Abstract
Objective
Carotid plaque and intima-media thickness are non-invasive arterial markers that are used as surrogate end points for cardiovascular disease. The aim was to assess the prevalence and severity of carotid plaque, and examine its determinant risk factors and their association to the common carotid artery intima-media thickness (CCA-IMT) in a general population.
Methods
We examined 6524 participants aged 25–69 years in the population-based REFINE (Risk Evaluation For INfarct Estimates)-Reykjavik study. Plaques at the bifurcation and internal carotid arteries were evaluated. Mean CCA-IMT was measured in the near and far walls of the common carotid arteries.
Results
The prevalence of minimal, moderate and severe plaque was 35.0%, 8.9% and 1.1%, respectively, and the mean CCA-IMT was 0.73 (SD 0.14) mm. Age, sex, smoking and type 2 diabetes mellitus (T2DM) were the strongest risk factors associated with plaque, followed by systolic blood pressure, total cholesterol, body mass index and family history of myocardial infarct. Low educational level was also strongly and independently associated with plaque. CCA-IMT shared the same risk factors except for a non-significant association with T2DM and family history of myocardial infarction (MI). Participants with T2DM had greater plaque prevalence, 2-fold higher in those <50 years and 17–30% greater in age groups 50–54 to 60–64, and more significant plaques (moderate or severe) were the difference in prevalence was 24% in age group 50–54 and ≥60% in older age groups, compared with non-T2DM.
Conclusions
Carotid plaque and CCA-IMT have mostly common determinants. However, T2DM and family history of MI were associated with plaque but not with CCA-IMT. Greater prevalence and more severe plaques in individuals with T2DM raise the concern that with increasing prevalence of T2DM we may expect an increase in atherosclerosis and its consequences.
Keywords: carotid arteries, atherosclerosis, risk factors, type 2 diabetes mellitus
Strengths and limitations of this study.
The strength of this study is the community-based design, including a large sample of men and women with a broad age range, and a high response rate.
The study describes the prevalence and severity of carotid plaque, and common carotid artery intima-media thickness (CCA-IMT) in the Icelandic population and their associated risk factors.
Standardised ultrasound protocols were used for image acquisition, and for plaque detection and CCA-IMT measurements.
The cross-sectional design precludes conclusions about causality, and therefore longitudinal studies are needed.
Introduction
Atherosclerosis is the major cause of cardiovascular diseases (CVDs), which are the leading cause of death and disability in western countries. It is an asymptomatic disease that may progress silently for decades before clinical manifestations that generally appear in middle and late adulthood.1 Reduction in the risk factor burden can decrease the risk of cardiovascular event2 3 and atherosclerosis progression.4 Therefore, an early identification of subclinical atherosclerosis and interventions may prevent or delay the onset of CVD.
Non-invasive imaging has been suggested as a method for estimating subclinical atherosclerosis, to improve cardiovascular risk assessment.5 B-mode ultrasound is an imaging method that has been widely used to detect and measure carotid plaques and carotid intima-media thickness (cIMT), which are arterial markers independently associated with CVD.6 7 These two arterial markers are correlated; however, they show differing patterns of association with risk factors8 and different predictive value for CVD.6 7 9
The majority of carotid ultrasound studies have used cIMT as a surrogate marker for CVD,10 but the usefulness of carotid plaque as a surrogate marker has been less investigated.7 The aim of this study was primarily to estimate the prevalence and severity of carotid plaque, and examine its determinant risk factors in participants in the REFINE (Risk Evaluation For INfarct Estimates)-Reykjavik study. The distribution of common carotid artery intima-media thickness (CCA-IMT) and its determinant risk factors was also studied.
Methods
Individuals in the present study are participants in the REFINE-Reykjavik study of the Icelandic Heart Association, which is an ongoing longitudinal population-based study. In the REFINE-Reykjavik study, a random sample of 9480 men and women born between 1935 and 1985 and living in the Reykjavik area in November 2005 was drawn from the Icelandic national registry.11 The first phase of the REFINE-Reykjavik study was carried out between 1 December 2005 and 18 March 2011. Of those, 6941 individuals attended (73%). All participants gave written informed consent.
This study was limited to participants aged 25–69 years (n=6661), and of those, 6652 had an ultrasound examination of the carotid arteries. Physical examination included standardised measurements of waist circumference, height and weight, and body mass index (BMI) was calculated. Blood pressure was measured with a computer-controlled device that automatically inflated the cuff to a user preset maximum pressure and then precisely controlled deflation at 2 mm Hg/s. Study participants were in a supine position for at least 15–20 min before the blood pressure measurement.12 Blood samples were drawn after overnight fasting for measuring blood parameters, including total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, triglycerides (TG), glucose and high-sensitivity C reactive protein (CRP). Low-density lipoprotein cholesterol was estimated using the Friedewald formula if the TG level was <4.5 mmol/L.13 Questionnaires were used to record smoking status (never, former, current), family history of myocardial infarction (MI; parents and siblings), educational level (elementary school, high school, junior college, university) and physical activity (participants were questioned whether they exercised regularly, yes/no).
For information on medication use, participants brought their medication to the visit. Hypertension was defined as the use of antihypertensive medications, self-report or measurements of systolic blood pressure (SBP) >140 mm Hg or diastolic blood pressure >90 mm Hg. Diabetes mellitus (DM) was defined as self-report, medication use or fasting serum glucose concentration ≥7.0 mmol/L.14 History of coronary heart disease (CHD) was defined as previous MI, coronary artery bypass graft or percutaneous transluminal coronary angioplasty and/or stent obtained from hospital records.15 Metabolic syndrome was defined as the presence of at least three of the following criteria: (1) waist circumference >102 cm in men and >88 cm in women; (2) TG≥1.7 mmol/L; (3) HDL <1.0 mmol/L in men and HDL<1.3 mmol/L in women; (4) blood pressure ≥130/≥85 mm Hg or antihypertensive medication use; (5) fasting glucose ≥6.1 mmol/L. After the exclusion of participants with missing information on TC, blood pressure, smoking status and medication use, the final analytical sample included 6524 participants.
Imaging
A detailed description of ultrasound imaging and reading protocols has been published.16 In brief, images of the right and left common carotid arteries (CCAs), bifurcation and internal carotid artery (ICA) were acquired with an Acuson Sequoia C256 with a two-dimensional 8 MHz linear array transducer. Images of the intima-media thickness (IMT) were acquired from a predefined 10 mm segment (extending from 10 to 20 mm proximal to the tip of the flow divider) at defined interrogation angles using the Meijers Arc.17 Standard images were obtained from four angles at each side. The mean IMT of the near and far walls was determined from a single image at each interrogation angle for the right and left CCAs. The mean of all these IMT values comprised the CCA-IMT outcome parameter.
