Abstract
Purpose
Pakistan is faced with an increasing prevalence of diabetes in addition to its existing high burden of tuberculosis (TB). Diabetes has a detrimental effect on treatment outcomes of patients with TB, which may hinder achieving the goals of the End-TB strategy by 2030. We conducted a prospective cohort study to determine difference between treatment outcomes among patients with diabetes and new pulmonary tuberculosis (PTB) and patients without diabetes and new PTB. This would help generate contextual and valid scientific evidence from a developing country like Pakistan with its unique interplay of sociocultural, economic and health system factors to inform policy and practice.
Participants
This paper outlines the baseline characteristics of 614 new cases of PTB, aged 15 years and older, which were followed up prospectively at 2nd, 5th and 6th months while on antituberculosis treatment and at 6 months after treatment completion.
Findings to date
We ascertained patients' diabetic status by conducting random and fasting blood glucose tests and their glycaemic control by determining glycosylated haemoglobin. Treatment outcomes were established using standardised definitions provided by WHO. The assessment of 614 respondents' diabetic status revealed that 113 (18%) were diabetic and 501 (82%) were non-diabetic. A greater proportion of patients with diabetes and PTB were illiterate (n=74/113, 65.5%) as compared to patients without diabetes and PTB (n=249/501, 50%) (p=0.035). More patients with diabetes and PTB gave a history of heart disease (n=14/113, 12%) and hypertension (n=26/113, 23%) as compared to patients without diabetes and PTB (n=2/501, 0.4% (heart disease) and n=13 501, 3% (hypertension)) (p<0.001). Unfavourable treatment outcome was more likely among patients with diabetes and PTB (n=23/93, 25%) as opposed to patients without diabetes and PTB (n=46/410, 11%) (p=0.001).
Future plans
We are negotiating with the government regarding funding for a further 2-year follow-up of the cohort to ascertain death and relapse in the post-treatment period and also differentiate between re-infection and recurrence among these patients with respect to their diabetic status.
Keywords: PUBLIC HEALTH, EPIDEMIOLOGY
Strengths and limitations of this study.
The diabetes-tuberculosis treatment outcome (DITTO) study is the first prospective cohort study which has been conducted in Pakistan to determine the effect of diabetes on the treatment outcome of patients with tuberculosis.
The ascertainment of diabetic status of DITTO cohort was based on two tests; one random and the other fasting blood glucose test.
The main limitations are the inability to conduct drug susceptibility testing of patients and to determine their HIV status at the time of recruitment into the DITTO cohort due to non-availability of funds.
Introduction
There is a resurgence of interest in the dual epidemic of diabetes and tuberculosis (TB) with the global increase in the diabetic population. This converging epidemic of diabetes and TB has many untoward and detrimental effects.1 The risk of development of TB is tripled by diabetes.2 It increases the severity related to TB and also slows the response to TB treatment.3 The clearance of Mycobacterium tuberculosis from the sputum, which is required to declare the patient non-infectious, is also delayed by diabetes.4 Diabetes increases the risk of treatment failure, death and relapse among patients with TB, which poses a challenge for the developed and developing world.1 5
Low-income and middle-income countries such as Pakistan are likely to be affected the most as they are struggling with their existing high burden of TB and the growing population of diabetics. Pakistan ranks fourth in terms of global burden of TB with 630 000 cases and an estimated incidence of 231 cases per 100 000 population.6 7 Pakistan is one of the 10 countries with the highest number of patients with diabetes and a prevalence ranging from 7.6% to 11%.8 9 It is feared that diabetes mellitus comorbidity will hinder the achievement of the long-term goal of eliminating TB. TB will be considered eliminated if by the year 2050 there is less than one incident case of the disease per one million population.3 10
Most of the scientific knowledge regarding association between diabetes and TB has been generated in industrialised countries.11 However, recently China and India have made valuable contributions.12 13 Currently, the published literature suffers from certain limitations. The majority of studies are either cross-sectional, case–control or retrospective cohort studies. The ascertainment of patient's diabetic status is based on past records, and a substantial number of studies fail to control for important confounders.5 14 15 Furthermore, good quality evidence needs to be generated from developing countries which can help in addressing this problem. Pakistan has a high burden of TB and a rapidly growing population of diabetics. Data are scarce regarding the impact of diabetes on TB treatment outcome in Pakistan. Therefore, this prospective cohort study was conducted to determine the difference between treatment outcomes in patients with diabetes and pulmonary tuberculosis (PTB) and patients without diabetes and PTB and identify the determinants of treatment outcomes in patients of PTB with and without diabetes mellitus.
