Abstract
Bordetella species utilize the BvgAS (Bordetella virulence gene) two-component signal transduction system to sense the environment and regulate gene expression among at least three phases: a virulent Bvg+ phase, a nonvirulent Bvg− phase, and an intermediate Bvgi phase. Genes expressed in the Bvg+ phase encode known virulence factors, including adhesins such as filamentous hemagglutinin (FHA) and fimbriae, as well as toxins such as the bifunctional adenylate cyclase/hemolysin (ACY). Previous studies showed that in the Bvgi phase, FHA and fimbriae continue to be expressed, but ACY expression is significantly downregulated. In this report, we determine that Bordetella bronchiseptica can form biofilms in vitro and that the generation of biofilm is maximal in the Bvgi phase. We show that FHA is required for maximal biofilm formation and that fimbriae may also contribute to this phenotype. However, expression of ACY inhibits biofilm formation, most likely via interactions with FHA. Therefore, the coordinated regulation of adhesins and ACY expression leads to maximal biofilm formation in the Bvgi phase in B. bronchiseptica.
Bordetella pertussis, Bordetella parapertussis, and Bordetella bronchiseptica are closely related gram-negative coccobacilli that colonize the upper respiratory tract of mammals. B. pertussis and most B. parapertussis strains are obligate human pathogens that usually cause acute respiratory diseases. B. bronchiseptica has a much broader host range and is considered to be representative of the evolutionary progenitor of all Bordetella spp. (10, 27). It naturally infects many laboratory animals, including mice, rats, and rabbits, and thus serves as an ideal model for studying bacterial pathogenesis in a natural infection setting. Although B. bronchiseptica has been associated with various respiratory diseases, infection by this organism generally leads to chronic and asymptomatic colonization in the host. This lifestyle indicates that the bacteria employ specific mechanisms to counteract host immune responses and also implies successful interactions with other commensal bacteria commonly found in the upper respiratory tract.
Most known virulence factors in Bordetella are regulated by the BvgAS (Bordetella virulence gene) two-component signal transduction system (21). In response to environmental stimuli, BvgAS undergoes a series of phosphorelay signal transduction events that ultimately lead to differential transcriptions of target genes (6). Bacteria grown in rich media at 37°C exhibit the virulent Bvg+ phase, and this phase is characterized by the expression of virulence factors, such as filamentous hemagglutinin (FHA), fimbriae, and bifunctional adenylate cyclase/hemolysin (ACY). Specific genes, such as those required for motility (2), are repressed in the Bvg+ phase but are expressed when the bacteria are grown in Bvg−-phase conditions. The BvgAS system is not a simple on/off switch, as a distinct intermediate Bvgi phase can be achieved with growth of the bacteria in phase-modulating conditions that are between that of the extreme Bvg+ and Bvg− phases. The Bvgi phase is characterized by expression of specific genes, e.g., bipA (30), that are highly expressed only in the Bvgi phase but not in the Bvg+ or Bvg− phases. However, some genes are highly expressed in both the Bvg+ and Bvgi phases (e.g., those encoding FHA and fimbriae), whereas others are expressed in the Bvg+ but not the Bvgi phase (e.g., ACY) (8). Although the actual environmental signal(s) sensed by BvgAS during infection has not yet been identified, certain laboratory growth conditions can be used to modulate the Bordetella expression profile to the Bvg− phase: growth at room temperature (<25°C) or in the presence of millimolar concentrations of nicotinic acid or MgSO4 (16). A semimodulating concentration of nicotinic acid concentration between 0.2 and 1.6 mM nicotinic acid in the growth medium leads to the Bvgi-phase phenotype (8). Furthermore, there are specific mutants of the BvgAS system that permanently lock the bacteria in each of the three phases, and they are insensitive to environmental modulations (7, 8).
FHA and fimbriae are two major adhesins that have been studied in Bordetella spp. FHA displays multiple attachment activities (20) and has been demonstrated to be important for adhesion of Bordetella spp. to cell surfaces (30) and also for the colonization of the trachea in animal models (9). Bordetella fimbriae has also been demonstrated to function as an adhesin in vitro and in vivo (22, 23). While both FHA and fimbriae are generally considered Bvg+-phase factors, they remain highly expressed in the Bvgi phase as well (6).
