Fermentas). CueO was purified as described previously (13). CueO was concentrated where applicable with Midi centrifuge filters (Nalgene). The protein concentration of purified CueO was determined at 280 nm (ɛCueO = 63063 M−1 cm−1). The β-galactosidase activity in permeabilized cells was determined as published previously (11, 20).
RESULTS
Oxidation of catecholate siderophores by CueO and copper.
When E. coli strain W3110 was grown in mineral salt medium under iron-restricted conditions and in the presence of elevated copper concentrations, spent medium and cells turned brownish gray (data not shown). Neither E. coli strain GR1 (ΔcueO::cat) nor GR417 (ΔentC::cat) (defective in enterobactin synthesis) was able to produce this colored compound (data not shown). The necessity for the presence of both CueO and EntC clearly indicated that colored-compound formation is strictly dependent on both the oxidase activity of CueO and the presence of enterobactin.
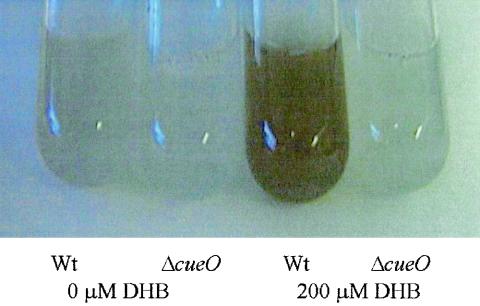
A similar finding was obtained with the enterobactin precursor 2,3-dihydroxybenzoic acid. 2,3-Dihydroxybenzoic acid and copper added to wild-type cultures resulted in enhanced production of a colored precipitate (Fig. 1). No colored precipitate was formed when 2,3-dihydroxybenzoic acid was added to E. coli strain GR1 (ΔcueO::cat) (Fig. 1).
FIG. 1.
Effect of 2,3-dihydroxybenzoic acid (DHB) on different E. coli strains. Colored precipitate formation in E. coli by CueO, CuCl2 and 2,3-dihydroxybenzoic acid in strains GR1 (ΔcueO::cat) and wild-type (Wt) W3110. Overnight cultures grown in Luria-Bertani medium were diluted 1:500 into minimal medium, and grown overnight, diluted 1:500 into fresh minimal medium with 500 μM CuCl2 added to all cultures, 2,3-dihydroxybenzoic acid was added where indicated, and growth at 37°C was continued for 16 h.
Deletion of cueO, encoding the multicopper oxidase of E. coli, leads to elevated biosynthesis of enterobactin under conditions of iron scarcity when copper is present.
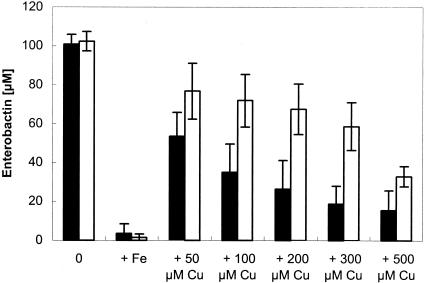
Determination of the enterobactin concentrations of spent medium from strain GR1 (ΔcueO::cat) and wild-type W3110 was performed in the presence of different concentrations of CuCl2 under iron deprivation (Fig. 2). We clearly demonstrated that in the absence of copper and thus no expression of cueO, both wild-type strain W3110 and GR1 (ΔcueO::cat) produced enterobactin at very similar concentrations. In contrast, under copper stress, i.e., when cueO is expressed in the wild-type strain, strain GR1 (ΔcueO::cat) exhibited a significant increase in enterobactin compared to the wild-type W3110 (Fig. 2). This difference is probably due to the oxidation of enterobactin by CueO (16) in the presence of copper rather than decreased enterobactin biosynthesis in the cueO deletion strain GR1 (ΔcueO::cat), since enterobactin levels were very much the same in the wild-type strain and the cueO mutant when copper was not present.
FIG. 2.
