Abstract
Background
The number of patients using second-line antiretroviral therapy (ART) has increased over time. In Ethiopia, 1.5% of HIV infected patients on ART are using a second-line regimen and little is known about its effect in this setting.
Objective
To estimate the rate and predictors of treatment failure on second-line ART among adults living with HIV in northwest Ethiopia.
Setting
An institution-based retrospective follow-up study was conducted at three tertiary hospitals in northwest Ethiopia from March to May 2015.
Participants
356 adult patients participated and 198 (55.6%) were males. Individuals who were on second-line ART for at least 6 months of treatment were included and the data were collected by reviewing their records.
Primary outcome measure
The primary outcome was treatment failure defined as immunological failure, clinical failure, death, or lost to follow-up. To assess our outcome, we used the definitions of the WHO 2010 guideline.
Result
The mean±SD age of participants at switch was 36±8.9 years. The incidence rate of failure was 61.7/1000 person years. The probability of failure at the end of 12 and 24 months were 5.6% and 13.6%, respectively. Out of 67 total failures, 42 (62.7%) occurred in the first 2 years. The significant predictors of failure were found to be: WHO clinical stage IV at switch (adjusted HR (AHR) 2.1, 95% CI 1.1 to 4.1); CD4 count <100 cells/mm3 at switch (AHR 2.0, 95% CI 1.2 to 3.5); and weight change (AHR 0.92, 95% CI 0.88 to 0.95).
Conclusions
The rate of treatment failure was highest during the first 2 years of treatment. WHO clinical stage, CD4 count at switch, and change in weight were found to be predictors of treatment failure.
Keywords: EPIDEMIOLOGY, INFECTIOUS DISEASES, PUBLIC HEALTH
Strengths and limitations of this study.
The study involved three diverse hospitals, and had a follow-up period longer than several other similar studies.
The data were collected in a retrospective fashion using secondary sources, with resulting incompleteness.
Information bias may have occurred due to under-reporting of clinical conditions that constitute clinical failure, and missing laboratory results which may have categorised patients as having immunological failure.
Considering treatment failure as a composite outcome for immunological failure, clinical failure, death and lost to follow-up might overestimate the rate of treatment failure.
Use of immunological and clinical criteria lacks both sensitivity and specificity to detect real treatment failure (ie, virological failure) and might underestimate treatment failure.
Introduction
HIV has been a global challenge for the past three decades. In 2013, 35 million people were living with HIV worldwide.1 Sub-Saharan Africa carries the highest burden with an estimated 24.7 million people living with HIV, nearly 71% of the global total.1 2 In Ethiopia the HIV prevalence among adults age 15–49 years was 1.5%,3 and in 2014 the estimated incidence was 35 002 cases with an overall estimated death toll of 52 405.
Since 1995, antiretroviral therapy (ART) has saved the lives of millions globally and has substantially decreased morbidity and mortality in people living with HIV/AIDS (PLWHA).4 Since ART first became freely available in Ethiopia in 2005 until 2013, death due to HIV/AIDS has decreased by 63%.2 Based on the 2014 report released from the United Nations Program on HIV/AIDS (UNAIDS), globally as many as 13 950 296 people were accessing ART.2 For the year 2013-14, the Federal Ministry of Health of Ethiopia (FMOH) reported that 1047 health facilities were providing ART, 805 948 PLWHA were enrolled in HIV/AIDS care, 492 649 PLWHA had started ART, and 344 344 people were using ART. Of these ART users, 1.5% were on second-line treatment. The Amhara regional state in northwest Ethiopia comprises the highest proportion of ART users, with 102 088 individuals.5
Most patients begin treatment for HIV/AIDS on a standard first-line regimen. The first-line treatment consists of a combination of two nucleoside/nucleotide reverse transcriptase inhibitors (NRTI) with one non-nucleoside reverse transcriptase inhibitor (NNRTI). Should failure of first-line treatment occur, a second-line treatment is implemented, utilising two NRTIs not previously used in first-line treatment, as well as one additional protease inhibitor (PI).6 7 Treatment failure of the initial first-line ART regimen is a common, though not inevitable, event.8 9 According to a study done in South Africa, the rate of first-line treatment failure was 13%,10 and another study by Roose et al reported an overall treatment failure rate of 46%.11 Second-line regimens are used after failure of first-line regimens, as measured by the patient's CD4 cell count, HIV viral load, or clinical features. Though frequently effective, standard first-line regimens do not work for everyone, particularly if they are infected with a drug-resistant strain of the virus12 and/or have poor adherence.13 14 Other predictors for treatment failure of first-line treatments include: older age,10 15 male sex,10 11 16 severe malnutrition,10 17 anaemia9 10 18 low baseline CD4 cell count,10 16 19 advanced baseline WHO clinical stage,16 20 21 longer duration of ART intake,22 and a negative change in body weight.23
According to different studies, switching patients after failure of first-line regimens reduces mortality,24 increases viral suppression, improves immune reconstitution,25 26 increases life expectancy,27 and decreases drug resistance.28 Though the number of patients being switched has been increasing over time,9 24 it remains small29 due to delays in switching from first-line therapy and30 31 challenges in drug availability. Little is known about the success of these second-line therapies. Although some studies have been conducted in other sub-Saharan countries, there is little information about second-line ART failure in Ethiopia.
