Abstract
Objectives
The aim of this pilot study was to determine the serum concentration of heparan sulfate, hyaluronan, chondroitin sulfate and syndecan-1 and if these serum concentrations can be used to identify women at 20 weeks' gestation who later develop gestational diabetes mellitus (GDM).
Design
Nested case–control study from Auckland, New Zealand participants in the prospective cohort Screening for Pregnancy Endpoints study.
Setting
Auckland, New Zealand.
Participants
20 pregnant women (70% European, 15% Indian, 10% Asian, 5% Pacific Islander) at 20 weeks' gestation without any hypertensive complications who developed GDM by existing New Zealand criteria defined as a fasting glucose ≥5.5 mmol/L and/or 2 hours ≥9.0 mmol/L after a 75 g Oral Glucose Tolerance Test. Women not meeting these criteria were excluded from this study. The patients with GDM were matched with 20 women who had uncomplicated pregnancies and negative screening for GDM and matched for ethnicity, maternal age and BMI.
Primary and secondary outcome measures
The primary measures were the serum concentrations of syndecan-1, heparan sulfate, hyaluronan and chondroitin sulfate determined by quantitative ELISA. There were no secondary outcome measures.
Results
Binary logistic regression was performed to determine if serum concentrations of endothelial glycocalyx layer constituents in women at 20 weeks' gestation would be useful in predicting the subsequent diagnosis of GDM. The model was not statistically significant χ2=12.5, df=8, p=0.13, which indicates that the model was unable to distinguish between pregnant women at 20 weeks' gestation who later developed GDM and those who did not.
Conclusions
Serum concentrations of syndecan-1, heparan sulfate, hyaluronan and chondroitin sulfate in pregnant women at 20 weeks' gestation were not associated with later development of GDM. To further explore whether there is any relationship between endothelial glycocalyx constituents and GDM, the next step is to evaluate serum concentrations at the time diagnosis of GDM.
Keywords: gestational diabetes mellitus, glycocalyx, glycosaminoglycans, endothelium
Strengths and limitations of this study.
This study used a nested case control study from Auckland, New Zealand participants in the prospective cohort Screening for Pregnancy Endpoints study.
To the best of our knowledge, this is the first study to investigate serum levels of endothelial glycocalyx layer constituents in women at 20 weeks’ gestation who later developed gestational diabetes mellitus compared to matched controls.
Two limitations of this pilot study were the study population was predominantly of European descent (70%) and the small sample size (n=20).
Introduction
More than 50% of women of reproductive age in New Zealand (NZ) are overweight or obese when they become pregnant1 and gestational diabetes mellitus (GDM) is now diagnosed in ∼18% of obese pregnant women using current NZ diagnostic criteria.2 Since there is a continuous relationship between increasing blood glucose on the Oral Glucose Tolerance Test (OGTT) and adverse maternal and infant outcomes,3 lower thresholds for international diagnostic criteria have been recommended to diagnose GDM.4 If adopted, these new criteria would identify up to 30% of obese women as having GDM.5 A simple blood test that enabled early and reliable diagnosis of GDM would improve antenatal care for women by replacing a complicated diagnostic test.