Cohort description
The recruitment and enrolment of new adult cases of PTB that were diagnosed, registered and received complete treatment at Gulab Devi Chest Hospital (GDH), Lahore, began in October, 2013. The diagnosis of PTB, sputum smear positive and sputum smear negative, was made according to the definition given by the National Tuberculosis Control Program (NTP).16 According to NTP, a patient having one or two sputum samples positive for acid-fast bacillus (AFB) is labelled as sputum smear-positive PTB patient. If both sputum samples are found negative, an antibiotic course of 7 days is prescribed to the patient. After 7 days, based on doctors’ assessment of the patient an X-ray chest, if required, is taken, which if compatible with active PTB helps to declare the patient sputum smear negative PTB. A new case was a patient with PTB, sputum smear positive or sputum smear negative, who had never taken TB drugs in the past or had taken TB drugs for <4 weeks in the past but was not registered with the NTP. An adult was a patient with PTB aged 15 years or older.16 Patients with a history of antituberculosis treatment (ATT), who were not sure or were unable to recall previous therapy with ATT and who were severely ill, disabled or mentally ill were excluded from the study. All eligible patients with PTB willing to participate after giving informed written consent were enrolled in the study cohort.
The enrolment period lasted up to March 2014, until our sample size of 614 participants was successfully achieved. We recruited 614 patients with PTB on the basis of our statistical calculations for cohort studies using WHO software for sample size calculation in health studies.17 The diabetic status of enrolled patients with PTB was ascertained. The patients with diabetes and PTB and the patients without diabetes and PTB were followed-up prospectively during standardised category I treatment consisting of fixed dose combinations for adults in accordance with current guidelines of NTP.18 The intensive phase of treatment was of 2 months in which rifampicin, isoniazid, ethambutol and pyrazinamide were given to all patients on a daily basis. This was followed by a continuation phase of 4 months in which a daily dose of isoniazid and rifampicin was given to all patients.
Follow-up
The two groups comprising exposed (diabetic) PTB patients and unexposed (non-diabetic) patients were followed-up prospectively at second, fifth and sixth months of ATT treatment and also at 6 months after treatment completion. The follow-up schedule was thoroughly explained to respondents at recruitment, and the follow-up visits were scheduled to coincide with patients' drug collection time from GDH. At the time of recruitment, patients' contact details were gathered which included home addresses and two telephone numbers (landline or mobile) belonging either to them or a family member or a neighbour to facilitate the follow-up process. Follow-up reminders were sent through telephone calls. The contact information of the respondents was reviewed at each subsequent visit to aid in efficient follow-up. The follow-up period was from December 2013 to March 2015.
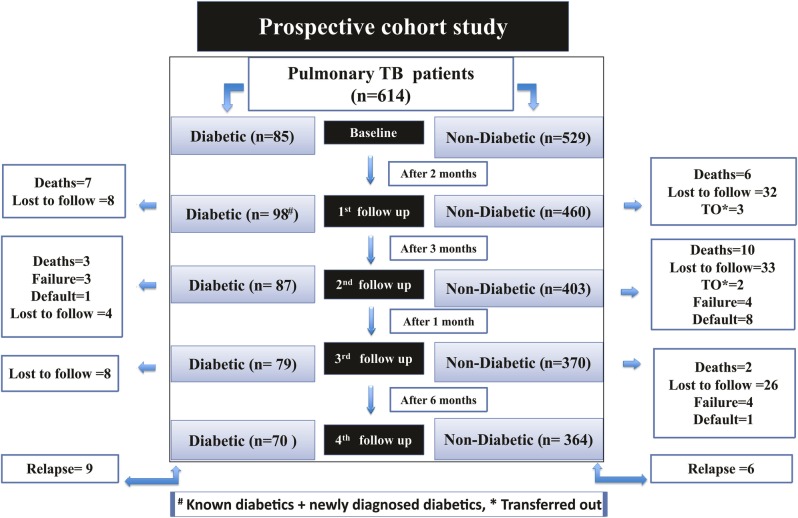
The first follow-up was scheduled at 2 months after the recruitment. The follow-up completion rate at this first follow-up was 93.4%, with 40 participants lost to follow-up. The total number of participants entering the second follow-up was 558 patients with PTB instead of 574, as 16 participants experienced a treatment outcome (13 deaths and 3 transferred out patients). The follow-up completion rate for the second phase was 93.3% with 37 patients with PTB lost to follow-up. For the third phase, the completion rate was 93% and 34 patients were lost to follow-up. The follow-up completion rate of the fourth phase was 100%; however, the overall follow-up completion rate of the 614 patients with PTB was 81.9% with 111 patients lost to follow-up. Figure 1 highlights the number of respondents lost to follow-up at each phase of the study and also the number who experienced a treatment outcome during and at the end of ATT and also after treatment completion.
