Abstract
Myxococcus xanthus uses extracellular signals during development to regulate gene expression. C-signaling regulates the expression of many genes induced after 6 h into development. FruA is a protein that is necessary for cells to respond to C-signaling, but expression of the fruA gene does not depend on C-signaling. Yet the fruA promoter region has a C box and a 5-bp element, similar to the promoter regions of several C-signal-dependent genes, where these sequences are crucial. Here, we show that the C box and 5-bp elements are important for expression of fruA, demonstrating for the first time that these sequences play a role in the expression of a gene that does not depend on C-signaling and is required for M. xanthus development.
Myxococcus xanthus is a gram-negative soil bacterium that provides a model system to study signaling between cells and resultant gene expression during a developmental process (9, 11, 29, 31). Upon starvation at a high cell density on a solid surface, rod-shaped cells move to aggregation centers, where they build mounds containing approximately 105 cells. Some cells within the mounds differentiate into dormant, spherical spores that are resistant to heat and desiccation. The spore-filled mounds are called fruiting bodies. Formation of fruiting bodies is controlled by a program of gene expression that is highly coordinated in response to cell-cell signaling (2, 6).
A-signaling involves the production of extracellular proteases, peptides, and amino acids, which are thought to provide a mechanism for cell density sensing that governs whether cells proceed further in development (12, 20, 21, 27). C-signaling involves the product of csgA, a 25-kDa protein that may have enzymatic activity and is believed to be cleaved to a 17-kDa form associated with the cell surface (16, 22, 25). C-signaling is essential for three events during M. xanthus development; a low level is sufficient for rippling (cells accumulate in parallel ridges that appear to travel as waves over time), a higher level is needed for aggregation into mounds, and an even higher level is necessary for sporulation within the fruiting body (15, 23). Transmission of the C-signal requires motility, presumably due to the need for cell-cell contact (13, 14, 17, 28). The response to C-signaling involves FruA, which is similar to the response regulator of two-component signal transduction systems that are common in bacteria (3, 26). FruA governs a branched pathway inside the recipient cell (32). One branch leads to rippling and aggregation through modification of the gliding movements of cells, and another branch includes expression of genes essential for sporulation, such as the dev operon and the locus identified by insertion Ω7536 (9, 31). Expression of other genes, which are not essential for development, also depends on C-signaling (18, 19, 24). These genes were originally identified as Tn5 lac insertions into the M. xanthus chromosome that fuse expression of the Escherichia coli lacZ reporter to developmentally regulated promoters. The Ω4403 (5), Ω4400 (1), and Ω4499 (4) promoter regions have been identified and important cis-acting DNA elements have been defined by mutational analyses (34-36). Both the Ω4403 and Ω4400 promoter regions have the sequence CATCCCT centered at −49 bp. Similar sequences are also found centered at −55 and −33 bp in the Ω4499 promoter region. These sequences, called C boxes, match the consensus CAYYCCY (Y represents C or T) and are important for promoter activity. Moreover, each of these C boxes is located 6 to 8 bp downstream of a 5-bp element with a consensus sequence of GAACA, which has also been shown to be important for promoter activity in each case. However, the C boxes and 5-bp elements in different promoter regions appear to function differently, because single-base-pair transversion mutations at the corresponding positions in these sequences had very different effects on promoter activity.
C boxes and 5-bp elements have been recognized in other M. xanthus developmental promoter regions (4, 34) but have not been subjected to detailed mutational analysis. Deletion of a C box in the fruA promoter region decreased promoter activity (8). This could mean that the C-box sequence is important for fruA promoter activity, but it could not be ruled out that C-box DNA is necessary for proper spacing between other critical cis-acting regulatory sequences. To distinguish between these possibilities and to identify important DNA sequence elements in the fruA promoter region, we performed a detailed mutational analysis.
Construction and testing of a fruA-lacZ fusion.