Enterobactin production in E. coli strains in the presence of copper. Overnight cultures grown in Luria-Bertani medium were diluted 1:500 into minimal medium, grown overnight, and diluted 1:500 into fresh minimal medium, and after 2 h of growth at 37°C the cells were diluted 1:500 into fresh minimal medium with the indicated concentrations of CuCl2 and 50 μM FeCl3. The strains were W3110 (solid bars) and GR1 (ΔcueO::cat) (open bars). Cell growth was continued for 16 h at 37°C with shaking before the enterobactin content was determined as described in Materials and Methods.
Enterobactin oxidation prevents copper-induced killing of E. coli.
We started out with the hypothesis that enterobactin can act as a Cu(II) reductant, shifting the balance from Cu(II) to Cu(I), increasing both uptake of Cu(I) and generation of reactive oxygen species by the prooxidant Cu(I). CueO could prevent this in two ways, oxidation of Cu(I) and oxidation of enterobactin.
In order to validate this hypothesis, we investigated whether enterobactin oxidation by CueO reduces the toxicity of the interaction of enterobactin with copper. Therefore, E. coli strain W3110 was challenged with spent medium supplemented with copper from either a cueO deletion strain, which is rich in enterobactin, or from strain GR417 (ΔentC::cat), which is unable to produce enterobactin (Table 2). No cells survived with spent medium of strain GR1 (ΔcueO::cat) (Table 2). Conversely, spent medium of strain GR417 (ΔentC::cat), whichcontained no enterobactin at all, was not toxic for E. coli cells in the presence of copper. This indicated that copper alone was lethal only in combination with enterobactin under the conditions used. Moreover, addition of purified CueO protein to the survival assay with spent medium rich in enterobactin resulted in survival of challenged cells (Table 2). This again demonstrated that oxidation of enterobactin by CueO rescued E. coli cells from copper- and enterobactin-induced killing. Conversely, when CueO was heat inactivated (10 min of boiling) prior to copper challenge, E. coli cells did not survive (data not shown). Thus, protection against copper toxicity could only be accomplished when CueO oxidized enterobactin.
TABLE 2.
Enterobactin toxicity to E. coli in the presence of Cu and the protective activity of CueO
| Source of spent mediuma | CFUb | Enterobactinc (μM) |
|---|---|---|
| ΔentC strain | >103 | 0 |
| ΔcueO strain | 0 | 57.2 |
| ΔcueO strain + CueOd | >103 | 57.2 |
Strains ΔentC, ΔcueO, and W3110 (wild type) were grown to stationary phase in iron-depleted minimal medium with subtoxic concentrations of CuCl2, and the supernatant filter was sterilized.
Stationary-phase cultures of E. coli strain W3110 were diluted into fresh minimal medium and, after growth for 2 h, diluted into the respective spent medium, challenged with 5 mM CuCl2 for 15 min, diluted into LB medium, and plated on LB plates.
Enterobactin content was determined from the spent supernatant as described in Materials and Methods.
Before addition of cells, CueO was added (2 μM final concentration) for 10 min at 37°C.
Oxidation of ferric enterobactin.
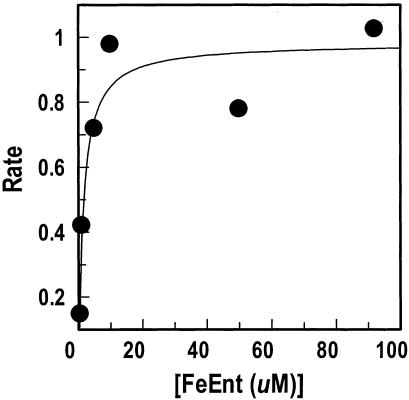
The ability of CueO to act on catecholate compounds, especially its oxidation of the native E. coli siderophore enterobactin, led us to test its recognition and oxidation of the iron complex ferric enterobactin. When suspended with ferric enterobactin at slightly acidic pH in the presence of copper ions, CueO rapidly oxidized the ferric siderophore (16). We reevaluated these experiments with slightly different conditions that better mimicked the physiological, periplasmic environment of CueO. Under these conditions, we determined a Km of 1.5 μM for ferric enterobactin oxidation by CueO (Fig. 3) compared to the 40 μM described by Kim et al. (16). This high affinity of ferric enterobactin indicates that it is a natural substrate of CueO in vivo.