Women with GDM have increased rates of pregnancy morbidity such as preeclampsia and caesarean section as well as a 50% lifetime risk of developing type-2 diabetes.6 GDM exposes the unborn baby to an abnormal metabolic environment with excessive nutrients, and consequently more infants are born excessively large with increased rates of birth trauma.7 Of great concern, GDM in pregnancy creates a vicious intergenerational cycle, which is further compounded when the mother is also obese. The resultant large infants are more likely to become obese children and adults who later develop type-2 diabetes with resultant lifelong increased healthcare costs.8 9 This cycle further promotes health inequalities in the next generation.3 Earlier diagnosis of GDM, before the usual screen at 24–28 weeks, might enable earlier intervention, such as with lifestyle advice and, if required, glucose-lowering agents, with the potential to reduce the adverse health outcomes for mother and child.10 11
A potential early marker of DM is endothelial dysfunction (impaired endothelium):12 13 the endothelium loses the ability to maintain homoeostasis and, thus vessel health is compromised. Fundamental to protecting vessel health is the interface between circulating blood and the endothelium. Strategically located at this interface is the endothelial glycocalyx layer (EGL).14 The EGL is a membranous gel-like layer of proteoglycans (eg, syndecans, glypicans, perlecan and versican), glycosaminoglycans (primarily hyaluronan (HA), heparan sulfate (HS), chondroitin sulfate (CS) and dermatan sulfate), glycoproteins and plasma proteins.14 15 Although the existence of the EGL has been known for around 70 years,16 for much of this time it was thought to be only a few nanometres thick and of little functional importance.17 However, this view has dramatically changed in recent years: (1) the full in vivo thickness of the EGL can even exceed that of the endothelium;18 and (2) the thickness and composition change as a function of the health of the cell—known as shedding.12 19 20 Thus, the thickness and composition of the EGL change as a function of cell health. Constituents of the EGL are shed into the circulation and the concentrations of these constituents in the circulation can be used as indicators for EGL and endothelium health.21
For example, Hofmann-Kiefer et al22 measured serum levels of syndecan-1, heparan sulfate and hyaluronan throughout pregnancy in women with HELLP (haemolysis, elevated liver enzymes and low platelets), as well as in healthy non-pregnant controls. Results showed increased serum concentrations of syndecan-1, heparan sulfate and hyaluronan in patients with HELLP syndrome compared to normal pregnancy at similar gestations.22 Lopez-Quintero et al23 showed that cultured endothelial cells exposed to hyperglycaemia decreased heparan sulfate content in the EGL. Nieuwdrop et al24 demonstrated by sublingual imaging of the microvascular glycocalyx and intravascular distribution volume of the glycocalyx that patients with type-1 diabetes have reduced EGL volume. In addition, plasma hyaluronan and hyaluranidase (an enzyme that degrades hyaluronan and indicates the capacity for EGL degradation) concentration have been shown to be higher in patients with type-2 diabetes mellitus25 and type-1 diabetes24 26 27 compared to healthy controls. Also, Wang et al28 showed that patients with diabetes had higher serum concentration of Syndecan-1 compared to healthy controls. These studies imply an alteration in EGL constituents of patients with diabetes.
This pilot study aims to extend previous research on EGL constituents as biomarkers for disease status by investigating whether serum concentrations of endothelial glycocalyx constituents, previously shown to shed during diabetes, can be used to identify women at 20 weeks' gestation who later develop GDM. The primary aim is to compare serum concentration levels of EGL constituents (syndecan-1 (S1), HS, HA and CS) between women who develop GDM and matched without GDM women with normal pregnancies.
Study design
Nested case–control study from Auckland participants in the prospective cohort Screening for Pregnancy Endpoints (SCOPE) study.29
Study participants, definition of GDM and matching criteria
We identified 20 participants without any hypertensive complications from the SCOPE study29 (http://www.scopestudy.net/) in Auckland, New Zealand who developed GDM by existing New Zealand criteria defined as a fasting glucose ≥5.5 mmol/L and/or 2 hours ≥9.0 mmol/L after a 75 g OGTT.30
The patients with GDM were matched with participants who had uncomplicated pregnancies and negative screening for GDM (using the definition of GDM above) and matched for (1) ethnicity (2) maternal age (age±5 years) and (c) body mass index (BMI; matched to ±3 kg/m2).
Sample size and power
No previous data existed on the differences in serum concentration of EGL constituents for pregnant women with and without GDM. However, since this was a pilot, we used all GDM cases available in the SCOPE study in Auckland, New Zealand.
Serum sample collection
Venepuncture was performed at 20±1 weeks' gestation in non-fasting participants. Serum samples were collected into BD plain serum vacutainer tubes, placed on ice and centrifuged at 2400 g at 4°C according to a standardised protocol. Serum was stored in 250 μL aliquots at −80°C within 4 hours of collection.
Experimental methods
To assess shedding of the EGL in the circulation in women with GDM and without GDM, we quantified the concentration of the main components of the EGL:15 S1, HS, HA and CS by quantitative ELISA measurements. For each EGL constituent, its concentration was determined using commercially available ELISA kits, as per the manufacturer's instructions (Syndecan-1, 950.640.096, Diaclone, Besancon Cedex, France; Heparan Sulfate, CSB-E09585h, CusaBio Biotech, Hubei Province, P.R. China; Hyaluronan, DHYAL0, R&D Systems, Minneapolis, Minnesota, USA; Chondroitin Sulfate, CSB-E09587h, CusaBio Biotech, Hubei Province, P.R. China). For each target, all samples were run in triplicate, while standards were run in duplicate; samples were randomly assigned to a triplicate block on the ELISA plate. GDM cases and their matched controls were run on the same ELISA plate. All laboratory staff performing the ELISA were blinded to GDM status and participant matches.