ACY is a bifunctional protein displaying both the adenylate cyclase and hemolytic activities. It can be translocated into infected host cells where it catalyzes the production of intracellular cyclic AMP, resulting in the suppression of various host cell functions (17). It also plays an important role in the interaction of the bacteria with neutrophils in vivo (11). However, ACY has also been shown to be associated with the cell surface of Bordetella and can bind specifically to FHA in vitro (36). ACY is highly expressed in the Bvg+ phase but is significantly downregulated in the Bvgi phase (8).
Biofilms are bacterial communities that adopt a surface-adapted, adherent multicellular lifestyle that appears to be fundamentally different from the free-living planktonic state (24, 33, 34). Biofilm communities may be the predominant lifestyle of most bacteria in nature and may also be that of bacteria that have adapted to adherent lifestyles on various artificial structures. The role of biofilms in the pathogenesis of various bacterial infections may be particularly important, as many chronic infections, such as cystic fibrosis airway infections by Pseudomonas aeruginosa, endocarditis, and periodontitis, are strongly associated with biofilm formation (5, 28). Regulation of biofilm formation in various bacterial species has been shown to be dependent on the expression of various cell surface structures and proteins (24). Furthermore, specific signaling pathways and cell-cell communication mechanisms are also important to the establishment of many well-studied biofilms and the dynamic equilibrium that is thought to exist between planktonic bacterial cells and biofilms (13).
In this report, we show that B. bronchiseptica can form biofilms in vitro and that the BvgAS system regulates this phenotype. We show that FHA and fimbriae contribute to the formation of biofilm, but ACY inhibits the generation of biofilm. We propose that the differential regulation of FHA, fimbriae, and ACY in various Bvg phases, coupled with the interaction between FHA and ACY, give rise to a strong biofilm phenotype in the Bvgi phase.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. bronchiseptica strains RB50 (wild type), RB53i (Bvgi phase-locked, bvgS R570H, T733M), RB58 (ΔcyaA, deleted of all but 61 codons at the 5′ end and 65 codons at the 3′ end, resulting in >97.5% of the gene deleted), RBX9 (ΔfhaB, deleted of all but four codons at the 5′ end and five codons at the 3′ end), and RB63 (ΔfimBCD, deleted from codon 72 of fimB to codon 327 of fimD of the fimBCD locus) were previously reported and well characterized (7-9, 11, 22). All of these mutants were in-frame deletions. A double in-frame deletion mutant in both fhaB and cyaA was constructed by an allelic exchange strategy as described previously (1), using the same vectors that were used for construction of RBX9 and RB58. All strains were cultured in Stainer-Scholte (SS) liquid medium (29) or on BG agar (Becton Dickinson) supplemented with defibrinated sheep blood at 37°C. For Bvg phase modulation, bacteria were grown in SS media with nicotinic acid (Sigma) added to appropriate final concentrations.
Microscopy.
Glass coverslips with attached biofilm from overnight cultures were stained with Syto Red 17 (a nucleic acid stain; Molecular Probes) for 30 min, which labels both live and dead cells. The coverslips were washed and then mounted onto microscope slides with antifade reagent (SlowFade Light Antifade kit; Molecular Probes). A Nikon MICROPHOT FXA epifluorescence microscope was used to observe the specimens. A deconvolution micrograph was taken with a Leica DM R epifluorescence microscope with deconvolution software (Improvision Volocity).
Quantitative assay of biofilm.
Biofilms were grown in non-tissue-culture-coated 96-well round-bottom polystyrene plates (Corning) essentially as described previously (26). Briefly, overnight cultures were inoculated to 1:20 dilutions (for single-strain biofilms) or 1:40 dilutions per strain (for dual-strain biofilms) and were added to 100 μl of SS/well supplemented with appropriate concentrations of nicotinic acid. After 24 h of incubation at 37°C, each well was washed with water and was stained with 150 μl of crystal violet solution (Becton Dickinson). The dye was then removed by thorough washing with water. For quantification of attached cells, crystal violet was solubilized in 200 μl of 33% acetic acid and the absorbance was measured at 595 nm. All strains were grown in triplicate for individual experiments, and the values were averaged with standard deviation of errors shown.
