Abstract
Introduction
Acute kidney injury (AKI) after cardiac surgery is common and results in increased morbidity and mortality. One possible mechanism for AKI is ischaemia–reperfusion injury caused by the extracorporeal circulation (ECC), resulting in an opening of the mitochondrial permeability transition pore (mPTP) in the kidneys, which can lead to cell injury or cell death. Ciclosporin may block the opening of mPTP if administered before the ischaemia–reperfusion injury. We hypothesised that ciclosporin given before the start of ECC in cardiac surgery can decrease the degree of AKI.
Methods and analysis
Ciclosporin to Protect Renal function In Cardiac Surgery (CiPRICS) study is an investigator-initiated double-blind, randomised, placebo-controlled, parallel design, single-centre study performed at a tertiary university hospital. The primary objective is to assess the safety and efficacy of ciclosporin to limit the degree of AKI in patients undergoing coronary artery bypass grafting surgery. We aim to evaluate 150 patients with a preoperative estimated glomerular filtration rate of 15–90 mL/min/1.73 m2. Study patients are randomised in a 1:1 ratio to receive study drug 2.5 mg/kg ciclosporin or placebo as an intravenous injection after anaesthesia induction but before start of surgery. The primary end point consists of relative P-cystatin C changes from the preoperative day to postoperative day 3. The primary variable will be tested using an analysis of covariance method. Secondary end points include evaluation of P-creatinine and biomarkers of kidney, heart and brain injury.
Ethics and dissemination
The trial is conducted in compliance with the current version of the Declaration of Helsinki and the International Council for Harmonisation (ICH) Good Clinical Practice guidelines E6 (R1) and was approved by the Regional Ethical Review Board, Lund and the Swedish Medical Products Agency (MPA). Written and oral informed consent is obtained before enrolment into the study.
Trial registration number
NCT02397213; Pre-results.
Strengths and limitations of this study.
Randomised, controlled, double-blind, prospective clinical trial.
Two Drug Safety Monitoring Board (DSMB) meetings have recommended that the study be continued.
Strictly standardised study population.
Possible selection bias because all possible patients may not be entered in the study.
Introduction
Acute kidney injury (AKI) is a common complication after cardiac surgery, with an incidence between 5% and 40% depending on the definition.1–5 A decreased renal function after cardiac surgery is associated with decreased long-term survival.2–5 Despite trials investigating several pharmaceutical agents,6–9 no effective prophylactic treatments have so far been found.
Cardiac surgery with extracorporeal circulation (ECC) may result in renal ischaemia–reperfusion injury, especially in the poorly oxygenated and metabolic active outer medulla. Thus, ECC-induced renal ischaemia–reperfusion injury is claimed to play a role in the resulting AKI.10
The proposed mechanism for AKI induced by renal ischaemia–reperfusion injury is opening of channels called the mitochondrial permeability transition pore (mPTP) during reperfusion. This can amplify or accelerate cell death, resulting in reperfusion-induced necrosis.11–17
The inner mitochondrial membrane is normally impermeable to most solutes, enabling efficient ATP production through oxidative phosphorylation. Under conditions of elevated Ca2+ levels and oxidative stress triggered by reperfusion after ischaemia, the mPTP in the inner mitochondrial membrane opens. On the mPTP opening, energy production is immediately halted and molecules smaller than ∼1500 Da equilibrate over the membrane. The osmotic force of matrix proteins results in matrix swelling, leading to rupture of the outer membrane and release into the cytosol of proapoptotic factors such as cytochrome C, further pushing the cell towards death.13 18 19
Cyclophilin-D is a key regulator of the mPTP, which has been confirmed in several independent cyclophilin-D knock-out studies. Ca2+ causes a conformational change in the mPTP from a closed to an open state.20–22 The opening of the mPTP can be inhibited pharmacologically by the immunosuppressive agent ciclosporin via inhibition of cyclophilin-D,23 24 and several reports in animals have indicated that it may limit ischaemia–reperfusion injury in various organs, including the kidneys.25–29 A cyclophilin-D activated mPTP has also been demonstrated in human mitochondria.30–33
Further, there are a number of animal studies showing cytoprotective preconditioning, antinecrotic and also antiapoptotic effects of ciclosporin against ischaemia–reperfusion injury in the kidneys.34–38 Importantly, ciclosporin has been administered before the kidney is exposed to ischaemia and subsequent reperfusion in these studies.
To the best of our knowledge, no clinical studies with the specific aim of investigating if ciclosporin has renoprotective effects if administered before the ischaemia–reperfusion episode have previously been performed. In contrast, ciclosporin is known to cause renal failure following high and/or long-term exposure. However, this side effect is caused by other mechanisms, discussed under ‘Safety considerations’ section.
