Abstract
The spores of Bacillus subtilis show remarkable resistance to many environmental stresses, due in part to the presence of an outer proteinaceous structure known as the spore coat. GerQ is a spore coat protein essential for the presence of CwlJ, an enzyme involved in the hydrolysis of the cortex during spore germination, in the spore coat. Here we show that GerQ is cross-linked into higher-molecular-mass forms due in large part to a transglutaminase. GerQ is the only substrate for this transglutaminase identified to date. In addition, we show that cross-linking of GerQ into high-molecular-mass forms occurs only very late in sporulation, after mother cell lysis. These findings, as well as studies of GerQ cross-linking in mutant strains where spore coat assembly is perturbed, lead us to suggest that coat proteins must assemble first and that their cross-linking follows as a final step in the spore coat formation pathway.
Bacillus subtilis is a gram-positive soil bacterium that has a number of ways to survive harsh environmental conditions. One survival mechanism that B. subtilis cells follow at the onset of nutrient limitation is to sporulate and give rise to spores that are both dormant and remarkably resistant to many stress factors (34). Detailed studies of sporulation have given us much insight into the spatial and temporal regulation of gene expression that occurs during this process (7, 31). Early in sporulation, the division septum is placed asymmetrically in the dividing cell instead of in the middle, as seen during vegetative cell growth. The small compartment generated after the asymmetric division develops into the spore, and the large compartment serves as the mother cell. Different sets of genes are switched on, and different proteins are produced at different times during spore development, as determined largely by the timing and compartmentation of the synthesis and activation of four RNA polymerase sigma factors (two active in the mother cell and two active in the developing spore).
One of the late events in sporulation is the formation of a complex, multilayer protein structure that surrounds the spore, known as the “coat.” In B. subtilis, there are two major coat layers, as shown by electron microscopy: the lamellar inner coat and the thicker outer coat (5, 6, 11). Coat protein assembly is a good system to learn how multiprotein complexes are formed, since the spore coat is composed of many different proteins. The first point of regulation of coat formation is at the level of gene expression. More than 30 coat proteins (18) are synthesized in the mother cell starting at the beginning of sporulation, and yet a functional coat begins to appear only several hours after the onset of sporulation, which implicates a second point of regulation during coat formation, that of protein assembly (5, 29). Very few of the coat proteins have any known function other than participating in coat assembly (5, 6, 11). The coat as a fully assembled structure, however, is essential for spore resistance to chemicals and lytic enzymes, as well as for spore germination.
Recent work has identified two coat proteins, CwlJ and GerQ, with specific roles in spore germination. When nutrients are available, the spores break dormancy through the process of germination and outgrowth (22, 28). CwlJ is needed (3, 12, 24, 33) to aid in the hydrolysis of the cortex peptidoglycan that surrounds the dormant spore and is found beneath the coat, and GerQ (initially called YwdL) is essential for the presence of CwlJ in the coats (33). Early biochemical analyses of the spore coat identified a fraction that makes up as much as 30% of the total coat protein that is resistant to solubilization by detergents, denaturants, and reducing agents combined (29). In this work, we show that GerQ is part of this insoluble coat protein fraction. We attempt to answer the following questions in this report. (i) What kind of posttranslational processing makes GerQ insoluble? (ii) How and when does GerQ become insoluble? (iii) What is the functional significance of generating insoluble GerQ for overall coat protein assembly?
Previous studies suggested that coat proteins may become cross-linked through the formation of di-tyrosine bridges (5, 6, 11). It was suggested that an as-yet-unidentified peroxidase generates the di-tyrosine cross-links and a superoxide dismutase was postulated to be essential for formation of these cross-links, presumably by providing the hydrogen peroxide necessary for the peroxidase (10). Recent studies also showed that the tyrosine-rich coat proteins CotB, CotC, and CotG are present as multimers in the spore coats, although the mechanism of this multimerization is not known (13, 42). Other coat proteins including CotE and CotT were also reported to form multimers by unknown mechanisms (2). The products of the cotVWXYZ cluster were the first proteins implicated in the formation of an insoluble coat lattice (40). It was also suggested that the CotY and CotZ coat proteins are cross-linked by disulfide bonds, while CotX may be a substrate for transglutaminase activity (40). Another coat protein suggested to be a substrate for transglutaminase is CotM, which appears to be related to the α-crystallin family of low-molecular-mass heat shock proteins, members of which can be cross-linked via a transglutaminase (9). A spore-associated transglutaminase has been extracted, and the gene (tgl) that encodes this enzyme was cloned by reverse genetics (14, 16). Transglutaminases catalyze various posttranslational reactions, mainly inter- or intraprotein cross-linking, and are abundant in all forms of life (19). The products of their activity are supramolecular structures with extra rigidity and resistance to degradation. Transglutaminases can cause protein cross-linking by forming an ɛ-(γ-glutamyl) lysine isopeptide bridge between a lysine donor residue in one protein and the acceptor glutamine residue from the same or another protein (19). In this work, we show that it is the transglutaminase encoded by tgl that is involved in GerQ incorporation into the insoluble coat protein fraction, most probably by mediating GerQ cross-linking in the spore coats. This is the first time that a spore coat protein has been shown to become cross-linked due to the spore's transglutaminase.
MATERIALS AND METHODS
Strains and plasmids used in this study.
The B. subtilis strains used in this study are listed in Table 1. All B. subtilis strains are isogenic with strain PS832, a prototrophic derivative of strain 168, except where indicated. B. subtilis strains were prepared by transformation with either chromosomal DNA or plasmid DNA as described previously (1). The genotypes of the strains arising from transformation with plasmid DNA were confirmed by PCR. Escherichia coli strains TG1 and DH5α were used for the production of plasmids (20). E. coli strain BL21 λ(DE3) (Novagen) (37) was used for protein expression.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Source (reference)a |
|---|---|---|
| Strains | ||
| B. subtilis | ||
| PS832 | Prototroph, wild-type | Laboratory stock |
| CVO1724b | gerQ-gfpc | 33 |
| FB111 | ΔcwlJ::tet | 24 |
| FB112 | ΔsleB::spc | 24 |
| KB29 | ΔgerQc::spc | 33 |
| KB81 | Δtgl::ermC | pKE80→PS832 |
| KB82 | ΔsleB::spc Δtgl::ermC | KB81→FB112 |
| KB83 | gerQ-gfpc Δtgl::ermC | CVO1724→KB81 |
| KB101 | Δtgl::cat | pPS2742→KB81 |
| KB102 | cwlJ-His tag Δtgl::cat | PS3449→KB101 |
| KB104 | ΔspoIVA::spc | RL2037→PS832 |
| PS2495 | sodA::cat | 4 |
| PS3328 | ΔcotE::tet | 24 |
| PS3449 | cwlJ-His tag | 3 |
| RL2037 | ΔspoIVA::spc | 32 |
| E. coli | ||
| KE96 | BL21 λ(DE3) pLysS pET11a-gerQc | pKE95→PS1404 |
| KE98 | BL21 λ(DE3) pLysS pET16b-gerQc | pKE97→PS1404 |
| PS1404 | BL21 λ(DE3) pLysS | 37 |
| PS2602 | BL21 λ(DE3) pLysS pET11a | 30 |
| Plasmids | ||
| pET11a | Protein expression vector | Novagen |
| pET16b | N-terminal His10 tag fusion protein expression vector | Novagen |
| pKE80 | Δtgl::ermC | This work |
| pKE95 | pET11a-gerQc | This work |
| pKE97 | pET16b-gerQc | This work |
| pPS2742 | ΔermC::cat | 35 |
DNA from the strain or plasmid to the left of the arrow was used to transform the strain to the right of the arrow.