RESULTS
B. bronchiseptica generates a maximal biofilm phenotype in the Bvgi phase in vitro.
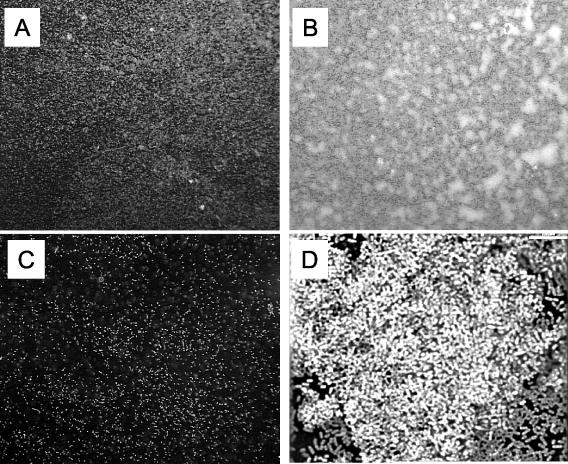
We had initially observed that wild-type B. bronchiseptica grown in Bvgi-phase conditions (e.g., in 0.8 mM nicotinic acid) or a Bvgi-phase-locked strain (RB53i, bvgS R570H, T733M point mutant; remains in Bvgi phase regardless of growth conditions) primarily formed thick aggregates and grew adherent to the polystyrene test tubes (Fig. 1) instead of the predominant suspension liquid cultures of bacteria grown in Bvg+ or Bvg− phases. The adherent aggregates were particularly pronounced at the liquid-air interface region of the cultures grown in tilted roller drums. We examined this phenotype by microscopy to determine the presence of microcolony formation (a hallmark of biofilm formation) under Bvgi conditions (Fig. 2). Wild-type bacteria grown in Bvg+ phase on glass coverslips formed a thin layer with small aggregates within the layer (Fig. 2A). Wild-type bacteria grown in Bvgi phase, however, formed more distinct and larger microcolonies (Fig. 2B). The three-dimensional architecture of a microcolony shown in Fig. 2B can be seen in Fig. 2D, which is a deconvoluted image of a microcolony at higher magnification. Bacteria grown in Bvg− phase did not attach significantly to the coverslip, and the attached bacteria did not show formation of microcolonies (Fig. 2C). Therefore, B. bronchiseptica appears to form a relatively weak biofilm at Bvg+ phase but a strong biofilm phenotype can be observed in the Bvgi phase.
FIG. 1.
Biofilm formation by B. bronchiseptica grown in the Bvgi phase. Overnight liquid cultures of B. bronchiseptica were grown in the Bvgi phase (0.8 mM nicotinic acid, left) or Bvg− phase (4 mM nicotinic acid, right) in polystyrene culture tubes in continuous rotation on roller drums. In the Bvgi phase, a majority of the bacteria were adherent to the test tube wall, in contrast to bacteria that was grown in Bvg− phase (or Bvg+ phase; data not shown) in which most bacterial cells remained in the liquid media.
FIG. 2.
Formation of microcolonies by B. bronchiseptica on glass coverslips. Wild-type B. bronchiseptica organisms were grown on glass coverslips and then were stained with Syto Red 17 and observed under a fluorescent microscope (20× objective and 10× eyepiece). (A) Culture medium with no nicotinic acid (Bvg+ phase). (B) Culture medium supplemented with 0.8 mM nicotinic acid (Bvgi phase). (C) Culture medium supplemented with 4 mM nicotinic acid (Bvg− phase). Bacteria grown in Bvg+ phase (A) appear to form small aggregates, whereas microcolonies formed by bacteria grown in Bvgi phase are large and distinct (B). Bacteria in Bvg− phase (C) displayed little adherence to the coverslip with no aggregative properties. (D) Deconvolution micrograph of a microcolony depicted in panel B displaying the cellular architecture of the microcolony. Bar, 7 μm.
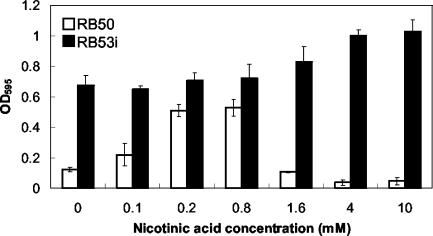
We quantitatively assayed the biofilm formation in polystyrene 96-well plates at various concentrations of nicotinic acid to determine the variation of this phenotype at different Bvg phases. Figure 3 shows that with increasing nicotinic acid concentrations, biofilm formation by wild-type B. bronchiseptica reached a maximum at 0.8 mM nicotinic acid and decreased with further increases of nicotinic acid concentration. A Bvgi-phase-locked mutant showed large amounts of biofilm formation regardless of nicotinic acid concentration in the growth medium. These observations confirm that B. bronchiseptica forms a strong biofilm phenotype primarily in the Bvgi phase. The absorbance values of crystal violet stains (used for quantitation of biofilm formation) was not a simple measure of bacterial growth, as they did not correlate with the total growth of the bacteria in these wells (i.e., bacterial growth was not maximal at 0.8 mM nicotinic acid; data not shown). While Fig. 3 is a representative result of several experiments, the absolute maximum absorbance values varied between 0.5 and 0.8 in experiments performed on different days, and the maximum biofilm phenotype was always observed in the range of nicotinic acid concentrations between 0.2 and 0.8 mM.