Hypothesis
On the basis of the aforementioned, we raised the hypothesis that ciclosporin, administered as a single intravenous bolus dose preoperatively in cardiac surgery with ECC, will reduce the level of renal dysfunction associated with this type of surgery.
Methods and analysis
Study design
We designed an investigator-initiated, clinical, double-blind, randomised, placebo-controlled, parallel design clinical trial based on a single centre aiming to detect if there is a role for ciclosporin in renal protection. Study patients will be randomised in a 1:1 ratio. We plan to have a total of 150 consecutive evaluable study patients with ∼75 patients in each arm. Approximately 170 patients are estimated to be enrolled, in order to have 150 evaluable patients for the two groups.
Definitions
Day numbering: Day 0 will be the surgery and study drug administration day. Day −1 is the day the study patients are included and is normally the day before surgery and day 1 the first day after surgery, etc. On day 0, the end of ECC is defined as time zero.
Data collection
Efficacy data will be collected on day −1 until, and including, day 4 as long as the patient is present at the hospital. Safety data will be collected from the time for distribution of study drug until, and including, day 4 as long as the patient is present at the hospital. From day 4 until day 30, serious adverse events (SAEs) and serious unexpected adverse reactions (SUSARs) will be reported.
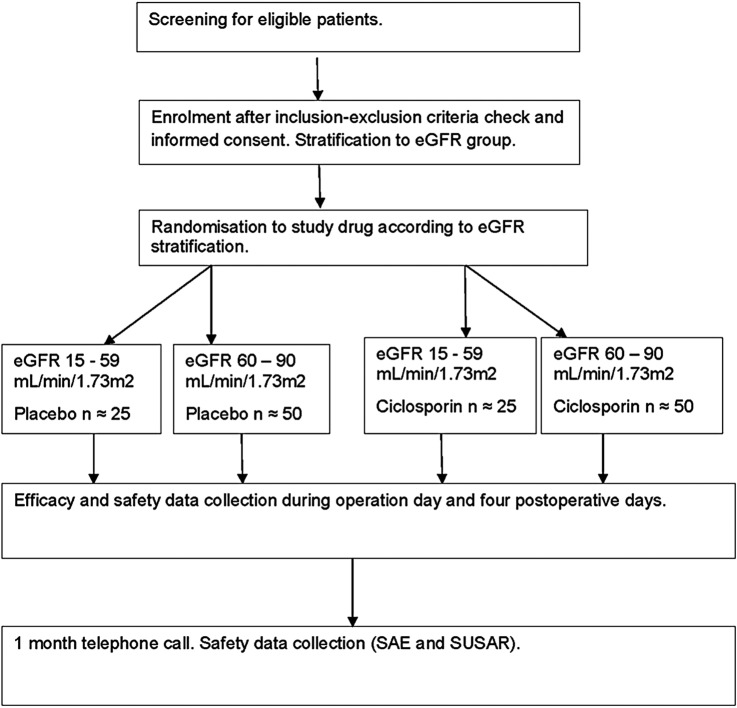
For details, see table 1 and figure 1.
Table 1.
Participant timeline
| Day number in relation to surgery day | −1 | 0 | 1 | 2 | 3 | 4 | 1 month telephone call |
|---|---|---|---|---|---|---|---|
| Informed consent | X | ||||||
| Inclusion/exclusion criteria | X | X | |||||
| Randomisation | X | ||||||
| Study drug | X | ||||||
| AE and SAE registration and report | X | X | X | X | X | X | |
| Blood ciclosporin concentration | X | X | |||||
| Blood tests efficacy: P-cystatin C, P-creatinine | X | X | X | X | X | ||
| Analysis U-TIMP-2, U-IGFBP7, U-albumin/creatinine | X | X | |||||
| Blood tests safety: Mg, K, urea, myoglobin, ASAT, ALAT, bilirubin, ALP, GT, leucocytes, CRP, CK, Hb, Trc | X | X | X | X | X | ||
| Exploratory immunological tests | X | X | |||||
| Blood tests cardiac: troponin T, CK MB | X | X | X | X | X | ||
| Blood test cerebral: S-S100B | X | X | X | ||||
| Documentation of: hourly diuresis, bleeding at 12 hours and total, time to extubation, time in ICU, fluid balance | X | X | |||||
| Temperature, blood pressure | X | X | X | X | X | ||
| Scoring leg wound infection. | X |
Day −1 illustrates the day before surgery, usually the same as admission day, day 0 surgery day, day 1 the day after surgery, etc.
AE, adverse event; ALAT, alanine aminotransferase; ALP, alkaline phosphatase; ASAT, aspartate aminotransferase; CK, creatine kinase; CRP, C reactive protein; GT, γ-glutamyl transferase; Hb, haemoglobin; ICU, intensive care unit; IGFBP7, insulin-like growth factor binding protein 7; K, potassium; Mg, magnesium; SAE, serious adverse event; TIMP-2, tissue inhibitor of metalloproteinase 2; Trc, thrombocytes.