Before the ELISA measurements were made for the cases with GDM and matched without GDM, serial dilution experiments were performed to determine an appropriate dilution factor for each target. Since the assay range for each kit was different, the corresponding serum dilutions for each EGL constituent was also different: HS—1:1, 1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128; HA—1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128; 1:256; CS: 1:100, 1:200, 1:300; 1:400, 1:500, 1:600; and S1–2:5, 1:5, 1:10, 1:20, 1:40, 1:80, 1:160, 1:320. Each sample was run in duplicate and average concentration for each dilution was calculated along with the SD. Serum collected at 20 weeks of gestation from four European women who had a negative GDM screen and an uncomplicated pregnancy was used.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics (V.22). Mean, SD of the mean, median and IQR were calculated for each EGL constituent measured. Since most data were not normally distributed, data are presented as the median (25th centile, 75th centile). For the analysis, logistic regression was used with GDM/without GDM as the binary outcome variable; the explanatory variables were BMI, maternal age and serum concentrations of S1, HS, HA and CS. For each explanatory variable, we obtained an OR for GDM. In addition to comparing serum concentration between patients with GDM/without GDM, the serum concentration data were analysed by t-tests for normally distributed data; non-normal data were log-transformed and t-tests were performed. A p value <0.05 was defined as statistically significant. All staff analysing the data were blinded to GDM status.
Results
Study population
The study population consisted of 20 pregnant women at 20 weeks of gestation who later developed GDM and 20 controls with a negative screen for GDM and with uncomplicated pregnancies (table 1). Seventy per cent identified themselves as being of European ethnicity.
Table 1.
Maternal age, ethnicity and BMI for the cases with GDM and matched without GDM
| Cases with GDM (n=20) |
Matched cases without GDM (n=20) |
|||||
|---|---|---|---|---|---|---|
| Maternal age (years) | Ethnicity | BMI (kg/m2) | Maternal age (years) | Ethnicity | BMI (kg/m2) | |
| 1 | 34 | European | 24.5 | 38 | European | 26.4 |
| 2 | 27 | Indian | 23.8 | 26 | Indian | 21.4 |
| 3 | 28 | European | 23.7 | 27 | European | 21.9 |
| 4 | 33 | European | 32.4 | 28 | European | 32.3 |
| 5 | 26 | Pacific Islander | 34.3 | 21 | Pacific Islander | 32.2 |
| 6 | 35 | European | 30.4 | 35 | European | 30.4 |
| 7 | 40 | European | 22.4 | 40 | European | 24.5 |
| 8 | 35 | European | 28.5 | 39 | European | 26 |
| 9 | 38 | European | 31.4 | 40 | European | 29 |
| 10 | 29 | European | 22.8 | 31 | European | 21.3 |
| 11 | 25 | European | 19.9 | 27 | European | 22 |
| 12 | 26 | Indian | 26 | 26 | Indian | 23.6 |
| 13 | 27 | European | 23.8 | 32 | European | 23 |
| 14 | 19 | European | 37.3 | 24 | European | 36.7 |
| 15 | 33 | European | 24.4 | 30 | European | 23.5 |
| 16 | 32 | European | 27.2 | 32 | European | 28.7 |
| 17 | 29 | Asian | 20.4 | 29 | Asian | 19.7 |
| 18 | 28 | Indian | 25.8 | 24 | Indian | 22.8 |
| 19 | 33 | European | 24.2 | 29 | European | 21.2 |
| 20 | 33 | Asian | 27.5 | 31 | Asian | 35.4 |
All women were at 20 weeks of gestation.
BMI, body mass index; GDM, gestational diabetes mellitus.