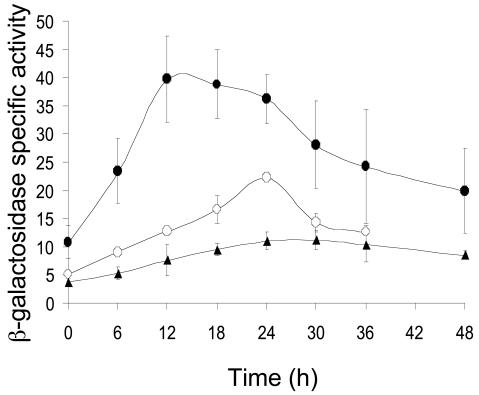
To analyze the fruA promoter region, we amplified a segment extending from −185 to +50 bp relative to the transcriptional start site, with wild-type M. xanthus DK1622 chromosomal DNA as a template for PCR. The primers for the PCR were designed to produce an XhoI restriction site at the upstream end and a BamHI restriction site at the downstream end of the product. The PCR product was cloned into pCR2.1-TOPO (Invitrogen) to form pDS12.T (plasmids and strains used in this study are listed in Table 1), which was recovered after electroporation into E. coli DH5α. Ampicillin-resistant transformants were selected and plasmid DNA was sequenced at the Michigan State University Genomics Technology Support Facility to confirm the sequence and end points of the M. xanthus DNA insert. The insert was gel purified from XhoI-BamHI-digested pDS12.T and subcloned into pREG1727 digested with the same enzymes to create pDS12.P, in which the fruA promoter region is fused to lacZ, as verified by PCR. After transformation into wild-type M. xanthus DK1622 and screening for developmental lacZ expression as described previously (34), three kanamycin-resistant transformants, expected to have pDS12.P integrated at the Mx8 phage attachment site (designated attB; Table 1), were designated MDS12. β-Galactosidase specific activity was measured during development of the MDS12 strains as described previously (19). Induction of β-galactosidase activity was observed by 6 h and rose to a maximum of about 40 U at 12 h (Fig. 1). The pattern of developmental lacZ expression was similar to that observed previously with translational (8, 26, 33) and transcriptional (3) fusions between fruA and lacZ, but the maximum activity of our transcriptional fusion was lower.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| E. coli strain | ||
| DH5α | φ80 lacZΔM15 ΔlacU169 recA1 endA1 hsdR17 supE44 thi-1 gyrA relA1 | 7 |
| M. xanthus strain | ||
| DK1622 | Wild type | 10 |
| DK5208 | csgA::Tn5-132 (Tcr) Ω205 | 30 |
| MDS1727 | attB::pREG1727 | This study |
| MDS5 | attB::pDS5.P (pREG1727 with 150-bp XhoI-BamHI fragment from pDS5.T)a | This study |
| MDS12 | attB::pDS12.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS12.T) | This study |
| MDS21 | attB::pDS21.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS21.T) | This study |
| MDS22 | attB::pDS22.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS22.T) | This study |
| MDS23 | attB::pDS23.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS23.T) | This study |
| MDS24 | attB::pDS24.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS24.T) | This study |
| MDS25 | attB::pDS25.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS25.T) | This study |
| MDS26 | attB::pDS26.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS26.T) | This study |
| MDS27 | attB::pDS27.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS27.T) | This study |
| MDS28 | attB::pDS28.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS28.T) | This study |
| MDS29 | attB::pDS29.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS29.T) | This study |
| MDS30 | attB::pDS30.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS30.T) | This study |
| MDS31 | attB::pDS31.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS31.T) | This study |
| MDS32 | attB::pDS32.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS32.T) | This study |
| MDS33 | attB::pDS33.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS33.T) | This study |
| MDS34 | attB::pDS34.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS34.T) | This study |
| MDS35 | attB::pDS35.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS35.T) | This study |
| MDS36 | attB::pDS36.P (pREG1727 with 235-bp XhoI-BamHI fragment from pDS36.T) | This study |
| Plasmid | ||
| pCR2.1-TOPO | Apr KmrlacZα | Invitrogen |
| pREG1727 | Apr Kmr P1-inc attP lacZ | 5 |