Genetic background is PY79 (prototroph, wild type).
gerQ is the new name for the ywdL gene identified as described in reference 33.
Plasmid pKE80, used to generate a tgl deletion mutant, was constructed in two steps. The 3′ region of tgl (from 45 bp upstream to 212 bp downstream of the translation stop codon) was amplified by PCR (all primer sequences are available upon request) from chromosomal DNA of strain PS832, cloned into plasmid pCR2.1 (Invitrogen, Carlsbad, Calif.), and the insert was sequenced and then recovered as an XbaI-EagI fragment (sites present in the PCR primers). The fragment was inserted between the XbaI and EagI sites downstream of the ermC resistance cassette in plasmid pFE140 (27), giving plasmid pKE79. The 5′ region of tgl (from 198 bp upstream to 33 bp downstream of the translation start codon) was amplified by PCR from chromosomal DNA of strain PS832 and cloned into plasmid pCR2.1. The insert was sequenced, recovered as a KpnI-XhoI fragment (KpnI site present in the 5′ PCR primer and XhoI site present in plasmid pCR2.1), and inserted between the same sites in plasmid pKE79 upstream of the ermC resistance cassette, giving plasmid pKE80. Plasmid pKE80 was used to transform B. subtilis strain PS832 to macrolide-lincosamide-streptogramin B resistance by a double-crossover event such that the internal part of the tgl open reading frame (ORF) is deleted and replaced by the ermC resistance cassette.
Plasmid pKE95, which was used to overexpress the gerQ ORF, was derived from plasmid pET11a (Novagen). The gerQ ORF was PCR amplified with primers gerQ-N-pET (5′-CATATGAAACCGAAAAAAAATCAATAT) and gerQ-C-pET (5′-GGATCCTTATCTTGGCGAATAGGACG) from plasmid pKE39 (33), which contains the complete transcription unit of gerQ (194 bp upstream of the translational start codon, the gerQ ORF, and 156 bp downstream of the translational stop codon). The gerQ-N-pET primer introduced an NdeI site (underlined) at the translation start codon (boldface) of gerQ, and the gerQ-C-pET primer introduced a BamHI site (underlined) right after the translation stop codon (boldface) of gerQ. The PCR product was cloned into plasmid pCR2.1, and the insert was sequenced and then recovered as an NdeI-BamHI fragment. This fragment was inserted between the NdeI and BamHI sites of plasmid pET11a, giving plasmid pKE95.
Plasmid pKE97, which was used to overexpress the gerQ ORF as a His10-tag fusion, was derived from plasmid pET16b (Novagen, Madison, Wis.). The gerQ ORF was recovered as an NdeI-BamHI fragment from plasmid pKE95 and inserted between the same sites in plasmid pET16b, giving plasmid pKE97.
Growth of strains and spore preparation.
E. coli and B. subtilis strains were grown at 37°C in rich medium (Luria-Bertani [LB] or 2× YT) (20), supplemented when necessary with the following antibiotics: ampicillin, 100 mg/liter; chloramphenicol, 5 mg/liter for B. subtilis and 20mg/liter for E. coli strains carrying plasmid pLysS; erythromycin, 1 mg/liter; and lincomycin, 25 mg/liter; kanamycin, 10 mg/liter; spectinomycin, 100mg/liter; or tetracycline, 7 mg/liter.
Spores were prepared by nutrient exhaustion on 2× SG medium agar plates at 37°C for 6 days and were harvested and purified by sonication and repeated washing with cold distilled water as described previously (23, 25). All spore preparations were free (>99%) of vegetative and sporulating cells and germinated spores as evaluated by phase-contrast microscopy. For study of GerQ cross-linking during sporulation, cells were induced to sporulate by the resuspension method (see reference 36 and below).
Spore germination.
B. subtilis spores were germinated by either nutrients or a 1:1 chelate of Ca2+ and pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]). For spore germination by nutrients, spores in water (optical density at 600 nm [OD600] of 50) were heat activated (70°C, 30 min) and cooled on ice. The heat-activated spores were diluted to an OD600 of 1 in 2× YT medium plus 10 mM l-alanine and incubated at 37°C, and changes in OD600 were monitored as described previously (21, 26). In other experiments, heat-activated spores were diluted to an OD600 of 1 in water, LB agar plates were spotted with 10-μl aliquots of serial dilutions of spores in water, and the colonies formed were counted after overnight incubation at 30°C (27). For germination with a 1:1 chelate of Ca2+-DPA, spores at an OD600 of 1 were incubated in 60 mM Ca2+-DPA (60 mM CaCl2, 60 mM DPA [pH 8.0]) at 25°C for 1 h (24, 33), and spore germination was monitored by phase-contrast microscopy. The germinated spores appear phase dark, in contrast to the dormant spores, which appear phase bright.
Spore decoating and spore protein extraction.
Spores at an OD600 of 50 were decoated by either of two methods: (i) treatment for 30 min at 70°C with 1 ml of 0.1 M NaCl-0.1 M NaOH-1% sodium dodecyl sulfate (SDS)-0.1 M dithiothreitol (DTT) (38) or (ii) treatment for 90 min at 37°C with 1 ml of 50 mM Tris-HCl (pH 8)-8 M urea-10 mM EDTA-1% SDS-50 mM DTT (29). We found the first method to be more efficient in extracting high-molecular-mass species of GerQ compared to the second extraction method. The coat extracts were dialyzed overnight against 0.33 M sodium acetate (pH 5.0) at 12°C and then dialyzed three times against water at 12°C for 4 h each time. The dialyzed extracts were lyophilized, and the dry material was resuspended in 100 μl of 1× SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer (62.5 mM Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 0.003% bromophenol blue, 1% β-mercaptoethanol) and boiled for 5 min before SDS-PAGE and Western blot analysis (reference 8 and see below). The decoated spores were centrifuged (13,000 × g, 1 min, 25°C) and washed 10 times with water at 25°C, and the spore pellet was lyophilized.