FIG. 3.
Oxidation of ferric enterobactin by purified CueO. The initial rates of ferric enterobactin (FeEnt) oxidation by CueO (1 μg/ml) were spectrophotometrically monitored at 393 nm; the data were analyzed and plotted with the enzyme kinetics algorithm of Grafit 4.013 (Erithacus Ltd., Middlesex, United Kingdom). The apparent Km of the plotted data for the enzymatic reaction was 1.5 μM.
Oxidation-product of 2,3-dihydroxybenzoic acid and CueO sequesters copper.
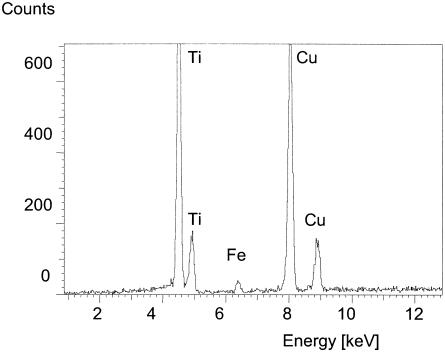
Incubation of 2,3-dihydroxybenzoic acid with CueO in the presence of copper resulted in the formation of an insoluble colored precipitate. The precipitate was found predominantly in the growth medium, indicating that, after oxidation, 2,3-dihydroxybenzoic acid is translocated to the outside of the cell. This oxidation product might act as a metal-chelating compound, and therefore the precipitate was examined by EDX spectral analysis. It was demonstrated that copper was bound to this polymeric 2,3-dihydroxybenzoic acid oxidation product (Fig. 4). Since 2,3-dihydroxybenzoic acid is also an excellent iron chelator, iron was also detected in the analysis. This suggests that oxidized 2,3-dihydroxybenzoic acid did not lose its ability to bind iron but gained the ability to complex copper. This could create a sink for copper outside the cell, thereby decreasing the overall solubilized copper concentration.
FIG. 4.
Spectrum of CueO oxidation product. 2,3-Dihydroxybenzoic acid was oxidized by CueO, and the oxidation product was analyzed. For EDX analysis, the colored oxidation product was harvested by centrifugation, and the resulting pellet was redissolved in water and recentrifuged to remove excess copper. The washed precipitate was subjected to EDX analysis with an EM 912 Omega electron transmission microscope (Leo, Oberkochen, Germany) equipped with an EDX system (energy-dispersible X-ray analysis Link eXIII; Oxford Instruments, High Wycombe, United Kingdom) in the spot mode (100-nm spot size at 80 keV and 20 μA emission current).
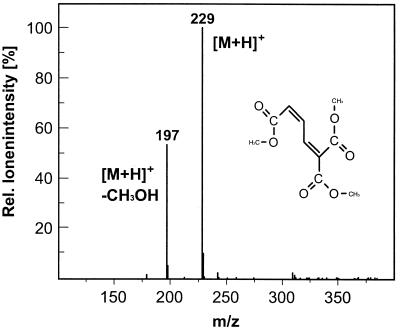
Short-term incubation of CueO with 2,3-dihydroxybenzoic acid resulted in an oxidation product in which the aromatic ring was opened by oxidation and oxygen was added in a stoichiometry of 1 mol of O2 per mol of 2,3-dihydroxybenzoic acid, as determined by oxygraph analysis (30). The reaction product was identified as 2-carboxymuconate by GC-MS and was able to complex Cu(II) ions. The product has a molecular weight of 229, indicating that all three carboxylic groups are methylated. The molecular weight of 197 corresponds to a fragment of the reaction product which is expected when one molecule of methanol is lost, as shown in Fig. 5.
FIG. 5.