The presence of plaque was assessed in the near and far walls of the bifurcation and ICA on the left and right sides. A plaque was defined as an isolated thickening at least two times the adjacent normal cIMT by visual assessment.18 The presence/absence of plaque was assessed during the ultrasound examination and the most severe lesion per segment was evaluated qualitatively as (1) no plaque, complete absence of plaque but cIMT thickening may be observed; (2) minimal plaque, small isolated thickening approximately two times the adjacent normal cIMT; (3) moderate plaque, clear and reasonably easy to visualise plaque, with or without calcifications; and may cause some diameter reduction; and (4) severe plaque, significant plaque formation very easy to image with or without calcifications, causing clear diameter reduction. Individuals with severe plaque were few or 72 (39 men and 33 women), and were therefore combined with the group of individuals with moderate plaque and termed significant plaque in the statistical analysis. The REFINE-Reykjavik study uses strict quality control procedures for monitoring and testing consistency in image acquisition and image analysis. The detailed protocol is in online supplementary material, including information on reader reproducibility.
bmjopen-2016-012457supp.pdf (123.8KB, pdf)
Statistical analyses
Risk factors and atherosclerotic measures were reported as means and SD or median with IQR. Categorical variables were reported in frequencies and percentages (%). Variables with skewed distribution were log transformed to achieve normal distribution, including TG and CRP. The t-test and the χ2 test were used to compare difference in risk factors and atherosclerosis between men and women. The association between carotid plaque severity (minimal or significant) and cardiovascular risk factors was evaluated with multinomial logistic regression models, where participants with no plaque were used as a reference category. The associations between CCA-IMT and cardiovascular risk factors were evaluated with general linear regression models. Analyses were adjusted for age and sex in a simple model (model 1), and the full set of cardiovascular risk factors (model 2) including age, sex, TC, log TG, log CRP, BMI, SBP, smoking status, type 2 diabetes mellitus (T2DM), family history of CHD, physical activity, statin and antihypertensive medication use, education status and CHD. These analyses were also performed for: (1) men and women separately, (2) participants free of known CHD and not using statins, and (3) participants 50 years and older (for carotid plaque only). Furthermore, CCA-IMT association with risk factors was also evaluated after adjusting for carotid plaque category.
All tests were two-sided and statistical significance was set at 0.05. All statistical analyses were carried out with SAS software V.9.3.
Results
A total of 6524 participants were included in the study. The clinical characteristics of participants are shown in table 1. The sample consisted of 49% men; the average age was 49.7 (SD 11.2) years, 3.4% had known CHD, 9% were taking statins and 24% antihypertensive medications. Of the participants examined, minimal plaques were detected in 2280 (35%), moderate plaques in 579 (8.9%) and severe plaques in 72 (1.1%). The average CCA-IMT was 0.73 (SD 0.14) mm.
Table 1.
Clinical characteristics of study participants
| Total | Men | Women | |
|---|---|---|---|
| n=6524 | n=3204 | n=3320 | |
| Sex, men (%) | 3204 (49.2) | – | |
| Age, years | 49.7 (11.2) | 49.8 (11.2) | 49.6 (11.2) |
| TC, mmol/L | 5.3 (1.0) | 5.2 (1.0) | 5.3 (1.0) |
| LDL cholesterol, mmol/L | 3.2 (0.9) | 3.3 (0.9) | 3.2 (0.9)*** |
| HDL cholesterol, mmol/L | 1.5 (0.4) | 1.3 (0.3) | 1.6 (0.4)*** |
| TG, mmol/L (IQR) | 1.0 (0.7–1.4) | 1.1 (0.8–1.7) | 0.9 (0.7–1.3)*** |
| Glucose, mmol/L | 5.5 (1.0) | 5.7 (1.1) | 5.3 (0.8)*** |
| CRP, mg/L (IQR) | 1.3 (0.7–2.8) | 1.3 (0.7–2.5) | 1.4 (0.7–3.0)* |
| BMI, kg/m2 | 27.5 (4.9) | 28.1 (4.4) | 27.0 (5.3)*** |
| Waist circumference, cm | 97.7 (13.3) | 102.3 (12.0) | 93.3 (13.0)*** |
| SBP, mm Hg | 122.2 (16.5) | 126.8 (15.5) | 117.8 (16.3)*** |
| DBP, mm Hg | 71.3 (10.3) | 72.6 (10.8) | 70.1 (9.5)*** |
| Hypertension (%) | 2214 (33.9) | 1178 (36.8) | 1036 (31.2)*** |
| T2DM (%) | 292 (4.5) | 195 (6.1) | 97 (2.9)*** |
| Smoking status (%) | |||
| Never | 2632 (40.3) | 1239 (38.7) | 1393 (41.9)** |
| Former | 2496 (38.3) | 1268 (39.6) | 1228 (37.0) |
| Current | 1396 (21.4) | 697 (21.8) | 699 (21.1) |
| CHD (%)† | 210 (3.4) | 160 (5.2) | 50 (1.6)*** |
| Family history of MI (%)‡ | 2355 (36.8) | 1062 (33.7) | 1293 (39.8)*** |
| Physical activity (%)§ | 3077 (47.2) | 1428 (44.6) | 1649 (49.7)*** |
| Metabolic syndrome (%) | 1290 (19.8) | 746 (23.3) | 544 (16.4)*** |
| Education (%) | |||
| Elementary school | 1306 (20.0) | 481 (15.0) | 825 (24.8)*** |
| High school | 2037 (31.2) | 1235 (38.6) | 802 (24.2)*** |
| Junior college | 803 (12.3) | 365 (11.4) | 438 (13.2)* |
| University | 2378 (36.5) | 1123 (35.0) | 1255 (37.8)* |
| Medication use (%) | |||
| Statin use | 592 (9.1) | 401 (12.5) | 191 (5.8)*** |
| Antihypertensive medication use | 1542 (23.6) | 779 (24.3) | 763 (23.0) |
| Glucose-lowering medication use | 164 (2.5) | 106 (3.3) | 58 (1.8)*** |
| CCA-IMT, mm | 0.73 (0.14) | 0.76 (0.15) | 0.71 (0.13)*** |
| Plaque severity (%) | |||
| No | 3593 (55.0) | 1614 (50.4) | 1979 (59.6)*** |
| Minimal | 2280 (35.0) | 1211 (37.8) | 1069 (32.2)*** |
| Moderate | 579 (8.9) | 340 (10.6) | 239 (7.2)*** |
| Severe | 72 (1.1) | 39 (1.2) | 33 (1.0) |
Data are presented as mean (SD), median (IQR) or number (%).
*p<0.05; **p<0.01 and ***p<0.001 refer to sex difference.
†Missing information on 329 participants (106 men and 223 women).
‡Missing information on 125 participants (54 men and 71 women).
§Missing information on five participants (one man and four women).
BMI, body mass index; CCA-IMT, common carotid artery intima-media thickness; CHD, coronary heart disease; CRP, C reactive protein; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MI, myocardial infarction; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglycerides.
Plaque burden and CCA-IMT by age groups
Carotid plaque prevalence and severity rose steeply from age 40 (figure 1A). The prevalence of minimal plaque increased from 4.5% in the age group 25–29 to 51.2% in the age group 65–69, and the prevalence of moderate plaque rose from 0.8% in the age group 30–34 to 25.0% in the age group 65–69 years. The prevalence of moderate plaque increased markedly (more than twofold) between age groups 50–54 and 55–59 years, where the prevalence was 6.6% and 13.5%, respectively. Severe plaques were rare in participants 54 years and younger, 0.2% in the age group 45–54. In the age group 55–59, the prevalence of severe plaque was 1.5% and rose to 5.0% in the age group 65–69 years. The pattern of carotid plaque severity with increasing age was similar for men and women (see online supplementary table S1 and figure S1), although with an age shift of 5–10 years later in women.