FIG. 3.
Quantitative assay of biofilm formation by wild-type (RB50) and Bvgi-phased-locked (RB53i) B. bronchiseptica at different nicotinic acid concentrations. Bacteria were grown in 96-well polystyrene plates, and biofilm formation was quantified by absorbance of solubilized crystal violet stains, as described in Materials and Methods. Biofilm formation in the wild-type bacteria peaked in the Bvgi phase (0.2 to 0.8 mM nicotinic acid). The Bvgi-phase-locked mutant formed high levels of biofilm at all nicotinic acid concentrations. Bvg+-phase growth condition is 0 to 0.1 mM nicotinic acid, 0.2 to 1.6 mM is Bvgi phase, and 4.0 mM (and above) is Bvg− phase. OD595, optical density at 595 nm.
FHA is required for maximum biofilm formation in B. bronchiseptica.
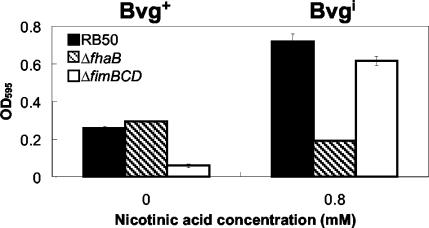
Various adhesins have been shown to be important for the formation of biofilm in other bacterial species (24). We examined the possible role of two Bvg-regulated adhesins expressed by Bordetella spp., FHA and fimbriae, in biofilm formation (Fig. 4). In a comparison of biofilm formation by wild-type B. bronchiseptica and a mutant with an in-frame deletion in the structural gene encoding FHA (ΔfhaB), the mutant formed significantly less biofilm in the Bvgi phase (at 0.8 mM nicotinic acid). However, there was no significant decrease of biofilm formation in the Bvg+ phase compared to that of the wild-type bacteria. On the other hand, a mutant that does not express fimbriae (ΔfimBCD) was highly attenuated in biofilm formation in the Bvg+ phase but did not show significant decreases in biofilm formation in the Bvgi phase. These results suggest that FHA plays a primary role in the formation of the strong biofilm phenotype in the Bvgi phase. Expression of fimbriae appears to be required for the weaker biofilm phenotype that is observed in the Bvg+ phase.
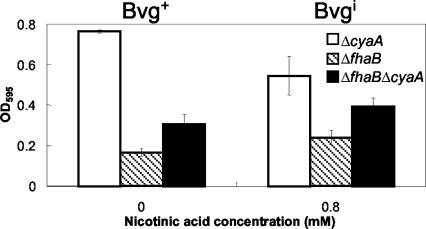
FIG. 4.
Quantitative assay of biofilm formation in wild-type B. bronchiseptica (RB50), ΔfhaB mutant, and ΔfimBCD mutant in the Bvg+ phase (0 mM nicotinic acid) and Bvgi phase (0.8 mM nicotinic acid). Bacteria were grown in 96-well polystyrene plates, and biofilm formation was quantified by absorbance of solubilized crystal violet stains, as described in Materials and Methods. The amount of biofilm formed by the ΔfhaB mutant in the Bvg+ phase was similar to that of the wild-type but was significantly decreased in the Bvgi phase. The ΔfimBCD mutant appears to form almost no biofilm in the Bvg+ phase, but the amount of biofilm formed by this mutant in the Bvgi phase was comparable to that of the wild-type bacteria. OD595, optical density at 595 nm.
ACY inhibits biofilm formation in B. bronchiseptica.
