Abstract
The fluorescent dihydroxyquinoline chromophore of the pyoverdine siderophore in Pseudomonas is a condensation product of d-tyrosine and l-2,4-diaminobutyrate. Both pvdH and asd (encoding aspartate β-semialdehyde dehydrogenase) knockout mutants of Pseudomonas aeruginosa PAO1 were unable to synthesize pyoverdine under iron-limiting conditions in the absence of l-2,4-diaminobutyrate in the culture media. The pvdH gene was subcloned, and the gene product was hyperexpressed and purified from P. aeruginosa PAO1. PvdH was found to catalyze an aminotransferase reaction, interconverting aspartate β-semialdehyde and l-2,4-diaminobutyrate. Steady-state kinetic analysis with a novel coupled assay established that the enzyme adopts a ping-pong kinetic mechanism and has the highest specificity for α-ketoglutarate. The specificity of the enzyme toward the smaller keto acid pyruvate is 41-fold lower. The enzyme has negligible activity toward other keto acids tested. Homologues of PvdH were present in the genomes of other Pseudomonas spp. These homologues were found in the DNA loci of the corresponding genomes that contain other pyoverdine synthesis genes. This suggests that there is a general mechanism of l-2,4-diaminobutyrate synthesis in Pseudomonas strains that produce the pyoverdine siderophore.
Iron is a nutrient that is required by most bacteria. However, the bioavailability of iron in an aerobic environment at pH 7 is low since it is primarily available in its oxidized form (Fe3+), which is highly insoluble (∼10−9 M) (5). Under iron depletion conditions many bacteria can synthesize iron-chelating molecules called siderophores. Siderophores are also of particular importance to pathogens during infection due to iron sequestration by host iron-binding proteins (30).
The gram-negative bacterium Pseudomonas aeruginosa produces two siderophores, pyochelin (7) and pyoverdine (25, 41). This opportunistic pathogen infects both burn victims and individuals afflicted with cystic fibrosis, in which it is the leading cause of morbidity and mortality (10). Both pyoverdine and pyochelin are synthesized during pathogenesis (11, 40), although pyoverdine was found to have more prominent effects on virulence (24, 39). Recently, extracellular ferric pyoverdine has also been shown to upregulate the transcription of other Pseudomonas virulence genes, such as the exotoxin A and PrpL endoprotease genes (20).
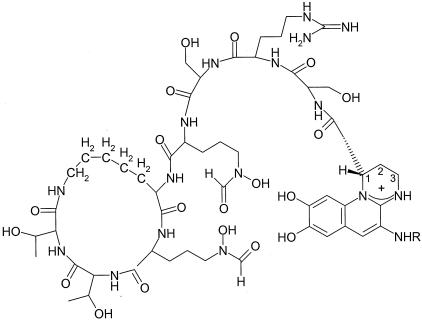
Structurally, pyoverdines produced by strains of Pseudomonas consist of a peptide chain containing 6 to 12 amino acids, a dihydroxyquinoline chromophore, and an amide-linked acyl side chain (Fig. 1). Both the peptide chain and the chromophore have been proposed to be assembled from amino acid precursors by nonribosomal peptide synthetases (1, 22, 23, 26). The chromophore, for example, is a condensation product of d-tyrosine and l-2,4-diaminobutyrate. Thus, it has been demonstrated that position 4 of the chromophore is labeled with 15N if the pyoverdine-producing bacteria are fed l-2,4-[4-15N]diaminobutyrate in the media (3). However, the precursor of l-2,4-diaminobutyrate and the enzyme(s) required for its biosynthesis in fluorescent pseudomonads have not been reported.
FIG. 1.
Structure of pyoverdine produced by P. aeruginosa PAO1 (41). Carbon atoms of the chromophore derived from l-2,4-diaminobutyrate are numbered. R indicates a variable acyl group.
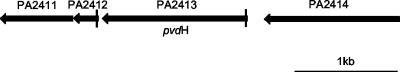
In a limited number of cases l-2,4-diaminobutyrate has also been found to serve both a structural role and a metabolic role in bacteria. For example, l-2,4-diaminobutyrate is a precursor of the compatible solute ectoine (28, 29) and a metabolite for the biosynthesis of 1,3-diaminopropane in Acinetobacter baumannii (14). In these cases, l-2,4-diaminobutyrate is formed from aspartate β-semialdehyde by the enzyme l-2,4-diaminobutyrate:α-ketoglutarate 4-aminotransferase. The gene encoding a homologue of this enzyme is found in P. aeruginosa PAO1 at a locus of the genome that contains other pyoverdine synthesis genes (Fig. 2). The homologous gene, termed pvdH or PA2413, has been inactivated by insertional mutation and has been shown to abolish the formation of fluorescent pyoverdine in P. aeruginosa PAO1 (21, 27). However, the reported knockout mutants have not been complemented with the intact gene in trans; thus, its role in pyoverdine synthesis is not unequivocal. Moreover, the biochemical activity of PvdH has not been demonstrated.
FIG. 2.
Locus of P. aeruginosa PAO1 genome containing the pvdH gene (PA2413). The promoters of pvdH and PA2412, indicated by vertical lines, contain iron starvation box consensus sequences, suggesting that they are both regulated by the sigma factor, PvdS (21).