| pDS5.T | pCR2.1-TOPO with 150-bp fragment from −100 to +50 bp of fruA DNA generated by PCR using chromosomal DNA from M. xanthus DK1622 as template, inserted as a XhoI-BamHI fragment | This study |
| pDS12.T | pCR2.1-TOPO with 235-bp fragment from −185 to +50 bp of fruA DNA generated by PCR using chromosomal DNA from M. xanthus DK1622 as template, inserted as a XhoI-BamHI fragment | This study |
| pDS21.T | pDS12.T with CACTCCC to ACAGAAA mutation from −54 to −48 bp | This study |
| pDS22.T | pDS12.T with GCACA to TACAC mutation from −64 to −60 bp | This study |
| pDS23.T | pDS12.T with TTCGCG to GGATAT mutation from −36 to −31 bp | This study |
| pDS24.T | pDS12.T with TAGGGT to GCTTTG mutation from −13 to −8 bp | This study |
| pDS25.T | pDS12.T with GGGGCTG to TTTTAGT mutation from −44 to −38 bp | This study |
| pDS26.T | pDS12.T with TGATTCAT to GTCGGACG mutation from −72 to −65 bp | This study |
| pDS27.T | pDS12.T with CGGGCTC to ATTTAGA mutation from −79 to −73 bp | This study |
| pDS28.T | pDS12.T with C to A mutation at −61 bp | This study |
| pDS29.T | pDS12.T with C to A mutation at −54 bp | This study |
| pDS30.T | pDS12.T with A to C mutation at −53 bp | This study |
| pDS31.T | pDS12.T with C to A mutation at −52 bp | This study |
| pDS32.T | pDS12.T with T to G mutation at −51 bp | This study |
| pDS33.T | pDS12.T with C to A mutation at −50 bp | This study |
| pDS34.T | pDS12.T with C to A mutation at −49 bp | This study |
| pDS35.T | pDS12.T with C to A mutation at −48 bp | This study |
Where possible, the plasmid description is given in parentheses after the strain description.
FIG. 1.
Deletion analysis of the fruA promoter region. Developmental lacZ expression was determined for M. xanthus DK1622 bearing integrated plasmids with fruA DNA from −185 to +50 bp (•) or −100 to +50 bp (○) fused to lacZ. Also shown is the vector, no-insert negative control (▴). The average β-galactosidase specific activity of at least three independent isolates is expressed as nanomoles of o-nitrophenyl phosphate per minute per milligram of protein. Error bars show 1 standard deviation of the data.
Since MrpC2 has been shown to bind to two sites located between −152 and −113 bp in the fruA promoter region and very likely activates transcription (33), we tested the effect of deleting these sites. By using the methods described above, we constructed MDS5 strains with the fruA promoter region from −100 to +50 bp fused to lacZ. These strains showed a different pattern of developmental lacZ expression than the MDS12 strains (Fig. l). The increase in β-galactosidase activity was much more gradual, rising slowly to a maximum of about 22 U at 24 h, which was about 10 U above the maximum observed for the integrated plasmid with no promoter DNA inserted (Fig. 1). This result confirms the importance of DNA between −185 and −100 bp for fruA promoter activity (33). Therefore, we performed our mutational analysis in the context of fruA DNA from −185 to +50 bp. The result also suggests that the fruA promoter exhibits a low level of developmental activity in the absence of the upstream MrpC2 binding sites.
Expression of a fruA-lacZ fusion was initially reported to depend absolutely on csgA (26); however, expression of a different fruA-lacZ fusion was subsequently reported to be csgA independent (3). To investigate the csgA dependence of our fusion, we transformed pDS12.P, which contains fruA DNA from −185 to +50 bp fused to lacZ, into csgA mutant DK5208 cells (Table 1) and measured developmental lacZ expression. The activity of the fruA promoter in the csgA mutant background (data not shown) was not significantly different than in the wild-type background (Fig. 1). We conclude that expression of our fruA-lacZ fusion is csgA independent, in agreement with the finding of Ellehauge et al. (3).
Effects of mutations in a C box centered at −51 bp.