Lyophilized intact or decoated spores were pulverized with 100 mg of glass beads in a dental amalgamator (Wig-L-Bug) for 20 pulses of 30 s each with 30-s intervals between pulses. Soluble proteins were extracted from the disrupted spores with 100 μl of 50 mM HEPES (pH 7.5)-5 mM EDTA-1 mM phenylmethylsulfonyl fluoride (PMSF) by incubation for 30 min on ice and a 5-min centrifugation (13,000 × g, 12°C) as described previously (3, 33). The soluble fraction was mixed with 100 μl of 2× SDS-PAGE sample buffer, and the insoluble fraction was resuspended in 100 μl of 1× SDS-PAGE sample buffer, and both fractions were boiled for 5 min before SDS-PAGE and Western blot analysis (reference 8 and see below). Note that together the soluble and insoluble fractions contain the total proteins from intact or decoated spores that have been mechanically disrupted. The soluble coat proteins are likely to be extracted with the coats as described above, and the insoluble coat proteins will be in the insoluble fraction of the mechanically disrupted spores along with other insoluble spore proteins.
Protein extraction from sporulating cells.
B. subtilis strains were induced to sporulate by the resuspension method (36). A 4-ml sample was collected every hour after the onset of sporulation, and the cell pellet (13,000 × g, 1 min, 25°C) was lyophilized. The lyophilized cells were pulverized with 50 mg of glass beads in a dental amalgamator as described above for protein extraction from intact or decoated spores. Proteins were extracted by resuspending the disrupted spores in 100 μl of 1× SDS-PAGE sample buffer (8), boiling for 5 min, and centrifugation (13,000 × g, 30 s, 25°C) to remove insoluble debris, and aliquots of equal volume were run on SDS-PAGE and subjected to Western blot analysis (reference 8 and see below).
Production of anti-GerQ antibodies.
The His10-GerQ protein was overexpressed in BL21 λ(DE3) E. coli cells as follows. Strain KE98, which carries plasmid pKE97, was grown at 37°C in 2× YT medium, and at an OD600 of ∼0.7, cells were induced by the addition of isopropyl-β-d-thiogalactoside (IPTG) to 1 mM. After 4 h of further growth, the cells were harvested (4,000 × g, 20 min, 4°C), resuspended in lysis buffer (100 mM NaH2PO4-10 mM Tris-HCl-8 M urea [pH 8.0]), and incubated for 30 min at 25°C. Cells were centrifuged at 25°C (10,000 × g, 20 min), and the supernatant fluid was mixed with Ni2+-nitrilotriacetic acid agarose (Qiagen). The mixture was loaded onto a column, and purification of His10-GerQ was performed under denaturing conditions according to Qiagen specifications. The overexpressed His10-GerQ is present in the insoluble fraction of the extracted protein from E. coli cells and most probably is in the inclusion bodies that were observed under the phase-contrast microscope. The purified His10-GerQ was dialyzed in 50 mM Tris-HCl (pH 8.5) at 12°C overnight, during which time the protein precipitated. After further dialysis against fresh buffer for 7 h, the precipitated protein was used for antibody production (Pocono Rabbit Farm and Laboratory, Canadensis, Pa.). Anti-His10-GerQ antibodies were detected in a bleed 2 months after antigen injection, the animals were exsanguinated, and the antiserum was collected and stored at −80°C. In later work, we managed to keep the purified His10-GerQ in solution by dialyzing the protein against 5% (vol/vol) acetic acid (adjusted to pH 5.5 by addition of NaOH).
Preparation of E. coli cell extracts.
Strain PS2602, which carries plasmid pET11a, and strain KE96, which carries plasmid pKE95, were grown in 2× YT medium, and at an OD600 of ∼0.5, the cell culture was induced by addition of IPTG to 1 mM. After 4 h of further growth, 1-ml samples were centrifuged (13,000 × g, 1 min, 25°C), the cell pellet was resuspended in 100 μl of 1× SDS-PAGE sample buffer (8) and boiled for 5 min, and aliquots (adjusted to contain the lysate from an equal number of cells) were run on SDS-PAGE followed by Western blot analysis (reference 8 and see below).
Western blot analysis.
For GerQ detection, E. coli and B. subtilis extracts were run on SDS-PAGE (10% polyacrylamide) and proteins were transferred to an Immobilon-P membrane (Millipore) (8). The membrane was treated with a 1:50,000 dilution of anti-GerQ antiserum and then with a 1:10,000 dilution of goat anti-rabbit immunoglobulin G-alkaline phosphatase conjugate (Southern Biotechnology Associates, Birmingham, Ala.) in 1× Tris-buffered saline (TBS) with 2% blocking reagent (Roche, Indianapolis, Ind.) and 0.1% Tween 20 added as described previously (8). For detection of the CwlJ-His tag, B. subtilis extracts were run on SDS-PAGE (12.5% polyacrylamide) and proteins were transferred to an Immobilon-P membrane and treated with a 1:666 dilution of anti-His tag monoclonal antibody (Novagen, Madison, Wis.) and then a 1:30,000 dilution of goat anti-mouse immunoglobulin G-alkaline phosphatase conjugate (Sigma, St. Louis, Mo.) in TBS with 2% blocking reagent (Roche) and 0.1% Tween 20 added as described previously (3, 8, 33). The alkaline phosphatase was detected with the chemiluminescent substrate disodium 3-(4-methoxyspiro {1,2-dioxetane-3,2′-(5′-chloro) tricyclo [3.3.1.13,7] decan}-4-yl) phenyl phosphate (Roche), and chemiluminescence was detected by exposure to X-ray film.
Other methods.
B. subtilis spores at an OD600 of 1 were treated with 0.25% sodium hypochlorite (pH 11.5) in water at 25°C, and spore viability was monitored on LB agar plates spotted with aliquots of serial dilutions as described previously (33, 39). For lysozyme resistance assays, spores were treated with lysozyme (1 mg/ml) in buffer (20 mM Tris-HCl [pH 8.0]-300 mM NaCl) for 20 min at 37°C, and LB agar plates were spotted with serial dilutions of aliquots (33).
RESULTS
Detection of GerQ.