Therefore, this study aimed to determine the rate and predictors of treatment failure on second-line ART. This research also evaluated the progress in Ethiopia's strategy against HIV/AIDS, and is to be used to inform respective stakeholders about the current state of second-line ART users and to assist in planning for the possible need of future, third-line regimens.
Methods
Study design
An institution-based retrospective follow-up study was conducted in three large hospitals in the Amhara regional state, northwest of Ethiopia's capital city, Addis Ababa (University of Gondar Teaching Hospital, Felege Hiwot referral Hospital, and Debretabor Hospital).
Settings
University of Gondar Teaching Hospital HIV care clinic is located in North Gondar administrative zone, Amhara National Regional state, which is about 750 km northwest of Addis Ababa. Currently, University of Gondar Teaching Hospital is serving more than five million people in the North Gondar zone and peoples of the neighbouring zones. Since 2005, when the hospital initiated ART, 7581 adults and 738 paediatrics patients have enrolled. Currently 4891 adults are actively being treated.
The second study area is Felege Hiwot Referral Hospital, located in Bahirdar, Amhara National Regional State, Ethiopia. Bahirdar is the capital city of Amhara National Regional State and it is located 562 km from Addis Ababa and 180 km from Gondar. The hospital serves a catchment population of 5–7 million. Since the initiation of ART in 2005, 16 314 adults and 1383 paediatrics patients have been enrolled. Currently 5401 adults are actively being treated.
The third study area was Debretabor Hospital which is located in Debretabor 665 km from Addis Ababa. It serves a catchment population of around 2 million. In addition to other services, Debretabor Hospital is currently providing HIV chronic care (both pre-ART and ART). Since 2005, when the hospital started ART, 9859 adults and 698 paediatrics patients have been enrolled. Currently 2401 adults are being treated.
Participants
The study population included all HIV positive adults, age 15 years and above, who started second-line ART at University of Gondar, Debre Tabor or Felge Hiwot referral hospitals. Patients who switched to a second-line treatment in the years between September 2006 and October 2014 were included and followed until April 2015 (figure 1). To be included, patients must have been on second-line ART therapy for at least 6 months. This inclusion criterion was based on the WHO treatment guideline which recommends allowing at least 6 months of treatment with a given regimen before diagnosing treatment failure.7 The study participants were managed using different treatment guidelines due to guideline updates over time. The drugs they were taking, the definition of treatment failure, and the criteria used to switch them to a second-line treatment are variable. As per the current recommendation, NRTIs such as lamivudine (3TC), zidovudine (AZT) and tenofovir (TDF); NNRTIs such as efavirenz (EFV) and nevirapine (NVP); and protease inhibitors (PI) such as atazanavir/ritonavir (ATV/r) and lopinavir/ritonavir (LPV/r), are being used. For first-line treatment a combination of two NRTIs with one NNRTI, and for second-line treatment two NRTIs with one PI,6 7 are being used. After baseline data are collected from patients, the CD4 count is repeated every 6–12 months and viral load is determined when there is suspicion of treatment failure.6
Figure 1.
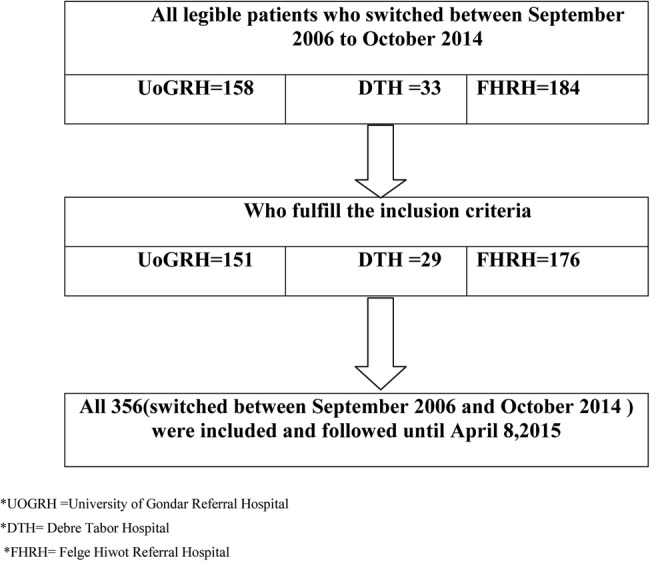
Schematic representation of the sampling procedure of HIV positive adults on second-line antiretroviral therapy at University of Gondar, Debretabor, and Felege Hiwot referral hospitals, 1 September 2006 to 8 April 2015.