Figure 1.
Flow diagram depicting the follow-up periods of the PTB cohort along with treatment outcome and loss to follow-up at Gulab Devi Chest Hospital, Lahore. TB, tuberculosis; PTB, pulmonary tuberculosis.
The common reasons for lost to follow-up included patients movement to another city, patients in denial of their disease status, patients attributing their signs and symptoms to black magic/supernatural power, patients claiming that they were incorrectly diagnosed and the inability to contact the patient through telephone calls and home visits.
Additional data
Interview
Trained male and female data collectors conducted the interviews. They were employed as full-time data collectors who worked 6 days a week in the out patient department (OPD) and directly observed treatment, short-course (DOTS). Data were collected on structured questionnaires from the study participants at the time of recruitment and at each follow-up visit. The variables studied included sociodemographic characteristics such as age, gender, education, occupation, income, area of residence and marital status. Lifestyle and behavioural characteristics that included smoking status, alcohol consumption status, history of imprisonment, body mass index (BMI) and drug abuse were also determined. Patients’ clinical presentation variables included cough longer than 3 weeks, prolonged fever, difficulty in breathing, presence of blood in sputum, night sweats, weight loss and type of PTB. Furthermore, history of comorbidities such as hypertension, heart disease, renal disease and asthma was inquired. Other variables studied were type of diabetes, glycaemic control among the diabetics, family history of diabetes, exposure to a household TB contact and adherence to DOTS therapy. Information on these variables was collected at baseline and at each subsequent visit. Additionally, at each follow-up patients were inquired about adverse effects related to ATT, and data were collected on favourable and unfavourable outcomes among them.
Measurement of height and weight
The height of the patients with PTB was measured with a stadiometer to the last complete 0.1 cm and rounded to the nearest whole number while patients were standing erect without shoes. The weight of these patients was measured on a standing scale with minimal clothing on to the last 0.1 kg and rounded to the nearest whole number. Throughout the study, the same instruments were used which were calibrated every day to ensure the validity of the results.19
Estimation of blood sugar
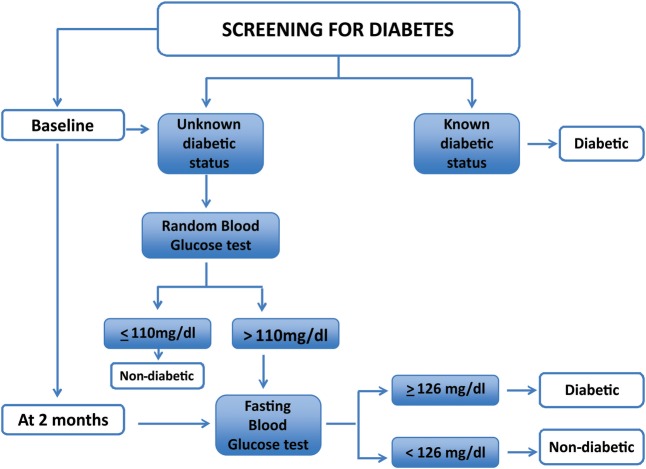
Patients were inquired about their diabetic status, and those who had a known diagnosis of diabetes (self-report) were labelled as diabetic. In those with unknown diabetic status, a random blood glucose (RBG) test was conducted at baseline and a fasting blood glucose (FBG) test was conducted at the first follow-up visit, which coincided with the second month of ATT treatment (figure 2). The protocol used to screen patients with TB for diabetes in China and India has been adhered to in this study.20–22
Figure 2.