Figure 1.
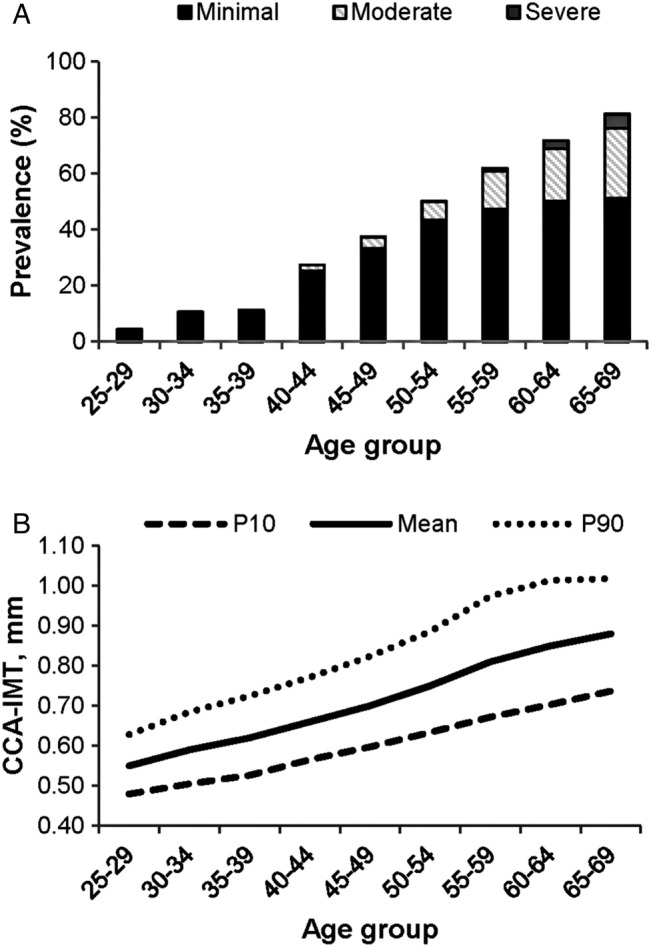
Carotid plaque prevalence and severity (A), and mean CCA-IMT, 10th (P10) and 90th (P90) centiles (B) by age group. CCA-IMT, common carotid artery intima-media thickness.
Mean CCA-IMT increased from 0.55 (SD 0.06) mm in the age group 25–29 to 0.88 (SD 0.13) in the age group 65–69, but the variation in CCA-IMT was greater among older participants (figure 1B). Men had greater CCA-IMT than women in all age groups (see online supplementary table S1 and figure S2).
Carotid plaque and associated risk factors
Carotid plaque severity was strongly related to age and sex, but the average age for no, minimal and significant (moderate+severe) plaque was 44.9 (SD 10.6), 54.5 (SD 8.9) and 59.7 (SD 7.0) years, respectively, and the percentage of men was 45%, 53% and 58%, respectively. The association between cardiovascular risk factors and carotid plaques are shown in table 2. In age-adjusted and sex-adjusted analysis, TC, log TG, log CRP, SBP, former smoker, current smoker, T2DM, family history of MI, physical activity (inverse), lower educational status, statin use and antihypertensive medication use were associated with both minimal and significant carotid plaque, but CHD was only associated with significant carotid plaque. In a multivariable analysis, the association of minimal plaque with log CRP and educational status, and the association of significant plaque with log TG disappeared. Physical activity was not associated with either minimal or significant plaque in multivariable analysis. However, BMI became inversely associated with both minimal and significant plaque in multivariable analysis. Excluding participants with known CHD and statin users did not alter the results with respect to direction or the magnitude of the association (data not shown), except that the association between T2DM and plaque became stronger, in particular for significant plaque (in multivariable analysis OR 3.33; 95% CI 1.97 to 5.63 and OR 1.61; 95% CI 1.06 to 2.43 for significant and minimal plaque, respectively). Furthermore, the log TG association with minimal plaque became weaker and borderline statistically significant in multivariable analysis (OR 1.17; 95% CI 1.00 to 1.36), p=0.053).
Table 2.
Carotid plaque association with cardiovascular risk factors (multinomial logistic regression analysis)
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| Significant plaque (vs no plaque) | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Sex, men | – | – | 1.53 (1.22 to 1.92) | <0.001 |
| Age, 5 years | – | – | 1.97 (1.83 to 2.12) | <0.001 |
| TC, mmol/L | 1.12 (1.03 to 1.23) | <0.05 | 1.38 (1.23 to 1.55) | <0.001 |
| Log TG, mmol/L | 1.86 (1.54 to 2.25) | <0.001 | 1.00 (0.78 to 1.28) | 0.99 |
| Log CRP, mg/L | 1.27 (1.15 to 1.39) | <0.001 | 1.14 (1.02 to 1.28) | <0.05 |
| BMI, 5 units | 0.98 (0.89 to 1.08) | 0.67 | 0.72 (0.64 to 0.82) | <0.001 |
| SBP, 10 mm Hg | 1.35 (1.27 to 1.43) | <0.001 | 1.36 (1.28 to 1.45) | <0.001 |
| Former smoker (vs never smoked) | 2.00 (1.58 to 2.52) | <0.001 | 1.78 (1.38 to 2.29) | <0.001 |
| Current smoker (vs never smoked) | 4.86 (3.74 to 6.31) | <0.001 | 3.90 (2.90 to 5.25) | <0.001 |
| T2DM | 3.20 (2.21 to 4.62) | <0.001 | 2.26 (1.49 to 3.44) | <0.001 |
| Family MI | 1.64 (1.36 to 1.99) | <0.001 | 1.37 (1.11 to 1.68) | <0.01 |
| Physically active | 0.63 (0.52 to 0.76) | <0.001 | 0.86 (0.69 to 1.08) | 0.19 |
| Elementary school (vs university education) | 3.02 (2.30 to 3.96) | <0.001 | 2.08 (1.53 to 2.83) | <0.001 |
| High school (vs university education) | 1.96 (1.53 to 2.51) | <0.001 | 1.55 (1.18 to 2.04) | <0.01 |
| Junior college (vs university education) | 1.40 (0.95 to 2.06) | 0.12 | 1.22 (0.80 to 1.85) | 0.35 |
| Statin use | 3.05 (2.34 to 3.98) | <0.001 | 2.38 (1.67 to 3.38) | <0.001 |
| Antihypertensive medication use | 2.28 (1.86 to 2.79) | <0.001 | 1.79 (1.41 to 2.29) | <0.001 |
| CHD | 3.75 (2.48 to 5.69) | <0.001 | 2.60 (1.60 to 4.24) | <0.001 |
| Minimal plaque (vs no plaque) | ||||
| Sex, men | – | – | 1.35 (1.18 to 1.55) | <0.001 |
| Age, 5 years | – | – | 1.48 (1.43 to 1.54) | <0.001 |
| TC, mmol/L | 1.26 (1.19 to 1.34) | <0.001 | 1.24 (1.15 to 1.33) | <0.001 |
| Log TG, mmol/L | 1.63 (1.45 to 1.83) | <0.001 | 1.21 (1.04 to 1.41) | <0.05 |
| Log CRP, mg/L | 1.11 (1.04 to 1.17) | <0.001 | 1.04 (0.97 to 1.12) | 0.25 |
| BMI, 5 units | 1.01 (0.95 to 1.07) | 0.86 | 0.86 (0.79 to 0.92) | <0.001 |
| SBP, 10 mm Hg | 1.21 (1.17 to 1.26) | <0.001 | 1.20 (1.15 to 1.26) | <0.001 |
| Former smoker (vs never smoked) | 1.22 (1.07 to 1.39) | <0.01 | 1.22 (1.05 to 1.40) | <0.01 |
| Current smoker (vs never smoked) | 1.80 (1.53 to 2.11) | <0.001 | 1.69 (1.42 to 2.02) | <0.001 |
| T2DM | 1.76 (1.28 to 2.41) | <0.001 | 1.44 (1.02 to 2.04) | <0.05 |
| Family MI | 1.25 (1.10 to 1.41) | <0.001 | 1.17 (1.02 to 1.33) | <0.05 |
| Physically active | 0.87 (0.78 to 0.98) | <0.05 | 1.04 (0.91 to 1.19) | 0.55 |
| Elementary school (vs university education) | 1.36 (1.16 to 1.61) | <0.001 | 1.15 (0.95 to 1.37) | 0.15 |
| High school (vs university education) | 1.18 (1.02 to 1.36) | <0.05 | 1.06 (0.90 to 1.23) | 0.50 |
| Junior college (vs university education) | 1.22 (1.01 to 1.49) | <0.05 | 1.09 (0.88 to 1.35) | 0.42 |
| Statin use | 1.40 (1.11 to 1.76) | <0.01 | 1.53 (1.16 to 2.02) | <0.01 |
| Antihypertensive medication use | 1.43 (1.24 to 1.66) | <0.001 | 1.30 (1.10 to 1.53) | <0.01 |