Data were collected by four health officers and five BSc nurses who had experience of working in ART clinics. All data collectors were trained in the data collection process which included a standardised data extraction checklist prepared in English. Trained data collectors, other than the investigators, reviewed and extracted data from patient charts and registries about sociodemographic characteristics (age, sex), clinical characteristics (WHO clinical staging, CD4 count at switch of ART, length of time on first-line ART, change in weight, presence of opportunistic infection (OI), calendar year of starting second-line ART), and treatment related factors (drug regimen, OI prophylaxis, cotrimoxazole preventive therapy (CPT), isoniazid (INH) prophylaxis, history of first-line modification, treatment duration, number of NRTIs tried, and drug side effect). Additionally, for the cards that had incomplete information, computer databases were used to supplement the information where possible.
To control the data quality, a data extraction checklist was pre-tested for consistency of understanding the review tools and completeness of data items. The necessary adjustments were made on the final data extraction format and the filled formats were checked daily by the supervisors. Investigators were involved in the supervision of the data collection. The patients’ names and their unique ART numbers were not included during data collection to ensure privacy. To protect patient identity, supervisors linked the patient's card number with a study identification number.
Second-line regimen was defined as a boosted PI-based regimen, following a first-line regimen of one NNRTI and two NRTIs. According to the WHO criteria, clinical failure was defined as a new or recurrent clinical event indicating severe immunodeficiency: WHO clinical stage 4 conditions (eg, Kaposi's sarcoma, pneumocystis pneumonia) and certain WHO clinical stage 3 conditions (eg, pulmonary tuberculosis (TB), severe bacterial infections) after 6 months of effective treatment. Immunological failure was defined as fulfilment of at least one of the following criteria: follow-up CD4 count at or below baseline values, a 50% fall from peak value while on treatment, or persistent CD4 levels below 100 cells/mm3. Death was defined as the recorded death in a patient who was on second-line ART. Transferred out referred to patients who were transferred to other healthcare facilities. Transferred in referred to patients who were transferred from other facilities and accepted by one of the three study hospitals. Loss to follow-up (LTFU) was defined as a patient who had not received repeat ART for a period of 3 months or longer, and was not yet classified as ‘dead’ or ‘transferred-out’. In the setting of HIV drug resistance, genotyping and follow-up viral load of patients on second-line treatment is not being done. Because of this, even if it is less reliable, we assessed treatment failure by using the available information of clinical and immunological criteria. In this study, our primary outcome was treatment failure, defined as a composite outcome of immunological failure, clinical failure, death and LTFU. If a patient had one of the four outcomes, he/she was considered to have had treatment failure. As shown by other studies, most patients who were categorised as LTFU ended up having treatment failure and subsequently died after being lost1 23 32 33 due to rebound viral replication following their treatment discontinuation. Because of these reasons we considered LTFU as a treatment failure. In the setting, since there is only one regional laboratory to undertake viral load determination for more than 20 million people, viral load was not performed for all patients. We did not have any follow-up viral load data to assess virological failure after switching treatment. The reported viral load data are documented at the time of switch to diagnose first-line treatment failure. Regarding the definition of virological failure of first-line treatment, there was no single definition. Since our participants were switched over a variable period of time, there were guideline changes and because of that the definition of virological failure also varied from participant to participant. Therefore, we just recorded the presence of documented virological failure at the time of switch (provided that the definitions were different from time to time). Some patients might switch to second-line treatment based on their clinical and immunological condition regardless of the viral load. Change in weight was defined as the difference between the participant's last weight and the baseline weight at switch to second-line ART.
The data were entered into Epi info V.7 and transferred to STATA V.12.0 for analysis. Descriptive and summary statistics were performed. The rate of failure of the composite outcome (treatment failure) and each of the other potential outcomes separately were observed. Person-time at risk was measured starting from the time of switch to a second-line regimen until each patient ended the follow-up. Patients who switched between September 2006 and October 2014 were included in the analysis. Life table analysis was used to estimate the cumulative failure of patients and log rank tests were used to compare failure curves between the different categories of the explanatory variables. Patients who transferred out or remained alive in care at the end of follow-up were considered as censored observations. Schoenfeld residuals test (both global and scaled) and graphical methods were used to check the Cox proportional hazard assumption. Both bivariable and multivariable Cox proportional hazards models were used to identify predictors of failure. Variables having p values of 0.2 or less in the bivariable analysis were fitted into the multivariable model. The 95% CI of HR was computed and variables having a value of p<0.05 in the multivariable Cox proportional hazards model were considered to be significantly associated with treatment failure.
Results
Baseline characteristics
Of the 356 participants, 198 (55.6%) were male with a mean±SD age of 36±8.9 years; 167 (46.9%) of the participants were between 30 and 39 years of age.
Characteristics during first-line treatment
During the start of first-line treatment, 306 (86%) participants were at WHO clinical stage III or IV and 213 (59.8%) had CD4 counts below 100 cells/mL with a median CD4 count of 78.5 cells/mL (interquartile range (IQR) 37−151). One hundred and seventy-six (49.4%) of the participants were eligible to start ART by both clinical and immunological (CD4 count) criteria. In regard to NRTIs, at initiation of first-line ART, 162 (45.5%) of the study participants were on zidovudine (AZT), 133 (37.4%) were on stavudine (d4T), and 61 (17.1%) were on tenofovir (TDF). Two hundred and fifty-seven (72.2%) of the study participants were taking NVP as an NNRTI drug.