The protocol used in screening patients with PTB for diabetes. PTB, pulmonary tuberculosis.
RBG test
Patients' RBG was estimated using the Accu-Check Active glucometer by Roche. All patients having a RBG above the cut-off value were offered FBG testing.
FBG test
Patients were asked to fast overnight, that is, 8 hours before coming for their first follow-up visit at the second month of ATT. They were reminded of the fast through telephone calls twice before their arrival at the health facility; first call a week before their date of visit to the hospital and the second call a day before the visit to ensure their eligibility for the FBG test. For FBG estimation, the same procedure mentioned above was carried out. However, before conducting the test, the patients were again inquired regarding their overnight fast to confirm their fasting status. In case of any suspicion of food intake, the patient was asked to come the next day for the FBG test. Diagnosis of diabetes was based on WHO guidelines.23 The diabetics were referred to a specialist for the management of their newly diagnosed disease.22 24
Sputum smear examination
The diagnosis of patients with PTB and assessment of treatment outcomes were established by performing sputum smear microscopy using the Ziehl-Neelson (ZN) staining technique by the laboratory staff of GDH under the guidelines of NTP. The presence of AFB was checked in the sputum sample after preparing a sputum smear on a glass slide followed by its staining and fixation. The reporting of AFB was performed as depicted in table 1.
Table 1.
Reporting AFB through sputum smear microscopy16
| Seen on slide | Result | Positive (grading) | Bacterial load |
|---|---|---|---|
| More than 10 AFB per field | POS | 3+ | Heavy |
| 1–10 AFB per field | POS | 2+ | Medium |
| 10–99 AFB in 100 fields | POS | 1+ | Low |
| 1–9 AFB in 100 fields | POS | Record actual number | Very low |
| No AFB in 100 fields | NEG | 0 | Nil/not seen |
AFB, acid-fast bacillus; NEG, negative; POS, positive.
The results of the sputum sample were communicated by the laboratory personnel to OPD staff, who recorded it on the patients' treatment card and in hospitals’ TB registers. The results were acquired by the data collectors of the study from the patient treatment card and were verified with the hospital TB registers.
Estimation of glycosylated haemoglobin
At the second follow-up visit which coincided with the fifth month of ATT, a blood sample was drawn from all known and the newly diagnosed patients with diabetes and PTB for the estimation of gylcosylated haemoglobin. The glycaemic assessment was performed at a time when the effect of transient hyperglycaemia due to TB disease was probably negligible. This would provide an unbiased estimate of the association between patients' glycaemic control and treatment outcome. A 3 cc sample of blood was obtained through venipuncture by the data collectors using a disposable syringe and an aseptic technique. The blood was immediately transferred to a tube containing ethylenediaminetetraacetic acid, which was gently inverted to ensure the mixing of the components. The specimen was transported on a daily basis to the pathology laboratory of Punjab Institute of Cardiology (PIC), where the samples were analysed for haemoglobin A1c (HbA1c) using high-performance liquid chromatography (HPLC). A HbA1c value of ≤7% was considered to be a normal value, and a HbA1c value >7% was considered to be an abnormal value in the study.25
Treatment outcomes
All the patients were followed-up to determine treatment outcomes. The standardised treatment outcome definitions given by the NTP, Pakistan, and the WHO were followed in the study.26 27 The treatment outcomes are as follows.
Cured: A sputum smear positive patient, who had completed 6 months of treatment and became sputum smear negative at the end of treatment and on at least one previous occasion.
Treatment completed: A sputum smear positive patient, who completed 6 months of treatment and had at least one follow-up smear negative result and none at the end of treatment due to any reason or smear negative cases who completed 6 months of treatment successfully.
Death: A patient who died for any reason during the course of treatment.
Failure: A sputum smear positive patient who remained positive or again became positive at 5 months or a sputum smear negative patient found to be smear positive at the end of 2 months.
Default: A patient whose treatment was interrupted for two consecutive months or more after registration (according to new definition, this treatment outcome is called ‘loss to follow-up’).
Transferred out: A patient who was transferred to another centre and for whom the treatment outcome was not known (according to new definition, this treatment outcome is called ‘not evaluated’).
