Abstract
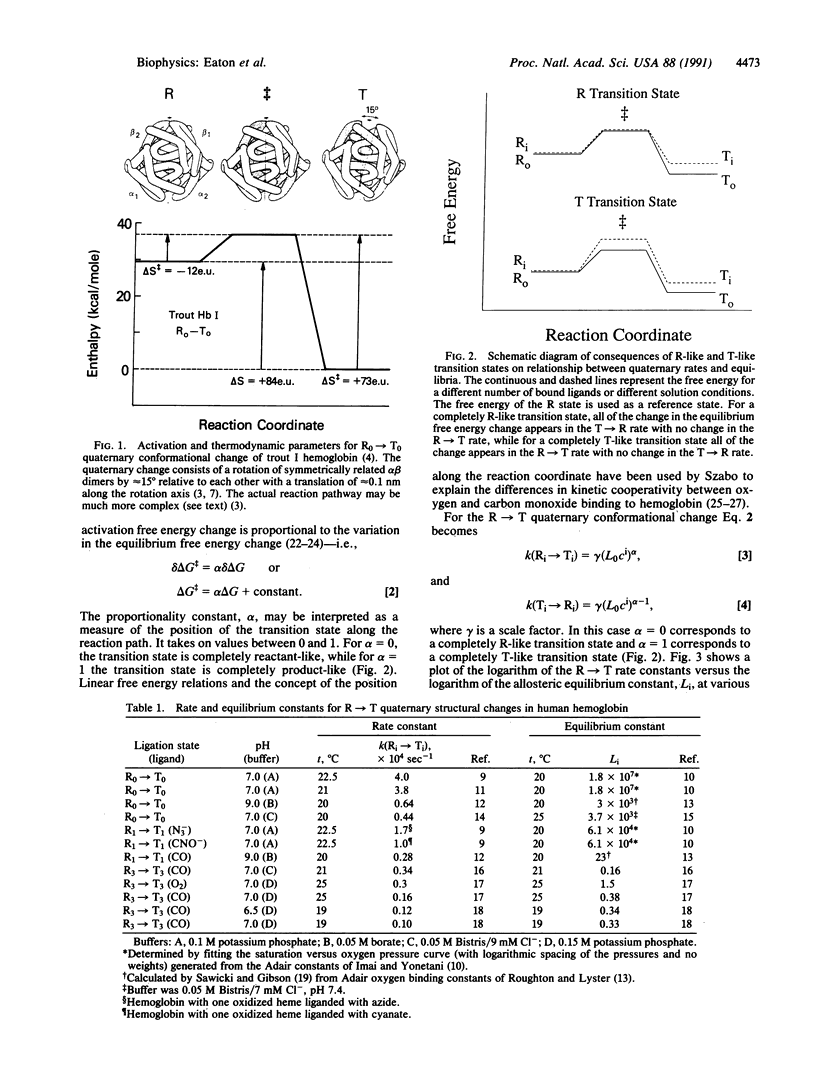
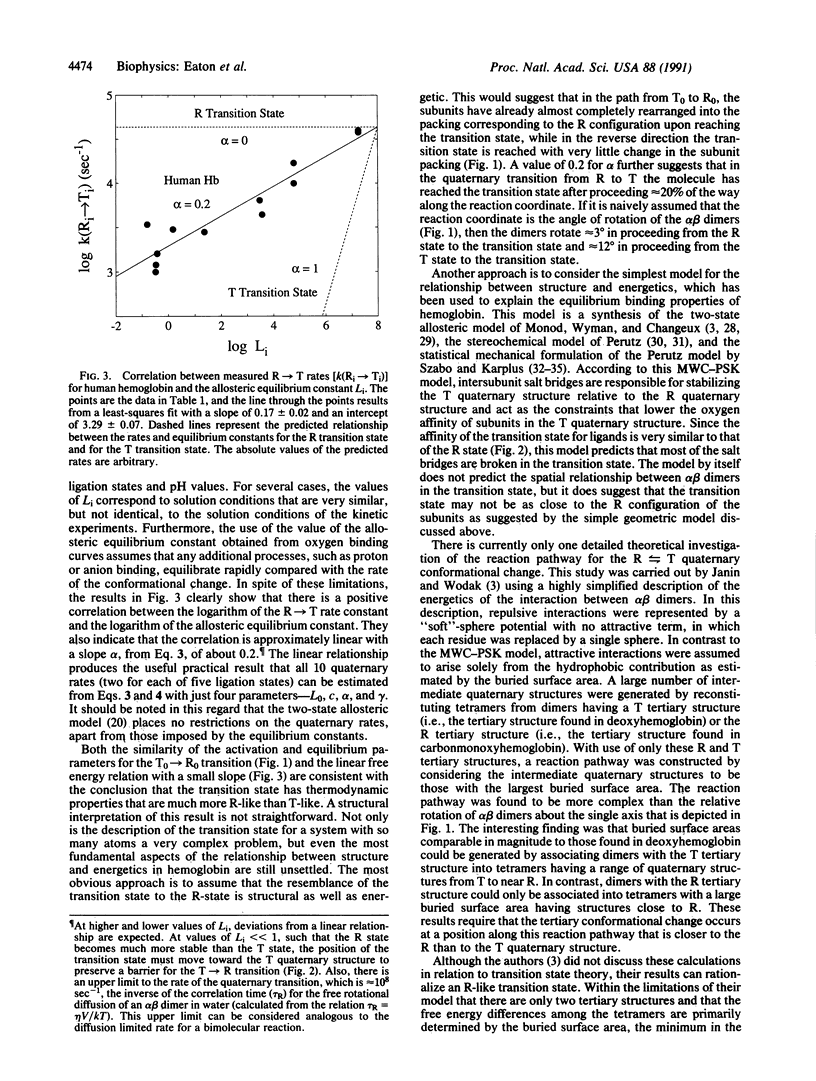
The transition state for the R in equilibrium with T quaternary conformational change of hemoglobin has thermodynamic properties much closer to those of the R conformation than to those of the T conformation. This finding is based on a comparison of activation and equilibrium enthalpy and entropy changes and on the observation of a linear free energy relationship between quaternary rate and equilibrium constants. A previous theoretical study [Janin, J. & Wodak, S. J. (1985) Biopolymers 24, 509-526], using a highly simplified energy function, suggests that the R-like transition state is the result of a reaction pathway with the maximum buried surface area between alpha beta dimers.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K. Energetics of subunit assembly and ligand binding in human hemoglobin. Biophys J. 1980 Oct;32(1):331–346. doi: 10.1016/S0006-3495(80)84960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin J., Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J Mol Biol. 1979 Apr 5;129(2):175–220. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- Barisas B. G., Gill S. J. Thermodynamic analysis of carbon monoxide binding by hemoglobin trout I. Biophys Chem. 1979 Mar;9(3):235–244. doi: 10.1016/0301-4622(79)85006-1. [DOI] [PubMed] [Google Scholar]
- Cho K. C., Hopfield J. J. Spin equilibrium and quaternary structure change in hemoglobin A. Experiments on a quantitative probe of the stereochemical mechanism of hemoglobin cooperativity. Biochemistry. 1979 Dec 25;18(26):5826–5833. doi: 10.1021/bi00593a012. [DOI] [PubMed] [Google Scholar]
- Chothia C. Structural invariants in protein folding. Nature. 1975 Mar 27;254(5498):304–308. doi: 10.1038/254304a0. [DOI] [PubMed] [Google Scholar]
- Edelstein S. J. Cooperative interactions of hemoglobin. Annu Rev Biochem. 1975;44:209–232. doi: 10.1146/annurev.bi.44.070175.001233. [DOI] [PubMed] [Google Scholar]
