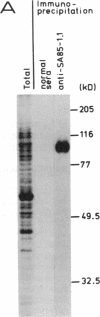
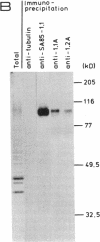
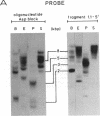
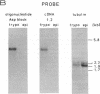
Abstract
Trypanosoma cruzi, an intracellular protozoan parasite infecting a wide variety of vertebrates, is the agent responsible for Chagas disease in humans. An estimated 15-20 million people in South and Central America are infected with the parasite. Chagas disease often results in severe autoimmune and inflammatory pathology and is the major cause of heart failure in endemic areas. Nevertheless, little is known about the host-parasite interactions that lead to this pathology. We have previously cloned several members of a large gene family (SA85-1) and shown that these genes encode 85-kDa T. cruzi, mammalian-stage-specific, surface antigens. Here we report that members of the SA85-1 family possess sialidase activity and are shed by the parasite. We suggest that the sialidases may contribute to the pathology during T. cruzi infection by cleaving sialic acid from cells of the immune system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M., Laver W. G., Luo M., Stray S. J., Legrone G., Webster R. G. Antigenic, sequence, and crystal variation in influenza B neuraminidase. Virology. 1990 Aug;177(2):578–587. doi: 10.1016/0042-6822(90)90523-t. [DOI] [PubMed] [Google Scholar]
- Air G. M., Ritchie L. R., Laver W. G., Colman P. M. Gene and protein sequence of an influenza neuraminidase with hemagglutinin activity. Virology. 1985 Aug;145(1):117–122. doi: 10.1016/0042-6822(85)90206-5. [DOI] [PubMed] [Google Scholar]
- Air G. M., Webster R. G., Colman P. M., Laver W. G. Distribution of sequence differences in influenza N9 neuraminidase of tern and whale viruses and crystallization of the whale neuraminidase complexed with antibodies. Virology. 1987 Oct;160(2):346–354. doi: 10.1016/0042-6822(87)90005-5. [DOI] [PubMed] [Google Scholar]
- Andrews N. W., Katzin A. M., Colli W. Mapping of surface glycoproteins of Trypanosoma cruzi by two-dimensional electrophoresis. A correlation with the cell invasion capacity. Eur J Biochem. 1984 May 2;140(3):599–604. doi: 10.1111/j.1432-1033.1984.tb08144.x. [DOI] [PubMed] [Google Scholar]
- Andrews N. W., Robbins E. S., Ley V., Hong K. S., Nussenzweig V. Developmentally regulated, phospholipase C-mediated release of the major surface glycoprotein of amastigotes of Trypanosoma cruzi. J Exp Med. 1988 Feb 1;167(2):300–314. doi: 10.1084/jem.167.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Remington J. S. Characterization of stages and strains of Trypanosoma cruzi by analysis of cell membrane components. J Immunol. 1981 Sep;127(3):855–859. [PubMed] [Google Scholar]
- Brandley B. K., Swiedler S. J., Robbins P. W. Carbohydrate ligands of the LEC cell adhesion molecules. Cell. 1990 Nov 30;63(5):861–863. doi: 10.1016/0092-8674(90)90487-y. [DOI] [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Cavallesco R., Pereira M. E. Antibody to Trypanosoma cruzi neuraminidase enhances infection in vitro and identifies a subpopulation of trypomastigotes. J Immunol. 1988 Jan 15;140(2):617–625. [PubMed] [Google Scholar]
- Concannon P., Kwolek C. J., Salser W. A. Nucleotide sequence of the influenza virus A/USSR/90/77 neuraminidase gene. J Virol. 1984 May;50(2):654–656. doi: 10.1128/jvi.50.2.654-656.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988 Nov 25;16(22):10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale B., Brown R., Miller J., White R. T., Air G. M., Cordell B. Nucleotide and deduced amino acid sequence of the influenza neuraminidase genes of two equine serotypes. Virology. 1986 Dec;155(2):460–468. doi: 10.1016/0042-6822(86)90207-2. [DOI] [PubMed] [Google Scholar]
- Fields S., Winter G., Brownlee G. G. Structure of the neuraminidase gene in human influenza virus A/PR/8/34. Nature. 1981 Mar 19;290(5803):213–217. doi: 10.1038/290213a0. [DOI] [PubMed] [Google Scholar]