For 118 (33.2%) participants, the first drug regimen was modified before switching to second-line ART, and among them, 102 (86.4%) patients went through only one modification. In 82 (70.7%) participants, drug side effects were the reason for regimen modifications. TB comorbidity was the reason for modification in 24 (20.7%) participants. The median time of stay on first-line regimen was 42.6 months (IQR 26.3−64.1) months. One hundred and twenty-one (34%) participants had a previous history of TB treatment before switching to second-line ART.
Characteristics during and after switch to second-line treatment
At the start of second-line ART, 172 (48.3%) of the patients were at WHO clinical stage III. The median CD4 count at switch was 79 cells/mm3 (IQR 37−155) for all participants. The mean±SD weight of participants at the start of second-line ART was 52.5±10.8 kg. In regard to NRTIs, 206 (57.9%) of the patients were taking TDF based second-line regimens. For PIs, 289 (81.2%) were taking lopinavir boosted with ritonavir (table 1).
Table 1.
Sociodemographic, clinical and immunological characteristics of HIV positive adults at start of second-line ART at University of Gondar, Debretabor, and Flege Hiwot referral hospitals, September 2006 to April 2015
| Variable | Category | Frequency | % |
|---|---|---|---|
| Age (years) | 15–29 | 78 | 21.9 |
| 30–39 | 167 | 46.9 | |
| 40–49 | 82 | 23.0 | |
| >50 | 29 | 8.2 | |
| Sex | Male | 198 | 55.6 |
| Female | 158 | 44.4 | |
| Marital status | Unmarried | 154 | 43.3 |
| Married | 157 | 44.1 | |
| Not recorded | 45 | 12.6 | |
| Residence | Urban | 260 | 73.0 |
| Rural | 76 | 21.4 | |
| Not recorded | 20 | 5.6 | |
| WHO clinical stage at switch | Stage I/II | 127 | 35.7 |
| Stage III | 172 | 48.3 | |
| Stage IV | 57 | 16.0 | |
| Number of changed NRTI at switch | None | 19 | 5.3 |
| One | 217 | 61.0 | |
| Two | 120 | 33.7 | |
| Protease inhibitor | Lopinavir/ritonavir | 289 | 81.2 |
| Atazanavir/ritonavir | 64 | 18.0 | |
| Nelfinavir | 3 | 0.84 | |
| NRTI backbone at switch | Tenofovir | 206 | 58.0 |
| Abacavir | 102 | 28.6 | |
| Zidovudine | 41 | 11.5 | |
| †Others (d4t, ddi) | 7 | 2.0 | |
| CD4 count at switch | <100 cells/mm3 | 222 | 62.4 |
| ≥100 cells/mm3 | 134 | 37.6 | |
| CPT | Yes | 306 | 86.0 |
| No | 50 | 14.0 | |
| INH prophylaxis | Yes | 33 | 9.3 |
| No | 323 | 90.7 | |
| Total | 356 | 100.00 |
ART, antiretroviral therapy; CPT, cotrimoxazole preventive therapy; d4t, stavudine; ddi, didanosine; INH, isoniazid; NRTI, nucleoside reverse transcriptase inhibitors.
The reasons for patients switching to second-line treatments were virological failure, immunological failure, clinical failure and drug toxicity of first-line treatment. The most common causes were a combination of immunological and virological failure (40.5%), followed by immunological failure only (37.1%), and a combination of clinical, immunological and virological failure (8.9%). The less common reasons were immunological and clinical failure in combination, drug toxicity only, virological failure only, and clinical failure only (6.5%, 4.8%, 1.4%, and 0.8%, respectively) (table 2).
Table 2.
Reasons for switching to second-line ART, at University of Gondar, Debretabor, and Flege Hiwot referral hospitals, 1 September 2006 to 8 April 2015
| Reasons for switching | Frequency | % |
|---|---|---|
| Immunological and virological failure | 144 | 40.5 |
| Immunological failure only | 132 | 37.1 |
| Clinical, immunological and virological failure | 32 | 8.9 |
| Immunological and clinical failure | 23 | 6.5 |
| Drug toxicity only | 17 | 4.8 |
| Virological failure only | 5 | 1.4 |
| Clinical failure only | 3 | 0.8 |
| Total | 356 | 100.00 |
ART, antiretroviral therapy.
Two hundred and ten (59%) of the participants had a recorded viral load before the start of second-line treatment, of which 177 (84.3%) had virological failure; 331(93%) of the participants also had an immunological failure at the start of second-line ART (table 2). During the follow-up period, 24 (6.7%) participants developed opportunistic infections other than those classified in the clinical failure category. Eighteen (5.1%) of the participants modified their second-line treatment, of whom 12 (66.7%) modified due to drugs being out of stock. Drug adverse effect were recorded in 23 (6.5%) participants.