Chemical ionization-MS spectrum of the 2,3-dihydroxybenzoate oxidation product catalyzed by CueO in the presence of 0.5 mM CuCl2. The m/z of 229 represents the protonized trimethylated reaction product 2-carboxymuconate, and the m/z of 197 represents a fragment after the loss of one molecule of methanol.
Enterobactin may facilitate copper uptake.
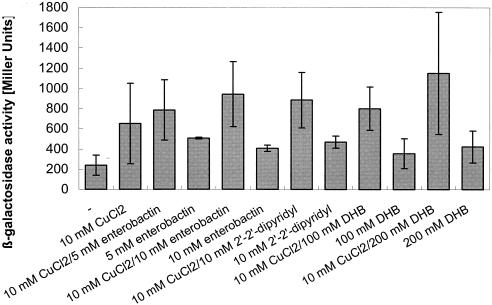
Previously, Outten et al. (23) had shown that cueO expression is induced by the cytoplasmic regulator of transcription CueR in a copper-dependent fashion. Lysogen E. coli strain WOII260B (22) expressing a Φ(cueO-lacZ) operon fusion was also used to elucidate induction of cueO in this study. Compared to copper alone, elevated lacZ expression in E. coli Φ(cueO-lacZ) was observed when 2,3-dihydroxybenzoic acid or enterobactin in combination with copper was added (Fig. 6). This may be due to increased copper uptake as a result of Cu(II) reduction by enterobactin or 2,3-dihydroxybenzoic acid.
FIG. 6.
Induction of cueO in E. coli strain WOII260B Φ(cueO-lacZ) under copper stress. Cells of E. coli strain WOII260B Φ(cueO-lacZ) containing a cueO-lacZ operon fusion on the bacterial chromosome grown in LB medium were diluted 15-fold into fresh minimal medium and induced after 2 h of growth. Incubation was continued with shaking at 37°C for 2 h, and the β-galactosidase activity was determined. Each experiment was performed in triplicate, and the average and standard deviation were calculated.
It is thought that copper imposes oxidative stress by catalyzing Fenton-like reactions (26). This raised the question of whether expression of cueO was also induced by oxidative stress independent of copper. However, compounds generating oxidative stress, such as hydrogen peroxide, t-butyl peroxide, and paraquat, did not induce the reporter, and neither did a radical-generating system comprising hematin and peroxide (data not shown). Menadione induced cueO only in the presence of copper (data not shown). However, Φ(cueO-lacZ) was induced in the presence of low added copper concentrations (10 μM) by 2′,2′-dipyridyl, a strong chelator of iron and copper [Martell's critical stability constants log K = 8.5 for Cu(II), log K = 16.3 for Fe(III)] (Fig. 6). There was also a slight increase in Φ(cueO-lacZ) induction in medium without added copper, possibly due to residual copper in the medium. This could indicate that copper bound to 2′,2′-dipyridyl is able to enter the cell and might increase the cytoplasmic copper concentration. This all suggested that expression of cueO is directly dependent on copper and not indirectly on copper-generated oxidative stress.
Deletion of fur leads to copper hypersensitivity.
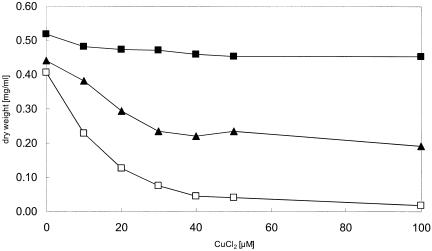
The Fur protein of E. coli is the global regulator for iron uptake systems, defense against oxidative stress, and iron storage (3). Thus, a strain deleted of fur is also derepressed for enterobactin biosynthesis. We demonstrated that deletion of the fur gene rendered E. coli hypersensitive to copper (Fig. 7). The presence of ascorbate at the initial setup of the experiment was essential for the copper-sensitive phenotype of the fur mutants, indicating that the production of the prooxidant Cu(I) is the first step in creating toxic oxygen intermediates.
FIG. 7.