- Harley V. R., Ward C. W., Hudson P. J. Molecular cloning and analysis of the N5 neuraminidase subtype from an avian influenza virus. Virology. 1989 Mar;169(1):239–243. doi: 10.1016/0042-6822(89)90063-9. [DOI] [PubMed] [Google Scholar]
- Harth G., Haidaris C. G., So M. Neuraminidase from Trypanosoma cruzi: analysis of enhanced expression of the enzyme in infectious forms. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8320–8324. doi: 10.1073/pnas.84.23.8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Wallace J. C., Brown J. P. Finding protein similarities with nucleotide sequence databases. Methods Enzymol. 1990;183:111–132. doi: 10.1016/0076-6879(90)83009-x. [DOI] [PubMed] [Google Scholar]
- Hiti A. L., Nayak D. P. Complete nucleotide sequence of the neuraminidase gene of human influenza virus A/WSN/33. J Virol. 1982 Feb;41(2):730–734. doi: 10.1128/jvi.41.2.730-734.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn S., Van Voorhis W. C., Eisen H. The major 85-kD surface antigen of the mammalian form of Trypanosoma cruzi is encoded by a large heterogeneous family of simultaneously expressed genes. J Exp Med. 1990 Aug 1;172(2):589–597. doi: 10.1084/jem.172.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanar D. E., Manning J. E. Major surface proteins and antigens on the different in vivo and in vitro forms of Trypanosoma cruzi. Mol Biochem Parasitol. 1984 Apr;11:119–131. doi: 10.1016/0166-6851(84)90059-8. [DOI] [PubMed] [Google Scholar]
- Libby P., Alroy J., Pereira M. E. A neuraminidase from Trypanosoma cruzi removes sialic acid from the surface of mammalian myocardial and endothelial cells. J Clin Invest. 1986 Jan;77(1):127–135. doi: 10.1172/JCI112266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Nishida Y., Hisajima H., Miyata T., Kumahara Y., Nerome K., Oya A., Fukui T., Ohtsuka E., Ikehara M. The complete nucleotide sequence of the influenza virus neuraminidase gene of A/NJ/8/76 strain and its evolution by segmental duplication and deletion. Mol Biol Med. 1983 Nov;1(4):401–413. [PubMed] [Google Scholar]
- Myers R. W., Lee R. T., Lee Y. C., Thomas G. H., Reynolds L. W., Uchida Y. The synthesis of 4-methylumbelliferyl alpha-ketoside of N-acetylneuraminic acid and its use in a fluorometric assay for neuraminidase. Anal Biochem. 1980 Jan 1;101(1):166–174. doi: 10.1016/0003-2697(80)90056-1. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Chaplan S., Tydings J. D., Unkeless J., Cohn Z. Trypanosoma cruzi. Surface antigens of blood and culture forms. J Exp Med. 1981 Mar 1;153(3):629–639. doi: 10.1084/jem.153.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. E. A developmentally regulated neuraminidase activity in Trypanosoma cruzi. Science. 1983 Mar 25;219(4591):1444–1446. doi: 10.1126/science.6338592. [DOI] [PubMed] [Google Scholar]
- Peterson D. S., Fouts D. L., Manning J. E. The 85-kd surface antigen gene of Trypanosoma cruzi is telomeric and a member of a multigene family. EMBO J. 1989 Dec 1;8(12):3911–3916. doi: 10.1002/j.1460-2075.1989.tb08571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. S., Wrightsman R. A., Manning J. E. Cloning of a major surface-antigen gene of Trypanosoma cruzi and identification of a nonapeptide repeat. Nature. 1986 Aug 7;322(6079):566–568. doi: 10.1038/322566a0. [DOI] [PubMed] [Google Scholar]
- Petry K., Eisen H. Chagas disease: a model for the study of autoimmune diseases. Parasitol Today. 1989 Apr;5(4):111–116. doi: 10.1016/0169-4758(89)90052-5. [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Plata F., Garcia Pons F., Eisen H. Antigenic polymorphism of Trypanosoma cruzi: clonal analysis of trypomastigote surface antigens. Eur J Immunol. 1984 May;14(5):392–399. doi: 10.1002/eji.1830140503. [DOI] [PubMed] [Google Scholar]
- Prioli R. P., Mejia J. S., Pereira M. E. Monoclonal antibodies against Trypanosoma cruzi neuraminidase reveal enzyme polymorphism, recognize a subset of trypomastigotes, and enhance infection in vitro. J Immunol. 1990 Jun 1;144(11):4384–4391. [PubMed] [Google Scholar]