It was noted previously that the fruA promoter region has the sequence CACTCCC centered at −51 bp, which matches the C-box consensus sequence (4). To test whether this sequence is important for fruA promoter activity, it was changed to ACAGAAA in the context of DNA from −185 to +50 bp. A Quikchange site-directed mutagenesis kit (Stratagene) was used to create the mutation using pDS12.T as the template for PCR with mutagenic primers. The M. xanthus DNA insert was sequenced to ensure that only the proper mutation had been created. The insert was subcloned into pREG1727 in order to fuse expression of the mutant promoter region to lacZ, and this plasmid (pDS21.P) was introduced into wild-type M. xanthus DK1622 as described above. Three independent transformants were induced to develop, and β-galactosidase specific activity was measured. In parallel, developmental lacZ expression was measured for the wild-type promoter region and for the integrated plasmid (pREG1727) with no promoter fused to lacZ. Table 2 lists the average maximum β-galactosidase specific activity during a 48-h developmental time course for all determinations made for each construct. It also shows how that value compared to the maximum β-galactosidase specific activity observed for the wild-type promoter in the same experiment, after subtracting from both values the background activity observed for the plasmid with no promoter fused to lacZ. The multiple-base-pair mutation in the C box decreased fruA promoter activity to nearly the background level (Table 2).
TABLE 2.
Summary of activities of mutant fruA promoters
| Promoter assayed | Avg maximum β-galactosidase sp act during developmenta | % Wild-type activity measured in the same exptb |
|---|---|---|
| Vector (no insert) | 11 ± 1 | |
| Wild-type fruA promoter | 40 ± 8 | |
| Deletion: (−100 to +50 bp) | 22 ± 2 | 28 ± 4 |
| Mutationc | ||
| TAGGGT −13 to −8 GCTTTG | 15 ± 0.3 | 12 ± 1 |
| TTCGCG −36 to −31 GGATAT | 19 ± 0.7 | 25 ± 3 |
| GGGGCTG −44 to −38 TTTTAGT | 76 ± 12 | 240 ± 23 |
| CACTCCC −54 to −48 ACAGAAA | 13 ± 5 | 5 ± 3 |
| C −54 A | 13 ± 0.7 | 5 ± 2 |
| A −53 C | 15 ± 0.5 | 12 ± 1 |
| C −52 A | 62 ± 8 | 163 ± 26 |
| T −51 G | 29 ± 4 | 56 ± 12 |
| C −50 A | 115 ± 18 | 330 ± 44 |
| C −49 A | 136 ± 19 | 406 ± 63 |
| C −48 A | 112 ± 3 | 328 ± 9 |
| GCACA −64 to −60 TACAC | 19 ± 2 | 26 ± 2 |
| C −61 A | 93 ± 14 | 267 ± 43 |
| TGATTCAT −72 to −65 GTCGGACG | 41 ± 5 | 83 ± 12 |
| CGGGCTC −79 to −73 ATTTAGA | 32 ± 3 | 61 ± 3 |
The maximum β-galactosidase specific activity in nanomoles of o-nitrophenyl phosphate per minute per milligram of protein (average plus or minus 1 standard deviation) is listed for three independently isolated M. xanthus transformants (one determination each) in the case of mutant promoters and for one isolate (five determinations) in the case of the wild-type promoter and vector (no insert) controls. Samples were assayed at 0, 6, 12, 18, 24, 30, 36, and 48 h during development.
The wild-type promoter and vector (no insert) strains were included in each experiment. The maximum for each mutant promoter is expressed as a percentage of the maximum observed for the wild-type promoter in the same experiment, after subtracting from both values the maximum observed for vector (no insert) in that experiment. The average percentage plus or minus 1 standard deviation is listed.
For example, mutant TAGGGT −13 to −8 GCTTTG has a mutation changing TAGGGT at positions −13 to −8 to GCTTTG, and mutant C −54 A has a mutation changing C at position −54 to A.