Here we characterized PvdH and showed that it is an aminotransferase that catalyzes the formation of l-2,4-diaminobutyrate from aspartate β-semialdehyde and is required for pyoverdine synthesis. We also developed a convenient assay that allows us to determine the true Km and kcat values for this type of aminotransferase.
MATERIALS AND METHODS
Chemicals.
α-Ketobutyrate was purchased from ICN Biomedicals, Inc. meso-Diaminopimelate, l-homoserine, l-2,4-diaminobutyrate, NAD+, NADP+, NADPH, NADH, pyridoxal phosphate, α-ketoglutarate, pyruvate, and oxaloacetate were purchased from Sigma-Aldrich.
Bacterial strains and plasmids.
The strains used for protein expression or DNA propagation were Escherichia coli BL21(λDE3), JM109, and SM10 and P. aeruginosa PAO1. asd::Gmr P. aeruginosa (13) was obtained from H. P. Schweizer, Colorado State University. The plasmids used in this work were pT7-7 (38), pVLT31 (8), pEX18Tc (12), and pUCGm (34). E. coli and P. aeruginosa were grown at 37°C. Recombinant E. coli strains were propagated in media supplemented with the appropriate antibiotics at concentrations of 100 μg/ml (for ampicillin), 12.5 μg/ml (for tetracycline), and 15 μg/ml (for gentamicin). Recombinant P. aeruginosa PAO1 was grown in media supplemented with the appropriate antibiotics at concentrations of 110 μg/ml (for tetracycline) and 300 μg/ml (for gentamicin).
General DNA manipulations.
DNA was purified, digested, and ligated by using standard protocols (33). PCR fragments were purified by using the CONCERT rapid PCR purification system (Life Technologies, Inc.). DNA fragments were purified from agarose gels by using a QIAEX II gel extraction kit (QIAGEN, Inc.) according to the manufacturer's instructions. The CONCERT rapid plasmid miniprep system (Life Technologies, Inc.) was used according to the manufacturer's instructions to isolate plasmid DNA for DNA sequencing. DNA sequencing was performed by the Guelph Molecular Supercentre, University of Guelph.
Cloning of pvdH.
The forward primer 5′-GAGCCATATGCACGTCGCCACCAGCGTC-3′ and the reverse primer 5′-GAGGCTGCAGTTAGCCGGCCAGCGCCGCGC-3′ were used to PCR amplify the pvdH gene by using P. aeruginosa PAO1 chromosomal DNA as the template (introduced NdeI and PstI restriction sites are underlined in the forward and reverse primers, respectively). The PCR mixtures consisted of 1× Expand High Fidelity buffer containing 1.5 mM MgCl2 (Roche Diagnostics), 1× Pfx enhancer solution (Invitrogen), each deoxynucleoside triphosphate at a concentration of 0.4 mM, 1 pmol of each primer, 22 ng of P. aeruginosa PAO1 chromosomal DNA, and 0.5 U of Platinum Pfx DNA polymerase (Invitrogen). The following amplification profile was employed: hot start at 94°C for 2 min, followed by 30 cycles of 94°C for 30 s, 50°C for 30 s, and 68°C for 1 min and finally 68°C for 10 min. PCR fragments were purified and digested with NdeI and PstI. Following gel purification, the amplified gene was cloned into pT7-7 that had been digested with the same enzymes. The resulting construct was transformed into E. coli BL21(DE3). Positive transformants were verified by restriction enzyme digestion, as well as nucleotide sequencing. The pT7-7 construct containing pvdH was also transformed into E. coli BL21(DE3) RP (Stratagene). pvdH was excised from pT7-7 by using XbaI and PstI and then subcloned into pVLT31, a broad-host-range plasmid. The resultant recombinant plasmid was transformed into P. aeruginosa PAO1. P. aeruginosa transformants were plated onto Luria-Bertani (LB) agar containing tetracycline.
Construction of a pvdH::Gmr insertion mutant.
The gene replacement strategy of Schweizer and Hoang (35) was used for creation of knockout mutations in pvdH. Briefly, a 855-bp gentamicin resistance gene cassette was excised by SmaI digestion from pUCGm and ligated to the MscI restriction site of pvdH. The gentamicin cassette was inserted at position 1019 of the 1,440-bp pvdH gene. The pvdH::Gmr construct in pT7-7 was then subcloned into pEX18Tc by using XbaI and HindIII, transformed into E. coli JM109, and subsequently transformed into E. coli SM10. The pEX18Tc (pvdH::Gmr) plasmid was introduced into P. aeruginosa PAO1 cells by biparental mating, and gentamicin-resistant, sucrose-sensitive P. aeruginosa strains were selected. Correct gene replacement was ascertained by PCR of the pvdH gene and Southern blotting of digested genomic DNA by using a DIG High Prime DNA labeling and detection starter kit I (Roche) according to the manufacturer's instructions.
Characterization of pvdH::Gmr and asd::Gmr mutants.
Pyoverdine production by P. aeruginosa pvdH::Gmr and asd::Gmr mutants was assessed by growing the mutants in iron-limiting Casamino Acids (CAA) broth (17). The same mutants were also grown in CAA broth supplemented with 1.5 mM l-2,4-diaminobutyrate. The presence of pyoverdine in culture supernatant was detected spectrophotometrically at 405 nm (37) and visually by green fluorescence under UV light. Pyoverdine production was also assessed in the pvdH::Gmr mutant that contained a wild-type copy of pvdH inserted into the expression vector pVLT31. The same mutant transformed only with the vector pVLT31 served as a negative control. The abilities of the pvdH::Gmr mutant and the complemented mutant to grow on LB agar containing the iron chelator 2,2′-dipyridyl (0.6 mM) were also determined.