The cases with GDM had a mean age of 30.5 (SD 4.98) years and a mean BMI of 26.5 (SD 4.6) kg/m2. The cases without GDM had a mean age of 31.2 (SD 5.4) years and mean BMI of 25.6 (SD 4.4) kg/m2. Random glucose median (IQR) measures in the GDM and control groups were 6.0 (5.0–6.5) mmol/L and 5.3 (5.0–6.3) mmol/L (p=0.49), respectively.
Dilution factor experiment
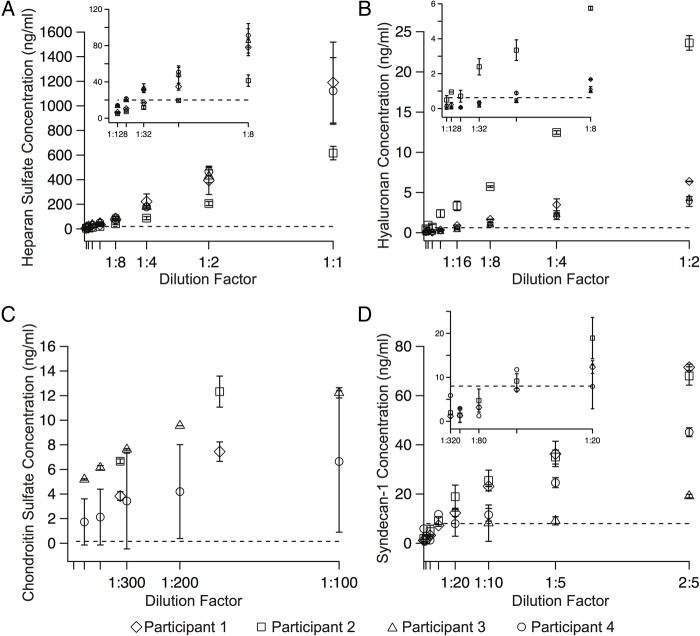
A dilution factor was recommended by the ELISA kit manufacturers for HA (1:4), CS (1:20) and S1 (1:5); however, the HS ELISA kit manufacturer did not recommend a dilution factor. To determine the appropriate HS ELISA kit dilution factor for the pilot study, and to confirm the recommended dilution factor for the other ELISA kits, a series of serial dilution experiments were performed. The serum concentration of HS (figure 1A), HA (figure 1B), CS (figure 1C) and S1 (figure 1D) was determined by ELISA in four additional SCOPE participants of European ethnicity at 20 weeks' gestation with an average maternal age of 34.5 (SD 1.7) years and average BMI of 23.3 (SD 3.5) kg/m2. The appropriate dilution factor range that will (1) account for individual variations in serum concentration of each constituent in the pilot study participants and (2) ensure that the serum concentrations of each constituent were within the assay's detectable range was determined to be the following: HS—1:4, 1:8, 1:16; HA—1:2, 1:4; CS—1:300, 1:400, 1:500; and S1–2:5, 1:5, 1:10. In figure 1C, only two of the four participants are shown because the serum concentration of CS at these low concentrations (<1:100) was measured for only two participants. At the higher concentrations, the serum concentration was above the detectable range (10 ng/mL) of the ELISA kit.
Figure 1.
Serum concentration versus dilution factor for (A) heparan sulfate, (B) hyaluronan, (C) chondroitin sulfate and (D) syndecan-1 from the serum dilution experiments. The inset for (A), (B) and (D) shows the serum concentration versus dilution factor for the lower dilution factors used. For each dilution factor, the samples were run in duplicate and the error bars represent the standard deviation. The dashed horizontal lines represent the lower measurable range of the ELISA kit for that EGL constituent. (Note: each ELISA kit's upper measurable range is greater than the maximum value shown on the ordinate of that constituent). EGL, endothelial glycocalyx layer.