We induced the overexpression of GerQ in E. coli both as an untagged and as a His10-tagged fusion protein. In both cases, the protein was found in the insoluble fraction of the total cell lysate and inclusion bodies were observed in E. coli cells overexpressing either of the two forms of GerQ. This suggested that GerQ tends to form inclusion bodies when overexpressed in E. coli cells. Therefore, we purified the overexpressed, insoluble His10-GerQ under denaturing conditions by Ni2+ affinity chromatography. While purified His10-GerQ precipitated during subsequent dialysis, the precipitated protein was immunogenic when injected into rabbits and we were successful in production of anti-GerQ antiserum.
We investigated the specificity and sensitivity of anti-GerQ antiserum by Western blot analysis on cell extracts from E. coli strains that express the untagged GerQ and those carrying only the empty vector (Fig. 1, lanes B and C). We also ultimately managed to maintain the purified His10-GerQ in solution during dialysis and used the solubilized form to estimate the minimum amount of GerQ detectable by the anti-GerQ antiserum (≥30 ng) (Fig. 1, lane A) (data not shown). The antiserum recognizes a single band of ∼18 kDa, present only in the extract of E. coli cells expressing the untagged GerQ. The molecular mass of full-length GerQ is calculated to be 20.2 kDa. The discrepancy between the calculated molecular mass and the observed value could be due to the physical properties of the denatured GerQ when run on SDS-PAGE or some protein degradation or processing, but this was not studied further.
FIG. 1.
Specificity and sensitivity of the anti-GerQ antiserum. The samples in the various lanes are as follows: A, purified His10-GerQ (30 ng); B, extract from 2 × 104 E. coli cells of strain KE96 carrying plasmid pKE95 (pET11a-gerQ) induced to overexpress the untagged GerQ; C, extract from 2 × 104 cells of strain PS2602 carrying the empty vector pET11a. The cell extracts were prepared in parallel as described in Materials and Methods. Samples were run on SDS-PAGE (10% polyacrylamide), proteins were transferred to an Immobilon-P membrane, and GerQ was detected as described in Materials and Methods. The numbered bars to the right of the figure indicate the migration positions of mass markers in kilodaltons. The band at the position of the asterisk is the His10-GerQ product, and the band at the position of the dot is the untagged GerQ. Note that the anti-GerQ antiserum fails to cross-react with any E. coli host proteins.
We then examined GerQ in extracts from B. subtilis spores (Fig. 2). While there was no signal in Western blot analysis with extracts from gerQ spores, in wild-type spore extracts we observed a band of 18 kDa, similar to the size of the band detected in E. coli cell extracts, as well as a number of higher-molecular-mass bands and a possible breakdown product (Fig. 2). Although we detected the high-molecular-mass bands and the single 18-kDa band in all of our Western blots in this and subsequent experiments, the band just below the 18-kDa band was not detected in all experiments. This led us to assign it as a degradation product of GerQ. All of these bands were found only in the insoluble fraction from mechanically disrupted wild-type spores. Since neither the 18-kDa band, the higher-molecular-mass bands, nor any other bands were seen at significant levels in the gerQ spore extracts, the antiserum appears to be specific for GerQ. Moreover, GerQ appears to be present in wild-type spores largely as a part of an insoluble high-molecular-mass complex.
FIG. 2.
GerQ in extracts from spores of various strains. The insoluble (lanes A, C, and E) and soluble (lanes B, D, and F) proteins in extracts from identical amounts (5 OD600 units; ∼0.6 mg [dry weight]) of mechanically disrupted spores were run on SDS-PAGE (10% polyacrylamide), proteins were transferred to an Immobilon-P membrane, and GerQ was detected as described in Materials and Methods. The spores were from strains KB81 (tgl; lanes A and B), KB29 (gerQ; lanes C and D), and PS832 (wild type; lanes E and F). The bars on the right side of the figure indicate the migration positions of molecular mass markers in kilodaltons. The asterisk denotes the migration position of monomeric GerQ, and the band below it, at the position of the dot, is a possible degradation product of GerQ.
GerQ is cross-linked into a high-molecular-mass complex in the spore coats.
Since the anti-GerQ antiserum recognized a number of high-molecular-mass products in the insoluble fraction of wild-type spores (Fig. 2, lanes E and F), GerQ must be present in one or more higher-molecular-mass forms observed in samples from mechanically disrupted spores. GerQ is a spore coat protein (33), and analysis by fluorescence microscopy has shown that a GerQ-green fluorescent protein (GFP) fusion is localized on the spore periphery, with this localization dependent on the major coat morphogenetic proteins CotE and SpoIVA (33). Previous studies have suggested that approximately 30% of the spore coat protein remains insoluble even after treatment of spores with detergents and reducing agents (29). This observation, the detection of ɛ-(γ-glutamyl) lysine isopeptide bonds in the spore coats (15), and the cloning of some of the proteins that make up the insoluble coat protein lattice (40) have led to the idea that some spore coat proteins are covalently cross-linked into higher-molecular-mass complexes. Moreover, a number of tyrosine-rich coat proteins have been found to form multimers, supporting the suggestion that di-tyrosine bonds could be involved in cross-linking of proteins in the spore coat (5, 6, 10, 11, 13, 42). Indeed, the presence of such highly cross-linked complexes could render the spore coat and the spore itself rigid and resistant to mechanical disruption (5, 6, 11). In light of this idea, we analyzed GerQ in extracts from mechanically disrupted spores lacking either SodA, a superoxide dismutase associated with cross-linking of another protein into the coats (10), or Tgl, a spore-associated transglutaminase (14, 16). SodA could cross-link GerQ by generation of di-tyrosine bonds, while Tgl could cross-link GerQ by generation of isopeptide bonds. GerQ was detected in much smaller species in extracts from tgl spores, in contrast to the similar levels of higher-molecular-mass species observed in the extracts of wild-type or sodA spores (Fig. 2) (data not shown). Moreover, the low-molecular-mass species of GerQ in tgl spores were completely removed by a decoating treatment, in contrast to the higher-molecular-mass species present in wild-type spores that were still present in the extracts of decoated spores (Fig. 3). Presumably when the transglutaminase is not expressed, GerQ is still present in the spore coats, but because it is not part of a rigid, cross-linked complex, it is easily removed during standard coat removal procedures. Given the effect of Tgl on GerQ, it was surprising to find GerQ present in the coats of tgl spores. To confirm this observation, we examined the localization of GerQ-GFP in tgl mutant spores. Previous fluorescence microscopy has shown that GerQ-GFP localizes as a dot close to the developing forespore early in sporulation and assembles around the periphery of the spore only later during development (33). In the tgl mutant, GerQ-GFP was both localized and assembled as in the wild-type strain (data not shown). These results suggest that Tgl is not essential for the proper localization and assembly of GerQ in the spore coats.