Purification of recombinant homoserine dehydrogenase.
E. coli BL21(DE3) containing the hom6 gene, encoding homoserine dehydrogenase from Saccharomyces cerevisiae, was obtained from G. Wright, McMaster University. Homoserine dehydrogenase was expressed by using a previously reported protocol (16). The cell pellet from a 1-liter culture was resuspended in 10 ml of 20 mM HEPES buffer (pH 7.5) and passed through a French pressure cell three times. Buffers containing 20 mM sodium HEPES (pH 7.5) were used throughout the purification procedure. Chromatography was performed by using an ÄKTA Explorer with resins and columns from Amersham Pharmacia Biotech. Crude cell extracts were applied to a Source 15Q anion-exchange column and eluted with a linear gradient of a buffer containing 0 to 0.25 M NaCl in 10 column volumes. Homoserine dehydrogenase did not bind to the column and was eluted during the initial column wash. Fractions containing homoserine dehydrogenase activity were pooled and concentrated by using an Amicon cell equipped with a YM10 filter to 3 ml and then loaded onto a Superdex 200 column equilibrated with 20 mM HEPES buffer (pH 7.5) containing 0.15 M NaCl. Active fractions were pooled and concentrated as described above to 3 ml, divided into aliquots, and stored at −80°C in 20 mM HEPES buffer (pH 7.5) with 5% glycerol.
Purification of PvdH.
pvdH was overexpressed in P. aeruginosa PAO1 by using the vector pVLT31. Protein expression was induced at the log phase with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Mixtures (total volume, 1 ml) for standard coupled assays to determine fractions containing PvdH during purification contained 0.15 mM pyridoxal phosphate, 10 mM α-ketoglutarate, 0.2 mM NAD(P)H, 10 mM l-2,4-diaminobutyrate, 0.03 mg of homoserine dehydrogenase, partially purified PvdH, and 1 M phosphate buffer (pH 7.5). The decrease in absorbance at 340 nm due to NADH oxidation in the assay was monitored spectrophotometrically.
HEPES (20 mM, pH 7.5) was used throughout the purification procedure. Crude cell extracts from a 2-liter culture were applied to a Source 15Q anion-exchange column equilibrated with buffer containing 0.1 M NaCl. The enzyme was eluted with a 10-column-volume 0.15 to 0.35 M NaCl gradient. PvdH eluted at approximately 0.22 M NaCl. Fractions containing the expected activity were pooled and concentrated; the protein concentration was kept less than 1 mg/ml to prevent undesired precipitation of proteins. The concentrated fraction was loaded onto a Superdex 200 column equilibrated with buffer containing 0.15 M NaCl. Activity-containing fractions were pooled and concentrated as described above, divided into aliquots, and stored at −80°C in 20 mM HEPES (pH 7.5) containing 10% glycerol and 0.5 mM pyridoxal phosphate. The enzyme exhibited the same specific activity for at least 1 month when it was stored under these conditions.
Determination of protein concentration, purity, and molecular mass.
Protein concentrations were determined by the Bradford assay (4) by using bovine serum albumin as the standard. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) was performed and gels were stained with Coomassie blue by using established procedures (19). The BenchMark protein ladder (Invitrogen) containing proteins having molecular masses ranging from 10 to 220 kDa was used as a molecular weight marker. Gel filtration was performed with a Superdex 200 column (Amersham Pharmacia) to determine the relative molecular weight and possible quaternary structure of PvdH. HEPES buffer (20 mM, pH 7.5) containing 0.15 M NaCl was used as both the equilibration buffer and the elution buffer. The standard curve was produced with the following proteins (Sigma): cytochrome c (Mr, 12,400), carbonic anhydrase (Mr, 29,000), bovine serum albumin (Mr, 66,000), alcohol dehydrogenase (Mr, 150,000), and β-amylase (Mr, 200,000).
Enzyme kinetics assays.
All kinetics assays were performed in duplicate at 25°C by using a Varian Cary 3 spectrophotometer equipped with a thermojacketed cuvette holder. The extinction coefficient for the NADH and NADPH used was 6,200 M−1 cm−1.
The mixtures for standard assays used to determine the specific activity of homoserine dehydrogenase during purification contained 100 mM Tris-Cl (pH 9.0), 25 mM l-homoserine, and 0.2 mM NAD(P). The increase in absorbance at 340 nm due to NAD+ reduction was monitored spectrophotometrically.
The stoichiometry of the coupled assay was tested by using an assay mixture containing 1 mM α-ketoglutarate and 100 μM l-2,4-diaminobutyrate.
Assays to determine kinetic parameters for PvdH were performed in 1-ml (total volume) mixtures containing 1 M phosphate buffer (pH 7.5), 0.15 mM pyridoxal phosphate, 0.2 mM NADH, 0.03 mg of homoserine dehydrogenase, and 0.29 to 3.3 μg of PvdH. l-2,4-Diaminobutyrate was used as the amino donor at concentrations ranging from 50 μM to 1 mM. α-Ketoglutarate (100 μM to 1 mM), pyruvate (2 to 20 mM), oxaloacetate (30 mM), and α-ketobutyrate (10 to 20 mM) were tested as amino acceptors. NADH concentrations ranging from 0.2 to 0.6 mM and homoserine dehydrogenase amounts ranging from 0.03 to 0.2 mg were initially used to confirm that excess amounts of these components were present. Kinetic parameters were fitted to the Ping-Pong Bi Bi equation by using the software LEONORA (6).