| CHD | 1.15 (0.77 to 1.72) | 0.50 | 1.00 (0.64 to 1.56) | 0.98 |
Model 1, age-adjusted and sex-adjusted analysis.
Model 2, multivariable-adjusted analysis including age, sex, TC, log TG, log CRP, BMI, SBP, smoking status, T2DM, family MI, physical activity, education level, statin use, antihypertensive medication use, CHD.
BMI, body mass index; CHD, coronary heart disease; CRP, C reactive protein; MI, myocardial infarction; NS, not significant; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglycerides.
In sex-specific analysis, the patterns of association were similar in men and women, except that lower educational status and log CRP had a stronger association with plaque in men than in women (see online supplementary table S2). Elementary school education was independently associated with both significant (OR 2.69; 95% CI 1.72 to 4.21) and minimal (OR 1.41; 95% CI 1.06 to 1.88) plaque in men, but only with significant plaque in women (OR 1.70; 95% CI 1.10 to 2.63). High school education was associated with significant plaque in men (OR 1.76; 95% CI 1.23 to 2.51) but not in women (OR 1.34; 95% CI 0.86 to 2.10). Furthermore, log CRP was independently associated with significant (OR 1.18; 95% CI 1.01 to 1.38) and minimal (OR 1.16; 95% CI 1.05 to 1.29) plaque in men, but not in women (OR 1.12; 95% CI 0.95 to 1.33 and OR 0.93; 95% CI 0.84 to 1.03 for significant and minimal plaque, respectively).
Since the prevalence of significant plaque was low in the younger age groups, we performed an additional analysis only including participants aged 50 years and older. The associations were of similar magnitude to those observed for the total sample with few exceptions. In older participants, the association between significant plaque and current smoker was stronger (in multivariable analysis OR 4.87; 95% CI 3.45 to 6.88), whereas significant plaque association with log CRP was minimally reduced and not statistically significant (in multivariable analysis OR 1.11; 95% CI 0.98 to 1.26). Furthermore, minimal plaque association with T2DM (OR 1.25; 95% CI 0.84 to 1.85) and family history of MI (OR 1.13; 95% CI 0.96 to 1.34) were weaker and not statistically significant in multivariable analysis (data not shown).
CCA-IMT and associated risk factors
Across quintiles of CCA-IMT, the average age rose from 36.9 (SD 8.1) to 60.0 (SD 6.2) years and the percentage of men rose from 39% to 62% for quintiles 1–5. Table 3 shows results from linear regression analysis. When adjusted for age and sex, TC, log TG, log CRP, BMI, SBP, former smoking, current smoking, T2DM, elementary school education, high school education, statin use, antihypertensive medication use and CHD were associated with greater CCA-IMT. In multivariable-adjusted analysis, log TG, log CRP, T2DM and high school education were not independently associated with CCA-IMT. Furthermore, antihypertensive medication use that was positively associated with CCA-IMT in age-adjusted and sex-adjusted analysis was inversely associated with CCA-IMT in multivariable-adjusted analysis. Excluding participants with CHD and statin users did not alter these results, except in multivariable-adjusted analysis when the T2DM association became stronger and statistically significant (estimate 0.012 (SE 0.006), p<0.05, data not shown).
Table 3.
CCA-IMT association with cardiovascular risk factors
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| Estimate (SE) | p Value | Estimate (SE) | p Value | Estimate (SE) | p Value | |
| Sex, men | 0.030 (0.003) | <0.001 | 0.028 (0.003) | <0.001 | ||
| Age, 5 years | 0.039 (0.001) | <0.001 | 0.036 (0.001) | <0.001 | ||
| TC, mmol/L | 0.004 (0.001) | <0.01 | 0.004 (0.001) | <0.01 | 0.002 (0.001) | 0.08 |
| Log TG, mmol/L | 0.017 (0.002) | <0.001 | −0.002 (0.003) | 0.51 | −0.002 (0.003) | 0.44 |
| Log CRP, mg/L | 0.008 (0.001) | <0.001 | 0.000 (0.001) | 0.73 | 0.000 (0.001) | 0.79 |
| BMI, 5 units | 0.015 (0.001) | <0.001 | 0.011 (0.001) | <0.001 | 0.013 (0.001) | <0.001 |
| SBP, 10 mm Hg | 0.013 (0.001) | <0.001 | 0.012 (0.001) | <0.001 | 0.010 (0.001) | <0.001 |
| Former smoker (vs never smoked) | 0.017 (0.003) | <0.001 | 0.015 (0.003) | <0.001 | 0.013 (0.003) | <0.001 |
| Current smoker (vs never smoked) | 0.019 (0.003) | <0.001 | 0.020 (0.003) | <0.001 | 0.014 (0.003) | <0.001 |
| T2DM | 0.029 (0.006) | <0.001 | 0.008 (0.006) | 0.17 | 0.003 (0.006) | 0.61 |
| Family MI | 0.003 (0.003) | 0.26 | −0.001 (0.003) | 0.78 | −0.002 (0.002) | 0.53 |
| Physically active | −0.003 (0.002) | 0.19 | 0.005 (0.002) | 0.057 | 0.006 (0.002) | <0.05 |
| Elementary school (vs university education) | 0.017 (0.003) | <0.001 | 0.008 (0.003) | <0.05 | 0.005 (0.003) | 0.13 |
| High school (vs university education) | 0.010 (0.003) | <0.01 | 0.004 (0.003) | 0.21 | 0.003 (0.003) | 0.39 |
| Junior college (vs university education) | 0.003 (0.004) | 0.41 | −0.001 (0.004) | 0.90 | −0.002 (0.004) | 0.68 |
| Statin use | 0.018 (0.004) | <0.001 | 0.014 (0.005) | <0.01 | 0.008 (0.005) | 0.14 |
| Antihypertensive medication use | 0.007 (0.003) | <0.05 | −0.009 (0.003) | <0.01 | −0.012 (0.003) | <0.001 |
| CHD | 0.025 (0.007) | <0.001 | 0.023 (0.008) | <0.01 | 0.014 (0.008) | 0.057 |
| Minimal plaque | 0.029 (0.003) | <0.001 | ||||
| Significant plaque | 0.066 (0.005) | <0.001 | ||||
Model 1, age-adjusted and sex-adjusted analysis.