FIG. 3.
GerQ in extracts from spores of various strains. Spores of strains KB29 (gerQ; lanes A), KB81 (tgl; lanes B) and PS832 (wild-type; lanes C) were decoated by treatment for 30 min at 70°C with 0.1 M NaCl-0.1 M NaOH-1% SDS-0.1 M DTT as described in Materials and Methods. Extracted coat proteins and the insoluble fraction of extracts from mechanically disrupted, decoated, and intact spores were run on SDS-PAGE (10% polyacrylamide), proteins were transferred to an Immobilon-P membrane, and GerQ was detected as described in Materials and Methods. Samples from identical amounts of spores (5 OD600 units; ∼0.6 mg [dry weight]) were run in each lane. The bars on the right of the figure indicate the migration positions of molecular mass markers in kilodaltons.
Effect of transglutaminase on spore properties.
We were interested in determining whether there is some functional role of this transglutaminase-mediated cross-linking of GerQ. Consequently we studied a number of the properties of tgl spores. Initial experiments showed that germination of tgl spores in either nutrients or Ca2+-DPA was similar to that of wild-type spores (Fig. 4). GerQ is a spore coat protein essential for the presence of another coat protein, CwlJ, which together with SleB carries out spore cortex hydrolysis during germination (3, 12, 24, 33). CwlJ action is triggered by the Ca2+-DPA released early in germination, thus initiating spore cortex hydrolysis (24, 33). Given the role of GerQ in CwlJ localization, we therefore asked whether cross-linking of GerQ is essential for CwlJ function during germination. For this purpose, we used spores lacking both the cortex lytic enzyme, SleB, as well as Tgl. Spores of the sleB tgl strain germinated as well as wild-type spores, giving rise to similar numbers of colonies when applied as spots to nutrient agar plates, while colony formation by cwlJ sleB spores was more than 104-fold lower than that of wild-type spores (24, 33). The germination efficiency of the sleB (FB112) spores had been shown previously to be similar to that of wild-type spores (24, 33). For strains FB112 (sleB) and KB82 (sleB tgl), the numbers of spores germinated with nutrients were 3.1 × 107 and 3.0 × 107 CFU/ml, respectively. The percentage of germination with Ca2+-DPA was ≥99% for each of these strains. (For details, see Materials and Methods.) Moreover, CwlJ localization was also likely not affected by the absence of cross-linked GerQ, since sleB tgl spores germinated as well as wild-type spores in response to both internal and external sources of Ca2+-DPA. To confirm that the localization of CwlJ does not require cross-linked GerQ, we examined the levels of a His-tagged version of CwlJ in the coats of tgl spores by Western blot analysis (3, 33) and found that CwlJ was present in the coats of the tgl spores at levels similar to those in wild-type spores (Fig. 5). Thus transglutaminase-mediated cross-linking of GerQ is essential neither for the spore's ability to germinate nor for the proper localization of CwlJ.
FIG. 4.
Germination of spores of various strains in nutrients. Spores of the wild-type strain PS832 (○), the gerQ strain (KB29) (⧫), and the tgl strain (KB81) (▪) were heat activated and incubated in 2× YT medium with 10 mM l-alanine at 37°C. The OD600 of each sample was measured at various times, indicated as [OD600 (t)], and is plotted as the fraction of the initial OD600 at time zero [OD600 (t)/OD600 (t0)] versus time.
FIG. 5.
Detection of the CwlJ-His tag protein in the coats of wild-type and tgl spores. Spores of strains KB102 (cwlJ-His tag tgl; lanes A, B, and C) and PS3449 (cwlJ-His tag; lanes D, E, and F) were decoated by treatment for 90 min at 37°C with 50 mM Tris-HCl (pH 8)-8 M urea-10 mM EDTA-1% SDS-50 mM DTT as described in Materials and Methods. The extracted coat proteins (lanes C and D) and the soluble (lanes A and F) and insoluble (lanes B and E) proteins from mechanically disrupted decoated spores were run on SDS-PAGE (12.5% polyacrylamide), proteins were transferred to an Immobilon-P membrane, and CwlJ-His tag was detected as described in Materials and Methods. All lanes were loaded with extracts from identical amounts of spores (7.5 OD600 units; ∼0.9 mg [dry weight]). Note that proteins from the tgl coat extracts had a slightly different migration profile relative to the proteins from the wild-type coat extracts, most probably due to the different amounts and species of coat proteins extracted by the decoating of spores of the two strains. The bars on the right of the figure indicate the migration positions of molecular mass markers in kilodaltons.
Spores show remarkable resistance to chemical agents, and the coats are an important factor in spore chemical resistance (34). We therefore examined the ability of tgl spores to tolerate two agents that have been shown to affect spores with coat defects. One is lysozyme, which destroys spores without a proper outer coat due to the absence of the morphogenetic protein CotE (Table 2) (41). However, lysozyme-treated tgl spores showed similar numbers of survivors, as did gerQ and wild-type spores (Table 2) (33). The second agent tested, sodium hypochlorite, is particularly effective in inactivating spores with defective coats (Table 2) (39) However, tgl spores treated with sodium hypochlorite exhibited survival similar to that of wild-type spores (Table 2). Previous work has shown that absence of GerQ also does not reduce spore hypochlorite resistance (Table 2) (33).
TABLE 2.
Chemical resistance of spores of various strains
| Genotype (strain) | Result for treatment with:
|
|||||
|---|---|---|---|---|---|---|
| Lysozymea (survival [CFU/ml])
|
Sodium hypochloriteb (% survival with incubation time)
|
|||||
| Buffer | Buffer + lysozyme | 0 min | 15 min | 30 min | 45 min | |
| Wild type (PS832) | 3.4 × 107 | 3.1 × 107 | 100 | 38 | 14 | 0.4 |
| tgl (KB81) | 4.8 × 107 | 2.7 × 107 | 100 | 42 | 11 | 0.8 |
| gerQ (KB29) | 4.0 × 107 | 2.4 × 107 | 100 | 30 | 3.5 | 0.2 |
| cotE (PS3328) | 4.4 × 107 | 1.3 × 106 | 100 | 0 | 0 | 0 |
Spores were mixed at an OD600 of 1 with either buffer or buffer containing 1-mg/ml lysozyme and incubated at 37°C for 20 min, and the numbers of surviving spores (CFU per milliliter) were determined as described in Materials and Methods. The values shown are the averages of two independent experiments, with individual values within 25% of the average.
Spores were mixed at an OD600 of 1 with 0.25% sodium hypochlorite (pH 11.5) in water and incubated at 25°C for the times indicated, and the numbers of surviving spores (CFU per milliliter) were determined as described in Materials and Methods. The values shown are the averages of two independent experiments with individual values within 30% of the average.