RESULTS
Construction and analysis of pvdH insertion mutant.
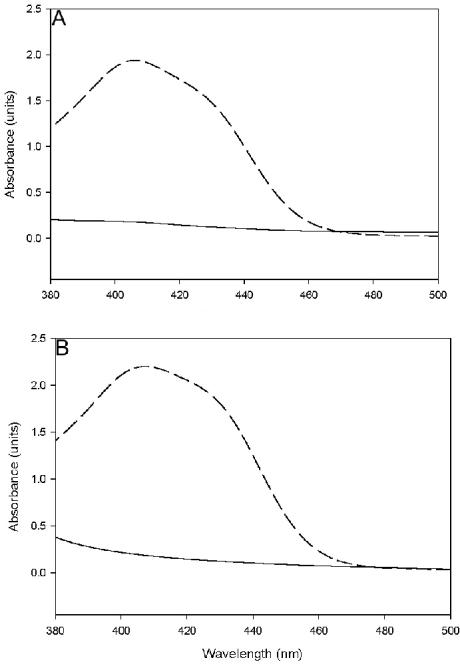
The pvdH gene from P. aeruginosa PAO1 was inactivated by insertion of a gentamicin cassette. When grown in iron-limiting CAA broth, the mutant did not produce pyoverdine, as determined by the absence of green fluorescence of the medium under UV light and the characteristic absorbance peak of pyoverdine at 405 nm (37). In addition, the mutant did not grow on LB agar containing the iron chelator 2,2′-dipyridyl. However, the mutant was able to produce pyoverdine and grow in media containing dipyridyl if it was complemented with an intact pvdH gene introduced in trans by using the broad-host-range plasmid pVLT31. Pyoverdine production was also restored in the mutant if it was grown in the presence of 1.5 mM pure l-2,4-diaminobutyrate in the medium (Fig. 3). Together, these results are consistent with the prediction that PvdH is involved in the production of l-2,4-diaminobutyrate for pyoverdine synthesis.
FIG. 3.
Spectra of culture supernatants of pvdH (A) and asd (B) knockout mutants grown in CAA media. The dashed lines are the spectra for culture supernatants of mutants grown in medium containing l-2,4-diaminobutyrate, and the solid lines are spectra for culture supernatants of mutants grown in medium without l-2,4-diaminobutyrate.
Analysis of asd insertion mutant.
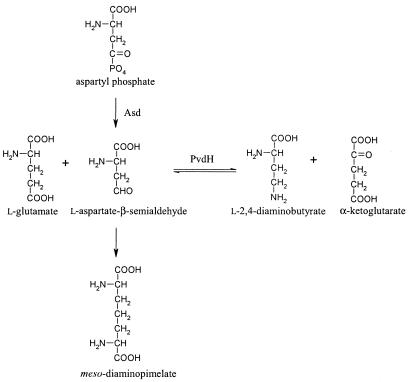
Aspartate β-semialdehyde dehydrogenase is an enzyme that catalyzes the formation of aspartate β-semialdehyde from aspartyl phosphate (Fig. 4) (13). Aspartate β-semialdehyde is a metabolic precursor for the amino acids lysine, threonine, isoleucine, methionine, and meso-diaminopimelate. The latter compound is a component of the peptidoglycan of P. aeruginosa. Accordingly, the asd knockout mutant is viable only if meso-diaminopimelate is added to CAA media. We reasoned that if l-2,4-diaminobutyrate is formed from aspartate β-semialdehyde, the asd knockout mutant should also be pyoverdine negative. Indeed, when the asd mutant was grown in CAA broth containing only meso-diaminopimelate, no pyoverdine was produced. Pyoverdine production could be restored if the same mutant was grown in CAA broth containing l-2,4-diaminobutyrate (Fig. 3). Surprisingly, in the presence of l-2,4-diaminobutyrate, meso-diaminopimelate did not have to be added to the media for growth of the mutant. This could be explained by a reverse reaction catalyzed by PvdH, converting a fraction of the l-2,4-diaminobutyrate in the media to aspartate β-semialdehyde, which could then be used for meso-diaminopimelate biosynthesis.
FIG. 4.
Metabolic pathway showing the relationship between the reaction catalyzed by PvdH and aspartate β-semialdehyde dehydrogenase (Asd). The enzymatic steps required to convert aspartate β-semialdehyde to meso-diaminopimelate in P. aeruginosa have not been elucidated.
Expression, purification, and determination of the molecular mass of PvdH.