Model 2, multivariable-adjusted analysis including age, sex, TC, log TG, log CRP, BMI, SBP, smoking status, T2DM, family MI, physical activity, education level, statin use, antihypertensive medication use, CHD.
Model 3, model 2 plus carotid plaque category.
BMI, body mass index; CCA-IMT, common carotid artery intima-media thickness; CHD, coronary heart disease; CRP, C reactive protein; MI, myocardial infarction; NS, not significant; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglycerides.
In addition, we also evaluated risk factor association with CCA-IMT when adjusted for carotid plaque severity (table 3). CCA-IMT association with TC, statin use and education disappeared and the association with CHD was borderline statistically significant (p=0.057) when adjusted for plaque category.
In sex-specific analysis (see online supplementary tables S3 and S4), TC (p<0.001) and statin use (p<0.05) were independently associated with CCA-IMT in women, but not in men. Low educational status (elementary education and high school education) was associated with CCA-IMT in men (p<0.05) but not in women. Furthermore, antihypertensive medication use was inversely associated with CCA-IMT in men (p<0.01), but this association was not detected in women.
T2DM and carotid ultrasound markers
Given the stronger association between T2DM and plaque than between T2DM and CCA-IMT, we explored further the association between T2DM and the carotid ultrasound markers. Figure 2 shows the prevalence and severity of carotid plaque and CCA-IMT stratified by T2DM status across age groups. In all age groups except in the oldest (65–69 years), the prevalence of carotid plaque was greater in those with T2DM. The difference in plaque prevalence was twofold in participants younger than 50 years and the difference ranged between 17% and 30% in age groups 50–54 to 60–64 years. In addition to greater prevalence, the plaques were also more severe in participants with T2DM. In the age group 50–54 years, significant plaque was 24% more prevalent in those with T2DM compared with those free of T2DM, but in older age groups the difference in prevalence was ≥60%. In participants younger than 50 years, the prevalence of significant plaque was 11.3% in those with T2DM, but 1.9% in those free of T2DM. In all age groups, CCA-IMT was greater in those with T2DM. The difference in CCA-IMT was greatest in the youngest age groups, 0.08 mm in those <40 years, but in the older age groups the difference varied between 0.02 and 0.05 mm.
Figure 2.
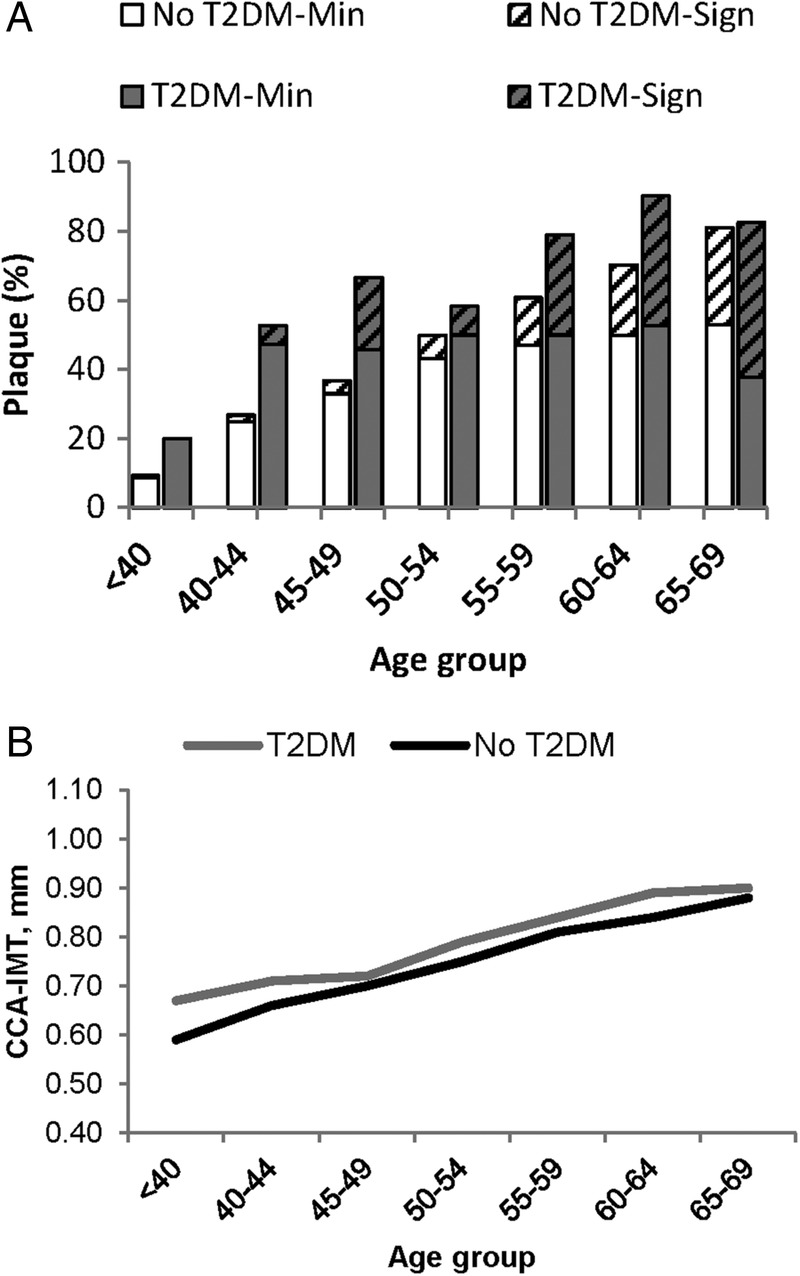
Carotid plaque prevalence and severity (A), and mean CCA-IMT (B) in participants with no T2DM and those with T2DM by age group. CCA-IMT, common carotid artery intima-media thickness; Min, minimal plaque; Sign, significant plaque (diagonal pattern fill); T2DM, type 2 diabetes mellitus.