Treatment failure of second-line treatment
Study subjects were followed for a median follow-up period of 32.3 months (IQR 15.4−53.2) after switching to second-line ART, with a total observation period of 1085.11 person-years. A total of 67 patients developed treatment failure, of which 19 (28.3%) and 42 (62.7%) were reported within the first and second years of follow-up, respectively. Among the total failures, 24 (35.8%) were immunological failure, 21 (31.3%) were deaths, 11 (16.4%) were clinical failure, and 11 (16.4%) were categorised as lost to follow-up.
The incidence of treatment failure of second-line treatment was 61.7 (95% CI 48.6 to 78.5) per 1000 person years of observation. From this, immunological failure, clinical failure, death, and lost to follow-up were 22.1, 10, 19, and 10 per 1000 person years, respectively.
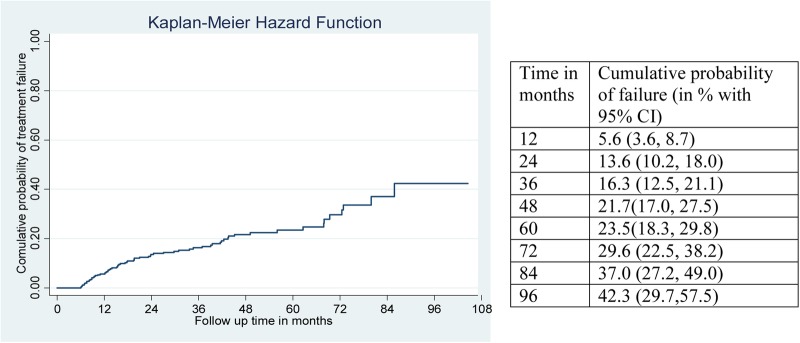
Considering the composite outcome, the cumulative probabilities of failure at 12 months was 5.6% (95% CI 3.6% to 8.7%), at 24 months it was 13.6% (95% CI 10.2% to 18%), at 60 months it was 23.5% (95% CI 18.3% to 29.8%), and at 96 months it was 42.3% (95% CI 29.7% to 57.5%) (figure 2). At the end of 24 and 60 months, the probability of failure for those who had CD4 counts <100 cells/mm3 at switch was 16.1% and 27.2%, respectively.
Figure 2.
Kaplan-Meier failure curve showing hazard from treatment failure of HIV positive adults on second-line antiretroviral therapy at University of Gondar, Debretabor, and Felege Hiwot referral hospitals, September 2006 to April 2015.
Predictors of second-line treatment failure
In the multivariable Cox regression analysis, the independent predictors of treatment failure of second-line ART were: a negative change in weight, a CD4 count <100 cells/mm3 at the start of second-line ART, and WHO clinical stage IV at the start of second-line ART (table 3). Being at WHO stage IV compared to WHO stage I/II was associated with a 2.1 times higher risk (adjusted HR (AHR) 2.1, 95% CI 1.1 to 4.1) of treatment failure.
Table 3.
Multivariable Cox regression analysis of predictors of second-line ART failure of adult HIV positive patients at University of Gondar, Debretabor, and Felege Hiwot referral hospitals, September 2006 to April 2015
| Treatment failure |
Crude HR (95% CI) | Adjusted HR (95% CI) | ||
|---|---|---|---|---|
| Variable | Yes | No | ||
| Age (years) | ||||
| 15–29 | 17 | 61 | 1 | * |
| 30–39 | 26 | 141 | 0.7 (0.4 to 1.3) | 0.7 (0.4 to 1.4) |
| 40–49 | 14 | 68 | 0.8 (0.4 to 1.5) | 0.9 (0.4 to 1.8) |
| ≥50 | 10 | 19 | 1.8 (0.8 to 4.0) | 1.7 (0.8 to 3.9) |
| INH prophylaxis | ||||
| Yes | 3 | 30 | 2.3 (0.7 to 7.2) | 1.7 (0.5 to 5.7) |
| No | 64 | 259 | 1 | |
| Weight change (per 1 kg increase) | 0.9 (0.89 to 0.96) | 0.916 (0.88 to 0.95) | ||
| WHO clinical staging at switch | ||||
| I/II | 20 | 107 | 1 | |
| III | 29 | 143 | 0.8 (0.5 to 1.5) | 1.0 (0.6 to 1.8) |
| IV | 18 | 39 | 1.6 (0.8 to 3.0) | 2.1 (1.1 to 4.1) |
| CD4 cell count | ||||
| <100 cells/mm3 | 47 | 175 | 1.8 (1.0 to 2.9) | 2 (1.2 to 3.5) |
| ≥100 cells/mm3 | 20 | 114 | 1 | |
| NRTI‡ at first-line ART start | ||||
| Stavudine (d4T) | 29 | 104 | 1 | |
| Zidovudine (AZT) | 15 | 137 | 0.9 (0 0.5 to 1.5) | 0.9 (0.5 to 1.6) |
| Tenofovir (TDF) | 13 | 48 | 1.8 (0.9 to 3.6) | 1.9 (0.9 to 3.8) |
*Non-significant from the multivariable Cox regression.
ART, antiretroviral therapy; AZT, as lamivudine (3TC), zidovudine; INH,isoniazid; NRTI, nucleoside reverse transcriptase inhibitors.