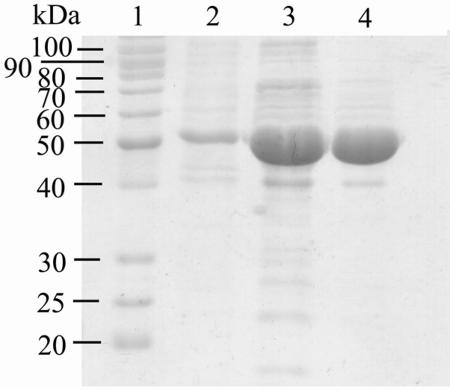
PvdH was initially expressed in E. coli BL21(λDE3) under control of the T7 promoter from plasmid pT7-7. However, the expression levels were low, and the PvdH protein was insoluble (data not shown). The low levels of expression may have been due to the presence of arginine (CGG and AGG), glycine (GGA), and proline (CCC) codons that are rare in E. coli (18). Introduction of the expression plasmid into E. coli BL21(λDE3) RP, which contains a greater number of copies of tRNA for the codons AGG and CCC, did not significantly improve PvdH expression, and the protein remained insoluble. pvdH was then transferred to P. aeruginosa PAO1 and expressed in a soluble form under control of the tac promoter from plasmid pVLT31. PvdH was purified from crude extracts by using anion-exchange and gel filtration chromatography (Table 1). The enzyme was purified up to 221-fold with a yield of 7%. The final enzyme preparation was judged to be more than 90% pure by SDS-PAGE analysis (Fig. 5).
TABLE 1.
Purification of PvdH from P. aeruginosa PAO1
| Step | Protein (mg) | Activity (U)a | Sp act (U/mg) | Recovery (%) | Purification (fold) |
|---|---|---|---|---|---|
| Cell extract | 796 | 1.42 | 0.01 | 100 | |
| Source 15Q | 44 | 0.2 | 1.72 | 14 | 172.2 |
| Superdex 200 | 26 | 0.09 | 2.21 | 1 | 220.7 |
One unit was the amount of enzyme required to oxidize 1 μmol of NAD(P)H/min at pH 7.5
FIG. 5.
Coomassie blue-stained SDS-PAGE gel of purified PvdH. The gel was loaded with samples of PvdH from the crude extract (lane 2), the preparation after anion exchange (lane 3), and the preparation after gel filtration (lane 4). The molecular masses of the proteins in the standard (lane 1) are indicated on the left.
During purification, fractions containing PvdH were faintly yellow, with an absorbance maximum at 410 nm. This is consistent with the presence of the cofactor pyridoxal phosphate.
PvdH has a molecular mass of 50 kDa as determined by SDS-PAGE. This is consistent with the calculated molecular mass based on the amino acid sequence, 50.2 kDa. The relative molecular mass of the native enzyme, as determined by gel filtration, was approximately 183 ± 6.6 kDa, suggesting that the enzyme forms a homotetramer.
Coupled assay to determine PvdH activity.
PvdH was predicted to catalyze the reversible transamination of l-2,4-diaminobutyrate (Fig. 4). l-Aspartate β-semialdehyde is not available commercially. Therefore, the activity of PvdH was measured in the reverse direction by using l-2,4-diminobutyrate as the amino donor. A novel coupled assay involving the use of homoserine dehydrogenase from S. cerevisiae was developed. Homoserine dehydrogenase catalyzes the reversible conversion of l-aspartate β-semialdehyde to l-homoserine, with concomitant oxidation of NADH, which can be monitored spectrophotometrically at 340 nm. Homoserine dehydrogenase from S. cerevisiae is a highly specific enzyme, accepting only the amino acids l-aspartate β-semialdehyde and l-homoserine as substrates (15), and thus it is suited for use in this coupled assay.
The reliability of this assay was assessed by using several criteria. The concentrations of homoserine dehydrogenase and NADH in each coupled assay were confirmed to be in excess, since addition of higher concentrations of these constituents did not result in higher PvdH activity. In addition, a linear relationship between the concentration of PvdH and the resultant PvdH activity was verified (data not shown). Finally, if the enzyme reaction was allowed to proceed to completion (end point assay), the amount of NADH consumed, calculated from the decrease in absorbance at 340 nm, corresponded to the total amount of l-2,4-diaminobutyrate in the assay mixture (99.7%), thus demonstrating that there was stoichiometric detection of products. This is in line with previous studies which showed that the equilibrium of the reaction catalyzed by homoserine dehydrogenase strongly favors l-aspartate β-semialdehyde reduction at pH 7.5 (16).
Kinetic analysis of PvdH.
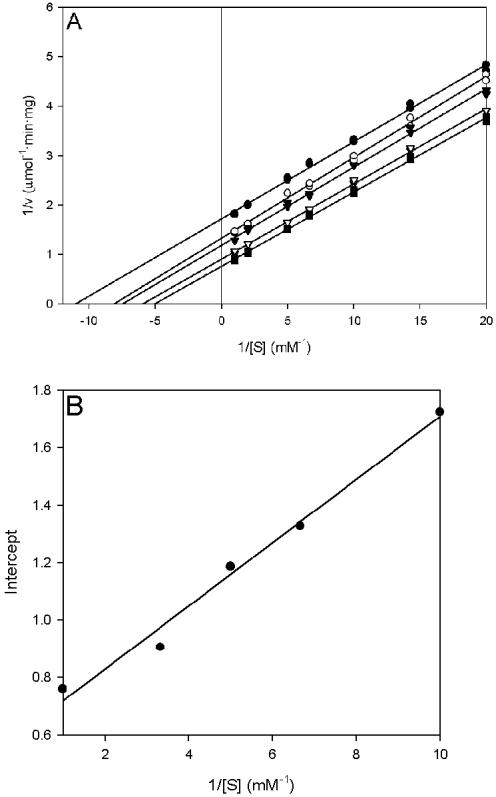
PvdH activity was measured by using l-2,4-diaminobutyrate as the amino donor and α-ketoglutarate, pyruvate, oxaloacetate, or α-ketobutyrate as the amino acceptor. A Lineweaver-Burk plot of the data obtained with α-ketoglutarate as the amino acceptor has a series of parallel lines characteristic of a ping-pong kinetic mechanism (Fig. 6) (9). A similar result was obtained with pyruvate as the amino acceptor. The ping-pong mechanism in pyridoxal phosphate-dependent aminotransferase can be explained by the initial binding and transfer of the amino group from the amino donor to the pyridoxal phosphate cofactor, forming a pyridoxamine phosphate. Binding of the amino acceptor by the enzyme occurs after the exit of the oxidized amino donor from the active site, thereby allowing the transfer of the amino group from pyridoxamine phosphate to the amino acceptor (36).