Relapse: A patient who was previously treated for TB was declared cured or treatment completed at the end of their treatment and was diagnosed with a recurrent episode of TB (either a true relapse or a new episode of TB caused by reinfection).27
Treatment outcomes were categorised into favourable and unfavourable treatment outcomes. The ‘unfavourable outcome’ included patients who defaulted, died, were transferred out, had treatment failure and had relapse. The category of ‘favourable outcome’ included patients who were cured and completed treatment.
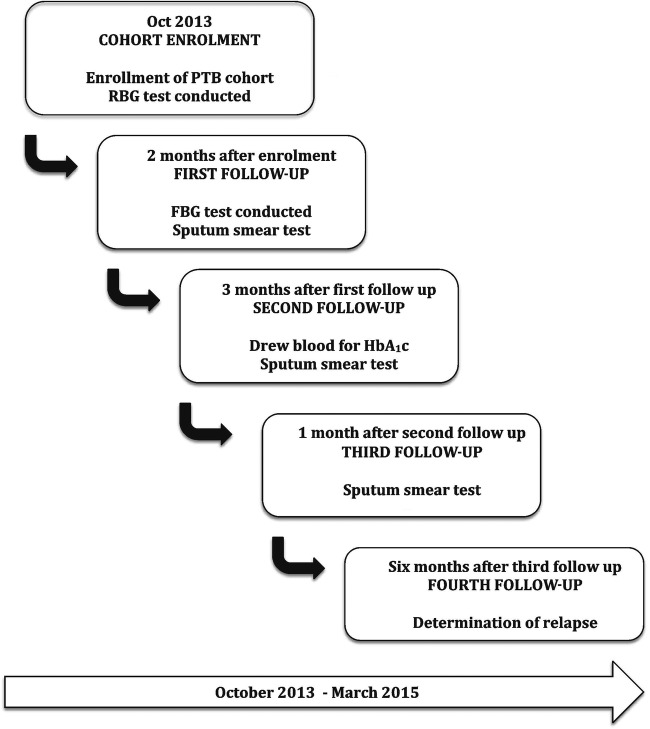
The various data collection activities undertaken by the DITTO study are depicted in figure 3.
Figure 3.
Flow diagram of data collection activity at Gulab Devi Hospital, Lahore.
Characteristics of the study population
Of the total 661 new PTB patients who fulfilled the inclusion criteria, 614 patients consented to participate in the study, whereas 47 refused participation. The assessment of 614 respondents' diabetic status revealed that 113 (18%) were diabetic and 501 (82%) were non-diabetic. The age distribution of the PTB cohort comprising of 113 (18%) diabetics and 501 (82%) non-diabetics shows the respondents with diabetes and PTB to be older (mean age=47.4) as compared to the respondents without diabetes and PTB (mean age=28.5) (p<0.001). A greater proportion of patients with diabetes and PTB were illiterate (n=74/113, 65.5%) as compared to patients without diabetes and PTB (n=249/501, 50%) (p=0.035). The majority of patients with diabetics and PTB were married (n=101/113, 89%) as opposed to patients without diabetes and PTB (n=243/501, 58 %) (p<0.001). The majority of the exposed patients with PTB were overweight (n=18/113, 17 %) and obese (n=9/113, 8%), whereas, the majority of unexposed respondents were underweight (n=289/501, 58 %) (p<0.001). More patients with diabetes and PTB gave a history of heart disease (n=14/11 3, 12%) and hypertension (n=26/113, 23%) as compared to patients without diabetes and PTB (n=2/501, 0.4% and n=13 501, 3%) (p<0.001), respectively (table 2).
Table 2.