A change in weight from initiation of the study to the end of follow-up had an inverse relation with failure. For a unit increase in weight in kilograms, the risk of treatment failure decreased by 8.4% (AHR 0.916, 95% CI 0.88 to 0.95). A CD4 count <100 cells/mm3 at the start of second-line ART increased the risk of developing treatment failure by 2.0 times as compared to a CD4 count >100 cells/mm3 (AHR 2, 95% CI 1.2 to 3.5).
Discussion
This study aimed to measure predictors of treatment failure of adult patients on second-line ART. The overall incidence of failure was 61.7 (95% CI 48.6 to 78.5) per 1000 person years, with the majority of failures (62.8%) occurring in the first 2 years of follow-up. This study found the significant predictors of treatment failure of second-line ART to include: a negative change in weight during the study period, a CD4 count <100 cells/mm3 at switch, and patients categorised as WHO clinical stage IV at switch.
The incidence of failure in this study is in agreement with studies conducted in Cambodia and Thailand and a meta-analysis done in developing countries.22 25 34 35 However, the incidence of failure found by this study is lower than a multicentred study conducted in Asia and Africa, which found an overall incidence of 195 per 1000 person-years.36 The reason for this might be due to the difference in follow-up periods. Because most failures occur soon after the switch is made to a second-line therapy, a shorter follow-up period is likely to find a higher probability of failure when compared to a study with a longer follow-up period. Another explanation may be differences in the diagnostic criteria for treatment failure. In another study, viral load was used to assess treatment failure, in addition to other criteria. Viral load increases before other immunologic and clinical markers, which can shorten the time to diagnosis. Due to its sensitivity, patients who have no immunological and clinical failure may already have virological failure. Finally, this study was prospective which allowed the investigators to ensure strict follow-up and diagnosis of failure criteria, unlike our current study which was retrospective.
In our study, being at WHO clinical stage IV at the time of switch to second-line therapy was one of the significant predictors of treatment failure. Patients who were at WHO clinical stage IV were 2.1 times more at risk of treatment failure than those patients who were at WHO clinical stage I/II. This result is consistent with studies done in Malawi and sub-Saharan Africa.13 37 This finding is likely due to the fact that patients who present with advanced disease stage are at higher risk of drug resistance, viral mutation and death. Additionally, having a comorbid advanced opportunistic disease which categorises a patient as WHO IV may lead to drug interactions between the treatment for OI and the ART which may further compromise their immunity. This may negatively affect their response to treatment after switch.
A CD4 count below 100 cells/mm3 was also a predictor of failure. A CD4 count <100 cells/mm3 at baseline increased the risk of developing treatment failure by 2.0 times compared to a CD4 count ≥100 cells/mm3. This finding is consistent with studies done in Thailand, Malawi, and South Africa.13 36 38 This might be due to the fact that patients with a very low CD4 count are more likely to have different opportunistic infections and the added burden of these diseases further complicates their response. This likely increases the possibility of treatment failure and/or death.
Change in weight was the other significant predictor of failure. For a unit increase in weight in kilograms, the risk of treatment failure decreased by 8.4%. This can be explained by understanding weight gain as an indicator of good response to treatment and having a positive effect for immunity.13 Decreasing weight may cause OI and vice versa. In most of cases weight change is linked to the clinical condition of the patient.
While the strengths of this study include the involvement of three diverse hospitals and a follow-up period longer than several other similar studies, it also has limitations. First, the data were collected in a retrospective fashion using secondary sources with resulting incompleteness, especially where treatment adherence of the participants was not assessed. Adherence to treatment is a known predictor of treatment response, and lack of adherence data is a limitation of the current study. Additionally, information bias may have occurred due to under-reporting of clinical conditions that constitute clinical failure, and missing laboratory results which may have categorised patients as having immunological failure. Use of immunological and clinical criteria lacks both sensitivity and specificity to detect real treatment failure (ie, virological failure) and it might underestimate treatment failure. Finally, regarding treatment failure as a composite outcome for immunological failure, clinical failure, death and lost to follow-up might overestimate the rate of treatment failure.
Conclusions
The rate of treatment failure was high during the first 2 years after switching to a second-line regimen. The significant predictors of second-line ART therapy failure were: being at WHO clinical stage IV; having a CD4 count <100 cells/mm3 at the time of switching to a second-line treatment; and a negative change in weight. Therefore, an alternative third-line ART regimen should be considered for those who are on a failing second-line regimen. This study may be generalised to patients who are using second-line ART in the Amhara region.
Acknowledgments
We would like to thank the University of Gondar, Debre Tabor and Felege Hiwot referral hospitals administrative bodies, data clerks and card room workers for their cooperation and permission to conduct the study. We are grateful to the University of Gondar for the financial support as well as the data collectors who participated in this study for their commitment. The authors also would like to extend their gratitude to Anna Bazinet and Khathrine Pfizenmaier for their manuscript editing.