Discussion
The results show a high prevalence of carotid plaques in this sample of the general population, where 35% had minimal, 9% moderate and 1% severe plaque. Both carotid ultrasound markers were associated with age, sex, TC, SBP, smoking status and BMI (although the direction of the association differed between them). Carotid plaques also showed a strong and graded association with T2DM and family history of MI, but CCA-IMT was not independently associated with those risk factors. In addition to conventional risk factors, low education level was strongly associated with carotid plaque but the association with CCA-IMT was weaker.
Several population-based studies have evaluated carotid plaque prevalence and cIMT with B-mode ultrasound.8 19–25 These studies vary considerably in plaque definition and cIMT measurements that makes direct comparison complicated, but the prevalence of carotid plaque, including minimal plaque, in this study is somewhat higher than that reported in previous ultrasound studies. Our definition of minimal plaque is the same as that used in clinical trials,26 representing significant atherosclerotic plaque in the carotids and also including some areas that are more intermediate atherosclerosis stages. This has been pointed out in the Mannheim Carotid Intima-Media Thickness and Plaque Consensus, where intermediate stages between increasing cIMT and significant atherosclerotic plaque formation cannot be reliably differentiated by either B-mode ultrasound or histological examination.27 This in turn may overestimate the atherosclerotic burden in our study using the definition of minimal plaque.
Both the Tromsø study19 and the San Daniele Project23 examined plaque prevalence across a wide age range in both sexes. In the Tromsø study, carotid plaques were only evaluated in the right carotid artery that may lead to an underestimation of plaque prevalence, which in men increased from 3.0% in the age group 25–34 years to 52.2% in the age group 55–64 years, and the corresponding prevalences for women were 1.7% and 40.3%. In the San Daniele Project,23 no plaques were found in participants <40 years but lesions defined as IMT>1.0 mm were detected in 1% of participants aged 30–39 years, and in the age group 60–69 years the prevalence of atherosclerotic lesions was 59% for men and 48% for women, but in this study the corresponding values were 80% and 71%. The plaque prevalence in this study is to some extent similar to that of the Finnish22 population, where the prevalence of carotid lesions was 14%, 32%, 68% and 82% in men aged 42, 48, 54 and 60 years, respectively, but in this study, the corresponding prevalence was 33%, 40%, 57% and 80%. In the Multi-Ethnic Study of Atherosclerosis (MESA), plaque prevalence was 46.7% in participants where the mean age was 60 years.25
Smoking is one of the most important risk factors for atherosclerosis, which in this study is reflected in a 3.9-fold and 1.8-fold greater odds of significant plaque in current and former smokers, respectively. Smoking prevalence varies between populations that might explain the difference in plaque prevalence, such as in the MESA study,25 where smoking prevalence in the USA is less (11% current and 36% former smokers) than in Iceland (21% current and 38% former smokers). When compared to the Tromsø study, the opposite was found where smoking prevalence is higher (31% current smoker) but plaque prevalence is lower than in the current study. However, only considering significant plaques in the current study the prevalence was lower compared with the Tromsø study. The difference in plaque prevalence might be explained by methodological differences, but they only measured plaque unilaterally as mentioned previously.19
The pattern of carotid plaque development is similar to other studies, where plaque prevalence and severity are considerably elevated in participants aged 50 years and older.19 20 22 23 The prevalence of minimal plaque plateaued in the age group 60–64 years, which coincides with substantial increase in moderate and severe carotid plaque. In sex-specific analysis, this transition in plaque prevalence appears to happen 5–10 years earlier in men than women. CCA-IMT increased with age and the variation in CCA-IMT was greater in older participants, which is in agreement with other studies21 28 29 and may be related to greater plaque burden.8 Our results also show graded association between CCA-IMT and plaque severity, where CCA-IMT was greatest in those with significant plaque.
The current results suggest that carotid plaque and CCA-IMT have a common pattern of risk factors but with a very important exception. Both ultrasound markers were positively associated with age, sex, TC, SBP and smoking status, whereas T2DM and family history of MI were only associated with the presence of plaque. Furthermore, association with BMI was in the opposite direction. BMI was strongly and positively associated with CCA-IMT, whereas it was inversely associated with carotid plaque when adjusted for other risk factors.
In this study, T2DM was associated with carotid plaque, but it was not independently associated with CCA-IMT after adjustment for conventional risk factors. An additional analysis showed that in all age groups plaque burden was greater in participants with T2DM compared with those free of T2DM. These results are consistent with those from previous carotid ultrasound studies.30–33 CCA-IMT is increased in individuals with DM but results from different studies are conflicting.31 33 34 DM is a major risk factor for CVD, but individuals with DM are two times more likely to have CHD than those without DM.35 36 In contrast to other cardiovascular risk factors, which have declined over the past three decades,2 the prevalence of T2DM has increased rapidly,37 38 which may offset the beneficial effects of changes in other major cardiovascular risk factors and stall the decline in CHD death rate.39
Family history of MI was associated with carotid plaque but not with CCA-IMT, which is in agreement with previous studies.40 41 Family history of CVD is an important risk factor for CVD42–44 and has been included in some risk scores. The predictive information of family history has been shown to be independent of established risk factors.45 46 In the Reykjavik study, ∼15% of MI and coronary revascularisations were explained by family history that could not be related to conventional risk factors.46
In addition to conventional risk factors, we also evaluated the association between education and the two carotid ultrasound markers. Low educational status was associated with both markers; however, the association was stronger for plaque than CCA-IMT, and it was stronger in men than women. Education is a widely used indicator of socioeconomic status, but several population-based studies have examined the association between carotid ultrasound markers and measures of socioeconomic status.47–51 The current results are similar to those reported by Deans et al48 where low socioeconomic status was associated with CCA-IMT and carotid plaque in men, but in women low socioeconomic status was only associated with carotid plaque. On the other hand, in the Malmö Diet and Cancer study, low education level was associated with carotid plaque in women but not in men, and no independent association was detected for CCA-IMT.49
The strength of this study is the community-based design, including a large sample of men and women with a broad age range. The overall response rate was high, but 73% of the invited participants attended. Our study has limitations. This is a cross-sectional study showing only an association between cardiovascular risk factors and carotid ultrasound markers, preventing conclusions about causality. Difference in education level is also a concern of this study, but our comparison with information from Statistics Iceland demonstrated that education level among participants is somewhat higher than reported for the general population in Iceland (in the year 2011).52 In the general population aged 25–64 years and living in the Reykjavik area, the prevalence of basic education (classified according to the International Standard Classification of Education—ISCED 1,2) was 25%, but in analysis for participants aged 25–64 years in this study, the prevalence of elementary education was 19%. In the general population, the prevalence of upper secondary education (ISCED 3, 4) was 36% and that of tertiary education (ISCED 5, 6) was 39%, but corresponding percentages for study participants were 43% and 38%. Such participation bias in this study may affect the evaluation on carotid plaque prevalence, in particular significant plaque, which may be underestimated where those with a lower educational level had substantially greater odds of significant carotid plaque.