Serum concentration of EGL constituents
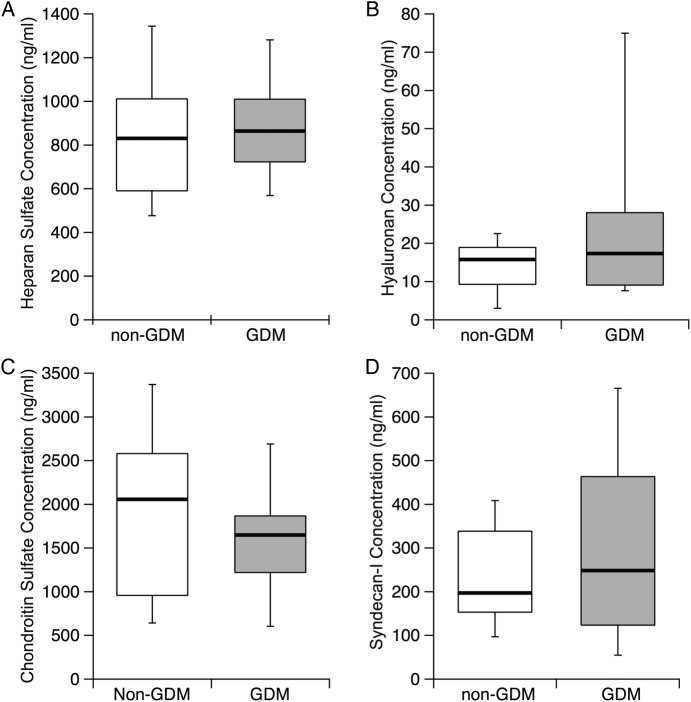
Serum concentration of HS, HA, CS (n=10) and S1 determined by ELISA for women at 20 weeks’ gestation who either later developed GDM, or did not, is shown in figure 2. Medians (25th centile, 75th centile) of serum concentration for each target are: (1) HS—867.7 (722.8, 1009.6) ng/mL for GDM cases versus 830.8 (590.9, 1011.4) ng/mL for matched cases without GDM; (2) HA—17.4 (9.09, 28.04) ng/mL for cases with GDM cases versus 15.81 (9.31, 18.96) ng/mL for matched without GDM; (3) CS—1648.6 (1219.8, 1866.2) ng/mL for cases with GDM versus 2056.6 (957.3, 2580.3) ng/mL for matched without GDM; and (4) syndecan-1–248.6 (123.7, 463.6) ng/mL for cases with GDM versus 197.2 (123.7, 338.4) ng/mL for matched without GDM.
Figure 2.
Serum concentration of (A) heparan sulfate, (B) hyaluronan, (C) chondroitin sulfate (n=10) and (D) syndecan-1 was determined by ELISA for women at 20 weeks’ gestation who either later developed GDM (grey box) or did not (white box). The black line represents the median; the top of the box represents the 75% percentile, while the bottom of the box represents the 25% percentile. No significant differences between GDM and without GDM were observed. GDM, gestational diabetes mellitus.
No differences were observed in the log-transformed serum concentration means of HS (p=0.69, two-tailed), HA (p=0.12, two-tailed), CS (p=0.60 two tailed) and S1 (p=0.72, two-tailed) of women who later developed GDM and those who did not. After data were log-transformed, all distributions were normal; normality was assessed using the Shapiro-Wilk test (p<0.05).
Binary logistic regression was performed to determine if serum concentrations of EGL constituents in women at 20 weeks' gestation would be useful in predicting the subsequent diagnosis of GDM. The model contained five explanatory variables: maternal age, BMI and serum concentration of HS, HA and S1. Since the CS concentration was determined in only 10 of the 20 participants, it was not included in the logistic regression analysis. In addition, ethnicity was not included in the logistic regression, due to the fact that 14 of 20 participants in the pilot study identified themselves as being of the same ethnicity (European), while 3 of the participants identified themselves as Indian, 2 as Asian and 1 as a Pacific Islander. With a larger sample size, it will be possible to include ethnicity as a possible explanatory variable in the logistic regression. No potential outliers were detected. The equation met the linearity assumption for logistic regression analysis. The GDM predictive equation was p=1/(1–e−x), where x=−3.207+0.015 (maternal age in years)+0.052 (BMI in kg/m2)+0.047 (HA concentration in ng/mL)+0.003 (S1 concentration in ng/mL). The model was not statistically significant χ2=12.5, df=8, p=0.13, which indicates that the model was unable to distinguish between pregnant women at 20 weeks' gestation who later developed GDM and those who did not. The model explained between 13.8% (Cox and Snell R2) and 18.3% (Nagelkerke R2) of the variation in the development of GDM. No independent variables made a unique statistically significant contribution to the model (table 2). The cut-off value that provided the highest overall percentage of correctly classified cases was 0.5. For that cut-off value, the sensitivity and specificity (with 95% CI) were, respectively, 60% (36% to 81%) and 80% (56% to 94%).