Footnotes
Contributors: ATT conceived the idea and the research designed by ATT, MW and TA. ATT coordinated the process. ATT and TA analysed the data. ATT, MW, TA and KAA wrote the paper. All authors read and approved the final manuscript.
Funding: The financial backing of this research was provided by the University of Gondar as a grant to its staff.
Competing interests: None declared.
Ethics approval: Ethical clearance was obtained from the Institutional Review Board of University of Gondar. A letter of support and a permission letter were obtained from the Amhara Regional State Health Bureau and the hospital's administration, respectively. Care was taken to keep all patient information confidential. Since we used secondary sources, informed consent was waived.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data supporting our findings will be shared on request.
References
- 1.World Health Organization. Global update on the health sector response to HIV 2014. http://apps.who.int/iris/bitstream/10665/128494/1/9789241507585_eng.pdf?ua=1
- 2.UNAIDS. The gap report 2014. http://www.aidsdatahub.org/sites/default/files/publication/UNAIDS_Gap_report_en.pdf
- 3.Central statistical agency. Ethiopian Demographic and Health Survey 2011. http://www.unicef.org/ethiopia/ET_2011_EDHS.pdf
- 4.Chan KC, Wong KH, Lee SS. Universal decline in mortality in patients with advanced HIV-1 disease in various demographic subpopulations after the introduction of HAART in Hong Kong, from 1993 to 2002. HIV Med 2006;7:186–92. 10.1111/j.1468-1293.2006.00352.x [DOI] [PubMed] [Google Scholar]
- 5.FMOH. Health Sector Development Programme IV Annual Performance Report 2013/14(1). http://www.moh.gov.et/documents/26765/0/Annual+Perfomance+Report+2006+EFY/4f5a6b33-3ef1-4430-a0a0-21fa13221343?version=1.0
- 6.Federal Ministry of Health Ethiopia. National Comprehensive HIV Care and Treatment Training for Health care Providers Participant Manual 2014.
- 7.World Health Organization. Consolidated Guidelines On The Use Of Antiretroviral Drugs For Treating And Preventing HIV Infection Recommendations for a Public Health Approach 2013. http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1
- 8.Johnston V, Cohen K, Wiesner L et al. . Viral suppression following switch to secondline antiretroviral therapy: associations with nucleoside reverse transcriptase inhibitor resistance and subtherapeutic drug concentrations prior to switch. J Infect Dis 2014;209:711–20. 10.1093/infdis/jit411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jourdain G, Le Cœur S, Ngo-Giang-Huong N et al. . Switching HIV treatment in adults based on CD4 count versus viral load monitoring: a randomized, noninferiority trial in Thailand. PLoS Med 2013;10:e1001494 10.1371/journal.pmed.1001494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox MP, Shearer K, Maskew M et al. . Treatment outcomes after seven years of public-sector HIV treatment at the Themba Lethu clinic in Johannesburg, South Africa. AIDS 2012;26:1823–8. 10.1097/QAD.0b013e328357058a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barth RE, Tempelman HA, Moraba R et al. . Long-term outcome of an HIV-treatment programme in rural Africa: viral suppression despite early mortality. AIDS Res Treat 2011;2011:434375 10.1155/2011/434375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panos Global AIDS Programme. Antiretroviral drugs for all? Obstacles to access to HIV/AIDS treatment. Lessons from Ethiopia, Haiti, India, Nepal and Zambia 2006. http://otp.unesco-ci.org/training-resource/community-empowerment-general/antiretroviral-drugs-all-obstacles-access-hivaids-tr
- 13.Hosseinipour MC, Kumwenda JJ, Weigel R et al. . Second-line treatment in the Malawi antiretroviral programme: high early mortality, but good outcomes in survivors, despite extensive drug resistance at baseline. HIV Med 2010;11:510–18. 10.1111/j.1468-1293.2010.00825.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy RA, Sunpath H, Castilla C et al. . Second-line antiretroviral therapy: long-term outcomes in South Africa. J Acquir Immune Defic Syndr 2012;61:158–63. 10.1097/QAI.0b013e3182615ad1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulissa Z, Jerene D, Lindtjørn B. Patients present earlier and survival has improved, but pre-ART attrition is high in a six-year HIV cohort data from Ethiopia. PLoS One 2010;5:e13268 10.1371/journal.pone.0013268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wandeler G, Keiser O, Pfeiffer K et al. . Outcomes of antiretroviral treatment programs in rural Southern Africa. J Acquir Immune Defic Syndr 2012;59:e9–16. 10.1097/QAI.0b013e31823edb6a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zachariah R, Fitzgerald M, Massaquoi M et al. . Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS 2006;20:2355–60. 10.1097/QAD.0b013e32801086b0 [DOI] [PubMed] [Google Scholar]
