Abstract
Background
Prostate cancer and its treatment may impact physically, psychologically and socially; affecting the health-related quality of life of men and their partners/spouses. The Life After Prostate Cancer Diagnosis (LAPCD) study is a UK-wide patient-reported outcomes study which will generate information to improve the health and well-being of men with prostate cancer.
Methods and analysis
Postal surveys will be sent to prostate cancer survivors (18–42 months postdiagnosis) in all 4 UK countries (n=∼70 000). Eligible men will be identified and/or verified through cancer registration systems. Men will be surveyed twice, 12 months apart, to explore changes in outcomes over time. Second, separate cohorts will be surveyed once and the design will include evaluation of the acceptability of online survey tools. A comprehensive patient-reported outcome measure has been developed using generic and specific instruments with proven psychometric properties and relevance in national and international studies. The outcome data will be linked with administrative health data (eg, treatment information from hospital data). To ensure detailed understanding of issues of importance, qualitative interviews will be undertaken with a sample of men who complete the survey across the UK (n=∼150) along with a small number of partners/spouses (n=∼30).
Ethics and dissemination
The study has received the following approvals: Newcastle and North Tyneside 1 Research Ethics Committee (15/NE/0036), Health Research Authority Confidentiality Advisory Group (15/CAG/0110), NHS Scotland Public Benefit and Privacy Panel (0516-0364), Office of Research Ethics Northern Ireland (16/NI/0073) and NHS R&D approval from Wales, Scotland and Northern Ireland. Using traditional and innovative methods, the results will be made available to men and their partners/spouses, the funders, the NHS, social care, voluntary sector organisations and other researchers.
Keywords: QUALITATIVE RESEARCH
Background
Context
Prostate cancer is the most common cancer in men (excluding non-melanoma skin cancer) in the UK.1 Increasing incidence and survival has resulted in a growing population of men living with and beyond prostate cancer: this is currently around 255 000 and predicted to rise to 831 000 by 2040.2
Physical, psychosocial and emotional sequelae following prostate cancer diagnosis may result from the disease itself or treatments.3 Specific physical consequences vary with type of treatment and can affect urinary, sexual, bowel and hormone-related functioning, with detrimental effects on health-related quality of life (HRQL) for men and their partners/spouses.4–7 Active surveillance is increasingly recommended for the management of localised forms of prostate cancer.8 Yet while this avoids potential side effects of treatment, anxiety can be a problem.9 Consequently, there is a major challenge for health and social care services to provide services to support men living with and beyond prostate cancer and their partners/spouses.
Current knowledge
The importance of capturing the patients' perspective on how prostate cancer affects everyday living is increasingly recognised, with many studies now incorporating patient-reported outcome measures (PROMs). In one US study, HRQL was assessed for men with localised disease, from pretreatment until 24 months.10 At 24 months, sexual function was a problem for 43% of surgery patients, 37% after external beam radiotherapy and 30% after brachytherapy. Urinary problems were reported by 7% of surgical patients, 11% after radiotherapy and 16% after brachytherapy. An Australian population-based study reported that men in all treatment groups had worse sexual function than a control population at 1, 2 and 3 years. All treatment groups reported greater urinary ‘bother’.11 In England, a survey of 1250 men between 1 and 5 years postdiagnosis found that 38.5% of respondents reported some degree of urinary leakage, 12.9% reported difficulty controlling their bowels and 58.4% reported being unable to have an erection.5 Urinary leakage was significantly associated with lower HRQL scores, while erectile dysfunction, though common, did not significantly impact on HRQL.5 In Northern Ireland, psychological distress in men with prostate cancer was shown to be predicted by cancer-related symptoms, including urinary and bowel incontinence, fatigue and insomnia.12
Current services do not meet all the needs of men living with and beyond prostate cancer or their partners/spouses.11 13–18 The results of one English survey suggested that areas of greatest need were psychological distress, sexuality-related issues and management of enduring urinary symptoms.13 Elsewhere in the UK, unmet needs were related to changes in sexual feelings and relationships, concerns over significant others and fears of a recurrence.19 Men with prostate cancer also report dissatisfaction with current follow-up care regimes and information provision.16 20 21 Additionally, the impact on the men's partners/spouses is significant.22–24
Policy
Improving outcomes has been at the heart of recent health service reforms in the UK.25–27 Robust collection of patient-reported outcomes (PROs) is essential to provide evidence to influence such reforms. The National Cancer Survivor Initiative identified the need for routine measurement of experience and outcomes for cancer survivors.28–30 The National Cancer PROMs Programme was established in England in 2010 by the Department of Health (DH). A successful methodology for population-based PROMs surveys was established,5 31 which in 2013 was extended to all individuals 12–36 months postcolorectal cancer diagnosis.32 33
The National Cancer PROMs Programme Pilot showed that men with prostate cancer were willing to participate (69% response; the highest of the four pilot cancer sites).5 A 12-month follow-up demonstrated the willingness of men to continue to engage with longitudinal PROMs data collection: >80% participating in subsequent data collection.34 In 2013, the largest cancer PROMs exercise in Europe was undertaken with a survey of 35 000 people 12–36 months following colorectal cancer diagnosis in England. A 63% participation rate was obtained.32 The Life After Prostate Cancer Diagnosis (LAPCD) study will build on these experiences to perform the largest prostate cancer PROMs programme to date in the world.
Study aims
Primary aims
To describe the HRQL (eg, physical, psychosocial) of men with prostate cancer using qualitative and quantitative methods;
To explore if and how their HRQL is associated with or is predicted by disease, treatment and/or patient characteristics with a view to inform development of healthcare policy and service delivery in ways that better meet the needs of such men and their families;
To describe the levels of patient empowerment and undertake preliminary exploration of the interaction between patient empowerment and HRQL.
To undertake a normative study of men without prostate cancer to determine community levels of symptoms for comparison.
Secondary aims
To undertake provider-level and health economic analyses, and explore methods for producing robust, meaningful comparisons of outcomes across the UK;
To explore the acceptability/options of electronic PROMs data collection;
To explore and check the psychometric properties (eg, reliability, validity) of the newer, less well-established questionnaire measures used in the study;
To investigate the possibility of developing an item-bank for HRQL assessment in men living with and beyond prostate cancer;
To identify ‘gaps’ within existing surveys that are of importance to patients and partners/spouses.
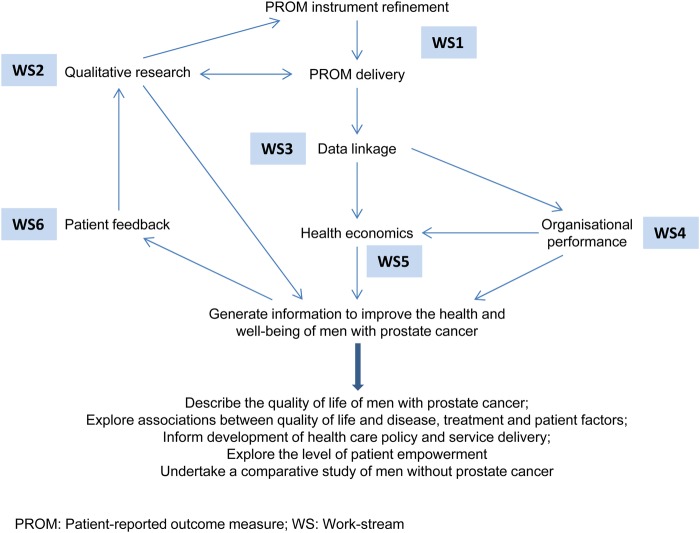
The study will achieve these aims through six interlinking work-streams centred round the collection of PROMs data and linkage with existing data sets (figure 1). The study will collect data from across all nations in the UK. While the survey questionnaire and analysis will be similar, the methodology differs in parts for each country in order to satisfy legal governance requirements; this is made clear throughout the protocol.
Figure 1.
Study overview.
Methods and analysis
Work-stream 1: survey development and delivery
A: survey development
A survey instrument has been developed which covers a range of generic and cancer-specific PROMs plus items covering treatments received, sociodemographic characteristics and the patient perspective on their disease, treatment and experiences. The survey content has been informed by a range of factors. These include the incorporation of questionnaire measures which will be used by international colleagues in similar surveys undertaken in their countries,35 three systematic reviews of questionnaires used in prostate cancer research36–38 and the International Consortium for Health Outcomes Measurement (ICHOM) recommendations for a minimum outcomes data set for men with localised prostate cancer.39 The experiences from other surveys undertaken by the coapplicants, including scope and response rates,40 the availability of routine demographic and health data (to avoid duplicate collection of information), questionnaire burden (length/number of items; suggested ≈100 acceptable), item duplication/ redundancy, costs and permission, and the priorities of different coapplicants and advisory group members, including service users were also considered. In addition to the survey items included, a free-text box is included at the end of each survey section for respondents to add further detail or to capture any other important relevant issues not covered in the section.