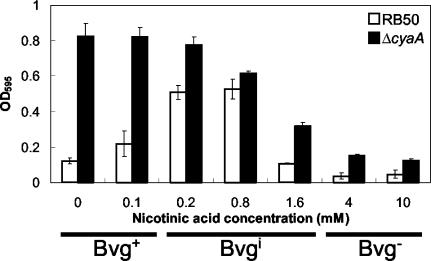
It was previously observed that a mutant B. bronchiseptica strain with an in-frame deletion of the cyaA gene (which codes for ACY) demonstrated a liquid culture phenotype of aggregation and adherence similar to that of a Bvgi-phase-locked mutant, even when the bacteria were grown in Bvg+ conditions. This indicates that ACY mutants may demonstrate a strong biofilm phenotype even in the Bvg+ phase, in contrast to wild-type bacteria, which have a maximal biofilm phenotype in the Bvgi phase. Figure 5 shows that the ACY deletion mutant formed a strong biofilm phenotype in both the Bvg+ and Bvgi phase but not in the Bvg− phase. This suggests that ACY suppresses biofilm formation in B. bronchiseptica. A previous report has demonstrated a direct protein-protein interaction of ACY with FHA (36). Because FHA appears to be a major contributor to biofilm formation in B. bronchiseptica, we propose that ACY may inhibit biofilm formation via its interaction with FHA. This result is also consistent with previous observations that FHA is highly expressed in both Bvg+ and Bvgi phases and that ACY is highly expressed in the Bvg+ but not in the Bvgi phase (8). We examined the biofilm formation of a double mutant strain with in-frame deletions in both cyaA and fhaB genes (Fig. 6). The amounts of biofilm formed by this double mutant in both Bvg+ and Bvgi phases were significantly lower than that of the single ACY mutant but were still higher than that of the single FHA mutant (Fig. 6). Overall, this observation is consistent with the hypothesis that the inhibition of biofilm formation by ACY is at least partially mediated via its interaction with FHA. However, because the double mutant forms more biofilm than the single FHA mutant, ACY may inhibit biofilm formation via other mechanisms besides its possible interaction with FHA.
FIG. 5.
Quantitative assay of biofilm formation by wild-type B. bronchiseptica (RB50) and ΔcyaA mutant at different nicotinic acid concentrations. Bacteria were grown in 96-well polystyrene plates, and biofilm formation was quantified by absorbance of solubilized crystal violet stains, as described in Materials and Methods. The ΔcyaA mutant formed high levels of biofilm in both Bvg+ and Bvgi phases compared to that of wild-type bacteria, which formed high levels of biofilm only in the Bvgi phase. OD595, optical density at 595 nm.
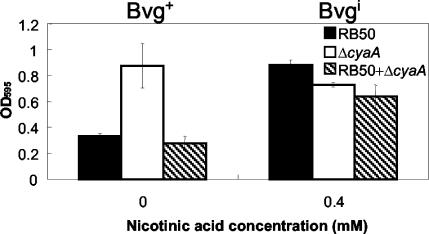
FIG. 6.
Comparative quantitative assay of biofilm formation by the ΔcyaA mutant, ΔfhaB mutant, and ΔfhaBΔcyaA double mutant in the Bvg+ phase (0 mM nicotinic acid) and Bvgi phase (0.8 mM nicotinic acid). Bacteria were grown in 96-well polystyrene plates, and biofilm formation was quantified by absorbance of solubilized crystal violet stains, as described in Materials and Methods. The double mutant formed higher levels of biofilm than the ΔfhaB mutant but formed lower levels than the ΔcyaA mutant. OD595, optical density at 595 nm.
ACY is secreted and also expressed on the cell surface by Bordetella spp. (36). We therefore tested if wild-type bacteria could complement in trans the inhibitory effect of ACY on biofilm formation by coculturing wild-type bacteria and ACY mutants. Wild-type B. bronchiseptica was cocultured with approximately equal numbers of the ACY mutant, and the overall level of biofilm formation was quantitatively assayed (Fig. 7). The coculture experiments showed that wild-type bacteria (which produce and secrete ACY mainly in the Bvg+ phase) were able to significantly reduce the level of total biofilm formation when cocultured with the ACY mutant in the Bvg+ phase. Moreover, this inhibition was not observed in cocultures in the Bvgi phase, when ACY expression by the wild-type bacteria is significantly decreased. In both conditions, both strains grow at similar rates in the cocultures (data not shown). This result further supports the idea that the strong biofilm phenotype observed in the ACY mutant is due to the absence of the inhibitory effect of ACY on biofilm formation.
FIG. 7.
Quantitative assay of biofilm formation in cocultures containing both wild-type B. bronchiseptica (RB50) and the ΔcyaA mutant in the Bvg+ phase (0 mM nicotinic acid) and Bvgi phase (0.4 mM nicotinic acid). Bacteria were grown in 96-well polystyrene plates, and biofilm formation was quantified by absorbance of solubilized crystal violet stains, as described in Materials and Methods. Coculture of RB50 with the ΔcyaA mutant results in a low level of biofilm (comparable to that of RB50 alone) in the Bvg+ phase, but no significant reduction of biofilm formation in the coculture was observed in the Bvgi phase. OD595, optical density at 595 nm.