- 18.Tadesse K, Haile F, Hiruy N. Predictors of mortality among patients enrolled on antiretroviral therapy in Aksum hospital, northern Ethiopia: a retrospective cohort study. PLoS One 2014;9:e87392 10.1371/journal.pone.0087392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao L, Xing H, Su B et al. . Impact of HIV drug resistance on virologic and immunologic failure and mortality in a cohort of patients on antiretroviral therapy in China. AIDS 2013;27:1815–24. 10.1097/QAD.0b013e3283611931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bussmann H, Wester CW, Ndwapi N et al. . Five-year outcomes of initial patients treated in Botswana's National Antiretroviral Treatment Program. AIDS 2008;22:2303–11. 10.1097/QAD.0b013e3283129db0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wandeler G, Keiser O, Mulenga L et al. . Tenofovir in second-line ART in Zambia and South Africa: collaborative analysis of cohort studies. J Acquir Immune Defic Syndr 2012;61:41–8. 10.1097/QAI.0b013e3182632540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ajose O, Mookerjee S, Mills EJ et al. . Treatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. AIDS 2012;26:929–38. 10.1097/QAD.0b013e328351f5b2 [DOI] [PubMed] [Google Scholar]
- 23.Rajasekaran S, Jeyaseelan L, Vijila S et al. . Predictors of failure of first-line antiretroviral therapy in HIV-infected adults: Indian experience. AIDS 2007;21(Suppl 4):S47–53. 10.1097/01.aids.0000279706.24428.78 [DOI] [PubMed] [Google Scholar]
- 24.Gsponer T, Petersen M, Egger M et al. . The causal effect of switching to second-line ART in programmes without access to routine viral load monitoring. AIDS 2012;26:57–65. 10.1097/QAD.0b013e32834e1b5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferradini L, Ouk V, Segeral O et al. . High efficacy of lopinavir/r-based second-line antiretroviral treatment after 24 months of follow up at ESTHER/Calmette Hospital in Phnom Penh, Cambodia. J Int AIDS Soc 2011;14:14 10.1186/1758-2652-14-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel D, Desai M, Shah AN et al. . Early outcome of second line antiretroviral therapy in treatment experienced human immunodeficiency virus positive patients. Perspect Clin Res 2013;4:216–20. 10.4103/2229-3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimmel AD, Weinstein MC, Anglaret X et al. . Laboratory monitoring to guide switching antiretroviral therapy in resource-limited settings: clinical benefits and cost effectiveness. J Acquir Immune Defic Syndr 2010;54:258–68. 10.1097/QAI.0b013e3181d0db97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols BE, Sigaloff KC, Kityo C et al. . Increasing the use of second-line therapy is a cost-effective approach to prevent the spread of drug-resistant HIV: a mathematical modelling study. J Int AIDS Soc 2014;17:19164 10.7448/IAS.17.1.19164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assefa Y, Kiflie A, Tesfaye D et al. . Outcomes of antiretroviral treatment program in Ethiopia: retention of patients in care is a major challenge and varies across health facilities. BMC Health Serv Res 2011;11:81 10.1186/1472-6963-11-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan S, Das M, Andries A et al. . Second-line failure and first experience with third-line antiretroviral therapy in Mumbai, India. Globa Health Action 2014;7:24861 10.3402/gha.v7.24861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long L, Fox M, Sanne I et al. . The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS 2010;24:915–19. 10.1097/QAD.0b013e3283360976 [DOI] [PubMed] [Google Scholar]
- 32.Dalal RP, MacPhail C, Mqhayi M et al. . Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr 2008;47:101–7. 10.1097/QAI.0b013e31815b833a [DOI] [PubMed] [Google Scholar]
- 33.Keiser O, Tweya H, Braitstein P et al. . Mortality after failure of antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health 2010;15:251–8. 10.1111/j.1365-3156.2009.02445.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boettiger DC, Nguyen VK, Durier N et al. . Efficacy of second-line antiretroviral therapy among people living with HIV/AIDS in Asia: results from the TREAT Asia HIV observational database. J Acquir Immune Defic Syndr 2015;68:186–95. 10.1097/QAI.0000000000000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siripassorn K, Manosuthi W, Chottanapund S et al. . Effectiveness of boosted protease inhibitor-based regimens in HIV type 1-infected patients who experienced virological failure with NNRTI-based antiretroviral therapy in a resource-limited setting. AIDS Res Hum Retroviruses 2010;26:139–48. 10.1089/aid.2009.0125 [DOI] [PubMed] [Google Scholar]
- 36.Pujades-Rodríguez M, Balkan S, Arnould L et al. . Treatment failure and mortality factors in patients receiving second-line HIV therapy in resource-limited countries. JAMA 2010;304:303–12. 10.1001/jama.2010.980 [DOI] [PubMed] [Google Scholar]
- 37.Sigaloff KC, Hamers RL, Wallis CL et al. . Second-line antiretroviral treatment successfully resuppresses drug-resistant HIV-1 after first-line failure: prospective cohort in Sub-Saharan Africa. J Infect Dis 2012;205:1739–44. 10.1093/infdis/jis261 [DOI] [PubMed] [Google Scholar]
- 38.Court R, Leisegang R, Stewart A et al. . Short term adherence tool predicts failure on second line protease inhibitor-based antiretroviral therapy: an observational cohort study. BMC Infect Dis 2014;14:664 10.1186/s12879-014-0664-3 [DOI] [PMC free article] [PubMed] [Google Scholar]