Survey measures
Generic HRQL
The included measures are: (1) EQ-5D: a generic measure of health for clinical and economic appraisal;41 (2) K-6: a measure of non-specific distress to discriminate cases of serious mental illness from non-cases;42 (3) the Short Warwick-Edinburgh Mental Well-being Scale (SWEBWEMS): a positive construct of emotional well-being;43 (4) the Social Difficulties Inventory (SDI): this assesses everyday problems experienced by patients with cancer, including difficulties with everyday living, money and employment and relationships.44–46 Three individual SDI items on difficulty with sexual matters (covered in detail elsewhere), housing (poorly endorsed in the pilot work) and any other difficulty (addressed in the free-text boxes) have been excluded.
Cancer-specific HRQL
These measures include: (1) the Expanded Prostate Cancer Index Composite short form (EPIC-26): Urinary Incontinence, Urinary Irritative/Obstructive, Bowel, Sexual, and Hormonal subscales;47 (2) European Organization for Research and Treatment of Cancer Prostate Cancer module (EORTC PR25): sexual subscale (two items);48 49 (3) medication/devices for erectile dysfunction:50 items amended to avoid the use of drug/trade names; (4) EORTC QLQ-C30: fatigue subscale (three items).51
Patient clinical and sociodemographic characteristics
These include: (1) Have you had a diagnosis of prostate cancer? (as part of the introduction, not part of main survey); (2) treatment items informed by prostate cancer clinicians and experts; (3) comorbidity item (a list of possible conditions); (4) standard sociodemographic items informed by the Office for National Statistics and other sources; (5) support for previous mental health problems, taken from National Comorbidity Survey;52 an item about carer status included in recognition of the growing number of carers.
Patient perspective measures
The included measures are: (1) the Decision Regret Scale which provides an indication of healthcare postdecision regret at a set moment in time53 and (2) the Bulsara Patient Empowerment Scale which taps into the construct of how much control patients feel they have over their experience of their illness and its diagnosis, treatment and follow-up.54
The survey has been piloted in a prostate cancer clinic in Leeds and in a group of service users. Cognitive testing has been carried out by the approved survey provider (Picker Institute Europe).
The Scottish version of the survey will also include a question asking respondents whether or not they give consent for their responses to be linked to other Scottish health and care data sets. This will be added to the end of the questionnaire, which is the standard approach used for patient experience surveys in Scotland.
B: survey delivery
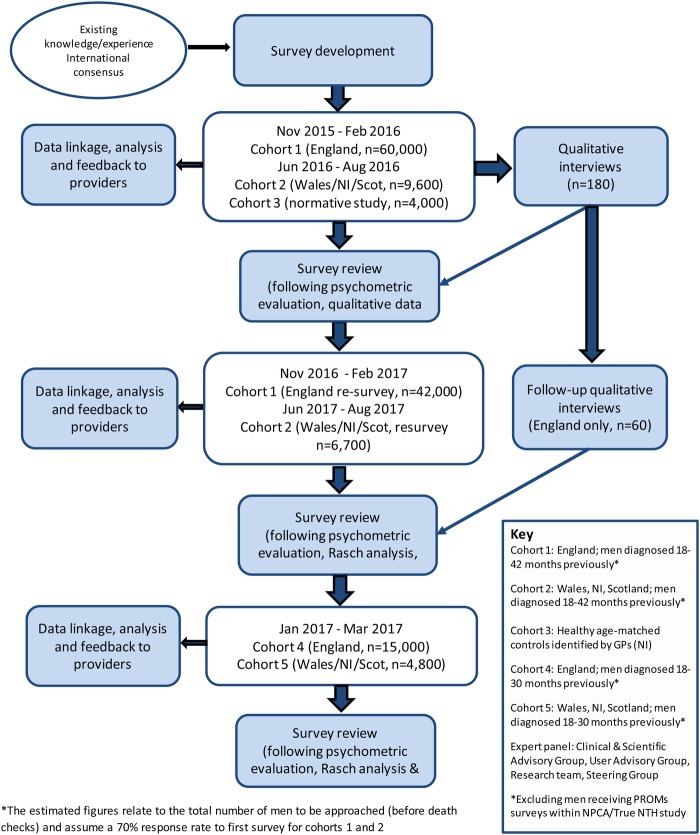
Men who are between 18 and 42 months postdiagnosis of prostate cancer will be eligible for inclusion in the study. Eligible men will be identified through cancer registration systems in England, Wales and Northern Ireland, and through hospital activity data in Scotland (with verification of a cancer diagnosis by cancer registration records). Two discrete cohorts of men will be surveyed in each UK nation (see table 1 and figure 2 for more information on the cohorts, timelines and expected numbers).
Table 1.
Overview of study methodology within each nation
| England (cohorts 1 and 4) | Wales (cohorts 2 and 5) | Northern Ireland (cohorts 2 and 5) | Scotland (cohorts 2 and 5) | Normative study (cohort 3) | |
|---|---|---|---|---|---|
| Data source | Cancer registry | Cancer registry | Cancer registry | Hospital admissions | BSO |
| Confirmation of diagnosis and eligibility | Prostate MDT lead | Prostate MDT lead | Prostate MDT lead plus nurse check for unstaged cases | Hospital admission for prostate cancer plus cancer registration in relevant time period | |
| Exclusions | Men eligible for NPCA /true NTH | Men eligible for NPCA | List from protocol | Men with previous prostate cancer | |
| Death checks | NHS Digital | NHS Digital | BSO | NRS/NHSCR/CHI | BSO |
| Survey mail-out | Picker | Picker | Cancer registry | Picker | Picker |
| Language | English | English/Welsh | English | English | |
| Survey dates | Cohort 1: November 2015–February 2016 Resurvey: November 2016–February 2017 Cohort 4: January 2017–March 2017 |
Cohort 2: June 2016–August 2016 Resurvey: June 2017–August 2017 Cohort 5: January 2017–March 2017 |
May 2016–July 2016 | ||
| Estimated survey numbers* | Cohort 1: n=60 000 Resurvey: n=42 000 Cohort 4: n=15 000 |
Cohort 2: n=4000 Resurvey: n=2800 Cohort 5: n=2000 |
Cohort 2: n=2000 Resurvey: n=1400 Cohort 5: n=1000 |
Cohort 2: n=3600 Resurvey: n=2500 Cohort 5: n=1800 |
n=4000 |
| Data linkages | Cancer registration; hospital admissions; radiotherapy; patient experience survey; end-of-life care |
Cancer registration; hospital admissions; radiotherapy |
Cancer registration; hospital admissions; radiotherapy |
Cancer registration; hospital admissions; radiotherapy (linkage will only be possible where responding patients have given their consent) |
|
| Telephone interviews | Cohort 1: n=120 Follow-up interviews: n=60 |
Cohort 2: n=20 | Cohort 2: n=20 | Cohort 2: n=20 | Not applicable to this cohort |
*Estimates represent the total number of men eligible for inclusion (before death checks); resurvey estimates are based on a 70% response to first surveys.
Figure 2.
Schematic outline of proposed patient-reported outcome measures data collection.
Cohort 1 (England) and cohort 2 (Wales/NI/Scotland)
Men living in England will be surveyed first, followed shortly by the devolved nations. The first cohorts for each nation will be resurveyed 12 months after the original survey to enable longitudinal assessment of outcomes. After review of the results from the first cohorts, minor modifications will be made to the survey instrument (if needed) and repeat cognitive testing will be undertaken (if changes made).
Cohort 3 (normative sample; NI)
A group of men without prostate cancer will be surveyed as a normative sample, using a similar version of the questionnaire (removing any prostate cancer-specific questions). This normative sample will be age and deprivation level matched with the prostate cancer group.
Cohort 4 (England) and cohort 5 (Wales/NI/Scotland)
A second new cohort will be surveyed in each nation, identified in the same way as the first cohort, but diagnosed during a later time period. The survey instrument will be the same unless review of the first cohort results suggests modifications should be made. The men included in this part of the study will be given the opportunity to complete the survey electronically, with online access via the study website. The sociodemographic and clinical characteristics of responders from both modes of administration will be compared. The response rate of the men in the second cohorts will be compared with the response rate of those in the first ‘paper only’ cohorts.