FIG. 6.
Lineweaver-Burk and secondary plots of PvdH activities with l-2,4-diaminobutyrate and α-ketoglutarate as substrates. (A) Lineweaver-Burk plot showing the relationship of PvdH activity and l-2,4-diaminobutyrate concentration. The α-ketoglutarate concentration was fixed at 100 μM (•), 150 μM (○), 200 μM (▾), 300 μM (▿), or 1 mM (▪). (B) y intercepts from the Lineweaver-Burk graph plotted against the reciprocal of the α-ketoglutarate concentration.
Kinetic parameters for PvdH obtained from Lineweaver-Burk and secondary plots are summarized in Table 2. The specificity constant (kcat/Km) for α-ketoglutarate is approximately 41-fold higher than that for pyruvate. This was attributed mainly to a lower Km value for α-ketoglutarate. The specific activity of PvdH when oxaloacetate was used as the amino acceptor was too low (0.14 ± 0.016 U/mg at 30 mM oxaloacetate) for determination of kinetic parameters. No activity was detected when α-ketobutyrate was used as the amino acceptor.
TABLE 2.
Steady-state kinetic parameters of purified PvdH from P. aeruginosa PAO1
| Amino acceptor | Km for amino acceptor (mM) | Km for l-2,4- diaminobutyrate (mM) | kcat (s−1) | kcat/Km for amino acceptor (mM−1 s−1) | kcat/Km for l-2,4-diaminobutyrate (mM−1 s−1) |
|---|---|---|---|---|---|
| α-Ketoglutarate | 0.18 ± 0.0075 | 0.26 ± 0.0076 | 1.39 ± 0.032 | 7.61 | 5.34 |
| Pyruvate | 6.195 ± 0.196 | 0.291 ± 0.012 | 1.15 ± 0.026 | 0.183 | 3.95 |
DISCUSSION
The chromophore of pyoverdine is produced from condensation of l-2,4-diaminobutyrate and d-tyrosine (3). We demonstrated in this study that l-2,4-diaminobutyrate is synthesized from aspartyl phosphate in P. aeruginosa. This conversion requires two enzymatic activities, aspartate β-semialdehyde dehydrogenase and an aminotransferase, which are encoded by asd and pvdH, respectively.
PvdH exhibits high amino acid sequence similarity (∼85%) to its homologues in Pseudomonas fluorescens, Pseudomonas syringae, and Pseudomonas putida. The genes encoding PvdH homologues in these Pseudomonas species are in a genome locus containing other pyoverdine synthesis genes (31). PvdH exhibits lower sequence identity with l-2,4-diaminobutyrate aminotransferases from A. baumannii (51% identity) and Halomonas elongata (33% identity). Neither of the enzymes from these bacteria has been associated with siderophore synthesis. The enzyme from A. baumannii is involved in the synthesis of 1,3-diaminopropane (14), while the enzyme from H. elongata catalyzes the first step in the ectoine biosynthesis pathway (28).
The molecular weight of native PvdH from P. aeruginosa PAO1 determined by gel filtration suggested that the protein forms a homotetramer. Similarly, the recombinant enzyme from A. baumannii has also been reported to be a homotetramer (14). However, this contrasts with the l-2,4-diaminobutyrate aminotransferase of H. elongata, which forms a homohexamer (28).
Both continuous (2, 32) and discontinuous (14, 28) assays have been used to test aminotransferase activity. In continuous reactions, glutamate and malate dehydrogenases have been coupled with aspartate aminotransferases from beef liver and pig heart, respectively. Solely discontinuous assays have been used to determine the activity of l-2,4-diaminobutyrate aminotransferases from A. baumannii and H. elongata (14, 28). The continuous assay described in this report provides a more convenient and rapid method to obtain substrate specificity constants for keto acids in the reverse reaction. This also allowed us to determine the true Km and kcat values for this aminotransferase.
Kinetic parameters for the l-2,4-diaminobutyrate aminotransferase from H. elongata have not been reported. A previous study of the enzyme from A. baumannii, on the other hand, yielded apparent Km values of 4.30 mM for l-2,4-diaminobutyrate and 1.46 mM for α-ketoglutarate (14). In contrast, the true Km values for l-2,4-diaminobutyrate and α-ketoglutarate were lower by an order of magnitude at 0.26 mM and 0.18 mM, respectively, for PvdH. The Km for pyruvate with the A. baumannii enzyme has not been reported; however, the specific activity for pyruvate was reported to be 4% of the activity obtained with α-ketoglutarate. This specific activity was determined by using 10 mM keto acid. In contrast, the kcat of PvdH when pyruvate was used as the amino acceptor is similar to the value obtained with α-ketoglutarate (Table 2). By using the kinetic parameters determined for PvdH, the specific activity of PvdH with 10 mM pyruvate and 10 mM l-2,4-diaminobutyrate was calculated to be 52% of the activity obtained with 10 mM α-ketoglutarate, which is significantly greater than the corresponding relative activities reported for the A. baumannii enzyme.