Copper resistance of different E. coli strains. Growth in the presence of different CuCl2 concentrations is shown. Overnight cultures were diluted 1:400 into fresh LB broth and, after 2 h of growth, diluted into fresh LB broth with the indicated concentrations of CuCl2 and 1 mM ascorbate. Cell growth after 6 h of incubation at 37°C with shaking was monitored as the optical density at 600 nm and converted to dry weight. The E. coli strains used were W3110 (▪), GG199 (Δfur::cat) (□), and GG213 (Δfur::cat ΔentC) (▴). Experiments were performed at least in triplicate, and the average is shown.
Because the combination of enterobactin and copper was shown to be detrimental for E. coli, the entC gene was deleted in addition to fur, preventing biosynthesis of enterobactin. The resulting strain tolerated copper much better than the single fur mutant (Fig. 7). An entC single deletion mutant exhibited copper tolerance comparable to that of the wild type at 100 μM (data not shown). These results support our hypothesis on the interaction of CueO with enterobactin that oxidation of enterobactin in the presence of copper is advantageous for E. coli. Moreover, this clearly indicated that the deregulation of iron uptake might lead to enhanced copper uptake through transporters such as FeoB, MntH, and ZupT, but additional deletion of enterobactin production rendered E. coli more copper tolerant than a single fur deletion, making enterobactin the major contributor to cytotoxicity under copper stress. These results are consistent with the data obtained for enterobactin- and copper-dependent killing of E. coli. Previously, no involvement of the global iron repressor Fur in copper tolerance had been observed. This emphasizes the interrelationship of copper and iron homeostasis in E. coli. The point of contact is probably enterobactin and CueO.
DISCUSSION
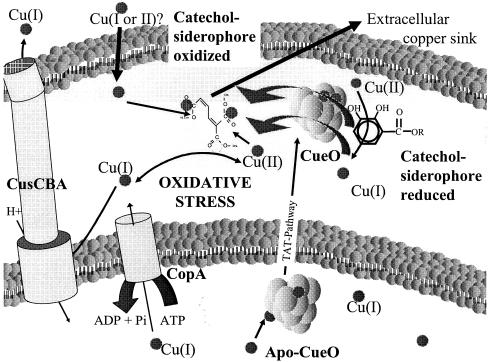
In our initial hypothesis (12), we and others proposed that CueO converts periplasmic Cu(I) to the less toxic Cu(II) (8, 13, 23, 28). This mode of copper protection was recently confirmed for the related multicopper oxidase Fet3 in S. cerevisiae. Fet3 was shown to possess cuprous oxidase activity (35). This activity was only necessary for protection from copper toxicity if a copper reductase was also present (34). We also believe that CueO-dependent cuprous oxidase activity may be important in conferring copper resistance in E. coli and recently demonstrated CueO-dependent Cu(I) oxidase activity (unpublished data). In addition to this activity, CueO appears to protect cells from the interaction of enterobactin with copper by oxidation of enterobactin. The current study suggests that CueO performs a catalytic reaction, oxidizing the iron chelator enterobactin under copper stress in E. coli. Figure 8 depicts known factors important for periplasmic copper homeostasis in E. coli and presents the most recent model of the central role of CueO as an interface between copper detoxification and iron homeostasis.
FIG. 8.
Model of copper homeostasis in E. coli and the central role of CueO. Copper enters the bacterial cell by an unknown mechanism and exerts its toxicity by generating reactive oxygen species via copper-mediated redox cycling. Cytoplasmic detoxification of copper is accomplished by the P-type ATPase CopA, while periplasmic copper is effluxed by the CusCBA complex. The multicopper oxidase CueO probably converts Cu(I) to the less toxic Cu(II). Additionally, when copper is present, CueO oxidizes the catechol siderophore enterobactin, avoiding enterobactin-mediated reduction of Cu(II) to Cu(I). The resulting oxidation product of enterobactin, 2-carboxymuconate, sequesters copper and might constitute a copper sink after export to the outside.