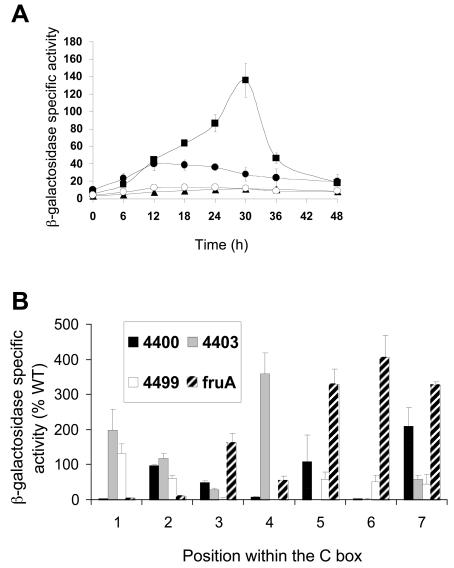
The effects of single-base-pair changes in the C box were examined in the same way. We chose to make drastic mutations; transversions that, for example, change a CG base pair to an AT base pair. Transversion mutations at −52 and −51 bp had relatively small effects on lacZ expression, increasing it 1.6-fold or decreasing it nearly twofold, respectively (Table 2). Figure 2A shows the β-galactosidase specific activity during development of two mutant promoter regions that exhibited large changes in activity. Changing C to A at −54 bp nearly abolished promoter activity (Fig. 2A and Table 2), as did changing A to C at −53 bp (Table 2). In contrast, changing C to A at −49 bp increased lacZ expression relative to the wild-type promoter by more than threefold (Fig. 2A and Table 2), as did changing C to A at −48 or −50 bp (Table 2). These results show that most changes in the fruA C box dramatically affect promoter activity.
FIG. 2.
Mutational analysis of the C box centered at −51 bp in the fruA promoter region. (A) Developmental lacZ expression was measured for M. xanthus strains with a C to A change at −49 bp (▪) or −54 bp (○). The wild-type fruA promoter region from −185 to +50 bp (•) and the vector with no insert (▴) were included as controls. The meaning of points and error bars is the same as is described in the legend to Fig. 1. (B) Comparison of the effects of single-base-pair transversion mutations in four different C boxes. The x axis represents the position in the C box corresponding to the consensus sequence CAYYCCY with the A being position 2, etc. The bars represent the average maximum developmental lacZ activity expressed as a percentage of the wild-type promoter activity for the C boxes centered at −49 bp in the Ω4400 and Ω4403 promoter regions or centered at −33 bp in the Ω4499 promoter region or centered at −51 bp in the fruA promoter region (Table 2). Error bars show 1 standard deviation of the data.
Figure 2B compares the pattern of effects of transversion mutations in the fruA C box centered at −51 bp with that of transversion mutations in C boxes centered at −49 bp in the Ω4400 (35) and Ω4403 (34) promoter regions and centered at −33 bp in the Ω4499 promoter region (36). A different pattern of effects on promoter activity is observed in each case. This indicates that despite their sequence similarity, each C box functions differently. For example, perhaps each sequence is recognized by a different transcription factor.
Effects of mutations in the 5-bp element.
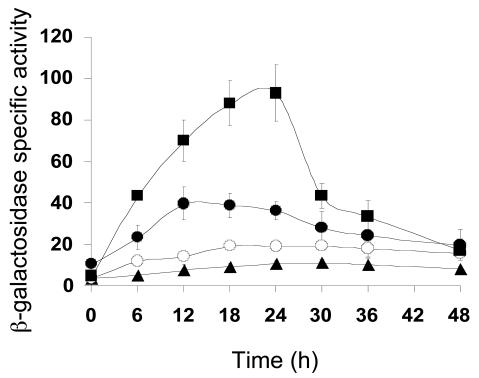
A 5-bp element was shown to be essential for Ω4403 promoter activity (34). Sequence comparison revealed a 5-bp sequence with the consensus GAACA located 5 to 7 bp upstream of C boxes in the Ω4400, Ω4499, csgA, and fruA promoter regions. Subsequent mutational analysis has demonstrated that 5-bp elements are important for Ω4400 (35) and Ω4499 (36) promoter activity (the importance of the 5-bp element in the csgA promoter region has not been tested). In the fruA promoter region, the 5-bp element is −64 GCACA −60. To determine if this element is important for expression of the fruA promoter, we made a 5-bp mutation of GCACA to TACAC. This multiple-base-pair transversion mutation caused a fourfold decrease in fruA promoter activity (Fig. 3 and Table 2).
FIG. 3.
Mutational analysis of the 5-bp element. Developmental lacZ expression was measured for M. xanthus strains with a multiple-base-pair mutation in the 5-bp element of the fruA promoter region (○) or a single-base-pair change of C to A at −61 bp (▪). The wild-type fruA promoter region from −185 to +50 bp (•) and the vector with no insert (▴) were included as controls. The meaning of points and error bars is the same as is described in the legend to Fig. 1.