Figure 1.
Schematic flow chart of the CiPRICS study. CiPRICS, Ciclosporin to Protect Renal function In Cardiac Surgery; eGFR, estimated glomerular filtration rate; SAE, serious adverse event; SUSAR, serious unexpected adverse reaction.
Study drug
The study drug is CicloMulsion, a 5 mg/mL ready-to-use lipid emulsion of ciclosporin (NeuroVive Pharmaceutical AB, Lund, Sweden) or its matching placebo, given as a single intravenous bolus dose of 0.5 mL/kg. The qualitative composition of CicloMulsion and its placebo only differ in the presence or absence of ciclosporin, so the final emulsions will be visually indistinguishable.
Eligibility criteria
This study will be eligible for patients planned for coronary artery bypass grafting (CABG), a standardised operation including the use of ECC.
Inclusion criteria
The study patient is scheduled for non-emergent (decision to operate more than 1 hour before start of surgery) CABG surgery.
Preoperative cystatin C estimated glomerular filtration rate (eGFR) or the Modification of Diet for Renal Disease (MDRD) eGFR is 15–90 mL/min/1.73/m2. eGFR will be calculated using both creatinine based on the MDRD39 and the cystatin C based formula on the Chronic Kidney Disease Epidemiological collaboration (CKD-EPI).40 The lowest eGFR value will be used as inclusion criteria.
The patient has given his/her written consent to participate.
Exclusion criteria
Patients are excluded if they meet one or more of the following criteria:
The patient has an uncontrolled hypertension.
Hypersensitivity to the active drug or vehicle, including egg protein, soya protein or peanut protein.
The patient is pregnant or is a fertile woman.
The patient has been treated with ciclosporin within 4 weeks prior to the surgery.
The patient has a known ongoing malignancy.
The patient has ongoing immunosuppressive treatment.
The patient has severe hepatic dysfunction.
The patient is treated with dialysis.
The patient has preoperatively ongoing and/or increasing clinical infection with C reactive protein (CRP) levels of >50 mg/L. Clinical signs of infection may or may not be present. Increase in CRP due to signs of cardiac origin,41 according to the investigator, should not be considered as exclusion criteria.
The patient has a severe ongoing viral infection, including HIV, hepatitis C, current or history of hepatitis B.
For non-allowed and restricted ongoing and concomitant medications, see Protocol section 12.2.
The patient is planned for off-pump CABG surgery.
The patient is included in other ongoing clinical trials.
For any other reason, the patient is unsuitable to participate in the study, according to the investigator.
Recruitment and stratification
Potential participants are identified from patients planned for elective CABG and included in a screening log. Patients are checked against the inclusion/exclusion criteria, and thereafter oral and written information is given to the patient. When written informed consent is obtained by an investigator, the patient is enrolled in the study.
At enrolment, the investigator also stratifies the patient to a predefined subgroup with reference to preoperative eGFR (15–59 or 60–90 mL/min/1.73 m2). When several patients fulfil the inclusion criteria, those with preoperative eGFR 15–59 are selected over those with 60–90 mL/min/1.73 m2 with the aim of including ∼50 patients from the group with eGFR 15–59 mL/min/1.73 m2. The reason for this is that preoperative renal function impairment is an important risk factor for developing postoperative AKI,42 and we know that without stratification there will only be a few patients enrolled with eGFR 15–59 mL/min/1.73 m2. Thus, we also investigate if treatment effects are similar in patients with preoperative milder and more severe renal impairment. Also, within each eGFR group, the study drug and placebo will be stratified in a 1:1 ratio. In summary, the stratification will increase the proportion of patients with a higher risk of developing AKI.
Randomisation
For randomisation to study drug or placebo treatment, a blinded randomisation list was pregenerated by a statistician not included in the study group (FoU-centrum Skane, Skane University Hospital, Lund, Sweden), to assign a unique sequential treatment number to each bottle of the study drug/placebo. The unique treatment number was printed on the study medication bottle, where also a sticker for the case report form (CSF) was positioned. The bottles with the study drug/placebo were packed in boxes marked with eGFR 15–59 or 60–90 mL/min/1.73 m2 according to the stratification aforementioned. Study drug/placebo is kept in two separate sets, one set for patients with eGFR 15–59 mL/min/1.73 m2 and one set for patients with eGFR 60–90 mL/min/1.73 m2.
Following completion of the assessments listed above, eligible patients will be allocated to one of the two treatment groups in a 1:1 ratio, to receive either a single intravenous bolus injection of ciclosporin or matching placebo by the following action.