DISCUSSION
There is consensus that most bacterial species that thrive on solid surface environments grow in biofilms, and the number of species that have been characterized to form biofilms in vitro continues to increase. In this report, we show that B. bronchiseptica can form biofilms in vitro. We discovered that the biofilm phenotype is regulated by the BvgAS two-component signal transduction system. The BvgAS system regulates a majority of known virulence factors in Bordetella spp., and our results suggest that biofilm formation may play an important role during colonization and pathogenesis within the animal host. We determine that B. bronchiseptica forms the strongest biofilm phenotype in vitro in the Bvgi phase. This is demonstrated by the formation of a strong biofilm phenotype by wild-type bacteria primarily in Bvgi-phase growth conditions and also by the consistently high biofilm levels formed by a Bvgi-phase-locked mutant regardless of growth conditions. We show that the molecular mechanism for the BvgAS-dependent biofilm formation at least involves FHA, fimbriae, and ACY (all of which are regulated by BvgAS). FHA and fimbriae positively contribute to biofilm formation, while ACY inhibits biofilm formation, most likely by interacting with FHA.
Various adhesin molecules in other bacteria, pathogenic and nonpathogenic, have been reported to be important for biofilm formation (24). In Bordetella spp., FHA and fimbriae have previously been shown to be important for adhesion to host cells and, as a consequence, are known to be virulence factors important in their roles for host colonization and pathogenesis (9, 22, 30-32). It is therefore not surprising that both FHA and fimbriae also mediate biofilm formation, probably by promoting attachment to surfaces.
The finding that ACY mutants formed strong biofilms in the Bvg+ phase as well as the Bvgi phase led us to propose that ACY inhibits biofilm formation when it is expressed in the Bvg+ phase in wild-type Bordetella spp. Zaretzky et al. reported that ACY and FHA interact with each other by direct protein-protein binding on the outer membrane surface of B. pertussis (36). We suggest that this interaction also occurs in B. bronchiseptica and is at least partly responsible for the inhibition of biofilm formation by ACY. Indeed, in the double mutant strain that does not express both FHA and ACY, the amount of biofilm formed in both Bvg+ and Bvgi phases is significantly less than that observed in the single ACY mutant. This suggests that at least part of the mechanism of inhibition of biofilm formation by ACY involves its interaction with FHA. However, the double mutant still forms more biofilm than the single FHA mutant, suggesting that ACY may interact with other yet unidentified factors to suppress biofilm formation. The expression of ACY is limited to Bvg+ phase, and therefore the strong Bvgi-phase biofilm trait observed in wild-type B. bronchiseptica is most likely due to the absence of significant ACY expression in Bvgi phase. This is also supported by the observation that cocultures of both wild-type bacteria and ACY mutants led to a significant reduction in overall biofilm formation compared to that of ACY mutants alone. The ability of the wild-type bacteria to trans complement the biofilm inhibition phenotype suggests that either ACY secreted into the medium can interact with FHA in trans or ACY that is present on the cell surface of wild-type cells can interact with mutant cells in close proximity to limit overall biofilm formation. The reduction in biofilm formation in the cocultures is limited to cultures grown in the Bvg+ phase but not in the Bvgi phase, and this is consistent with the reduced expression of ACY in the Bvgi phase by wild-type bacteria.
The physiological relevance of Bvg-dependent biofilm formation has potential implications in understanding the lifestyle of B. bronchiseptica as a chronically colonizing pathogen. In the Bvg− phase, B. bronchiseptica does not appear to form biofilms in vitro. As the Bvg− phase is proposed to be important for survival outside of the host, our results suggest that biofilm formation may not be critical for this phase of the B. bronchiseptica life cycle. Both the Bvg+ and Bvgi phases are likely to be important for successful interactions of B. bronchiseptica with the host. The upper nasopharynx, particularly the nasal mucosa, is one of the primary colonization sites for B. bronchiseptica. The temperature in this area in mammals is measured to be between 30 and 34°C (19). Temperature is an environmental signal that can mediate Bvg regulation, and this range of temperature would modulate the bacteria into the Bvgi phase. Therefore, B. bronchiseptica organisms that colonize this region of the host may be predominantly in the Bvgi phase and may form biofilms. We cannot, however, exclude the possibilities that the bacteria are sensing other signals from the nasal cavity, from the host directly, or from other bacterial species residing in the area. Bacteria can detach from mature biofilms, and such planktonic cells are presumed to colonize other sites and form new biofilms (33). It is possible that detached cells from B. bronchiseptica biofilms in the nasopharynx of infected hosts might also contribute to the process of transmission to new hosts. Although we do not yet have direct evidence that B. bronchiseptica actually forms biofilms in vivo during infections, the fact that this phenotype is Bvg-regulated strongly indicates that it is involved in bacteria-host interactions.