- Roggentin P., Rothe B., Kaper J. B., Galen J., Lawrisuk L., Vimr E. R., Schauer R. Conserved sequences in bacterial and viral sialidases. Glycoconj J. 1989;6(3):349–353. doi: 10.1007/BF01047853. [DOI] [PubMed] [Google Scholar]
- Roggentin P., Rothe B., Lottspeich F., Schauer R. Cloning and sequencing of a Clostridium perfringens sialidase gene. FEBS Lett. 1988 Sep 26;238(1):31–34. doi: 10.1016/0014-5793(88)80219-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Rothe B., Roggentin P., Frank R., Blöcker H., Schauer R. Cloning, sequencing and expression of a sialidase gene from Clostridium sordellii G12. J Gen Microbiol. 1989 Nov;135(11):3087–3096. doi: 10.1099/00221287-135-11-3087. [DOI] [PubMed] [Google Scholar]
- Russo T. A., Thompson J. S., Godoy V. G., Malamy M. H. Cloning and expression of the Bacteroides fragilis TAL2480 neuraminidase gene, nanH, in Escherichia coli. J Bacteriol. 1990 May;172(5):2594–2600. doi: 10.1128/jb.172.5.2594-2600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier E., Roeske H., Driesel G., Künkel U., Petzold D. R., Berlinghoff R., Michel S. Complete nucleotide sequence of the neuraminidase gene of the human influenza virus A/Chile/1/83 (H1N1). Brief report. Arch Virol. 1988;99(3-4):271–276. doi: 10.1007/BF01311076. [DOI] [PubMed] [Google Scholar]
- Shaw M. W., Lamb R. A., Erickson B. W., Briedis D. J., Choppin P. W. Complete nucleotide sequence of the neuraminidase gene of influenza B virus. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6817–6821. doi: 10.1073/pnas.79.22.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto-Padrón T., Harth G., de Souza W. Immunocytochemical localization of neuraminidase in Trypanosoma cruzi. Infect Immun. 1990 Mar;58(3):586–592. doi: 10.1128/iai.58.3.586-592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Steuler H., Rohde W., Scholtissek C. Sequence of the neuraminidase gene of an avian influenza A virus (A/parrot/ulster/73, H7N1). Virology. 1984 May;135(1):118–124. doi: 10.1016/0042-6822(84)90122-3. [DOI] [PubMed] [Google Scholar]
- Takle G. B., Young A., Snary D., Hudson L., Nicholls S. C. Cloning and expression of a trypomastigote-specific 85-kilodalton surface antigen gene from Trypanosoma cruzi. Mol Biochem Parasitol. 1989 Nov;37(1):57–64. doi: 10.1016/0166-6851(89)90102-3. [DOI] [PubMed] [Google Scholar]
- Teixeira M. M., Yoshida N. Stage-specific surface antigens of metacyclic trypomastigotes of Trypanosoma cruzi identified by monoclonal antibodies. Mol Biochem Parasitol. 1986 Mar;18(3):271–282. doi: 10.1016/0166-6851(86)90085-x. [DOI] [PubMed] [Google Scholar]
- True D. D., Singer M. S., Lasky L. A., Rosen S. D. Requirement for sialic acid on the endothelial ligand of a lymphocyte homing receptor. J Cell Biol. 1990 Dec;111(6 Pt 1):2757–2764. doi: 10.1083/jcb.111.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Eisen H. Fl-160. A surface antigen of Trypanosoma cruzi that mimics mammalian nervous tissue. J Exp Med. 1989 Mar 1;169(3):641–652. doi: 10.1084/jem.169.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990 Nov 23;250(4984):1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- Wrightsman R. A., Leon W., Manning J. E. Variation in antigenic determinants specific to the infective stage of Trypanosoma cruzi. Infect Immun. 1986 Aug;53(2):235–239. doi: 10.1128/iai.53.2.235-239.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingales B., Carniol C., de Lederkremer R. M., Colli W. Direct sialic acid transfer from a protein donor to glycolipids of trypomastigote forms of Trypanosoma cruzi. Mol Biochem Parasitol. 1987 Nov;26(1-2):135–144. doi: 10.1016/0166-6851(87)90137-x. [DOI] [PubMed] [Google Scholar]
- de Titto E. H., Araujo F. G. Serum neuraminidase activity and hematological alterations in acute human Chagas' disease. Clin Immunol Immunopathol. 1988 Jan;46(1):157–161. doi: 10.1016/0090-1229(88)90016-5. [DOI] [PubMed] [Google Scholar]