Exclusions
Only men managed by a multidisciplinary team (MDT) within an NHS Hospital Trust/Health Board will be eligible for the study. Individuals will be excluded if they are eligible for inclusion in the National Prostate Cancer Audit (NPCA), which is surveying men living in England and Wales diagnosed with localised prostate cancer from 1 April 2014. Men treated at the four hospitals in England participating in the True NTH Supported Self-Management and Follow Up Care Programme55 will be excluded to avoid burdening men with repeated surveys.
Methodology
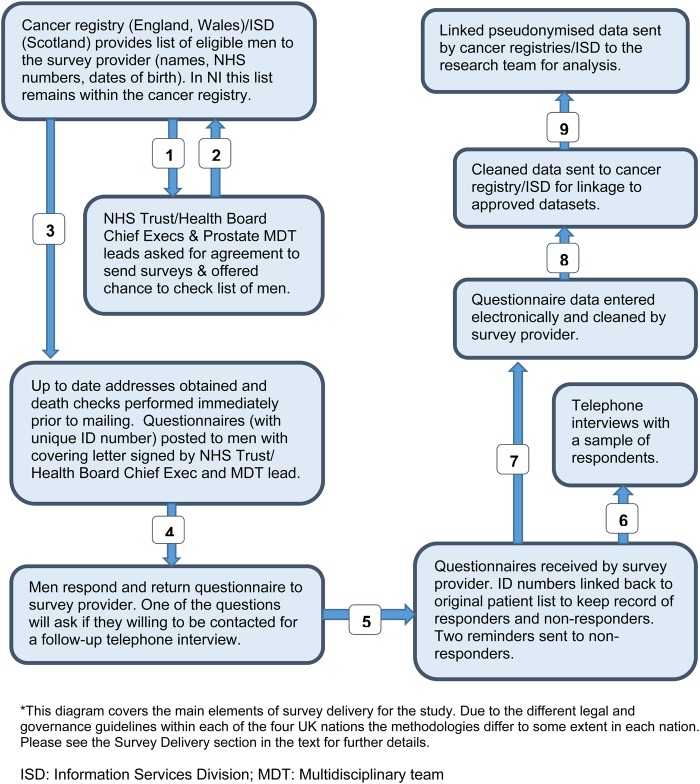
The methods for delivering the survey and the subsequent data flows are outlined in figure 3. These vary within each nation due to differing legal and governance processes and guidelines. The methodology for England (the largest portion of the survey) is outlined here. Deviations from this methodology in the other nations are summarised below and in table 1.
Figure 3.
Study data flows.
England
The methodology follows that successfully used by the National Colorectal PROMs Survey, England 2013.32 Briefly, the National Cancer Registration and Analysis Service (NCRAS), study team and funder will write to the Chief Executive and prostate cancer MDT lead of each Trust to seek their permission to survey men treated by their Trust. Trusts will be offered the chance to verify the list of identified patients and filter any patients where contact would be inappropriate. For the Trusts that agree to take part, the NCRAS will extract a list of eligible men and send this securely to NHS Digital for up-to-date address tracing and death checks (48 hours prior to mailing). On completion of these checks, the information will be passed on to the appointed survey provider, Picker Institute Europe. The survey will be sent out with a covering letter from the treating NHS Trust's Chief Executive and MDT lead and a participant information sheet. All documents will indicate that the survey is only to be completed if the patient has received a diagnosis of prostate cancer. A double-windowed envelope method will be used to reduce the chances of someone other than the addressee opening the survey. A translation sheet will be included which, in the 20 most spoken minority languages in the UK, informs participants that if they have any questions, or would like to speak to an interpreter, they can call the study helpline and they can then complete the survey over the phone in their preferred language.
Patients who agree to participate will complete the questionnaire which will be returned in prepaid envelopes to Picker Institute Europe. The questionnaires will not contain any personal information (ie, no names or addresses) but will be assigned a unique reference number (URN). The URN can be linked back to the original patient list in order to keep track of which men have returned the survey or have opted out (by returning the survey blank or phoning the dedicated survey helpline). Two reminders will be sent (with additional death checks performed each time). Picker Institute Europe will scan the completed surveys, transcribe any written ‘free-text’ comments and clean the data, including removing any identifying information where patients may have named specific Trusts or clinicians. The cleaned electronic data will be sent back to NCRAS using a secure transfer mechanism where they will be linked back to the necessary patient, disease and treatment information. The data set of pseudonymised survey responses, disease and treatment information will be forwarded, alongside a study ID number, to the research teams for analysis.
Wales
In Wales, the methodology follows that for England with a few minor changes. Approval will be sought at the Health Board level rather than individual Trusts. Following approval, eligible men will be identified through the Welsh Cancer Intelligence and Surveillance Unit (WCISU). The letters and patient information sheet will be provided in Welsh and English. However, the first survey will only be available in English, as not all of the items and scales included have been validated in Welsh. Those participants that wish to complete the questionnaire in Welsh will be able to do so by telephoning the survey helpline and articulating their responses to a Welsh speaker. It is hoped that subsequent surveys will also be available in Welsh once translation and validation of the items has been undertaken.
Northern Ireland
The methods for undertaking the survey in Northern Ireland follow those used for the International Cancer Benchmarking Partnership Module 4.56 Northern Ireland Cancer Registry (NICR) staff will compile a list of eligible men and will confirm diagnosis of prostate cancer using ‘stage of cancer’. Where stage is missing and a prostate cancer diagnosis cannot be confirmed by the NICR, a list of unconfirmed patients will be sent to research nurses for a final check. The lists of patients will also be available for MDT leads to view on request.
As information from the NICR cannot be passed to an external survey provider, Picker Institute Europe will provide pre made-up packs, containing the survey and cover letter, each with the same URN. The cover letter will have the logo of the Trust of residence at the time of diagnosis and the signatures of all three Northern Ireland Urology MDT leads. The NICR staff will print labels with the names and addresses of the eligible men and these labels will be cross checked against the URN before being applied to the cover letter. A death check will be carried out by the NICR staff (via Northern Ireland Business Services Organisation (BSO)) 24 hours before the surveys are posted. Patients will return the questionnaires to Picker Institute Europe in the prepaid envelope provided. On a fortnightly basis, Picker Institute Europe will supply the NICR staff with a list of the URNs for the patients who have responded and will also provide the associated reminder letters/packs. The NICR staff will carry out further death checks and send up to two reminders to the non-responders.
Scotland
In Scotland, patients identified from the Scottish Cancer Registry can only be approached through their doctor. In previous studies, this has resulted in low response rates (∼30%) and placed a high administrative burden on NHS National Services Scotland and GP practices. As such, this study will follow the methodology approved for the 2015 Scottish Cancer Patient Experience Survey.
Information Services Division (ISD: part of NHS National Services Scotland, Public Health and Intelligence) will identify eligible participants using hospital activity data, with cross checking against the Scottish Cancer Registry to confirm a diagnosis of prostate cancer in the required timeframe. This method means that only around 65% of Scottish men diagnosed with prostate cancer in the required timeframe will be sampled. The sample will also include a higher rate of men who have had surgery to treat their prostate cancer than the full population of men with prostate cancer. The sample will therefore be adjusted by removing a small number of men who have had surgery, using stratification to ensure that the sample otherwise retains the same profile as the full population of Scottish men with prostate cancer. ISD will carry out initial death checks against National Records for Scotland (NRS) deaths data and request current name and address for sampled patients from the Community Health Index (CHI) database. ISD will coordinate further death checking with the Scottish NHS Central Register (NHSCR) and the CHI database, to be run overnight before the day of each mail-out. ISD will pass the mailing lists and results of death checking to Picker Institute Europe who will post survey packs to eligible participants using URNs to track responses. The covering letters will be signed by the Medical Director for the NHS Board in which the patient currently lives (which may not be where some of their treatment was received).
Picker Institute Europe will pass the response data to ISD for further linkage (eg, cancer type and stage, treatment information). These data will only be added where the responding patient has given their consent for linkage. ISD will also provide basic demographic and treatment data for the men who have not responded to the survey (at an aggregated level), so that the full cohort can be described and the potential for bias in response fully assessed. The pseudonymised Scottish data set will then be passed to the research team.