CueO is only active aerobically because multicopper oxidases use oxygen as a terminal electron acceptor. Furthermore, copper slowly oxidizes catechols in the presence and/or absence of multicopper oxidases. One electron is transferred to molecular oxygen in copper-mediated oxidation of catechols, forming superoxide, which is subsequently reduced to hydrogen peroxide and hydroxyl radicals. In other words, enterobactin and other catechols reduce Cu(II) to Cu(I) and thereby change the steady-state level of Cu(I) (15, 18, 32). Increased redox cycling of copper could lead to both increased production of reactive oxygen species and increased Cu(I) uptake. CueO can therefore perform several beneficial reactions. CueO can reduce the amount of Cu(I), reduce the presence of unoxidized enterobactin, and prevent the accumulation of free intermediate reactive oxygen species by coupling the oxidation of substrates with the complete reduction of oxygen to water.
Given the relatively small increase in cueO expression in the presence of copper plus siderophore compared to that in the presence of copper alone, the effect of siderophore-mediated reduction and subsequent uptake of copper into the cytoplasm and, thus, increased copper concentration cannot be considered a major stress factor within the cytoplasm. Probably, the critical compartment for copper-siderophore toxicity is the periplasmic space, where CueO exerts its protective oxidase activity.
This situation is reminiscent of mammalian systems, in which ceruloplasmin, in addition to its ferrooxidase activity, is thought to oxidize catecholamines such as 6-hydroxydopamine and thereby prevent the formation of reactive oxygen species (9). For example, copper accelerated the autooxidation of 6-hydroxydopamine 61-fold (5). Copper neurotoxicity was also found to be dependent on dopamine-dependent copper uptake and may contribute to the death of dopaminergic neurons in Parkinson's disease. Interestingly, copper was implicated in the increased incidence of parkinsonism in subjects exposed to copper in mining operations in Chile (24). Since ceruloplasmin was also shown to have Cu(I) oxidase activity, it might have a bigger role in protection from neurodegenerative diseases than previously anticipated.
CueO might modify enterobactin so that it accumulates with sequestered copper in the periplasm and the extracellular medium. EDX analysis clearly demonstrated that the water-insoluble 2,3-dihydroxybenzoic acid oxidation product was able to bind copper. It was pointed out before that catechol-metal complexes are highly stable and that compounds containing catecholic nuclei can sequester metals from other complexes. This would also result in the prevention of redox cycling of those metals (4, 32).
In order to prevent an unfavorable constitutive oxidation of enterobactin by CueO, expression of cueO occurs only in the presence of copper. This control mechanism would ensure that the deleterious interaction between copper and enterobactin is avoided. CueO is regulated at the genetic and enzymatic levels by copper. CueO possesses a methionine-rich region that is involved in copper-dependent enzyme regulation. Recently, we showed that this region is essential for CueO-mediated siderophore oxidation (30).
E. coli strains deleted of fur were described as having a higher free iron concentration (3, 36). However, it was determined that such bacteria contain considerably less total iron, presumably by downregulating iron storage (1). Our results demonstrated that deletion of fur resulted in a severe copper-sensitive phenotype. Growth of a fur-deleted strain was almost completely inhibited at a CuCl2 concentration as low as 100 μM in the presence of ascorbate. The parental wild-type strain E. coli W3110 under the same growth conditions is only inhibited at much higher concentrations (12). About 5,000 copies of the Fur protein are usually present in an E. coli cell, but under redox stress this level is increased twofold (1). A fur mutant also produces an increased amount of enterobactin. That enterobactin with copper is toxic to cells is again supported by the ability of the Δfur ΔentC double deletion strain, which is unable to synthesize enterobactin, to tolerate higher copper concentrations than the Δfur single-deletion strain.
The fact that the interaction of copper and the natural iron chelator enterobactin is toxic indicates that CueO and enterobactin constitute a connecting link between copper and iron homeostasis in E. coli. These links between iron and copper homeostasis in E. coli provide a launching pad from which to tackle the physiological mechanisms of trace metal homeostasis in prokaryotes. It also might initiate studies of CueO-mediated protection from catecholate siderophore-enhanced copper toxicity as a model for copper damage to dopaminergic neurons.