GerQ cross-linking occurs very late in sporulation, after mother cell lysis.
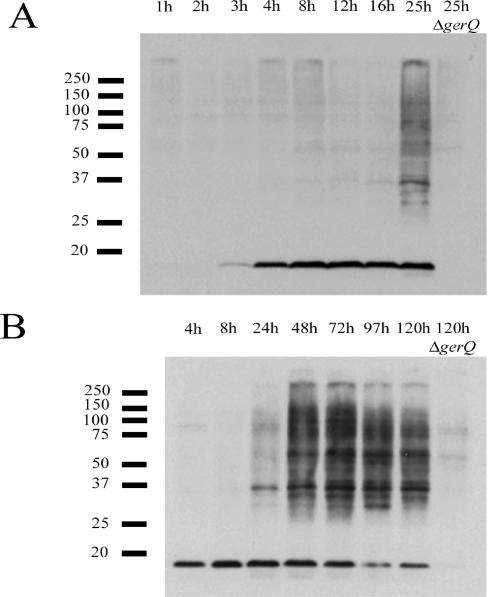
The next point we addressed was when GerQ cross-linking occurs during sporulation. Wild-type and mutant strains were induced to sporulate by the resuspension method (36), and the cross-linking of GerQ was monitored in sporulating cell extracts by Western blot analysis. Surprisingly, the high-molecular-mass GerQ species were detected only very late in sporulation (24 h after the initiation of sporulation) (Fig. 6A and B). Analysis by phase-contrast microscopy indicated that the appearance of the cross-linked GerQ species began only when the majority (>75%) of the spores had been released from their mother cells, which also was only ∼24 h after the initiation of sporulation (data not shown). These observations, in addition to the results noted above, reinforced the idea that cross-linking of GerQ occurs well after it is properly localized in the spore coat, a process that occurs 7 h after the initiation of sporulation (33). To confirm this point, we studied the appearance of cross-linked GerQ species in strains lacking either of the coat morphogenetic proteins, CotE and SpoIVA, previously shown to be essential for assembly and localization of GerQ on the spore coats (33). Fluorescence microscopy has shown that GerQ-GFP is targeted to the forespore normally in sporulating cotE cells, but fails to assemble properly around the spore periphery later in sporulation (33). In sporulating spoIVA cells, GerQ-GFP is not even targeted to the forespore and remains in the mother cell cytoplasm until cell lysis (33). As expected from these results, no cross-linked GerQ products were detected in extracts of sporulating cotE cells (Fig. 7A). Possibly the initial 18-kDa GerQ product normally expressed in the sporulating cell early in sporulation fails to remain associated with the released cotE spore after mother cell lysis (Fig. 7A). In the spoIVA mutant, the 18-kDa GerQ product accumulated early in sporulation, but as found in the cotE mutant, it was not properly assembled in the spore coat and hence did not form cross-linked species (Fig. 7B).
FIG. 6.
Detection of GerQ in extracts of sporulating cells. Strains PS832 (wild type) and KB29 (gerQ) were induced to sporulate by the resuspension method, samples were collected at various times after the initiation of sporulation (time zero), and protein extracts were prepared as described in Materials and Methods. A fraction (10%) of the extract from each sample was run on SDS-PAGE (10% polyacrylamide), proteins were transferred to an Immobilon-P membrane, and GerQ was detected as described in Materials and Methods. The bars on the left of the figure indicate the migration positions of molecular mass markers in kilodaltons. Note that the results in panels A and B are from two different experiments.
FIG. 7.
Detection of GerQ in extracts of sporulating cells of various strains. PS3328 (cotE) (A), KB104 (spoIVA) (B), KB81 (tgl) (C), KB29 (gerQ) (A, B, and C), and PS832 (wild type [WT]) (A, B and C) were induced to sporulate by the resuspension method, and samples were collected at various times. Protein extracts were prepared, a fraction (10%) was run on SDS-PAGE (10% polyacrylamide), proteins were transferred to an Immobilon-P membrane, and GerQ was detected as described in Materials and Methods. The bars to the left of the figure indicate the migration positions of molecular mass markers in kilodaltons. The three mutant strains were induced to sporulate in parallel with the wild-type strain (the results appear in Fig. 6B). Sporulation of strain KB104 (spoIVA) was blocked early, and no mature spores were released.
In the absence of the transglutaminase, a low level of high-molecular-mass GerQ species was still detectable in the spore extracts (Fig. 2, lane A). In agreement with our observation with the wild-type strain, the appearance of this low level of higher-molecular-mass GerQ species also occurred only after tgl spores were released from their mother cells late in sporulation (Fig. 7C).
DISCUSSION
There have been a number of reasons why the coats of B. subtilis spores have attracted study: (i) the ability of spores to resist the action of chemicals and lytic enzymes is due to the presence of an intact coat (5, 6, 11, 34), (ii) some proteins involved in spore germination are components of the coat (3, 12, 24, 33), and (iii) the formation of the spore coat is the result of the coordinated assembly of many proteins (5, 6, 11). Early studies suggested that some coat proteins are resistant to solubilization, perhaps due to their covalent cross-linking (29). In support of this suggestion, ɛ-(γ-glutamyl) lysine cross-links were discovered in spore coat protein fractions and a transglutaminase activity that could generate these cross-links was detected in spores and sporulating cells (15, 16). Only one transglutaminase has been identified in B. subtilis to date, and this enzyme is the product of the tgl gene, which is expressed in the mother cell late in sporulation under the control of the σK transcription factor (11, 14, 16; http://genolist.pasteur.fr/SubtiList/). While recent proteomic analysis has confirmed that Tgl is a spore component (17), it is still not known if it is present in the coat and no transglutaminase substrate has been identified. Previous studies suggested that CotX and CotM may be transglutaminase substrates, but no direct evidence for this suggestion has yet been presented (9, 40). Here we show that GerQ becomes cross-linked in the spore coat and that this cross-linking is mediated by Tgl, the spore transglutaminase. Since GerQ contains a large number of glutamine, lysine, and tyrosine residues and is resistant to extraction from spores by standard decoating procedures, it may be cross-linked in the spore coats either through formation of di-tyrosine or isopeptide cross-links or both. While Tgl is essential for GerQ cross-linking, there is no evidence that Tgl is directly responsible for generating the GerQ cross-links, as it is formally possible that this is an indirect effect of Tgl action, since we have not yet characterized the cross-link in GerQ. Thus it is formally possible that Tgl plays only a structural role in GerQ cross-linking. However, since GerQ is localized normally in the absence of Tgl, we find it difficult to conceive how Tgl is a structural component essential for GerQ cross-linking and not GerQ localization. We tested whether GerQ becomes cross-linked to cortex peptidoglycan by treating extracts of mechanically disrupted spores with lysozyme, and GerQ was still present in the high-molecular-mass species in these treated extracts (data not shown). The implication therefore is that GerQ is either cross-linked to itself or to some other coat proteins. Since the sizes of the cross-linked GerQ species are not multiples of GerQ itself, we suspect that GerQ becomes cross-linked to a number of other coat proteins, although the identity of the latter is not clear. Transglutaminases act on glutamine and lysine residues of proteins (19) and there are many coat proteins containing glutamine, lysine, or both, with which GerQ could become cross-linked. Early studies suggested that products of the cotVWXYZ gene cluster are involved in the formation of insoluble coat material (40). It is thus possible that GerQ is part of this material and is cross-linked to some or all of these proteins. Future work will identify if these or other candidate spore coat proteins are all cross-linked together.