Normative study
Many of the symptoms experienced by patients with prostate cancer are common in the general population. Therefore, to understand and document health and quality of life deficits experienced by patients with prostate cancer, we need to develop an understanding of the background levels of these symptoms in the population. To this end, a normative study will be conducted in Northern Ireland. Using GP registration data, BSO will generate a reference group of 10 000 men matched by 5-year age band and deprivation quintile to patients with prostate cancer. BSO will issue questionnaires with participants responding anonymously directly to Picker Institute Europe. The normative study questionnaire has been adapted from the main prostate cancer questionnaire, with exclusion of questions relating specifically to the prostate cancer diagnosis. Validated instruments were not amended. The tool was reviewed by the study advisory groups and by a focus group of older men in NI. A pilot survey of 500 men will be used to test response rates, bias and acceptability of the survey to the general public.
Assuming a 33% response rate, the sample size will allow (at 80% power and 5% confidence level) observation of a 6% difference in the proportion of 190 patients with prostate cancer aged 80+ reporting severe difficulty or inability to perform usual activities compared with the normative population. This will allow hypothesis testing that significant differences in health in patients with prostate cancer exist compared with the general population.
Free-phone helpline/symptoms process
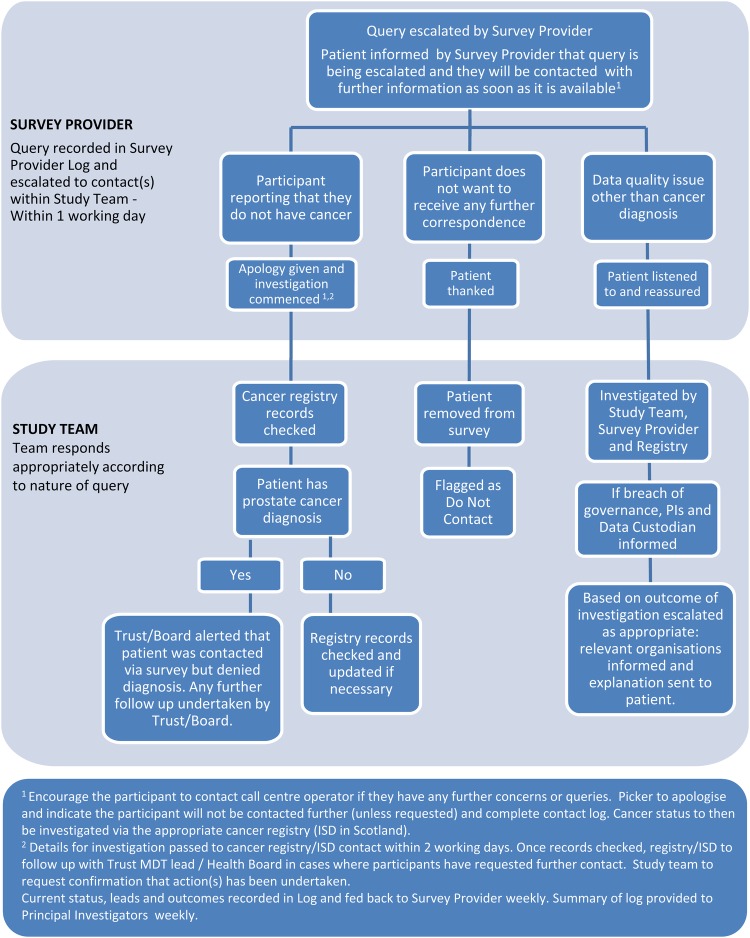
A 24 hour free-phone service will be provided during the times when surveys are live. Any queries relating to prostate cancer symptoms or disease management will be directed to the Prostate Cancer UK (PCUK) nurse-led telephone advice service. For other queries, for example, the patient wishes to report they do not have cancer or the patient does not wish to be contacted again, an escalation process has been developed (figure 4). Procedures to rapidly manage and report any symptoms/incidents arising from the survey have been established. It is not possible to foresee all possible queries that will be raised by the patients, but these processes have been developed to deal with the issues that have arisen in previous PROMs surveys.
Figure 4.
Patient query escalation process.
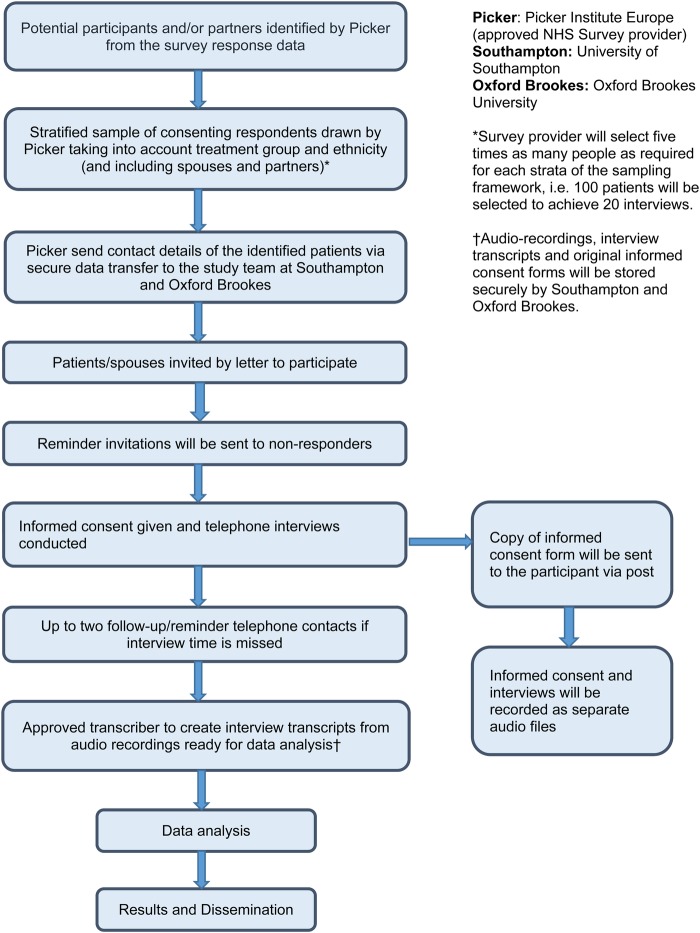
Work-stream 2: qualitative research
The qualitative element of the study will consist of cross-sectional telephone interviews in all four nations (n=180), longitudinal follow-up telephone interviews (England only, n=60) and analysis of free-text comments offered by respondents in each of the seven sections of the questionnaire.
Cross-sectional telephone interviews (year 1)
Sampling, recruitment and interviews will start ∼4–6 weeks after survey opening (figure 5). Survey participants will be asked to tick a box indicating their interest in taking part in a telephone interview. Using a sampling framework, Picker Institute Europe will randomly select individuals who have agreed to be interviewed. Sample groups comprise the four main treatment groups: radical prostatectomy; radical radiotherapy; systemic therapy (hormone therapy); active monitoring (active surveillance and watchful waiting), and a group of black and minority ethnic (BME) men from across the treatments groups. Approximately 100 men will be interviewed in England and 50 men from across the 3 devolved nations (NI /Wales/Scotland). There may be subtle differences in the processes for contacting men across the different nations.
Figure 5.
Outline of qualitative data collection process.
Approximately five times, the required number of men will be identified by Picker for each group in order to meet the target of completed interviews. Picker will then send the names and addresses of the selected men to the research team. From this randomised sample, the research team will then purposively select men for interview to include a range of interviewees in terms of age, marital status, time since diagnosis, sexual orientation and prostate cancer-related problems.
The research team will send selected men an invitation pack containing a letter, participant information sheet, consent form and reply slip (for them to respond with their telephone number and email address, should they wish to take part in the interview). A reminder letter will be sent to non-responders after 2 weeks. Researchers will contact responders by phone/email and arrange a date/time for the telephone interview for approximately a week (but more than 48 hours) later. If there is no reply at the set interview time, the researcher will try to contact the participant by telephone/email to arrange another time on up to two separate occasions over the following 2 weeks, after which the researcher will stop trying to contact the individual.
A further sample group comprising partners/spouses of men with prostate cancer will be interviewed (n=20 in England and n=10 across the three devolved nations). The survey will ask men to indicate on a tick box whether their partner/spouse (should they have one) would be interested in being interviewed. Partners will be sampled by Picker according to treatment type and sociodemographic characteristics of the respondent (five times the required number). Contact details will be sent by Picker to the research team. The research team will then purposively select a small group to be invited to be interviewed.
The research team will then write to the respondent informing them that the researchers would like to interview their partner. The respondent will be asked to give the enclosed invitation letter, participant information sheet, consent form and reply slip to their partner. A reminder letter will be sent to non-responders after 2 weeks. Once a partner reply slip is received, a researcher will contact them by phone/email and arrange a date/time for the telephone interview to take place (procedure as outlined above for the participants).