Acknowledgments
This work was supported by Hatch Project 136713 and NIEHS grant ESO4940 with funds from the EPA to C.R.
We thank Jennifer Crispin and Raina Maier for desferric enterobactin and Thomas V. O'Halloran for strain WOII260B. Thanks are due Jim Imlay and Dietrich H. Nies for suggestions and Dieter Neumann (Leipniz-Institut für Pflanzenbiochmie, Halle, Germany) for EDX analysis. We also thank Barry Rosen and Nigel Brown for carefully reading the manuscript and for suggestions.
REFERENCES
- 1.Abdul-Tehrani, H., A. J. Hudson, Y. S. Chang, A. R. Timms, C. Hawkins, J. M. Williams, P. M. Harrison, J. R. Guest, and S. C. Andrews. 1999. Ferritin mutants of Escherichia coli are iron deficient and growth impaired, and fur mutants are iron deficient. J. Bacteriol. 181:1415-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandre, G., and I. B. Zhulin. 2000. Laccases are widespread in bacteria. Trends Biotechnol. 18:41-42. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. [DOI] [PubMed] [Google Scholar]
- 4.Avdeef, A., S. R. Sofen, T. L. Bregante, and K. N. Raymond. 1978. Coordination chemistry of microbial iron transport compounds. 9. Stability constants for catechol models of enterobactin. J. Am. Chem. Soc. 100:5362-5370. [Google Scholar]
- 5.Bandy, B., P. B. Walter, J. Moon, and A. J. Davison. 2001. Reaction of oxygen with 6-hydroxydopamine catalyzed by Cu, Fe, Mn and V complexes: identification of a thermodynamic window for effective metal catalysis. Arch. Biochem. Biophys. 389:22-30. [DOI] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLisa, M. P., P. Lee, T. Palmer, and G. Georgiou. 2004. Phage shock protein PspA of Escherichia coli relieves saturation of protein export via the Tat pathway. J. Bacteriol. 186:366-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finney, L. A., and T. V. O'Halloran. 2003. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300:931-936. [DOI] [PubMed] [Google Scholar]
- 9.Floris, G., R. Medda, A. Padiglia, and G. Musci. 2000. The physiopathological significance of ceruloplasmin. A possible therapeutic approach. Biochem. Pharmacol. 60:1735-1741. [DOI] [PubMed] [Google Scholar]
- 10.Franke, S., G. Grass, and D. H. Nies. 2001. The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiology 147:965-972. [DOI] [PubMed] [Google Scholar]
- 11.Grass, G., B. Fan, B. P. Rosen, K. Lemke, H. G. Schlegel, and C. Rensing. 2001. NreB from Achromobacter xylosoxidans 31A is a nickel-induced transporter conferring nickel resistance. J. Bacteriol. 183:2803-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grass, G., and C. Rensing. 2001. Genes involved in copper homeostasis in Escherichia coli. J. Bacteriol. 183:2145-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grass, G., and C. Rensing. 2001. CueO is a multicopper oxidase that confers copper tolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 286:902-908. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa, H., M. Matsui, M. Suzuki, K. Naito, K. Ueda, and Y. Sohrin. 2001. The possibility of regulating the species composition of marine phytoplankton using organically complexed iron. Anal. Sci. 17:209-211. [DOI] [PubMed] [Google Scholar]
- 15.Kamau, P., and R. B. Jordan. 2002. Kinetic study of the oxidation of catechol by aqueous copper(II). Inorg. Chem. 41:3076-3083. [DOI] [PubMed] [Google Scholar]
- 16.Kim, C., W. W. Lorenz, J. T. Hoopes, and J. F. Dean. 2001. Oxidation of phenolate siderophores by the multicopper oxidase encoded by the Escherichia coli yacK gene. J. Bacteriol. 183:4866-4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klebba, P. E., M. A. McIntosh, and J. B. Neilands. 1982. Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J. Bacteriol. 149:880-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, Y., M. A. Trush, and J. D. Yager. 1994. DNA damage caused by reactive oxygen species originating from a copper-dependent oxidation of the 2-hydroxy catechol of estradiol. Carcinogenesis 15:1421-1427. [DOI] [PubMed] [Google Scholar]