One protein that GerQ could be cross-linked to is CwlJ. Since GerQ is essential for the localization of CwlJ in the spore coats (33), an obvious possibility is that CwlJ becomes cross-linked to GerQ. However, no differences were seen in GerQ localization or cross-linking in either the presence or absence of CwlJ (33; data not shown). In addition, previous results have shown that CwlJ can be readily extracted by decoating regimens (3, 24), indicating that the probability of CwlJ being cross-linked with GerQ in an insoluble complex is low. The lack of a role for Tgl in cross-linking of CwlJ to GerQ is also suggested by the normal germination of sleB tgl spores. Indeed, the relatively normal resistance of tgl spores to lysozyme and sodium hypochlorite indicates that cross-linking of GerQ or other coat proteins by Tgl is not essential for the integrity of the spore coat. Consequently, we are as yet unable to ascribe any functional significance to Tgl-mediated cross-linking of GerQ. Indeed, previous studies have shown that removal of a group of insoluble coat proteins encoded by the cotVWXYZ gene cluster had no effect on spore chemical resistance (40). Perhaps the cross-linking of coat proteins by Tgl is essential for building a spore coat structure that will render spores resistant to mechanical stress. Studies to date indicate that GerQ remains cross-linked in germinated spores (data not shown), and it is possible that the cross-linked coat also provides the germinated spore with some protection against mechanical disruption as well (5, 6). Studies on the role of Tgl in the mechanical resistance of spores are in progress.
Tgl may not be the only factor involved in GerQ cross-linking, since some GerQ species with higher molecular mass than the monomer are present in mechanically disrupted tgl spores. All extractions of GerQ were performed in the presence of disulfide reducing agents, and GerQ does not contain any cysteine residues. Therefore the high-molecular-mass species of GerQ in tgl spores cannot be the result of disulfide bridge formation. Coat protein cross-linking could also be mediated by di-tyrosine bond formation (5, 6, 11). Indeed, the tyrosine-rich coat proteins CotC, CotB, and CotG have been shown to form multimers (13, 42). Although the enzyme responsible for di-tyrosine formation has not been identified, studies have shown that SodA, a superoxide dismutase, is essential for the multimerization of CotG (10). GerQ is also rich in tyrosines and could in theory be a substrate for a coat peroxidase and become cross-linked via di-tyrosine bonds. However, the absence of SodA had no effect on GerQ cross-linking. Consequently, the factors in addition to Tgl that cross-link GerQ are not clear.
We were most surprised to find that the high-molecular-mass GerQ species appeared very late in sporulation. Since spore formation by the resuspension method is thought to be complete in ∼8 h, except for ultimate spore release from the mother cell (7; data not shown), it was surprising to detect GerQ cross-linking only 16 h after the apparent completion of spore formation. Two possible explanations for this apparent anomaly are that GerQ cross-linking is either coupled to the mother cell lysis and spore release that begins at this later time or that GerQ cross-linking is a time-dependent event in spore maturation that is not coupled to spore release from the mother cell. We favor the first possibility because we failed to detect any significant level of GerQ cross-linked intermediates between the time of apparent completion of spore formation (8th h) and spore release from the mother cell (24th h), indicating that spore release may be the triggering event for the cross-linking reaction. It is noteworthy that the cross-linking events occurred after mother cell lysis and spore release whether the cross-links were due to Tgl action or to some other mechanism. By the time of mother cell lysis, all proteins must be assembled on the spore and all the events that comprise the developmental pathway must have largely taken place (7, 31). Perhaps mother cell lysis signals the transglutaminase or other cross-linking factors somehow, indicating that all components have been positioned properly on the developing spore, and thus coat cross-linking can commence. Previous studies indicated that Tgl activity appears long before mother cell lysis, at ∼ 6 h after the initiation of sporulation (15, 16). However, these studies indicate merely the expression of the tgl gene and not Tgl activity per se, since the transglutaminase assay was performed on lysed sporulating cells. Experiments reported here show that GerQ cross-linking is blocked in mutants in which coat protein assembly is perturbed. Moreover, GerQ and CwlJ are properly assembled in the spore coats in the absence of Tgl activity. These findings reinforce the idea that proteins assemble on the spore coat first and become cross-linked later. This could be because GerQ cross-linking is blocked in the environment of the mother cell or is activated only once the mother cell lyses. However, the signals or requirements for GerQ cross-linking are not clear. Interestingly, GerQ, CwlJ, and Tgl are absent from the genomes of anaerobic spore-formers such as various Clostridium species (33), and perhaps GerQ cross-linking requires the oxidizing environment found after mother cell lysis in aerobic spore formers. However, it is not clear why an oxidizing environment might activate transglutaminase-dependent cross-linking.
This study supports the suggestion that some spore coat proteins become cross-linked through the function of a transglutaminase (15, 16). We provide evidence that in the absence of Tgl, the coat protein GerQ is no longer cross-linked into high-molecular-mass species and it is completely extracted by decoating procedures. Consequently, the tgl mutant strain could be of great use in proteomic studies of coat proteins, since those proteins that are no longer cross-linked in tgl spores could be easily extracted and analyzed. Moreover, such proteomic studies could serve to identify other proteins that are Tgl substrates. Finally, the results presented here lead us to suggest that cross-linking of coat components in general may be a very late event in spore coat formation and may not be essential for protein-protein interactions during the process of coat assembly.
Acknowledgments
We are grateful to Patrick Eichenberger and Adam Driks for motivating discussions. We also appreciate the comments of Adam Driks on the manuscript.
This work was supported by a grant from the NIH to P.S. (GM19698).