We also changed C to A at −61 bp in the fruA promoter region, because a C at the fourth position of the 5-bp element is perfectly conserved, yet when changed to A in the Ω4400 promoter region, activity was abolished (35), and the same change in the Ω4403 promoter region caused nearly a twofold increase in developmental lacZ expression (34). The corresponding change in the fruA promoter region increased expression nearly threefold (Fig. 3 and Table 2). We conclude that mutations in the 5-bp element, like those in the C box, can dramatically increase or decrease fruA promoter activity.
The different effects of single-base-pair mutations in C boxes and 5-bp elements of different C-signal-dependent promoters have led to the proposal that a C box and a 5-bp element, and in some cases the sequence in between, together constitute a transcription factor recognition site and that different transcription factors bind to this recognition site in the Ω4403, Ω4400, and Ω4499 promoter regions (36). Likewise, single-base-pair mutations in the fruA C box and 5-bp element show a unique pattern of effects on promoter activity (Fig. 2B and 3), suggesting that a different transcription factor might recognize these sequences. Alternatively, a single protein might bind differently to the putative recognition sites by adopting different conformations, possibly due to different states of posttranslational modification, interactions with other proteins, and/or the influence of DNA adjacent to the putative recognition site.
Effects of mutations in the −35 and −10 regions of the fruA promoter.
Promoter recognition by RNA polymerase holoenzyme in bacteria often involves interaction between the σ subunit and sequences centered at −35 and −10 bp relative to the transcription start site. To investigate whether this is the case for the fruA promoter, the sequence TTCGCG centered at −33.5 bp was changed to GGATAT, and separately, the sequence TAGGGT centered at −9.5 bp was changed to GCTTTG. The 6-bp change in the −35 region reduced developmental lacZ expression fourfold compared to that of the wild-type promoter (Table 2). The 6-bp change in the −10 region reduced expression even further (Table 2). Clearly the −10 and −35 regions are important for expression, which is consistent with the idea that the fruA promoter is recognized by a σ factor in the σ70 family.
Effect of a mutation between the C box and the promoter −35 region.
The C box centered at −51 bp in the fruA promoter region makes up nearly half of a palindromic sequence (CACTCCCATTGGGGCTG [inverted repeat sequences are underlined]) spanning from −54 to −38 bp. To test the importance of the other half of the palindromic sequence, we changed GGGGCTG to TTTTAGT in the fruA promoter region between −44 and −38 bp. This change led to more than a twofold increase in developmental lacZ expression (Table 2). Likewise, certain single-base-pair changes (at positions −48, −49, and −50) in the upstream half of the palindrome increased expression threefold to fourfold (Fig. 2 and Table 2). These results suggest that a dimeric transcriptional repressor might normally bind to the palindrome. On the other hand, single-base-pair changes at positions −53 and −54 in the palindrome greatly decreased expression (Fig. 2 and Table 2). Unless these mutations improve repressor binding, which seems unlikely since they weaken the palindrome, perhaps a transcriptional activator binding site (i.e., the 5-bp element and the C box) partially overlaps with a repressor binding site (i.e., the palindromic sequence) in the fruA promoter region.
Effects of mutations upstream of the 5-bp element.
In the promoter regions of C-signal-dependent genes that have been examined previously, cis-acting elements important for promoter activity have been found upstream of 5-bp elements, typically in the vicinity of −80 to −70 bp (34-36). To test the importance of this region for fruA promoter activity, we changed −72 TGATTCAT −65 to GTCGGACG and −79 CGGGCTC −73 to ATTTAGA. Neither mutation had a very large effect on developmental lacZ expression (Table 2).
Summary.
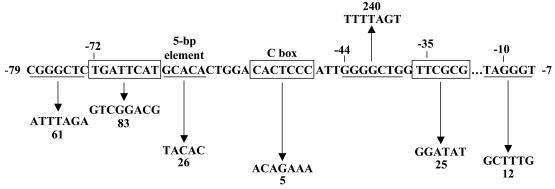
Figure 4 summarizes the effects of the multiple-base-pair changes we made in the fruA promoter region. Our results demonstrate that the 5-bp element and the C box are important cis-acting DNA sequences not only in C-signal-dependent promoter regions but also for fruA expression, which does not depend on C-signaling and is essential for development. Our future studies will aim to identify and characterize the trans-acting factor(s) that binds to the 5-bp element and the C box in the fruA promoter region, as well as the promoter regions of genes that depend on C-signaling for expression.