Table 2.
Logistic regression results (n=20), where B weights are the linear combination of the explanatory variables, SE, CI and OR is exp(B), −2LL is the negative two log likelihood, R2 is the proportion of variance in the outcome that the model successfully explains, χ2 is used to indicate how well the model fits the data, df is the degrees of freedom and p is the estimated probability of rejecting a true null hypothesis
| Variable | B (SE) | p Value | 95% CI for OR |
||
|---|---|---|---|---|---|
| Lower | OR | Upper | |||
| Constant | −3.207 (3.281) | 0.328 | – | 0.040 | – |
| Maternal age (years) | 0.015 (0.075) | 0.843 | 0.876 | 1.015 | 1.177 |
| BMI (kg/m2) | 0.052 (0.095) | 0.584 | 0.875 | 1.053 | 1.268 |
| Heparan sulfate (ng/mL) | 0.000 (0.095) | 0.887 | 0.997 | 1.000 | 1.003 |
| Hyaluronan (ng/mL) | 0.047 (0.036) | 0.184 | 0.978 | 1.049 | 1.125 |
| Syndecan-1 (ng/mL) | 0.003 (0.002) | 0.237 | 0.998 | 1.003 | 1.007 |
| −2LL | 49.530* | ||||
| R2 | 0.183 (Nagelkerke) | 0.138 (Cox and Snell) | |||
χ2=12.499, df=8, p=0.130.
*Estimation terminated at iteration number 6 because parameter estimate changed <0.001. Initial −2LL=55 452.
df, degrees of freedom.
Discussion
This is the first study to report HS, HA, S1 and CS serum concentration data in pregnant women at 20 weeks' gestation who either later developed GDM, compared with a control group matched by BMI and age who did not develop GDM. This pilot study showed that serum concentrations of HS, HA and S1 alone, and in combination with maternal age and BMI, were not associated with the later development of GDM.
Serum concentrations of HS, HA, S1 and CS were used for two reasons. First, these are the most prominent components of the EGL.14 21 31 32 Second, the selection was based on previous studies of glycocalyx shedding in clinical settings.33 For instance, Nieuwdorp et al24 showed plasma levels of HA to be significantly (p<0.01) increased in male patients with type-1 diabetes compared with male patients without type-1 diabetes. In addition, Hofmann-Kiefer et al34 showed that pregnant women with HELLP syndrome had more pronounced shedding of EGL components (eg, S1, HS and HA). Finally, plasma concentration of S1, HS and HA has been demonstrated to increase after coronary artery bypass grafting.35 36
The organisation and workflow for this pilot worked well and was divided among three different researchers. The first researcher (RT) organised the serum samples. The second researcher (DL) organised the sample layout on the ELISA plates and performed the statistical analysis (matches known, blind to GDM status). The third researcher (WH) performed the ELISA experiments and quantified the serum concentration (blind to matches and GDM status).
A limitation of this pilot study was the small sample size (n=20). To the best of our knowledge, these are the first data on serum concentration of these EGL constituents for women at 20 weeks' gestation who later developed GDM. Thus, the sample size could not have been calculated accurately a priori. The serum samples used in this pilot were from women at 20 weeks' gestation— 4–8 weeks before GDM is typically diagnosed with the OGTT. An increase in the sample size could possibly change the results/conclusions of this pilot study. As a result, conclusions drawn from these results should be interpreted bearing this in mind. However, we believe the next step should be to measure the serum concentration of EGL constituents at the time of diagnosis of GDM (time-of-disease samples). Our next step will be to measure the serum concentration of these EGL constituents later in pregnancy after diagnosis of GDM. These studies will help establish whether serum concentrations of EGL constituents are involved in the pathophysiology of GDM, at the time of disease, a necessary step before considering whether a larger study is justified.
Acknowledgments
The authors would like to thank all the pregnant women who participated in the SCOPE study. The authors would also like to thank Fiona Clow and the Fraser Laboratory (Faculty of Medical Health Science, University of Auckland) for use of laboratory space.
Footnotes
Contributors: DSL, RST and LMEM designed the study. WH performed the ELISA assays. DSL performed the statistical analysis and drafted the manuscript. All authors were involved in the interpretation of data and critical revision of the manuscript. DSL (manuscript's guarantor) affirms that the manuscript is an honest and accurate account of the study and no aspects of the study have been omitted.