The consent form will be read through with the individual (patient or partner) over the phone immediately prior to the interview taking place. Verbal consent to participate in the study and for audio-recording of the interview will be obtained. The interviewer will initial the tick boxes on the consent forms as they read them through, date and sign two copies and offer to send one copy to the patient/partner. Completed consent forms will be stored in a locked filing cabinet within a locked office in the University of Southampton or Oxford Brookes University.
Two separate recordings will be made, one for consent, the other for the interview. Audio files recording consent will be labelled and stored in the study's secure data repository. Researchers will ensure that interview recordings are anonymised by deleting any identifiable information that interviewees may have inadvertently disclosed. The recordings will then be transcribed verbatim by professional transcribers who have signed confidentiality agreements with either the University of Southampton or Oxford Brookes University.
Data collection and analysis will be synchronous, allowing the interview team to be aware of emerging themes while data collection continues. Three trained and experienced researchers will conduct the telephone interviews. Regular meetings will take place throughout the data collection process to review progress, interview techniques and discuss preliminary findings.
Longitudinal interviews (year 2)
At the completion of the first interview, the interviewer will ascertain whether the participant might be willing to take part in a second interview 12 months later. Those who agree to a second interview will be contacted by telephone 12 months later and willingness to be interviewed again confirmed. If so, a date/time will be set for the interview following the same methodology outlined for the year 1 interviews. Baseline interviews with each participant will be read by researchers prior to the second interview to ensure that issues of concern can be revisited to ascertain whether those issues have improved, worsened or been supplanted by other concerns during the intervening period. Owing to time constraints, the longitudinal interviews will take place in England only.
Topic guides
A literature review and meta-synthesis of qualitative studies exploring the experiences of men with prostate cancer and their partners has been undertaken.57 First-order, second-order and third-order constructs from this analysis have informed interview topic guides and ensure that data collected include important concerns previously identified while allowing further issues to emerge. The topic guides will be pilot tested with user representatives.
PhD substudy
As the basis of a PhD studentship, a substudy will aim to explore the experiences and needs of younger men with prostate cancer and their partners, in order to identify ways that couples can be better supported. The substudy will seek to recruit and interview 25 younger men with prostate cancer and their partners, and to conduct a second interview 9–12 months later with those who agree.
Free-text questions
At the end of each of the seven sections of the questionnaire, participants will be invited to provide free-text comments expanding on their responses to the closed questions. At the end of the survey, a final free-text question will ask participants whether there is anything else they would like to comment on regarding their life since diagnosis, which was not covered by the survey. The responses to these questions will be analysed using a variety of methods to identify insights and determine patterns in participant experiences (see the Data analysis and reporting section for more details).
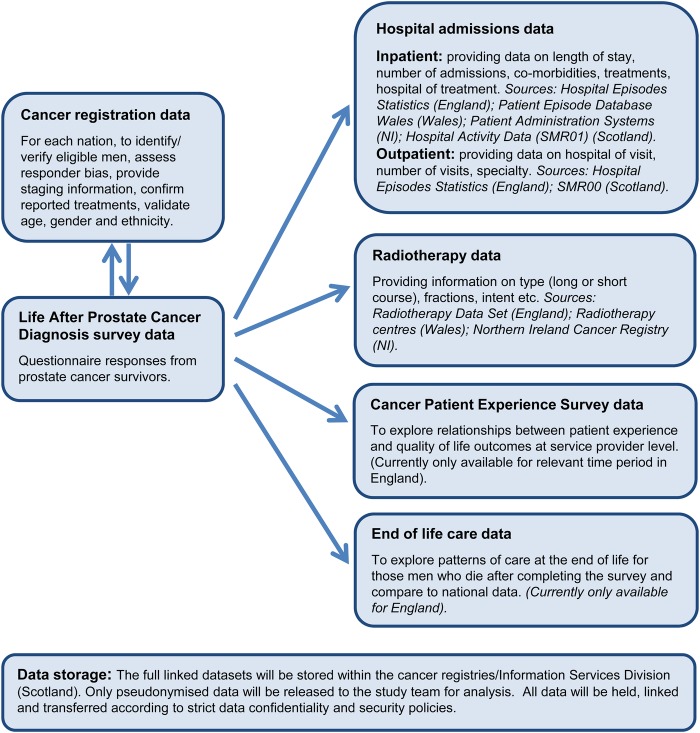
Work-stream 3: data linkage
The study will use a number of routine data sets in order to maximise the amount of clinical and treatment information available (see figure 6):
Figure 6.
Data linkages.
Cancer registration: Questionnaire data from respondents will be linked back to the cancer registration data within the relevant nation to provide staging information, confirmation of reported treatments and validation of age, gender and ethnicity. The cancer registration data will cover all eligible men in order to identify responder bias (comparison of the respondents and non-respondents in terms of age, deprivation etc).
Hospital admissions: These data provide information on inpatient admissions, including treatments, hospital of treatment, length of stay and comorbidities. Outpatient admission data may be available for some of the nations, and generally allows analysis of hospital visited and specialty seen. The specific data sets are listed in figure 6.
Radiotherapy: These data can provide information on type of radiotherapy (long or short course), number of fractions, intent etc. These data may not be available for all nations (see figure 6).
Patient experience survey: In England, the annual National Cancer Patient Experience Survey (NCPES) investigates patients' experiences of treatment and aftercare.58 Trust-level linkage with NCPES will allow exploration of the relationships between patient experience and quality-of-life outcomes at the service provider level. Patient experience surveys are underway in the devolved nations but will not cover the necessary timeframe (ie, men diagnosed between 1 April 2012 and 31 March 2014).
End-of-life care: In England, linkage with the data held by the National End of Life Care Intelligence Network (part of Public Health England) will be explored. These data would provide information, for example, healthcare usage and place of death for those men who die after completing the survey and allow exploration of patterns of care at the end of life.59
All linkage will be undertaken by trained staff with approvals to work with identifiable data. Linkage will be performed using combinations of identifiers, for example, date of birth, sex, postcode. Once linked, the data will be pseudonymised (names, addresses, dates of birth, NHS numbers removed) and securely transferred to the study team for analysis. In Scotland, linkage of survey responses to other health and care data sets will only be possible where responding patients have given their consent.
Work-stream 4: benchmarking and organisational performance
Comparison across countries (benchmarking)
The HRQL and other health-related outcomes of the respondents will be compared, within the UK and internationally, where possible (eg, comparing with Ireland and Australia where similar PROMs work is being undertaken). In this study, information on generic health outcomes will be collected through EQ-5D and cancer-specific outcomes through EPIC-26. A common methodology of data collection would allow meaningful comparisons to be made. Such analyses will require robust adjustment for casemix (age, deprivation level of the population) and other confounding factors to ensure that fair comparisons are made. This will depend on the amount and quality of information across the different countries.
Comparison across providers (organisational performance)
Performing robust comparison across provider organisations, such as hospitals, throws up a number of methodological issues,60 including correct allocation of patients to the institution that provided their main treatment, ensuring a sufficient number of respondents per hospital to allow meaningful comparison, differing response rates by hospital and robust adjustment for casemix. The feasibility of comparison across organisations, taking into account these issues, will be explored. Members of the study team are experienced in analysing the results of large-scale surveys and in the robust assessment of cancer outcomes.32 61–63
Feedback of information to providers
Initial results will be reported at national and provider/organisational level after each data collection (within 6 months of completion of data collection). This will be performed using an electronic toolkit, already developed by the team for the colorectal PROMs work, providing a national overview and organisational-level data compared with national averages. This will allow providers to see the responses from their patients and to quickly identify any areas of concern. These results will not, however, be adjusted for differences in casemix. More detailed results taking into account the issues described above will be disseminated to providers through specific topic-focused reports, as well as presentations and academic papers.
Work-stream 5: health economic analysis
Given the significant volume of PROMs data collected in this study, it is logical to explore their potential value in contributing to more focused health economic evaluation. The exploratory analyses undertaken as part of this work-stream will be split into three areas: (1) recalibration of EQ-5D health outcomes using patients' own self-assessed values (VAS 0–100 ratings) in order to make more meaningful comparisons with other relevant reference groups, including other cancer groups and the general population; (2) analysis of the relationship between EQ-5D (generic HRQL/health status) and other (condition-specific) measures to identify any descriptive ‘gaps’ within EQ-5D, establish the extent of any mis-measurement and examine the potential for remedial action; (3) examine the potential use of EQ-5D as an indicator of performance in treatment of patients with prostate cancer (this links in with work-stream 4).