FIG. 4.
Summary of the effects of mutations in the fruA promoter region. DNA subjected to mutagenesis is alternately underlined or boxed. Upward and downward arrows indicate that developmental lacZ expression was increase or decreased, respectively, by the given change in DNA sequence, and numbers indicate the maximum β-galactosidase specific activity observed for the mutant, expressed as a percentage of wild-type promoter activity measured in the same experiment (Table 2).
Acknowledgments
We thank D. Yoder, P. Viswanathan, and K. Viswanathan for suggestions on the manuscript.
This research was supported by a Strategic Partnership Grant from the Michigan State University Foundation to the Gene Expression in Development and Disease Group and by the Michigan Agricultural Experiment Station.
REFERENCES
- 1.Brandner, J. P., and L. Kroos. 1998. Identification of the Ω4400 regulatory region, a developmental promoter of Myxococcus xanthus. J. Bacteriol. 180:1995-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Downard, J., S. V. Ramaswamy, and K. Kil. 1993. Identification of esg, a genetic locus involved in cell-cell signaling during Myxococcus xanthus development. J. Bacteriol. 175:7762-7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellehauge, E., M. Norregaard-Madsen, and L. Sogaard-Andersen. 1998. The FruA signal transduction protein provides a checkpoint for the temporal co-ordination of intercellular signals in Myxococcus xanthus development. Mol. Microbiol. 30:807-817. [DOI] [PubMed] [Google Scholar]
- 4.Fisseha, M., D. Biran, and L. Kroos. 1999. Identification of the Ω4499 regulatory region controlling developmental expression of a Myxococcus xanthus cytochrome P-450 system. J. Bacteriol. 181:5467-5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisseha, M., M. Gloudemans, R. Gill, and L. Kroos. 1996. Characterization of the regulatory region of a cell interaction-dependent gene in Myxococcus xanthus. J. Bacteriol. 178:2539-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagen, D. C., A. P. Bretscher, and D. Kaiser. 1978. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev. Biol. 64:284-296. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 8.Horiuchi, T., T. Akiyama, S. Inouye, and T. Komano. 2003. Regulation of FruA expression during vegetative growth and development of Myxococcus xanthus. J. Mol. Microbiol. Biotechnol. 5:87-96. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser, D. 2003. Coupling cell movement to multicellular development in myxobacteria. Nat. Rev. Microbiol. 1:45-54. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser, D. 1979. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 76:5952-5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan, H. 2003. Multicellular development and gliding motility in Myxococcus xanthus. Curr. Opin. Microbiol. 6:572-577. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan, H. B., and L. Plamann. 1996. A Myxococcus xanthus cell density-sensing system required for multicellular development. FEMS Microbiol. Lett. 139:89-95. [DOI] [PubMed] [Google Scholar]
- 13.Kim, S. K., and D. Kaiser. 1990. Cell alignment required in differentiation of Myxococcus xanthus. Science 249:926-928. [DOI] [PubMed] [Google Scholar]
- 14.Kim, S. K., and D. Kaiser. 1990. Cell motility is required for the transmission of C-factor, an intercellular signal that coordinates fruiting body morphogenesis of Myxococcus xanthus. Genes Dev. 4:896-905. [DOI] [PubMed] [Google Scholar]
- 15.Kim, S. K., and D. Kaiser. 1991. C-factor has distinct aggregation and sporulation thresholds during Myxococcus development. J. Bacteriol. 173:1722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, S. K., and D. Kaiser. 1990. C-factor: a cell-cell signaling protein required for fruiting body morphogenesis of M. xanthus. Cell 61:19-26. [DOI] [PubMed] [Google Scholar]
- 17.Kroos, L., P. Hartzell, K. Stephens, and D. Kaiser. 1988. A link between cell movement and gene expression argues that motility is required for cell-cell signaling during fruiting body development. Genes Dev. 2:1677-1685. [DOI] [PubMed] [Google Scholar]