- 19.Mergeay, M., D. Nies, H. G. Schlegel, J. Gerits, P. Charles, and F. van Gijsegem. 1985. Alcaligenes eutrophus is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J. Bacteriol. 162:328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Munson, G. P., D. L. Lam, F. W. Outten, and T. V. O'Halloran. 2000. Identification of a copper-responsive two-component system on the chromosome of Escherichia coli K-12. J. Bacteriol. 182:5864-5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Outten, F. W., C. E. Outten, J. Hale, and T. V. O'Halloran. 2000. Transcriptional activation of an E. coli copper efflux regulon by the chromosomal MerR homologue, CueR. J. Biol. Chem. 275:31024-31029. [DOI] [PubMed] [Google Scholar]
- 23.Outten, F. W., D. L. Huffman, J. A. Hale, and T. V. O'Halloran. 2001. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J. Biol. Chem. 276:30670-30677. [DOI] [PubMed] [Google Scholar]
- 24.Paris, I., A. Dagnino-Subiabre, K. Marcelain, L. B. Bennett, P. Caviedes, R. Caviedes, C. O. Azar, and J. Segura-Aguilar. 2001. Copper neurotoxicity is dependent on dopamine-mediated copper uptake and one-electron reduction of aminochrome in a rat substantia nigra neuronal cell line. J. Neurochem. 77:519-529. [DOI] [PubMed] [Google Scholar]
- 25.Payne, S. M. 1994. Detection, isolation, and characterization of siderophores. Methods Enzymol. 235:329-344. [DOI] [PubMed] [Google Scholar]
- 26.Pena, M. M. O., J. Lee, and D. J. Thiele. 1999. A delicate balance: homeostatic control of copper uptake and distribution. J. Nutr. 129:1251-1260. [DOI] [PubMed] [Google Scholar]
- 27.Rensing, C., B. Fan, R. Sharma, B. Mitra, and B. P. Rosen. 2000. CopA: an Escherichia coli Cu(I)-translocating P-type ATPase. Proc. Natl. Acad. Sci. USA 97:652-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rensing, C., and G. Grass. 2003. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 27:197-213. [DOI] [PubMed] [Google Scholar]
- 29.Roberts, S. A., A. Weichsel, G. Grass, K. Thakali, J. T. Hazzard, G. Tollin, C. Rensing, and W. R. Montfort. 2002. Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli. Proc. Natl. Acad. Sci. USA 99:2766-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts, S. A., G. F. Wildner, G. Grass, A. Weichsel, A. Ambrus, C. Rensing, and W. R. Montfort. 2003. A labile regulatory copper ion lies near the T1 copper site in the multicopper oxidase CueO. J. Biol. Chem. 278:31958-31963. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Schweigert, N., A. J. B. Zehnder, and R. I. L. Eggen. 2001. Chemical properties of catechols and their molecular mode of toxic action in cells, from microorganisms to mammals. Environ. Microbiol. 3:81-91. [DOI] [PubMed] [Google Scholar]
- 33.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 34.Shi, X., C. Stoj, A. Romeo, D. J. Kosman, and Z. Zhu. 2003. Fre1p Cu2+ reduction and Fet3p Cu1+ oxidation modulate copper toxicity in Saccharomyces cerevisiae. J. Biol. Chem. 278:50309-50315. [DOI] [PubMed] [Google Scholar]
- 35.Stoj, C., and D. J. Kosman. 2003. Cuprous oxidase activity of yeast Fet3p and human ceruloplasmin: implication for function. FEBS Lett. 554:422-426. [DOI] [PubMed] [Google Scholar]
- 36.Touati, D., M. Jaques, B. Tardat, L. Bouchard, and S. Despied. 1995. Lethal oxidative damage and mutagenesis are generated by iron in Δfur mutants of Escherichia coli: protective role of superoxide dismutase. J. Bacteriol. 177:2305-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]