Biofilms appear to be more resistant to antibiotics and host immunity than are planktonic cells (12, 18). B. bronchiseptica infections are characterized by long-term chronic colonization of the upper respiratory tract, and biofilm formation may be a primary mechanism for their survival in the host and in successful interactions with other bacteria. Tuomanen et al. reported that other bacteria can utilize B. pertussis FHA to attach to host cells (31), and B. pertussis infection is often associated with superinfections of other respiratory pathogens. The possible interactions between various respiratory pathogens, such as those within multispecies biofilms, may be critical for the pathogenesis of bacterial respiratory infections. We are presently investigating the possible influence of other common respiratory commensal bacteria on biofilm formation by B. bronchiseptica (and vice versa) in coinfection models in vitro.
The developmental biology of biofilm formation can be characterized into three stages: the initial attachment, development of microcolony formation, and detachment (24). The initial attachment is often mediated by various adhesins, such as fimbriae in Salmonella enteritidis (4) and type IV pili in P. aeruginosa (25). Cell proliferation and type IV pili-driven twitching motility appear to be important for further microcolony formation (14, 15). It is not clear yet at which stages fimbriae and FHA participate in B. bronchiseptica biofilm formation. In addition, the detachment of bacterial cells from biofilm microcolonies is not well understood, but the possible roles of polysaccharide lyase (3) and cell death and survival within microcolonies (35) have been proposed. We are presently conducting experiments to understand and characterize the developmental aspects of B. bronchiseptica biofilm formation and the molecular mechanisms of these processes.
Acknowledgments
We thank Peggy Cotter and the Cotter laboratory for helpful discussions and for providing us with bacterial strains. We also thank Emmanuelle Binet and Marjan van der Woude for technical and scientific assistance and Andy Piefer for help with deconvolution microscopy.
This work was supported in part by NIH grant AI04936 to M.H.Y.
REFERENCES
- 1.Akerley, B. J., P. A. Cotter, and J. F. Miller. 1995. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell 80:611-620. [DOI] [PubMed] [Google Scholar]
- 2.Akerley, B. J., D. M. Monack, S. Falkow, and J. F. Miller. 1992. The bvgAS locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. J. Bacteriol. 174:980-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison, D. G., B. Ruiz, C. SanJose, A. Jaspe, and P. Gilbert. 1998. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. FEMS Microbiol. Lett. 167:179-184. [DOI] [PubMed] [Google Scholar]
- 4.Austin, J. W., G. Sanders, W. W. Kay, and S. K. Collinson. 1998. Thin aggregative fimbriae enhance Salmonella enteritidis biofilm formation. FEMS Microbiol. Lett. 162:295-301. [DOI] [PubMed] [Google Scholar]
- 5.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 6.Cotter, P. A., and A. M. Jones. 2003. Phosphorelay control of virulence gene expression in Bordetella. Trends Microbiol. 11:367-373. [DOI] [PubMed] [Google Scholar]
- 7.Cotter, P. A., and J. F. Miller. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter, P. A., and J. F. Miller. 1997. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol. Microbiol. 24:671-685. [DOI] [PubMed] [Google Scholar]
- 9.Cotter, P. A., M. H. Yuk, S. Mattoo, B. J. Akerley, J. Boschwitz, D. A. Relman, and J. F. Miller. 1998. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect. Immun. 66:5921-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings, C. A., M. M. Brinig, P. W. Lepp, S. van de Pas, and D. A. Relman. 2004. Bordetella species are distinguished by patterns of substantial gene loss and host adaptation. J. Bacteriol. 186:1484-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvill, E. T., P. A. Cotter, M. H. Yuk, and J. F. Miller. 1999. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect. Immun. 67:1493-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jesaitis, A. J., M. J. Franklin, D. Berglund, M. Sasaki, C. I. Lord, J. B. Bleazard, J. E. Duffy, H. Beyenal, and Z. Lewandowski. 2003. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J. Immunol. 171:4329-4339. [DOI] [PubMed] [Google Scholar]