These data provide a description of the distribution of carotid plaque and CCA-IMT in a general population across a wide age range. Our findings suggest that the prevalence of carotid plaque is high in this population sample and that the formation of moderate and severe carotid plaque increases substantially in participants aged 50 years or older. Current results show that carotid plaque and CCA-IMT share some of the risk factors associated with CVD. However, T2DM and family history of MI appear to be more strongly associated with carotid plaque than CCA-IMT, which may reflect the fact that CCA-IMT is not as clear evidence for atherosclerotic infiltration in the arterial wall as significant plaque is. With the rapidly rising prevalence of T2DM, we may expect an increase in extended atherosclerosis with the potential consequences of MI and stroke. Finally, this study also shows that low educational status is associated with carotid plaque and CCA-IMT independent of conventional risk factors, suggesting that atherosclerosis may have social class stratification in Iceland.
Acknowledgments
The authors thank the participants in the REFINE-Reykjavik study for their valuable contribution.
Footnotes
Contributors: RS, VG and TA drafted the manuscript. RS and TA performed the statistical analysis. VG, TA, BT, GE, SS and GB designed the study and collected the data. All authors reviewed and revised the manuscript, and approved the final version.
Funding: This study was supported by grants from RANNÍS (The Icelandic Research Fund 090452) and Hjartavernd (Icelandic Heart Association).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: National Bioethics Committee (05-112-S1) and the Data Protection Authority.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Hegele RA. The pathogenesis of atherosclerosis. Clin Chim Acta 1996;246:21–38. 10.1016/0009-8981(96)06224-9 [DOI] [PubMed] [Google Scholar]
- 2.Aspelund T, Gudnason V, Magnusdottir BT et al. Analysing the large decline in coronary heart disease mortality in the Icelandic population aged 25–74 between the years 1981 and 2006. PLoS ONE 2010;5:e13957 10.1371/journal.pone.0013957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford I, Murray H, Packard CJ et al. Long-term follow-up of the West of Scotland coronary prevention study. N Engl J Med 2007;357:1477–86. 10.1056/NEJMoa065994 [DOI] [PubMed] [Google Scholar]
- 4.Spence JD, Hackam DG. Treating arteries instead of risk factors: a paradigm change in management of atherosclerosis. Stroke 2010;41:1193–9. 10.1161/STROKEAHA.110.577973 [DOI] [PubMed] [Google Scholar]
- 5.Spence JD, Eliasziw M, DiCicco M et al. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke 2002;33:2916–22. 10.1161/01.STR.0000042207.16156.B9 [DOI] [PubMed] [Google Scholar]
- 6.Mathiesen EB, Johnsen SH, Wilsgaard T et al. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the Tromsø Study. Stroke 2011;42:972–8. 10.1161/STROKEAHA.110.589754 [DOI] [PubMed] [Google Scholar]
- 7.Plichart M, Celermajer DS, Zureik M et al. Carotid intima-media thickness in plaque-free site, carotid plaques and coronary heart disease risk prediction in older adults. The Three-City Study. Atherosclerosis 2011;219:917–24. 10.1016/j.atherosclerosis.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 8.Ebrahim S, Papacosta O, Whincup P et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in Men and Women: the British regional heart study. Stroke 1999;30:841–50. 10.1161/01.STR.30.4.841 [DOI] [PubMed] [Google Scholar]
- 9.Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging 2014;7:1025–38. 10.1016/j.jcmg.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 10.Stein JH, Korcarz CE, Hurst RT et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American society of echocardiography carotid intima-media thickness task force. Endorsed by the society for vascular medicine. J Am Soc Echocardiogr 2008;21:93–111. 10.1016/j.echo.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 11.Svansdottir E, Denollet J, Thorsson B et al. Association of type D personality with unhealthy lifestyle, and estimated risk of coronary events in the general Icelandic population. Eur J Prev Cardiol 2013;20:322–30. 10.1177/2047487312441723 [DOI] [PubMed] [Google Scholar]
- 12.Mitchell GF, van Buchem MA, Sigurdsson S et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility—Reykjavik Study. Brain 2011;134:3398–407. 10.1093/brain/awr253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Olafsdottir E, Aspelund T, Sigurdsson G et al. Effects of statin medication on mortality risk associated with type 2 diabetes in older persons: the population-based AGES-Reykjavik Study. BMJ Open 2011;1:e000132 10.1136/bmjopen-2011-000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gudmundsson EF, Gudnason V, Sigurdsson S et al. Coronary artery calcium distributions in older persons in the AGES-Reykjavik study. Eur J Epidemiol 2012;27:673–87. 10.1007/s10654-012-9730-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornsdottir G, Sigurdsson S, Sturlaugsdottir R et al. Longitudinal changes in size and composition of carotid artery plaques using ultrasound: adaptation and validation of methods (inter- and intraobserver variability). J Vasc Ultrasound 2014;38:198–208. [Google Scholar]
- 17.Oren A, Vos LE, Uiterwaal CSPM et al. Cardiovascular risk factors and increased carotid intima-media thickness in healthy young adults: the Atherosclerosis Risk in Young Adults (ARYA) Study. Arch Intern Med 2003;163:1787–92. 10.1001/archinte.163.15.1787 [DOI] [PubMed] [Google Scholar]
- 18.Gronholdt ML. Ultrasound and lipoproteins as predictors of lipid-rich, rupture-prone plaques in the carotid artery. Arterioscler Thromb Vasc Biol 1999;19:2–13. 10.1161/01.ATV.19.1.2 [DOI] [PubMed] [Google Scholar]
- 19.Joakimsen O, Bonaa KH, Stensland-Bugge E et al. Age and sex differences in the distribution and ultrasound morphology of carotid atherosclerosis: the Tromsø study. Arterioscler Thromb Vasc Biol 1999;19:3007–13. 10.1161/01.ATV.19.12.3007 [DOI] [PubMed] [Google Scholar]
- 20.Li R, Duncan BB, Metcalf PA et al. B-Mode-detected carotid artery plaque in a general population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke 1994;25:2377–83. 10.1161/01.STR.25.12.2377 [DOI] [PubMed] [Google Scholar]
- 21.Howard G, Sharrett AR, Heiss G et al. Carotid artery intimal-medial thickness distribution in general populations as evaluated by B-mode ultrasound. ARIC Investigators. Stroke 1993;24:1297–304. 10.1161/01.STR.24.9.1297 [DOI] [PubMed] [Google Scholar]
- 22.Salonen R, Seppänen K, Rauramaa R et al. Prevalence of carotid atherosclerosis and serum cholesterol levels in eastern Finland. Arterioscler Thromb Vasc Biol 1988;8:788–92. 10.1161/01.ATV.8.6.788 [DOI] [PubMed] [Google Scholar]