PvdH can also utilize oxaloacetate as an amino acceptor, although the specific activity is 20-fold lower than that with α-ketoglutarate. No activity was observed with α-ketobutyrate. It appears that carbon chain length and the presence of the distal carboxyl group of the keto acids have important bearing on substrate specificity. For example, α-ketobutyrate lacks a distal carboxyl group and is one carbon shorter than α-ketoglutarate. Although oxaloacetate is also one carbon shorter than α-ketoglutarate, it possesses a distal carboxyl group, and the enzyme shows some activity with this amino acceptor. It is, however, possible that a bulky carboxyl group at the β carbon position of the keto acid, like that in oxaloacetate, also results in an unfavorable steric interaction; therefore, the specific activity with this substrate is lower than that with α-ketoglutarate. Thus, higher activity was observed with pyruvate, which does not contain a distal carboxyl group or a β-substituent. The absence of a distal carboxyl group combined with a shorter side chain has a prominent effect on the Km for pyruvate, which is 34-fold higher than that for α-ketoglutarate.
We found that l-2,4-diaminobutyrate, which is required for pyoverdine synthesis, is produced by the aminotransferase PvdH. It has been predicted that during the synthesis of the pyoverdine chromophore, PvdL, a nonribosomal peptide synthetase, catalyzes the peptide linkage between l-2,4-diaminobutyrate and d-tyrosine (26). The enzyme required for cyclization of l-2,4-diaminobutyrate to form the pyrimidine ring of the pyoverdine chromophore remains to be discovered.
Pyoverdine is an important virulence factor in P. aeruginosa (24, 39). Therefore, the development of novel antimicrobial compounds directed toward specific pyoverdine synthesis proteins, such as PvdH, would be beneficial. In this regard, the specificity data obtained in this study and the continuous assay developed for this enzyme may facilitate the design and kinetic analysis of potential inhibitors.
Acknowledgments
This research was supported by grants from the Canadian Institutes of Health Research (grant MOP-62879), the Canadian Foundation for Innovation, and Ontario Innovation Trust.
We thank Herbert Schweizer for providing the asd mutant and Gerard Wright for providing the E. coli strain containing hom6. We also thank Joseph Lam and members of his laboratory for guidance on the construction of P. aeruginosa knockout mutants.
REFERENCES
- 1.Ackerley, D. F., T. T. Caradoc-Davies, and I. L. Lamont. 2003. Substrate specificity of the nonribosomal peptide synthetase PvdD from Pseudomonas aeruginosa. J. Bacteriol. 185:2848-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrio-Dupont, M., P. R. Coulet, and D. C. Gautheron. 1985. Coupled reaction of immobilized aspartate aminotransferase and malate dehydrogenase. A plausible model for the cellular behaviour of these enzymes. Biochim. Biophys. Acta 829:58-68. [DOI] [PubMed] [Google Scholar]
- 3.Böckmann, M., K. Taraz, and H. Budzikiewicz. 1997. Biogenesis of the pyoverdin chromophore. Z. Naturforsch. C 52:319-324.
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Chipperfield, J. R., and C. Ratledge. 2000. Salicylate is not a bacterial siderophore: a theoretical study. Biometals 13:165-168. [DOI] [PubMed] [Google Scholar]
- 6.Cornish-Bowden, A. 1995. Analysis of enzyme kinetic data. Oxford University Press, New York, N.Y.
- 7.Cox, C. D., K. L. Rinehart, Jr., M. L. Moore, and J. C. Cook, Jr. 1981. Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 78:4256-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lorenzo, V., L. Eltis, B. Kessler, and K. N. Timmis. 1993. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene 123:17-24. [DOI] [PubMed] [Google Scholar]
- 9.Ferscht, A. 1999. Structure and mechanism in protein science: a guide to enzyme catalysis and protein folding. W.H. Freeman and Company, New York, N.Y.