- 18.Kroos, L., and D. Kaiser. 1987. Expression of many developmentally regulated genes in Myxococcus depends on a sequence of cell interactions. Genes Dev. 1:840-854. [DOI] [PubMed] [Google Scholar]
- 19.Kroos, L., A. Kuspa, and D. Kaiser. 1986. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev. Biol. 117:252-266. [DOI] [PubMed] [Google Scholar]
- 20.Kuspa, A., L. Plamann, and D. Kaiser. 1992. Identification of heat-stable A-factor from Myxococcus xanthus. J. Bacteriol. 174:3319-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuspa, A., L. Plamann, and D. Kaiser. 1992. A-signalling and the cell density requirement for Myxococcus xanthus development. J. Bacteriol. 174:7360-7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, B.-U., K. Lee, J. Mendez, and L. Shimkets. 1995. A tactile sensory system of Myxococcus xanthus involves an extracellular NAD(P)+-containing protein. Genes Dev. 9:2964-2973. [DOI] [PubMed] [Google Scholar]
- 23.Li, S.-F., B. Lee, and L. J. Shimkets. 1992. csgA expression entrains Myxococcus xanthus development. Genes Dev. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 24.Li, S.-F., and L. J. Shimkets. 1993. Effect of dsp mutations on the cell-to-cell transmission of CsgA in Myxococcus xanthus. J. Bacteriol. 175:3648-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lobedanz, S., and L. Sogaard-Andersen. 2003. Identification of the C-signal, a contact-dependent morphogen coordinating multiple developmental responses in Myxococcus xanthus. Genes Dev. 17:2151-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa, M., S. Fujitani, X. Mao, S. Inouye, and T. Komano. 1996. FruA, a putative transcription factor essential for the development of Myxococcus xanthus. Mol. Microbiol. 22:757-767. [DOI] [PubMed] [Google Scholar]
- 27.Plamann, L., A. Kuspa, and D. Kaiser. 1992. Proteins that rescue A-signal-defective mutants of Myxococcus xanthus. J. Bacteriol. 174:3311-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sager, B., and D. Kaiser. 1994. Intercellular C-signaling and the traveling waves of Myxococcus. Genes Dev. 8:2793-2804. [DOI] [PubMed] [Google Scholar]
- 29.Shimkets, L. J. 1999. Intercellular signaling during fruiting-body development of Myxococcus xanthus. Annu. Rev. Microbiol. 53:525-549. [DOI] [PubMed] [Google Scholar]
- 30.Shimkets, L. J., and S. J. Asher. 1988. Use of recombination techniques to examine the structure of the csg locus of Myxococcus xanthus. Mol. Gen. Genet. 211:63-71. [DOI] [PubMed] [Google Scholar]
- 31.Sogaard-Andersen, L., M. Overgaard, S. Lobedanz, E. Ellehauge, L. Jelsbak, and A. A. Rasmussen. 2003. Coupling gene expression and multicellular morphogenesis during fruiting body formation in Myxococcus xanthus. Mol. Microbiol. 48:1-8. [DOI] [PubMed] [Google Scholar]
- 32.Sogaard-Andersen, L., F. Slack, H. Kimsey, and D. Kaiser. 1996. Intercellular C-signaling in Myxococcus xanthus involves a branched signal transduction pathway. Genes Dev. 10:740-754. [DOI] [PubMed] [Google Scholar]
- 33.Ueki, T., and S. Inouye. 2003. Identification of an activator protein required for the induction of fruA, a gene essential for fruiting body development in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 100:8782-8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viswanathan, P., and L. Kroos. 2003. cis elements necessary for developmental expression of a Myxococcus xanthus gene that depends on C signaling. J. Bacteriol. 185:1405-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoder, D., and L. Kroos. 2004. Mutational analysis of the Myxococcus xanthus Ω4400 promoter region provides insight into developmental gene regulation by C signaling. J. Bacteriol. 186:661-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoder, D., and L. Kroos. 2004. Mutational analysis of the Myxococcus xanthus Ω4499 promoter region reveals shared and unique properties in comparison with other C-signal-dependent promoters. J. Bacteriol. 186:3766-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]