Funding: This work was supported by the Performance-Based Research Fund of the Auckland Bioengineering Institute (DSL), the Department of Engineering Science (DSL), the New Enterprise Research Fund, Foundation for Research Science and Technology; Health Research Council 04/198; Evelyn Bond Fund, Auckland District Health Board Charitable Trust and the Nurture Foundation. The funders had no role in the study design; collection, analysis and interpretation of data; or preparation of this manuscript.
Competing interests: None declared.
Patient Consent: Obtained.
Ethics approval: Ethical approval for the SCOPE Study was obtained from the New Zealand Health and Disability Ethics/Northern A Health and Disability Ethics Committees (20 Aitken Street, Wellington, New Zealand) (number AKX/02/00/364).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Extra data can be accessed via the Dryad data repository at http://datadryad.org/ with the doi:10.5061/dryad.5h2s7.
References
- 1.Report on Maternity, 2012 Wellington, New Zealand: Ministry of Health, 2015; http://www.health.govt.nz/publication/report-maternity-2012 [Google Scholar]
- 2.2013. National Women's Annual Clinical Report. ISSN 1175-6667; http://nationalwomenshealth.adhb.govt.nz.
- 3.Group HSCR. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes 2009;58:453–9. 10.2337/db08-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Association of Diabetes and Pregnancy Study Groups Consensus Panel Metzger BE, Gabbe SG, Persson B et al. . International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes care 2010;33:676–82. 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poston L, Briley AL, Barr S et al. . Developing a complex intervention for diet and activity behaviour change in obese pregnant women (the UPBEAT trial); assessment of behavioural change and process evaluation in a pilot randomised controlled trial. BMC Pregnancy Childbirth 2013;13:148 10.1186/1471-2393-13-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes care 2002;25:1862–8. 10.2337/diacare.25.10.1862 [DOI] [PubMed] [Google Scholar]
- 7.Crowther CA, Hiller JE, Moss JR et al. . Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477–86. 10.1056/NEJMoa042973 [DOI] [PubMed] [Google Scholar]
- 8.Boney CM, Verma A, Tucker R et al. . Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290–6. 10.1542/peds.2004-1808 [DOI] [PubMed] [Google Scholar]
- 9.Catalano PM, Thomas A, Huston-Presley L et al. . Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol 2003;189:1698–704. 10.1016/S0002-9378(03)00828-7 [DOI] [PubMed] [Google Scholar]
- 10.Simmons D. Prevention of gestational diabetes mellitus: where are we now? Diabetes Obes Metab 2015;17:824–34. 10.1111/dom.12495 [DOI] [PubMed] [Google Scholar]
- 11.Kelley KW, Carroll DG, Meyer A. A review of current treatment strategies for gestational diabetes mellitus. Drugs Context 2015;4:212282 10.7573/dic.212282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemkes BA, Nieuwdorp M, Hoekstra JB et al. . The glycocalyx and cardiovascular disease in diabetes: should we judge the endothelium by its cover? Diabetes Technol Ther 2012;14(Suppl 1):S3–10. 10.1089/dia.2012.0011 [DOI] [PubMed] [Google Scholar]
- 13.Perrin RM, Harper SJ, Bates DO. A role for the endothelial glycocalyx in regulating microvascular permeability in diabetes mellitus. Cell Biochem Biophys 2007;49:65–72. 10.1007/s12013-007-0041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 2007;9:121–67. 10.1146/annurev.bioeng.9.060906.151959 [DOI] [PubMed] [Google Scholar]
- 15.Reitsma S, Slaaf DW, Vink H et al. . The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 2007;454:345–59. 10.1007/s00424-007-0212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danielli JF. Capillary permeability and oedema in the perfused frog. J Physiol (Lond) 1940;98:109–29. 10.1113/jphysiol.1940.sp003837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Teeffelen JW, Brands J, Stroes ES et al. . Endothelial glycocalyx: sweet shield of blood vessels. Trends Cardiovasc Med 2007;17:101–5. 10.1016/j.tcm.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 18.Gouverneur M, Berg B, Nieuwdorp M et al. . Vasculoprotective properties of the endothelial glycocalyx: effects of fluid shear stress. J Intern Med 2006;259:393–400. 10.1111/j.1365-2796.2006.01625.x [DOI] [PubMed] [Google Scholar]