- Ferrone F. A., Martino A. J., Basak S. Conformational kinetics of triligated hemoglobin. Biophys J. 1985 Aug;48(2):269–282. doi: 10.1016/S0006-3495(85)83780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H. The photochemical formation of a quickly reacting form of haemoglobin. Biochem J. 1959 Feb;71(2):293–303. doi: 10.1042/bj0710293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Henry E. R., Sommer J. H., Deutsch R., Ikeda-Saito M., Yonetani T., Eaton W. A. Nanosecond optical spectra of iron-cobalt hybrid hemoglobins: geminate recombination, conformational changes, and intersubunit communication. Biochemistry. 1985 May 21;24(11):2667–2679. doi: 10.1021/bi00332a012. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Sommer J. H., Henry E. R., Eaton W. A. Nanosecond absorption spectroscopy of hemoglobin: elementary processes in kinetic cooperativity. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2235–2239. doi: 10.1073/pnas.80.8.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Yonetani T. PH dependence of the Adair constants of human hemoglobin. Nonuniform contribution of successive oxygen bindings to the alkaline Bohr effect. J Biol Chem. 1975 Mar 25;250(6):2227–2231. [PubMed] [Google Scholar]
- Janin J., Wodak S. J. Reaction pathway for the quaternary structure change in hemoglobin. Biopolymers. 1985 Mar;24(3):509–526. doi: 10.1002/bip.360240307. [DOI] [PubMed] [Google Scholar]
- Lee A. W., Karplus M., Poyart C., Bursaux E. Analysis of proton release in oxygen binding by hemoglobin: implications for the cooperative mechanism. Biochemistry. 1988 Feb 23;27(4):1285–1301. doi: 10.1021/bi00404a031. [DOI] [PubMed] [Google Scholar]
- Leffler J. E. Parameters for the Description of Transition States. Science. 1953 Mar 27;117(3039):340–341. doi: 10.1126/science.117.3039.340. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Eaton W. A. Optically detected conformational changes in haemoglobin single crystals. Nature. 1974 Jan 4;247(5435):62–64. doi: 10.1038/247062a0. [DOI] [PubMed] [Google Scholar]
- Marden M. C., Hazard E. S., Gibson Q. H. Testing the two-state model: anomalous effector binding to human hemoglobin. Biochemistry. 1986 Nov 18;25(23):7591–7596. doi: 10.1021/bi00371a049. [DOI] [PubMed] [Google Scholar]