Work-stream 6: patient and public involvement
It is important that service users (ie, patients, along with their partners, family and carers) are involved, through active partnership with the project team, in contributing as lay advisors to all aspects of this research project. This study has incorporated a high level of patient and public involvement (PPI) from the outset with the establishment of a User advisory group (UAG) and reference group.
User advisory group
The UAG comprises six service user members plus a limited number of (1) health professionals and (2) researchers, with commitment to, as well as detailed expertise and research knowledge and experience of, user concerns and priorities. This Group has adopted Terms of Reference and a modus operandi based on the NIHR ‘PPI Research Cycle Model’.64 The UAG will meet every 3 months and the Chair is a full member of the study team and named Coinvestigator.
Reference group
The UAG's work will be supported by a reference group consisting of prostate cancer service users, partners and family members who will be invited, as appropriate, to provide information and views on particular issues. Members of PCUK's ‘On-Line Community’ (an open forum of PCUK volunteers and bloggers) will be kept up to date about the study and will be appraised of opportunities to contribute to advising the project on specific matters, as and when topics requiring additional input are identified. Those service users who express an interest in offering views on the identified topic will then act, de facto, as a member of the reference group.
Data analysis and reporting
Quantitative data analysis
Descriptive statistics will be used to report the survey results and ‘describe’ the health outcomes of men with prostate cancer. The outcome variables, that is, EQ-5D, EPIC-26 and SDI, will be analysed according to stage/severity of disease (TNM and Gleason Score, where available), treatment type, comorbidity, age, ethnic and sociodemographic group (and other relevant variables). These descriptive analyses will identify potential relationships of interest which can be investigated further. Regression modelling will be used to investigate associations and to identify statistically and clinically significant risk factors and predictors of health outcomes. In order to be robust, analyses will require appropriate adjustment for casemix and other confounding factors and may require more complex techniques, such as the modelling of hierarchies within the data (multilevel modelling) and post hoc weighting to overcome response bias. Multiple imputation methods may be used to deal with missing data. A similar methodology would be used for international comparison of health outcomes, depending on the comparability of the survey instruments used.
Respondents from the first cohorts (Cohorts 1 and 2) will be resurveyed 12 months after the initial survey, which will allow measurement of any changes in their outcomes over time. For example, differences in EQ-5D scores could be calculated between the two time points and this would allow assessment of whether outcomes improve, decline or remain static. Interpretation is difficult, however, as there is no information regarding the individuals' health before their cancer diagnosis. Normative data from the general population will be used, where these are available, in order to compare the health of men with prostate cancer with those in the general population and to assess whether their health returns to a ‘normal’ level over time.
New instrument development is not being undertaken as part of this work. However, there is the opportunity to explore and check the psychometric properties of the newer, less well-established questionnaires and to determine the most fitting instruments for future prostate cancer PROMs work using the Rasch analysis.65
Qualitative data analysis
Telephone interviews (cross-sectional and longitudinal)
Interviews will be transcribed verbatim and managed within NVivo software (QSR International. NVivo qualitative data analysis Software: version 11, 2016). A framework analysis approach will be adopted: a matrix-based approach for collating, reviewing and understanding data.66 The researchers will read interview transcripts from each of the groups to ensure a deep familiarisation with the data. An initial coding framework will be developed, drawn from the interview schedule but informed by emerging themes incorporating the experiences of the four treatment sample groups and the BME sample. Another coding framework will be developed for the partner's sample. Analysis and data collection will occur simultaneously and new data will be compared with that already coded to identify further themes. Specific themes within the data will be mapped and patterns, relationships and associations will be identified. Inter-rater comparability testing will take place at several points throughout the process.
Free-text comments
Free-text data, provided by survey respondents, will be analysed using NVivo (QSR International. NVivo qualitative data analysis Software: version 11, 2016) and ‘R’ software (R Core Team). All comments will be indexed and entered into NVivo (QSR International Pty. NVivo qualitative data analysis Software: version 11, 2016). Analysis will follow three phases in a way similar to a previous study conducted by members of the research team.67 First, random samples of comments will be read and coded to develop a thematic framework that comprehensively categorises issues and identifies ‘hot topics’. Second, machine learning algorithms will be trained and tested to retrieve comments within the larger data set pertaining to the categories of interest. Third, a deeper level of qualitative analysis will be conducted relating to issues of particular interest to identify insights and determine patterns in participant experiences.
Management and oversight
A Clinical/Scientific Advisory Group (CSAG) will be used to provide expert knowledge for study design, interpretation, analysis and reporting. The project team will work closely with the Clinical/Scientific and User advisory groups as well as clinical and methodological opinion leaders who have agreed to collaborate. In addition, a steering group has been established by PCUK with responsibility for oversight of active performance delivery.
The Principal Investigators, Project Managers and other relevant team members (depending on the phase of the study) will have weekly telephone meetings, while the full study team will meet monthly to review progress. The CSAG and steering group will meet every 3 months.
Discussion
It is intended that the study will provide detailed data on which to drive forward service improvements, produce information to help patients and their clinical teams choose the most appropriate treatment option, optimise the provision of post-treatment support and inform future research. The success of this study relies on correctly identifying and contacting the eligible men without causing undue distress, and obtaining a high response rate from a representative sample of prostate cancer survivors. The study results must be disseminated widely and effectively in order to have the maximum impact.
Ethics approval
The study has received the following approvals: Newcastle and North Tyneside 1 Research Ethics Committee (15/NE/0036), Health Research Authority Confidentiality Advisory Group (15/CAG/0110), NHS Scotland Public Benefit and Privacy Panel (0516-0364), Office of Research Ethics Northern Ireland (16/NI/0073) and NHS R&D approval from Wales, Scotland and Northern Ireland.
Ethical and safety considerations
The methodology will follow that adopted in previous surveys,5 32 where the number of adverse events/symptoms was very low. In addition, in England, Wales and Northern Ireland, approval will be sought from the treating Trust/MDT and they will be offered the chance to check the list of eligible men. The first question on the survey will ask men whether they have ever been diagnosed with prostate cancer. If not, they can tick no and return the survey and will not be contacted again. Death checks will be carried out immediately prior to survey mail-out; however, it must be acknowledged that even with the most stringent checks, a small number of individuals may have died very close to the time of survey mailing and these will receive a survey. A double envelope method will be used for the mailings to mitigate against someone other than the intended recipient opening the survey. Despite all of these measures, it is not possible to predict the reaction of the men who receive a survey, for example, whether they will become angry or upset at being contacted. The information accompanying the survey has been carefully worded and checked with service users and the ethics committee in order to optimise positive reactions. In order to deal with any adverse events/symptoms, a procedure for rapid and timely response to, and support of, affected individuals has been developed.
Maximising response rates
A number of methods will be employed to achieve as high a participation rate as possible, including a media campaign to coincide with survey mail out, the use of social media and the PCUK online forum to promote the survey, sending two reminder letters, which has been shown to increase response rates, and the option to complete the survey in a range of the most spoken minority languages in the UK. It is known from previous PROMs surveys that there tend to be differences in the characteristics of those who do and do not respond, with the elderly, ethnic minorities and those from more socioeconomically deprived areas being less likely to participate.5 32 If, after using the methods above, there are differences between the responders and non-responders, statistical techniques can be used to adjust for variation in participation rates.
The use of electronic data collection will be explored during the second surveys in each nation. Response rates will be carefully examined to look at variation by age, and other sociodemographic factors, and to see whether response rates can be increased using electronic methods.
Dissemination plan
The study findings will be disseminated through a series of reports, academic papers (open-access) and conference presentations, and all findings will be available on the dedicated study website as well as the PCUK website and online forum. These outputs will provide qualitative and quantitative empirical knowledge of key clinical, sociodemographic, psychosocial and service/organisational factors that predict patients with prostate cancer generic and cancer-specific HRQL. A public access online toolkit will provide detailed anonymised information. The toolkit will enable each provider (NHS Trust/Health Board, Commissioning Group, Clinical Network or equivalent) to visualise the results for their organisation and to compare them against the national ‘average’. The study will produce a validated survey tool for the collection of health outcomes of prostate cancer survivors. This would be made available for use by other organisations and researchers (dependent on appropriate conditions of use).
Participant anonymity
Publications/reports on the findings of the study will make no reference to the identities of the patients who participated. When describing the clinical and sociodemographic characteristics of the sample, care will be taken to ensure that, if any values are small numbers, for instance, this information does not allow individuals to be identified. Similarly, if any direct quotations are used for illustrative purposes, they will be anonymised and care taken to ensure that they are not inadvertently identifiable.