Profile of 614 new pulmonary tuberculosis patients with (n=113) or without diabetes mellitus (n=501) presenting at Gulab Devi Chest Hospital, Lahore
| PTB with diabetes n=113 |
PTB without diabetes n=501 |
Total n=614 |
||||
|---|---|---|---|---|---|---|
| n | Percent | n | Percent | n (%) | p value | |
| Age group (in years) | <0.001 | |||||
| 15–19 | 1 | 1 | 134 | 27 | 135 (22) | |
| 20–24 | 4 | 3.5 | 138 | 27 | 142 (23) | |
| 25–29 | 4 | 3.5 | 63 | 13 | 67 (11) | |
| 30–39 | 16 | 14 | 74 | 15 | 90 (15) | |
| 40–49 | 30 | 27 | 37 | 7 | 67 (11) | |
| >50 | 58 | 51 | 55 | 11 | 113 (18) | |
| Gender | 0.357 | |||||
| Male | 53 | 47 | 259 | 52 | 312 (51) | |
| Female | 60 | 53 | 242 | 48 | 302 (49) | |
| Sputum smear status | 0.232 | |||||
| Positive | 67 | 59 | 266 | 53 | 333 (54) | |
| Negative | 46 | 41 | 235 | 47 | 281 (46) | |
| Residence | 0.179 | |||||
| Urban | 84 | 74 | 340 | 68 | 424 (69) | |
| Rural | 29 | 26 | 161 | 32 | 190 (31) | |
| Educational qualification | 0.035 | |||||
| Illiterate | 74 | 65.5 | 249 | 50 | 323 (52) | |
| Primary | 13 | 11.5 | 71 | 14 | 84 (14) | |
| Matriculation | 20 | 18 | 126 | 25 | 146 (24) | |
| Intermediate | 5 | 4 | 25 | 5 | 30 (5) | |
| Bachelors | 1 | 1 | 17 | 3 | 18 (3) | |
| Masters and above | 0 | 0 | 13 | 3 | 13 (2) | |
| Income category (rupees) | 0.113 | |||||
| Nil* | 73 | 65 | 311 | 62 | 384 (63) | |
| <5000 | 5 | 5 | 38 | 8 | 43 (7) | |
| 5100–8000 | 6 | 5 | 61 | 12 | 67 (11) | |
| 8100–11 000 | 10 | 9 | 44 | 9 | 54 (9) | |
| 11 100–14 000 | 7 | 6 | 19 | 4 | 26 (4) | |
| 14 100–17 000 | 6 | 5 | 15 | 3 | 21 (3) | |
| >17 100 | 6 | 5 | 13 | 2 | 19 (3) | |
| Marital status | <0.001 | |||||
| Married | 101 | 89 | 243 | 48.4 | 344 (56) | |
| Single | 12 | 11 | 255 | 51 | 267 (43.5) | |
| Divorced | 0 | 0 | 1 | 0.2 | 1 (0.2) | |
| Widowed | 0 | 0 | 2 | 0.4 | 2 (0.3) | |
| BMI† | <0.001 | |||||
| <18.50 | 18 | 17 | 289 | 58 | 307 (51) | |
| 18.50–24.99 | 63 | 58 | 194 | 39 | 257 (42) | |
| 25–29.99 | 18 | 17 | 9 | 2 | 27 (4) | |
| 30 and above | 9 | 8 | 8 | 1 | 17 (3) | |
| Heart disease | <0.001 | |||||
| Yes | 14 | 12 | 2 | 0.4 | 16 (3) | |
| No | 99 | 88 | 499 | 99.6 | 598 (97) | |
| Hypertension | <0.001 | |||||
| Yes | 26 | 23 | 13 | 3 | 39 (6) | |
| No | 87 | 77 | 488 | 97 | 575 (94) | |
*Income in the form of loans/help from relatives/extended family/friends.
†Body mass index, of 608 patients.
BMI, body mass index; PTB, pulmonary tuberculosis.
Findings to date
The treatment outcome analysed as a binary variable shows that 69 (14%) patients had an unfavourable outcome and 434 (86%) had a favourable outcome. In univariate logistic regression analysis, patients with diabetes were more likely to experience an unfavourable outcome than patients without diabetes (OR=2.6, 95% CI 1.48 to 4.56, p=0.001). Other studies conducted in Taiwan and South Korea have also reported an increased risk of unfavourable treatment outcome among patients with diabetes and PTB as compared to patients without diabetes and PTB, that is, an OR of 1.46 (95% CI of 1.03 to 2.08)28 and 1.78 (95% CI 1.07 to 2.95),29 respectively.