- 19.Fu BM, Tarbell JM. Mechano-sensing and transduction by endothelial surface glycocalyx: composition, structure, and function. Wiley Interdiscip Rev Syst Biol Med 2013;5:381–90. 10.1002/wsbm.1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker BF, Chappell D, Bruegger D et al. . Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res 2010;87:300–10. 10.1093/cvr/cvq137 [DOI] [PubMed] [Google Scholar]
- 21.Tarbell JM, Cancel LM. The glycocalyx and its significance in human medicine. J Intern Med 2016;280:97–113. 10.1111/joim.12465 [DOI] [PubMed] [Google Scholar]
- 22.Hofmann-Kiefer KF, Knabl J, Martinoff N et al. . Increased serum concentrations of circulating glycocalyx components in HELLP syndrome compared to healthy pregnancy: an observational study. Reprod Sci 2013;20:318–25. 10.1177/1933719112453508 [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Quintero SV, Cancel LM, Pierides A et al. . High glucose attenuates shear-induced changes in endothelial hydraulic conductivity by degrading the glycocalyx. PLoS ONE 2013;8:e78954 10.1371/journal.pone.0078954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieuwdorp M, Mooij HL, Kroon J et al. . Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 2006;55:1127–32. 10.2337/diabetes.55.04.06.db05-1619 [DOI] [PubMed] [Google Scholar]
- 25.Broekhuizen LN, Lemkes BA, Mooij HL et al. . Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 2010;53:2646–55. 10.1007/s00125-010-1910-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieuwdorp M, van Haeften TW, Gouverneur MC et al. . Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 2006;55:480–6. 10.2337/diabetes.55.02.06.db05-1103 [DOI] [PubMed] [Google Scholar]
- 27.Nieuwdorp M, Holleman F, de Groot E et al. . Perturbation of hyaluronan metabolism predisposes patients with type 1 diabetes mellitus to atherosclerosis. Diabetologia 2007;50:1288–93. 10.1007/s00125-007-0666-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang JB, Guan J, Shen J et al. . Insulin increases shedding of syndecan-1 in the serum of patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2009;86:83–8. 10.1016/j.diabres.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 29.North RA, McCowan LM, Dekker GA et al. . Clinical risk prediction for preeclampsia in nulliparous women: development of model in international prospective cohort. BMJ 2011;342:d1875 10.1136/bmj.d1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmons D, Rowan J, Reid R et al. . National GDMWP. Screening, diagnosis and services for women with gestational diabetes mellitus (GDM) in New Zealand: a technical report from the National GDM Technical Working Party. N Z Med J 2008;121:74–86. [PubMed] [Google Scholar]
- 31.Gao L, Lipowsky HH. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc Res 2010;80:394–401. 10.1016/j.mvr.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pries AR, Kuebler WM. Normal endothelium. Handb Exp Pharmacol 2006. (176 Pt 1):1–40. 10.1007/3-540-32967-6_1 [DOI] [PubMed] [Google Scholar]
- 33.Becker BF, Jacob M, Leipert S et al. . Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol 2015;80:389–402. 10.1111/bcp.12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofmann-Kiefer KF, Chappell D, Knabl J et al. . Placental syncytiotrophoblast maintains a specific type of glycocalyx at the fetomaternal border: the glycocalyx at the fetomaternal interface in healthy women and patients with HELLP syndrome. Reprod Sci 2013;20:1237–45. 10.1177/1933719113483011 [DOI] [PubMed] [Google Scholar]
- 35.Bruegger D, Rehm M, Abicht J et al. . Shedding of the endothelial glycocalyx during cardiac surgery: on-pump versus off-pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 2009;138:1445–7. 10.1016/j.jtcvs.2008.07.063 [DOI] [PubMed] [Google Scholar]
- 36.Svennevig K, Hoel T, Thiara A et al. . Syndecan-1 plasma levels during coronary artery bypass surgery with and without cardiopulmonary bypass. Perfusion 2008;23:165–71. 10.1177/0267659108098215 [DOI] [PubMed] [Google Scholar]