Data storage and security
A 15-year data retention policy will be adopted for the hard-copy data (questionnaire responses) and electronic records held by Picker Institute Europe, with a review at the halfway point as to whether or not ongoing retention is justified. The records will be identified by an ID number with only the cancer registries (and ISD in Scotland) able to identify participants.
For the period of the study, the pseudonymised survey data and interview recordings and transcripts will be stored in a secure environment provided by the Leeds Institute of Data Analytics at the University of Leeds. The data will be accessed by approved members of the research team who will adhere to the agreed data security protocol and follow the relevant codes of practice concerning confidentiality, information security and records management.
The electronic survey data will be stored long term by the appropriate cancer registry (in England, Wales or NI) or by ISD in Scotland and held according to their respective information governance arrangements.
Acknowledgments
The authors thank the following people for their contribution to the development, setting up and running of the study: Heather Kinnear, Linda Roberts, Adrian Slater, the User Advisory Group, the Clinical and Scientific Advisory Group, Picker Institute Europe and Business Services Organisation (Northern Ireland). The authors thank the members of the Oxfordshire Prostate Cancer Support Group, the patients attending a prostate cancer clinic in Leeds and the volunteers who took part in cognitive testing for providing feedback on the survey content and layout. Data for this study are based on information collected and quality assured by the PHE National Cancer Registration and Analysis Service. Access to the data was facilitated by the PHE Office for Data Release.
Footnotes
Twitter: Follow Penny Wright at @DrPennyWright, Dyfed Huws at @BydDyfed and Carol Rivas at @wirebird50
Contributors: AWG and AG are the principal investigators. AD, PW, RW, EW, HB, LH, CD, PS, PK, JWHC and JV are coinvestigators. Together, these authors conceived and designed the study. TK, RM, MA, VC, OM, LM, CR, JN and MH have contributed to the design and running of the study. JWHC and WC are members of the Clinical and Scientific Advisory Group and contributed to the design of the survey. All authors have contributed to the writing of the study protocol and have approved the final version of this manuscript.
Funding: This 3-year study has been funded by the Movember Foundation, through Prostate Cancer UK (HO-LAPCD-14-001), and is sponsored by the University of Leeds.
Competing interests: None declared.
Ethics approval: Newcastle and North Tyneside 1 Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cancer Research UK. Prostate cancer incidence statistics 2015. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/incidence
- 2.Maddams J, Utley M, Møller H. Projections of cancer prevalence in the United Kingdom, 2010–2040. Br J Cancer 2012;107:1195–202. 10.1038/bjc.2012.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilt TJ, MacDonald R, Rutks I et al. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med 2008;148:435–48. 10.7326/0003-4819-148-6-200803180-00209 [DOI] [PubMed] [Google Scholar]
- 4.Ross KM, Ranby KW, Wooldridge JS et al. Effects of physical and mental health on relationship satisfaction: a dyadic, longitudinal examination of couples facing prostate cancer. Psychooncology 2016;25:898–904. 10.1002/pon.3931 [DOI] [PubMed] [Google Scholar]
- 5.Glaser AW, Fraser LK, Corner J et al. Patient-reported outcomes of cancer survivors in England 1–5 years after diagnosis: a cross-sectional survey. BMJ Open 2013;3:pii: e002317 10.1136/bmjopen-2012-002317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gavin AT, Drummond FJ, Donnelly C et al. Patient-reported ‘ever had’ and ‘current’ long-term physical symptoms after prostate cancer treatments. BJU Int 2015;116:397–406. 10.1111/bju.13036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drummond FJ, Kinnear H, O'Leary E et al. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J Cancer Surviv 2015;9:361–72. 10.1007/s11764-014-0419-6 [DOI] [PubMed] [Google Scholar]
- 8.National Institute of Health and Care Excellence. Prostate cancer: diagnosis and treatment. Update of Clinical Guidelines 58 (Clinical Guideline 175), 2014. [Google Scholar]
- 9.Kazer MW, Psutka SP, Latini DM et al. Psychosocial aspects of active surveillance. Curr Opin Urol 2013;23:273–7. 10.1097/MOU.0b013e32835eff24 [DOI] [PubMed] [Google Scholar]
- 10.Sanda MG, Dunn RL, Michalski J et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250–61. 10.1056/NEJMoa074311 [DOI] [PubMed] [Google Scholar]
- 11.Smith DP, King MT, Egger S et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ 2009;339:b4817 10.1136/bmj.b4817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharp L, O'Leary E, Kinnear H et al. Cancer-related symptoms predict psychological wellbeing among prostate cancer survivors: results from the PiCTure study. Psychooncology 2016;25:282–91. 10.1002/pon.3909 [DOI] [PubMed] [Google Scholar]
- 13.Ream E, Quennell A, Fincham L et al. Supportive care needs of men living with prostate cancer in England: a survey. Br J Cancer 2008;98:1903–9. 10.1038/sj.bjc.6604406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boberg EW, Gustafson DH, Hawkins RP et al. Assessing the unmet information, support and care delivery needs of men with prostate cancer. Patient Educ Couns 2003;49:233–42. 10.1016/S0738-3991(02)00183-0 [DOI] [PubMed] [Google Scholar]
- 15.O'Brien R, Rose P, Campbell C et al. ‘I wish I'd told them’: a qualitative study examining the unmet psychosexual needs of prostate cancer patients during follow-up after treatment. Patient Educ Couns 2011;84:200–7. 10.1016/j.pec.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 16.O'Brien R, Rose PW, Campbell C et al. Experiences of follow-up after treatment in patients with prostate cancer: a qualitative study. BJU Int 2010;106:998–1003. 10.1111/j.1464-410X.2010.09292.x [DOI] [PubMed] [Google Scholar]
- 17.Paterson C, Robertson A, Smith A et al. Identifying the unmet supportive care needs of men living with and beyond prostate cancer: a systematic review. Eur J Oncol Nurs 2015;19:405–18. 10.1016/j.ejon.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 18.King AJ, Evans M, Moore TH et al. Prostate cancer and supportive care: a systematic review and qualitative synthesis of men's experiences and unmet needs. Eur J Cancer Care (Engl) 2015;24:618–34. 10.1111/ecc.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson E, Shinkins B, Frith E et al. Symptoms, unmet needs, psychological well-being and health status in survivors of prostate cancer: implications for redesigning follow-up. BJU Int 2015;116:E10–19. [DOI] [PubMed] [Google Scholar]
- 20.Prostate Cancer UK. Men's Views on Quality Care in Prostate Cancer—What does Quality Care Mean for Men with Prostate Cancer? National Survey Report 2012 (updated 14 June 2012).
- 21.Lamers RE, Cuypers M, Husson O et al. Patients are dissatisfied with information provision: perceived information provision and quality of life in prostate cancer patients. Psychooncology 2016;25:633–40. 10.1002/pon.3981 [DOI] [PubMed] [Google Scholar]
- 22.Sinfield P, Baker R, Ali S et al. The needs of carers of men with prostate cancer and barriers and enablers to meeting them: a qualitative study in England. Eur J Cancer Care (Engl) 2012;21:527–34. 10.1111/j.1365-2354.2012.01341.x [DOI] [PubMed] [Google Scholar]
- 23.Ervik B, Nordøy T, Asplund K. In the middle and on the sideline: the experience of spouses of men with prostate cancer. Cancer Nurs 2013;36:E7–E14. 10.1097/NCC.0b013e31824fe1ef [DOI] [PubMed] [Google Scholar]
- 24.Gray RE, Fitch M, Phillips C et al. Managing the impact of illness: the experiences of men with prostate cancer and their spouses. J Health Psychol 2000;5:531–48. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/213789/dh_123138.pdf [DOI] [PubMed] [Google Scholar]
- 25.Department of Health. Improving outcomes. A Strategy for Cancer, 2011. [Google Scholar]
- 26.Department of Health. The NHS Outcomes Framework 2011/12. London, 2010. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/213789/dh_123138.pdf, 2013.
- 27.The Scottish Government. Beating cancer: ambition and action. Edinburgh, 2016. http://www.gov.scot/Resource/0049/00496709.pdf [Google Scholar]
- 28.Department of Health. Radiotherapy Dataset Annual Report 2009/2010, 2010.
- 29.Jefford M, Rowland J, Grunfeld E et al. Implementing improved post-treatment care for cancer survivors in England, with reflections from Australia, Canada and the USA. Br J Cancer 2013;108:14–20. 10.1038/bjc.2012.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Department of Health, Macmillan Cancer Support, NHS Improvement. Living with & beyond cancer: taking action to improve outcomes. London: Department of Health, 2013. http://www.ncsi.org.uk/wp-content/uploads/Living-with-and-beyond-2013.pdf [Google Scholar]
- 31.Department of Health. Quality of life of cancer survivors in England: report on a pilot survey using patient reported outcome measures (PROMS), 2012.