- 23.Prati P, Vanuzzo D, Casaroli M et al. Prevalence and determinants of carotid atherosclerosis in a general population. Stroke 1992;23:1705–11. 10.1161/01.STR.23.12.1705 [DOI] [PubMed] [Google Scholar]
- 24.Fabris F, Zanocchi M, Bo M et al. Carotid plaque, aging, and risk factors. A study of 457 subjects. Stroke 1994;25:1133–40. [DOI] [PubMed] [Google Scholar]
- 25.Gepner AD, Young R, Delaney JA et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging 2015;8:1–8. 10.1161/CIRCIMAGING.114.002262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters SA, Dogan S, Meijer R et al. The use of plaque score measurements to assess changes in atherosclerotic plaque burden induced by lipid-lowering therapy over time: the METEOR study. J Atheroscler Thromb 2011;18:784–95. 10.5551/jat.8169 [DOI] [PubMed] [Google Scholar]
- 27.Touboul PJ, Hennerici MG, Meairs S et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis 2012;34:290–6. 10.1159/000343145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stensland-Bugge E, Bønaa KH, Joakimsen O. Age and sex differences in the relationship between inherited and lifestyle risk factors and subclinical carotid atherosclerosis: the Tromsø study. Atherosclerosis 2001;154:437–48. 10.1016/S0021-9150(00)00486-X [DOI] [PubMed] [Google Scholar]
- 29.Homma S, Hirose N, Ishida H et al. Carotid plaque and intima-media thickness assessed by b-mode ultrasonography in subjects ranging from young adults to centenarians. Stroke 2001;32:830–5. [DOI] [PubMed] [Google Scholar]
- 30.Högberg D, Kragsterman B, Björck M et al. Carotid artery atherosclerosis among 65-year-old Swedish Men—a population-based screening study. Eur J Vasc Endovasc Surg 2014;48:5–10. 10.1016/j.ejvs.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 31.Mostaza JM, Lahoz C, Salinero-Fort MA et al. Carotid atherosclerosis severity in relation to glycemic status: a cross-sectional population study. Atherosclerosis 2015;242:377–82. 10.1016/j.atherosclerosis.2015.07.028 [DOI] [PubMed] [Google Scholar]
- 32.Sigurdardottir V, Fagerberg B, Hulthe J. Preclinical atherosclerosis and inflammation in 61-year-old men with newly diagnosed diabetes and established diabetes. Diabetes Care 2004;27:880–4. 10.2337/diacare.27.4.880 [DOI] [PubMed] [Google Scholar]
- 33.Ostling G, Hedblad B, Berglund G et al. Increased echolucency of carotid plaques in patients with type 2 diabetes. Stroke 2007;38:2074–8. 10.1161/STROKEAHA.106.480830 [DOI] [PubMed] [Google Scholar]
- 34.Kowall B, Ebert N, Then C et al. Associations between blood glucose and carotid intima-media thickness disappear after adjustment for shared risk factors: the KORA F4 study. PLoS ONE 2012;7:e52590 10.1371/journal.pone.0052590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarwar N, Gao P, Seshasai SR et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–22. 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seshasai SR, Kaptoge S, Thompson A et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–41. 10.1056/NEJMoa1008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danaei G, Finucane MM, Lu Y et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2 7 million participants. Lancet 2011;378:31–40. 10.1016/S0140-6736(11)60679-X [DOI] [PubMed] [Google Scholar]
- 38.Thórsson B, Aspelund T, Harris TB et al. [Trends in body weight and diabetes in forty years in Iceland]. Laeknabladid 2009;95:259–66. [PubMed] [Google Scholar]
- 39.Thorolfsdottir RB, Aspelund T, Capewell S et al. Population assessment of future trajectories in coronary heart disease mortality. PLoS ONE 2014;9:e85800 10.1371/journal.pone.0085800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang TJ, Nam BH, D'Agostino RB et al. Carotid intima-media thickness is associated with premature parental coronary heart disease: the Framingham heart study. Circulation 2003;108:572–6. 10.1161/01.CIR.0000081764.35431.DE [DOI] [PubMed] [Google Scholar]
- 41.Zureik M, Touboul PJ, Bonithon-Kopp C et al. Differential association of common carotid intima-media thickness and carotid atherosclerotic plaques with parental history of premature death from coronary heart disease: the EVA study. Arterioscler Thromb Vasc Biol 1999;19:366–71. 10.1161/01.ATV.19.2.366 [DOI] [PubMed] [Google Scholar]
- 42.Marenberg ME, Risch N, Berkman LF et al. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med 1994;330:1041–6. 10.1056/NEJM199404143301503 [DOI] [PubMed] [Google Scholar]
- 43.Lloyd-Jones DM, Nam BH, D'Agostino RB Sr et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults. JAMA 2004;291:2204–11. 10.1001/jama.291.18.2204 [DOI] [PubMed] [Google Scholar]
- 44.Calling S, Ji J, Sundquist J et al. Shared and non-shared familial susceptibility of coronary heart disease, ischemic stroke, peripheral artery disease and aortic disease. Int J Cardiol 2013;168:2844–50. 10.1016/j.ijcard.2013.03.149 [DOI] [PubMed] [Google Scholar]
- 45.Chow CK, Islam S, Bautista L et al. Parental history and myocardial infarction risk across the world: the INTERHEART study. J Am Coll Cardiol 2011;57:619–27. 10.1016/j.jacc.2010.07.054 [DOI] [PubMed] [Google Scholar]
- 46.Andresdottir MB, Sigurdsson G, Sigvaldason H et al. Fifteen percent of myocardial infarctions and coronary revascularizations explained by family history unrelated to conventional risk factors. The Reykjavik Cohort Study. Eur Heart J 2002;23:1655–63. 10.1053/euhj.2002.3235 [DOI] [PubMed] [Google Scholar]
- 47.Lutsey PL, Diez Roux AV, Jacobs DR et al. Associations of acculturation and socioeconomic status with subclinical cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Am J Public Health 2008;98:1963–70. 10.2105/AJPH.2007.123844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deans KA, Bezlyak V, Ford I et al. Differences in atherosclerosis according to area level socioeconomic deprivation: cross sectional, population based study. BMJ 2009;339:b4170 10.1136/bmj.b4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosvall M, Ostergren PO, Hedblad B et al. Occupational status, educational level, and the prevalence of carotid atherosclerosis in a general population sample of middle-aged Swedish men and women: results from the Malmö Diet and Cancer Study. Am J Epidemiol 2000;152:334–46. 10.1093/aje/152.4.334 [DOI] [PubMed] [Google Scholar]
- 50.Lynch J, Kaplan GA, Salonen R et al. Socioeconomic status and carotid atherosclerosis. Circulation 1995;92:1786–92. 10.1161/01.CIR.92.7.1786 [DOI] [PubMed] [Google Scholar]
- 51.Lynch J, Kaplan GA, Salonen R et al. Socioeconomic status and progression of carotid atherosclerosis. Prospective evidence from the Kuopio Ischemic Heart Disease Risk Factor Study. Arterioscler Thromb Vasc Biol 1997;17:513–19. [DOI] [PubMed] [Google Scholar]
- 52.Statistics-Iceland Reykjavík: 2015. http://www.statice.is/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-012457supp.pdf (123.8KB, pdf)