- 10.Govan, J. R. W., and T. Bergan. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handfield, M., Lehoux, D. E., F. Sanschagrin, M. J. Mahan, D. E. Woods, and R. C. Levesque. 2000. In vivo-induced genes in Pseudomonas aeruginosa. Infect. Immun. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 13.Hoang, T. T., S. Williams, H. P. Schweizer, and J. S. Lam. 1997. Molecular genetic analysis of the region containing the essential Pseudomonas aeruginosa asd gene encoding aspartate-β-semialdehyde dehydrogenase. Microbiology 143:899-907. [DOI] [PubMed] [Google Scholar]
- 14.Ikai, H., and S. Yamamoto. 1997. Identification and analysis of a gene encoding l-2,4-diaminobutyrate:2-ketoglutarate 4-aminotransferase involved in the 1,3-diaminopropane production pathway in Acinetobacter baumannii. J. Bacteriol. 179:5118-5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacques, S. L., L. J. Ejim, and G. D. Wright. 2004. Homoserine dehydrogenase from Saccharomyces cerevisiae: kinetic mechanism and stereochemistry of hydride transfer. Biochim. Biophys. Acta 1544:42-54. [DOI] [PubMed] [Google Scholar]
- 16.Jacques, S. L., C. Nieman, D. Bareich, G. Broadhead, R. Kinach, J. F. Honek, and G. D. Wright. 2001. Characterization of yeast homoserine dehydrogenase, an antifungal target: the invariant histidine 309 is important for enzyme integrity. Biochim. Biophys. Acta 1544:28-41. [DOI] [PubMed] [Google Scholar]
- 17.Julich, M., K. Taraz, H. Budzikiewicz, V. Geoffroy, J. M. Meyer, and L. Gardan. 2001. The structure of the pyoverdin isolated from various Pseudomonas syringae pathovars. Z. Naturforsch. C 56:687-694. [DOI] [PubMed]
- 18.Kane, J. F. 1995. Effects of rare codon clusters on high-level expression of heterologous proteins in Escherichia coli. Curr. Opin. Biotechnol. 6:494-500. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Lamont, I. L., P. A. Beare, U. Ochsner, A. I. Vasil, and M. L. Vasil. 2002. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 99:7072-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamont, I. L., and L. W. Martin. 2003. Identification and characterization of novel pyoverdine synthesis genes in Pseudomonas aeruginosa. Microbiology 149:833-842. [DOI] [PubMed] [Google Scholar]
- 22.Lehoux, D. E., F. Sanschagrin, and R. C. Levesque. 2000. Genomics of the 35-kb pvd locus and analysis of novel pvdIJK genes implicated in pyoverdine biosynthesis in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 190:141-146. [DOI] [PubMed] [Google Scholar]
- 23.Merriman, T. R., M. E. Merriman, and I. L. Lamont. 1995. Nucleotide sequence of pvdD, a pyoverdine biosynthetic gene from Pseudomonas aeruginosa: PvdD has similarity to peptide synthetases. J. Bacteriol. 177:252-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer, J. M., A. Neely, A. Stintzi, C. Georges, and I. A. Holder. 1996. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 64:518-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer, J.-M., and M. A. Abdallah. 1978. The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physicochemical properties. J. Gen. Microbiol. 107:319-328. [Google Scholar]
- 26.Mossialos, D., U. Ochsner, C. Baysse, P. Chablain, J. P. Pirnay, N. Koedam, H. Budzikiewicz, D. U. Fernandez, M. Schafer, J. Ravel, and P. Cornelis. 2002. Identification of new, conserved, non-ribosomal peptide synthetases from fluorescent pseudomonads involved in the biosynthesis of the siderophore pyoverdine. Mol. Microbiol. 45:1673-1685. [DOI] [PubMed] [Google Scholar]
- 27.Ochsner, U. A., P. J. Wilderman, A. I. Vasil, and M. L. Vasil. 2002. GeneChip expression analysis of the iron starvation response in Pseudomonas aeruginosa: identification of novel pyoverdine biosynthesis genes. Mol. Microbiol. 45:1277-1287. [DOI] [PubMed] [Google Scholar]
- 28.Ono, H., K. Sawada, N. Khunajakr, T. Tao, M. Yamamoto, M. Hiramoto, A. Shinmyo, M. Takano, and Y. Murooka. 1999. Characterization of biosynthetic enzymes for ectoine as a compatible solute in a moderately halophilic eubacterium, Halomonas elongata. J. Bacteriol. 181:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters, P., E. A. Galinski, and H. G. Trüper. 1990. The biosynthesis of ectoine. FEMS Microbiol. Lett. 71:162. [Google Scholar]
- 30.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 31.Ravel, J., and P. Cornelis. 2003. Genomics of pyoverdine-mediated iron uptake in pseudomonads. Trends Microbiol. 11:195-200. [DOI] [PubMed] [Google Scholar]
- 32.Salerno, C., J. Ovádi, T. Keleti, and P. Fasella. 1982. Kinetics of coupled reactions catalyzed by aspartate aminotransferase and glutamate dehydrogenase. Eur. J. Biochem. 121:511-517. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Schweizer, H. P. 1993. Small broad-host-range gentamycin resistance cassettes for site-specific insertion and deletion mutagenesis. BioTechniques 15:831-834. [PubMed] [Google Scholar]
- 35.Schweizer, H. P., and T. T. Hoang. 1995. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158:15-22. [DOI] [PubMed] [Google Scholar]
- 36.Silverman, R. B. 2000. The organic chemistry of enzyme catalyzed reactions. Academic Press, San Diego, Calif.
- 37.Stintzi, A., P. Cornelis, D. Hohnadel, J. M. Meyer, C. Dean, K. Poole, S. Kourambas, and V. Krishnapillai. 1996. Novel pyoverdine biosynthesis gene(s) of Pseudomonas aeruginosa PAO. Microbiology 142:1181-1190. [DOI] [PubMed] [Google Scholar]
- 38.Tabor, S., and C. C. Richardson. 1985. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 82:1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takase, H., H. Nitanai, K. Hoshino, and T. Otani. 2000. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect. Immun. 68:1834-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, J., A. Mushegian, S. Lory, and S. Jin. 1996. Large-scale isolation of candidate virulence genes of Pseudomonas aeruginosa by in vivo selection. Proc. Natl. Acad. Sci. USA 93:10434-10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wendenbaum, S., P. Demange, A. Dell, J.-M. Meyer, and M. A. Abdallah. 1983. The structure of pyoverdine Pa, the siderophore of Pseudomonas aeruginosa. Tetrahedron Lett. 24:4877-4880. [Google Scholar]