- Martino A. J., Ferrone F. A. Rate of allosteric change in hemoglobin measured by modulated excitation using fluorescence detection. Biophys J. 1989 Oct;56(4):781–794. doi: 10.1016/S0006-3495(89)82725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L. P., Hofrichter J., Henry E. R., Ikeda-Saito M., Kitagishi K., Yonetani T., Eaton W. A. The effect of quaternary structure on the kinetics of conformational changes and nanosecond geminate rebinding of carbon monoxide to hemoglobin. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2151–2155. doi: 10.1073/pnas.85.7.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. Mechanisms of cooperativity and allosteric regulation in proteins. Q Rev Biophys. 1989 May;22(2):139–237. doi: 10.1017/s0033583500003826. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. Quaternary conformational changes in human hemoglobin studied by laser photolysis of carboxyhemoglobin. J Biol Chem. 1976 Mar 25;251(6):1533–1542. [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. Quaternary conformational changes in human oxyhemoglobin studied by laser photolysis. J Biol Chem. 1977 Aug 25;252(16):5783–5788. [PubMed] [Google Scholar]
- Shulman R. G., Hopfield J. J., Ogawa S. Allosteric interpretation of haemoglobin properties. Q Rev Biophys. 1975 Jul;8(3):325–420. doi: 10.1017/s0033583500001840. [DOI] [PubMed] [Google Scholar]
- Szabo A., Karplus M. A mathematical model for structure-function relations in hemoglobin. J Mol Biol. 1972 Dec 14;72(1):163–197. doi: 10.1016/0022-2836(72)90077-0. [DOI] [PubMed] [Google Scholar]
- Szabo A. Kinetics of hemoglobin and transition state theory. Proc Natl Acad Sci U S A. 1978 May;75(5):2108–2111. doi: 10.1073/pnas.75.5.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman J., Gill S. J., Noll L., Giardina B., Colosimo A., Brunori M. The balance sheet of a hemoglobin. Thermodynamics of CO binding by hemoglobin trout I. J Mol Biol. 1977 Jan 15;109(2):195–205. doi: 10.1016/s0022-2836(77)80029-6. [DOI] [PubMed] [Google Scholar]
- Zhang N. Q., Ferrone F. A., Martino A. J. Allosteric kinetics and equilibria differ for carbon monoxide and oxygen binding to hemoglobin. Biophys J. 1990 Aug;58(2):333–340. doi: 10.1016/S0006-3495(90)82380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]