- 32.Downing A, Morris EJ, Richards M et al. Health-related quality of life after colorectal cancer in England: a patient-reported outcomes study of individuals 12 to 36 months after diagnosis. J Clin Oncol 2015;33:616–24. 10.1200/JCO.2014.56.6539 [DOI] [PubMed] [Google Scholar]
- 33.NHS England. Quality of life of colorectal cancer survivors in England. London: NHS England, 2015. [Google Scholar]
- 34.Quality Health. Quality of Life of Cancer Survivors in England: One Year On from the 2011 Survivorship Survey Pilot 2013. https://www.quality-health.co.uk/resources/surveys/cancer-survivorship-survey
- 35. International Consortium for Health Outcomes Measurement (ICHOM). Localized Prostate Cancer Data Collection Reference Guide. Boston, 2014. http://www.ichom.org/medical-conditions/localized-prostate-cancer/
- 36.Morris C, Gibbons E, Fitzpatrick R. A structured review of patient-reported outcome measures for men with prostate cancer. A report to the Department of Health, 2009. [Google Scholar]
- 37.Rnic K, Linden W, Tudor I et al. Measuring symptoms in localized prostate cancer: a systematic review of assessment instruments. Prostate Cancer Prostatic Dis 2013;16:111–22. 10.1038/pcan.2013.1 [DOI] [PubMed] [Google Scholar]
- 38.Schmidt S, Garin O, Pardo Y et al. Assessing quality of life in patients with prostate cancer: a systematic and standardized comparison of available instruments. Qual Life Res 2014;23:2169–81. 10.1007/s11136-014-0678-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin NE, Massey L, Stowell C et al. Defining a standard set of patient-centered outcomes for men with localized prostate cancer. Eur Urol 2015;67:460–7. 10.1016/j.eururo.2014.08.075 [DOI] [PubMed] [Google Scholar]
- 40.Drummond FJ, Kinnear H, Donnelly C et al. Establishing a population-based patient-reported outcomes study (PROMs) using national cancer registries across two jurisdictions; The Prostate Cancer Treatment, your experience (PiCTure) Study. BMJ Open 2015;5:e006851 10.1136/bmjopen-2014-006851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herdman M, Gudex C, Lloyd A et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kessler RC, Barker PR, Colpe LJ et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry 2003;60:184–9. 10.1001/archpsyc.60.2.184 [DOI] [PubMed] [Google Scholar]
- 43.Stewart-Brown S, Tennant A, Tennant R et al. Internal construct validity of the Warwick-Edinburgh Mental Well-being Scale (WEMWBS): a Rasch analysis using data from the Scottish Health Education Population Survey. Health Qual Life Outcomes 2009; 7:15 10.1186/1477-7525-7-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright EP, Kiely M, Johnston C et al. Development and evaluation of an instrument to assess social difficulties in routine oncology practice. Qual Life Res 2005;14:373–86. 10.1007/s11136-004-5332-4 [DOI] [PubMed] [Google Scholar]
- 45.Wright P, Smith A, Roberts K et al. Screening for social difficulties in cancer patients: clinical utility of the Social Difficulties Inventory. Br J Cancer 2007;97:1063–70. 10.1038/sj.bjc.6604006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright P, Smith AB, Keding A et al. The Social Difficulties Inventory (SDI): development of subscales and scoring guidance for staff. Psychooncology 2011;20:36–43. 10.1002/pon.1705 [DOI] [PubMed] [Google Scholar]
- 47.Wei JT, Dunn RL, Litwin MS et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 2000;56:899–905. 10.1016/S0090-4295(00)00858-X [DOI] [PubMed] [Google Scholar]
- 48.O'Leary E, Drummond FJ, Gavin A et al. Psychometric evaluation of the EORTC QLQ-PR25 questionnaire in assessing health-related quality of life in prostate cancer survivors: a curate's egg. Qual Life Res 2015;24:2219–30. 10.1007/s11136-015-0958-y [DOI] [PubMed] [Google Scholar]
- 49.van Andel G, Bottomley A, Fosså SD et al. An international field study of the EORTC QLQ-PR25: a questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur J Cancer 2008;44:2418–24. 10.1016/j.ejca.2008.07.030 [DOI] [PubMed] [Google Scholar]
- 50.Miller DC, Wei JT, Dunn RL et al. Use of medications or devices for erectile dysfunction among long-term prostate cancer treatment survivors: potential influence of sexual motivation and/or indifference. Urology 2006;68:166–71. 10.1016/j.urology.2006.01.077 [DOI] [PubMed] [Google Scholar]
- 51.Aaronson NK, Ahmedzai S, Bergman B et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 52.Kessler RC, Zhao S, Katz SJ et al. Past-year use of outpatient services for psychiatric problems in The National Comorbidity Survey. Am J Psychiatry 1999;156:115–23. 10.1176/ajp.156.1.115 [DOI] [PubMed] [Google Scholar]
- 53.Brehaut JC, O'Connor AM, Wood TJ et al. Validation of a decision regret scale. Med Decis Making 2003;23:281–92. 10.1177/0272989X03256005 [DOI] [PubMed] [Google Scholar]
- 54.Bulsara C, Styles I. Development of a cancer related patient empowerment scale using the polytomous Rasch measurement model. Cancer Clin Oncol 2013;2:87–102. 10.5539/cco.v2n1p87 [DOI] [Google Scholar]
- 55.University of Southampton. Research project: development, implementation and evaluation of the True NTH Supported Self Management and Follow Up Care Programme 2016. http://www.southampton.ac.uk/healthsciences/research/projects/development-impl-true-nth.page
- 56.Weller D, Vedsted P, Anandan C et al. , ICBP Module 4 Working Group. An investigation of routes to cancer diagnosis in ten international jurisdictions, as part of the International Cancer Benchmarking Partnership; survey development and implementation. BMJ Open 2016;6:e009641 10.1136/bmjopen-2015-009641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivas C, Matheson L, Wagland R et al. Exploring the quality of life and wellbeing of men with prostate cancer and their partners or carers, and related care needs and gaps in service: protocol for qualitative meta-synthesis. PROSPERO International prospective register of systematic reviews 2015;CRD42015017836. http://www.crd.york.ac.uk/PROSPERO_REBRANDING/display_record.asp?ID=CRD42015017836
- 58.Quality Health. National Cancer Patient Experience Survey 2015. http://www.quality-health.co.uk/surveys/national-cancer-patient-experience-survey
- 59.Public Health England. National End of Life Care Intelligence Network 2013. http://www.endoflifecare-intelligence.org.uk/home
- 60.Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013;346:f167 10.1136/bmj.f167 [DOI] [PubMed] [Google Scholar]
- 61.Corner J, Wagland R, Glaser A et al. Qualitative analysis of patients’ feedback from a PROMs survey of cancer patients in England. BMJ Open 2013;3:e002316 10.1136/bmjopen-2012-002316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright P, Downing A, Morris EJ et al. Identifying social distress: a cross-sectional survey of social outcomes 12–36 months following a colorectal cancer diagnosis. J Clin Oncol 2015;33:3423–30. 10.1200/JCO.2014.60.6129 [DOI] [PubMed] [Google Scholar]
- 63.Downing A, Aravani A, Macleod U et al. Early mortality from colorectal cancer in England: a retrospective observational study of the factors associated with death in the first year after diagnosis. Br J Cancer 2013;108:681–5. 10.1038/bjc.2012.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.National Institute of Health Research. Patient involvement in health and social care research: a handbook for researchers 2014. http://www.nihr.ac.uk/funding/how-we-can-help-you/RDS-PPI-Handbook-2014-v8-FINAL.pdf
- 65.Rasch G. Probabilistic models for some intelligence and attainment tests. Copenhagen: Paedagogike Institut, 1960. [Google Scholar]
- 66.Ritchie J, Lewis J. Qualitative research practice: a guide for social science students and researchers. London: SAGE Publications, 2003. [Google Scholar]
- 67.Wagland R, Recio-Saucedo A, Simon M et al. Development and testing of a text-mining approach to analyse patients’ comments on their experiences of colorectal cancer care. BMJ Qual Saf 2016;25:604–14. 10.1136/bmjqs-2015-004063 [DOI] [PubMed] [Google Scholar]