Strengths and limitations
The strength of our study is a rigorous study design, that is, the prospective cohort study design, which generates valid results as opposed to other observational epidemiological study designs in our endeavour to determine treatment outcomes among patients with diabetes and TB. The data collection tool gathered information on all possible confounders identified through literature review and having biological plausibility. These confounders will thus be adjusted for in the analysis producing valid results. The exposure status of patients with PTB was based on two tests; one random and the other FBG test. The confirmatory FBG test was conducted 2 months after the initiation of ATT to rule out the bias associated with transient stress-induced hyperglycaemia attributed to TB. Finally, standardised treatment outcome definitions provided by WHO were followed in the study. To the best of our knowledge, no previous study has been conducted in Pakistan to determine the effect of diabetes on the treatment outcome of TB patients.
The study found it beneficial to employ a male data collector and a female data collector who were trained for gender-matched data collection considering the prevailing cultural environment. We ensured negligible data collector turnover, which helped develop a good rapport between the researcher and respondents. Additionally, we provided a 24-hour helpline, which was very popular among the patients. It was greatly appreciated by them and helped develop sustained relationships with them, thus maximising our response rate.
However, there were certain limitations in our study. The drug susceptibility testing was not carried out among the PTB cohort at the time of enrolment or during the course of ATT, which could have led to bias in the results. However, because of our inclusion criteria of recruiting only the new patients with PTB with no prior history of ATT intake, drug resistance may not be an issue. The difference in drug resistance patterns between the two groups was unlikely to have contributed to the observed results. Second, HIV status which has been identified as a strong risk factor for adverse treatment outcome among patients with TB was not determined. Finally, we were unable to study the effect of glucose control on TB treatment outcome as HbA1c values for the entire cohort were not available. Owing to resource constraints, glycosylated haemoglobin blood analyses were performed only on the diabetics in the study. If treatment outcome among patients with diabetes and PTB is modified by glucose control, our results could be affected. However, according to Mi et al,30 2 and 6 month FBG levels among patients with PTB did not have statistically significant association with adverse outcomes.
Footnotes
Contributors: FM contributed to conception and design of the work, acquisition, analysis and interpretation of data and write-up. ZAB contributed to conception of work, analysis of data, revised the work for intellectual content and approved the final version to be published.
Competing interests: None declared.
Ethics approval: Ethical approval was obtained from the Institutional Ethical Review Committee of Health Services Academy, Islamabad, on 17th September 2013 (F. No. 107/2013-IERC/HSA). Permission was also taken from the administration of the Gulab Devi Chest Hospital, Lahore, where data collection was undertaken. All patients gave written informed consent before recruitment in the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data are not available freely, however we welcome specific and detailed proposals for collaboration. Enquiries and requests for further information should be made to fatimamukhtar@doctor.com.
References
- 1.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 2009;9:737–46. 10.1016/S1473-3099(09)70282-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jimenez-Corona ME, Cruz-Hervert LP, Garcia-Garcia L et al. . Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax 2013;68:214–20. 10.1136/thoraxjnl-2012-201756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brostrom RJ. Summary of the impact of diabetes on tuberculosis control and Submission of draft standards for diabetes and tuberculosis in the US-affiliated Pacific Islands, Meeting Paper: 6. Fifth Pacific Stop TB Meeting 4–7 May 2010 Nadi, Fiji Islands. [Google Scholar]
- 4.Restrepo BI, Fisher-Hoch SP, Smith B et al. . Mycobacterial clearance from sputum is delayed during the first phase of treatment in patients with diabetes. Am J Trop Med Hyg 2008;79:541–4. [PMC free article] [PubMed] [Google Scholar]
- 5.Baker MA, Harries AD, Jeon CY et al. . The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 2011;9:81 10.1186/1741-7015-9-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO report 2011. Global Tuberculosis Control Geneva: WHO, 2011. [Google Scholar]