REFERENCES
- 1.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson, A. I., L. Ekanayake, and P. C. Fitz-James. 1992. Protein filaments may initiate the assembly of the Bacillus subtilis spore coat. Biochimie 74:661-667. [DOI] [PubMed] [Google Scholar]
- 3.Bagyan, I., and P. Setlow. 2002. Localization of the cortex lytic enzyme CwlJ in spores of Bacillus subtilis. J. Bacteriol. 184:1219-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casillas-Martinez, L., and P. Setlow. 1997. Alkyl hydroperoxide reductase, catalase, MrgA, and superoxide dismutase are not involved in resistance of Bacillus subtilis spores to heat or oxidizing agents. J. Bacteriol. 179:7420-7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driks, A. 1999. Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 63:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driks, A. 2002. Proteins of the spore core and coat, p. 527-535. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 7.Errington, J. 1993. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol. Rev. 57:1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 9.Henriques, A. O., B. W. Beall, and C. P. Moran, Jr. 1997. CotM of Bacillus subtilis, a member of the α-crystallin family of stress proteins, is induced during development and participates in spore outer coat formation. J. Bacteriol. 179:1887-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henriques, A. O., L. R. Melsen, and C. P. Moran, Jr. 1998. Involvement of superoxide dismutase in spore coat assembly in Bacillus subtilis. J. Bacteriol. 180:2285-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henriques, A. O., and C. P. Moran, Jr. 2000. Structure and assembly of the bacterial endospore coat. Methods 20:95-110. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa, S., K. Yamane, and J. Sekiguchi. 1998. Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores. J. Bacteriol. 180:1375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isticato, R., G. Esposito, R. Zilhão, S. Nolasco, G. Cangiano, M. De Felice, A. O. Henriques, and E. Ricca. 2004. Assembly of multiple CotC forms into the Bacillus subtilis spore coat. J. Bacteriol. 186:1129-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi, K., K. Hashiguchi, K. Yokozeki, and S. Yamanaka. 1998. Molecular cloning of the transglutaminase gene from Bacillus subtilis and its expression in Escherichia coli. Biosci. Biotechnol. Biochem. 62:1109-1114. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi, K., Y. Kumazawa, K. Miwa, and S. Yamanaka. 1996. ɛ-(γ-Glutamyl) lysine cross-links of spore coat proteins and transglutaminase activity in Bacillus subtilis. FEMS Microbiol. Lett. 144:157-160. [Google Scholar]
- 16.Kobayashi, K., S. I. Suzuki, Y. Izawa, K. Miwa, and S. Yamanaka. 1998. Transglutaminase in sporulating cells of Bacillus subtilis. J. Gen. Appl. Microbiol. 44:85-91. [DOI] [PubMed] [Google Scholar]
- 17.Kuwana, R., Y. Kasahara, M. Fujibayashi, H. Takamatsu, N. Ogasawara, and K. Watabe. 2002. Proteomics characterization of novel spore proteins of Bacillus subtilis. Microbiology 148:3971-3982. [DOI] [PubMed] [Google Scholar]
- 18.Lai, E.-M., N. D. Phadke, M. T. Kachman, R. Giorno, S. Vazquez, J. A. Vazquez, J. R. Maddock, and A. Driks. 2003. Proteomic analysis of the spore coats of Bacillus subtilis and Bacillus anthracis. J. Bacteriol. 185:1443-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorand, L., and R. M. Graham. 2003. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 4:140-156. [DOI] [PubMed] [Google Scholar]
- 20.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Moir, A., E. Lafferty, and D. A. Smith. 1979. Genetic analysis of spore germination mutants of Bacillus subtilis 168: the correlation of phenotype and map location. J. Gen. Microbiol. 111:165-180. [DOI] [PubMed] [Google Scholar]
- 22.Moir, A., and D. A. Smith. 1990. The genetics of bacterial spore germination. Annu. Rev. Microbiol. 44:531-553. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination, and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons Ltd., Chichester, England.
- 24.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paidhungat, M., and P. Setlow. 1999. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant d-alanine. J. Bacteriol. 181:3341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paidhungat, M., and P. Setlow. 2000. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paidhungat, M., and P. Setlow. 2002. Spore germination and outgrowth, p. 537-548. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 29.Pandey, N. K., and A. I. Aronson. 1979. Properties of the Bacillus subtilis spore coat. J. Bacteriol. 137:1208-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen, L. B., T. Murray, D. L. Popham, and P. Setlow. 1998. Characterization of dacC, which encodes a new low-molecular-weight penicillin-binding protein in Bacillus subtilis. J. Bacteriol. 180:4967-4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piggot, P. J., and R. Losick. 2002. Sporulation genes and intercompartmental regulation, p. 483-515. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 32.Price, K. D., and R. Losick. 1999. A four-dimensional view of assembly of a morphogenetic protein during sporulation in Bacillus subtilis. J. Bacteriol. 181:781-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragkousi, K., P. Eichenberger, C. van Ooij, and P. Setlow. 2003. Identification of a new gene essential for germination of Bacillus subtilis spores with Ca2+-dipicolinate. J. Bacteriol. 185:2315-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Setlow, P. 1994. Mechanisms which contribute to the long-term survival of spores of Bacillus species. J. Appl. Bacteriol. Symp. Suppl. 76:49S-60S. [DOI] [PubMed] [Google Scholar]
- 35.Steinmetz, M., and R. Richter. 1994. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene 142:79-83. [DOI] [PubMed] [Google Scholar]
- 36.Sterlini, J. M., and J. Mandelstam. 1969. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem. J. 113:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 38.Vary, J. C. 1973. Germination of Bacillus megaterium spores after various extraction procedures. J. Bacteriol. 116:797-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young, S. B., and P. Setlow. 2003. Mechanisms of killing of Bacillus subtilis spores by hypochlorite and chlorine dioxide. J. Appl. Microbiol. 95:54-67. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, J., P. C. Fitz-James, and A. I. Aronson. 1993. Cloning and characterization of a cluster of genes encoding polypeptides present in the insoluble fraction of the spore coat of Bacillus subtilis. J. Bacteriol. 175:3757-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng, L., W. P. Donovan, P. C. Fitz-James, and R. Losick. 1988. Gene encoding a morphogenic protein required in the assembly of the outer coat of the Bacillus subtilis endospore. Genes Dev. 2:1047-1054. [DOI] [PubMed] [Google Scholar]
- 42.Zilhão, R., M. Serrano, R. Isticato, E. Ricca, C. P. Moran, Jr., and A. O. Henriques. 2004. Interactions among CotB, CotG, and CotH during assembly of the Bacillus subtilis spore coat. J. Bacteriol. 186:1110-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]