- 13.Kjelleberg, S., and S. Molin. 2002. Is there a role for quorum sensing signals in bacterial biofilms? Curr. Opin. Microbiol. 5:254-258. [DOI] [PubMed] [Google Scholar]
- 14.Klausen, M., A. Aaes-Jorgensen, S. Molin, and T. Tolker-Nielsen. 2003. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 50:61-68. [DOI] [PubMed] [Google Scholar]
- 15.Klausen, M., A. Heydorn, P. Ragas, L. Lambertsen, A. Aaes-Jorgensen, S. Molin, and T. Tolker-Nielsen. 2003. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48:1511-1524. [DOI] [PubMed] [Google Scholar]
- 16.Lacey, B. W. 1960. Antigenic modulation of Bordetella pertussis. J. Hyg. (London) 58:57-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ladant, D., and A. Ullmann. 1999. Bordetella pertussis adenylate cyclase: a toxin with multiple talents. Trends Microbiol. 7:172-176. [DOI] [PubMed] [Google Scholar]
- 18.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindemann, J., R. Leiacker, G. Rettinger, and T. Keck. 2002. Nasal mucosal temperature during respiration. Clin. Otolaryngol. 27:135-139. [DOI] [PubMed] [Google Scholar]
- 20.Locht, C., P. Bertin, F. D. Menozzi, and G. Renauld. 1993. The filamentous haemagglutinin, a multifaceted adhesion produced by virulent Bordetella spp. Mol. Microbiol. 9:653-660. [DOI] [PubMed] [Google Scholar]
- 21.Mattoo, S., A. K. Foreman-Wykert, P. A. Cotter, and J. F. Miller. 2001. Mechanisms of Bordetella pathogenesis. Front. Biosci. 6:E168-E186. [DOI] [PubMed] [Google Scholar]
- 22.Mattoo, S., J. F. Miller, and P. A. Cotter. 2000. Role of Bordetella bronchiseptica fimbriae in tracheal colonization and development of a humoral immune response. Infect. Immun. 68:2024-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mooi, F. R., W. H. Jansen, H. Brunings, H. Gielen, H. G. van der Heide, H. C. Walvoort, and P. A. Guinee. 1992. Construction and analysis of Bordetella pertussis mutants defective in the production of fimbriae. Microb. Pathog. 12:127-135. [DOI] [PubMed] [Google Scholar]
- 24.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 25.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 26.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 27.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeno-Tarraga, L. Temple, K. James, B. Harris, M. A. Quail, M. Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35:32-40. [DOI] [PubMed] [Google Scholar]
- 28.Parsek, M. R., and P. K. Singh. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57:677-701. [DOI] [PubMed] [Google Scholar]
- 29.Stainer, D. W., and M. J. Scholte. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 63:211-220. [DOI] [PubMed] [Google Scholar]
- 30.Stockbauer, K. E., B. Fuchslocher, J. F. Miller, and P. A. Cotter. 2001. Identification and characterization of BipA, a Bordetella Brg-intermediate phase protein. Mol. Microbiol. 39:65-78. [DOI] [PubMed] [Google Scholar]
- 31.Tuomanen, E., A. Weiss, R. Rich, F. Zak, and O. Zak. 1985. Filamentous hemagglutinin and pertussis toxin promote adherence of Bordetella pertussis to cilia. Dev. Biol. Stand. 61:197-204. [PubMed] [Google Scholar]
- 32.van den Akker, W. M. 1998. The filamentous hemagglutinin of Bordetella parapertussis is the major adhesin in the phase-dependent interaction with NCI-H292 human lung epithelial cells. Biochem. Biophys. Res. Commun. 252:128-133. [DOI] [PubMed] [Google Scholar]
- 33.Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webb, J. S., M. Givskov, and S. Kjelleberg. 2003. Bacterial biofilms: prokaryotic adventures in multicellularity. Curr. Opin. Microbiol. 6:578-585. [DOI] [PubMed] [Google Scholar]
- 35.Webb, J. S., L. S. Thompson, S. James, T. Charlton, T. Tolker-Nielsen, B. Koch, M. Givskov, and S. Kjelleberg. 2003. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 185:4585-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaretzky, F. R., M. C. Gray, and E. L. Hewlett. 2002. Mechanism of association of adenylate cyclase toxin with the surface of Bordetella pertussis: a role for toxin-filamentous haemagglutinin interaction. Mol. Microbiol. 45:1589-1598. [DOI] [PubMed] [Google Scholar]