- 7.World Health Organization. Global tuberculosis report 2015. 20th ed France: World Health Organization; 2015. [Google Scholar]
- 8.Restrepo BI, Camerlin AJ, Rahbar MH et al. . Cross-sectional assessment reveals high diabetes prevalence among newly-diagnosed tuberculosis cases. Bull WHO 2011;89: 352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakeem R, Fawwad A. Diabetes in Pakistan: epidemiology, determinants and prevention. Inter J Diabetol 2011;3:4. [Google Scholar]
- 10.World Health Organisation. Collaborative framework for care and control of tuberculosis and diabetes. Geneva: WHO, Stop TB Department, 2011. [PubMed] [Google Scholar]
- 11.Wang CS, Yang CJ, Chen HC et al. . Impact of type 2 diabetes on manifestations and treatment outcome of pulmonary tuberculosis. Epidemiol Infect 2009;137:203–10. 10.1017/S0950268808000782 [DOI] [PubMed] [Google Scholar]
- 12.Lin Y, Li L, Mi F et al. . Screening patients with diabetes mellitus for tuberculosis in China. Trop Med Int Health 2012;17:1302–8. 10.1111/j.1365-3156.2012.03069.x [DOI] [PubMed] [Google Scholar]
- 13.Indian Diabetes Mellitus-Tuberculosis Study Group. Screening of patients with diabetes mellitus for tuberculosis in India. Trop Med Int Health 2013;18:646–54. 10.1111/tmi.12083 [DOI] [PubMed] [Google Scholar]
- 14.Sullivan T, Ben Amor Y. The co-management of tuberculosis and diabetes: challenges and opportunities in the developing world. PLoS Med 2012;9:e1001269 10.1371/journal.pmed.1001269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008;5:e152 10.1371/journal.pmed.0050152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuberculosis Control Programme Pakistan. Doctors Training course on community-based TB care—DOTS. Department for International Development (DFID), World Health Organisation (WHO), 2012. [Google Scholar]
- 17.Lwanga SK, Lemeshow S. Sample size determination in health studies—a practical manual. Software version by KC Lun, P Chiam. Software version by the National University of Singapore Geneva: 1991; World Health Organization. [Google Scholar]
- 18.Ministry of Health, Government of Pakistan. Refresher module for doctors. Provincial TB control programme Punjab, 2008.
- 19.Dodor EA. Evaluation of nutritional status of new tuberculosis patients at the effia-nkwanta regional hospital. Ghana Med J 2008;42:22–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Y, Li L, Mi F et al. . Screening patients with tuberculosis for diabetes mellitus in China. Trop Med Int Health 2012;17:1294–301. 10.1111/j.1365-3156.2012.03068.x [DOI] [PubMed] [Google Scholar]
- 21.Indian Diabetes Mellitus-Tuberculosis Study Group. Screening of patients with tuberculosis for diabetes mellitus in India. Trop Med Int Health 2013;18:636–45. 10.1111/tmi.12084 [DOI] [PubMed] [Google Scholar]
- 22.Prakash BC, Ravish KS, Prabhakar B et al. . Tuberculosis-diabetes mellitus bidirectional screening at a tertiary care centre, South India. Public Health Action 2013;3:S18–22. 10.5588/pha.13.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organisation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Geneva: WHO/International Diabetes Federation, 2006. [Google Scholar]
- 24.Raghraman S, Vasudevan KP, Govindarajan S et al. . Prevalence of diabetes mellitus among tuberculosis patients in Urban Puducherry. N Am J Med Sci 2014;6:30–4. 10.4103/1947-2714.125863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balasubramanian R, Ramanathan U, Thyagarajan K et al. . Evaluation of an intermittent six-month regimen in new pulmonary tuberculosis patients with diabetes mellitus. Indian J Tuberc 2007;54:168–76. [PubMed] [Google Scholar]
- 26.Desk Guide for Doctors on Management of Tuberculosis. National Tuberculosis Control Program, Pakistan, 2015. [Google Scholar]
- 27.World Health Organisation. Definitions and reporting framework for tuberculosis—2013 revised. Geneva: WHO, Stop TB Department, 2013. [Google Scholar]
- 28.Chiang CY, Bai KJ, Lin HH et al. . The influence of diabetes, glycemic control, and diabetes-related comorbidities on pulmonary tuberculosis. PLoS One 2015;10:e0121698 10.1371/journal.pone.0121698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi H, Lee M, Chen RY et al. . Predictors of pulmonary tuberculosis treatment outcomes in South Korea: a prospective cohort study, 2005–2012. BMC Infect Dis 2014;14:360 10.1186/1471-2334-14-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mi F, Tan S, Liang L et al. . Diabetes mellitus and tuberculosis: pattern of tuberculosis, two-month smear conversion and treatment outcomes in Guangzhou, China. Trop Med Int Health 2013;18:1379–85. 10.1111/tmi.12198 [DOI] [PubMed] [Google Scholar]