Once the study patient has been admitted to the operation ward, a box with the allocated study drug/placebo is taken by the study nurse and the treatment number from that box and study drug/placebo bottle is attached in the CRF. The allocation to the study drug/placebo following the generated randomisation list will thus take place when the study nurse takes the next study medicine bottle, as described here, and prepares the syringe with the study drug/placebo.
Thus, the study treatment is blinded for all involved staff. Closed envelopes with unblinding information is available at the operating ward in case of emergency situations.
Interventions
After randomisation, the study drug/placebo will be administered as an intravenous single bolus dose injection, 0.5 mL/kg body weight (corresponding to 2.5 mg/kg ciclosporin), after anaesthetic induction has been performed and the patient is in a stable circulatory state before the surgery starts. The study drug/placebo will be given in a central venous catheter as an injection during 10 min, without concomitant administration of other drugs in this line. Anaesthesia is standardised using propofol, fentanyl and rocuronium. Anaesthetic gas is prohibited medicine in this study.
Sample size calculations
A total of 150–170 patients will be included to obtain a total of 150 evaluable study patients with ∼75 patients in each arm. The relative difference between groups in change from baseline P-cystatin C to the third postoperative day will serve as the primary end point. In a previous study at our department,7 the response within each participant group was normally distributed with an SD of 27%. The primary efficacy analysis will compare the ciclosporin group to placebo at a two-sided 5% level. A sample size equal to 75 in each arm will provide at least 80% power to detect a 13% units (0.5 SD) difference from placebo. The study will continue until the planned number of study patients have finalised the protocol.
Outcomes
Primary end point
Relative P-cystatin C change from day −1 to day 3.
Secondary end points
Secondary end points to evaluate ciclosporin’s effect on renal function include, but are not limited to, P-cystatin C day −1 versus days 1, 2 and 4, P-cystatin C area under curve day −1 to day 4, P-cystatin C and P-creatinine eGFR according to CKD-EPI, P-creatinine, MDRD eGFR. Biomarker for renal damage based on tissue inhibitor of metalloproteinases-2 (U-TIMP-2) and urine insulin-like growth factor binding protein 7 (U-IGFBP7) levels.43 U-albumin/creatinine. Incidence of AKI according to predefined eGFR stratification (15–59 and 60–90 mL/min/1.73/m2) and Risk, Injury, Failure, Loss and End-stage renal failure (RIFLE) criteria based on P-creatinine and/or eGFR.44
To evaluate ciclosporin’s possible effect on myocardial injury,45 plasma creatine kinase isoenzyme MB (P CK MB) and P-troponin T will be measured.
Ciclosporin also has a possible cerebral protective effect,46 and therefore S-S100B is evaluated from day −1 throughout day 2.
Clinical and procedural secondary variables include ECC time, operation time, respiratory time, blood pressure, temperature, etc.
B-ciclosporin will be measured after injection, after end of ECC and on the morning of day 1.
In addition, a number of immunological parameters will be measured on an exploratory basis; serum will be screened for cytokines related to the inflammatory cell pattern aiming for tissue aggressive inflammatory reaction (tumour necrosis factor-α, interleukin (IL)-1β, IL-6, IL-7, IL-12, IL-17, interferon-γ), regulatory/immune-suppressive function (IL-2, IL-10), neutrophil activation (IL-8) and tissue-lenient inflammatory responses (IL-2, IL-4, IL-13). Natural killer cells, T-cell populations (Th1, Th2, Th17) as well as B cells will be evaluated as well.
Monitoring
The study is monitored by the clinical research organisation at Skane University Hospital, Lund (FoU-centrum Skane, Skane university hospital, Lund, Sweden). This is an independent in-house Contract Research Organisation (CRO).
Data analysis plan
The statistical analysis plan (SAP) will be signed before database lock. Analyses not described here or in the SAP will be considered exploratory/post hoc analyses.
In general, descriptive statistics will be presented for all efficacy and safety variables, as appropriate. Continuous variables will be summarised by descriptive statistics (sample size (n), mean, SD, minimum, median and maximum values). Categorical data will be summarised in frequency tables showing the number of study patients, frequency and percentage of occurrence.
All statistical tests will be conducted at the two-sided 5% level unless otherwise specified. Where appropriate, model-based point estimates, together with their 95% CIs, will be presented along with the two-sided p value for the test.
The primary comparison will be to investigate difference in P-cystatin C change from baseline to the third postoperative day between ciclosporin and placebo. The treatment difference in the secondary and exploratory end points described earlier will also be tested.
The primary end point, P-cystatin C relative change from baseline to the third postoperative day, will be analysed using analysis of covariance (ANCOVA). The model will include treatment and baseline P-cystatin C as explanatory variables.
Secondary end points will mainly be analysed by using ANCOVA, following the same conventions as in the analysis of the primary end point.
Exploratory analyses will be performed of several quality indices including, for example, time on mechanical ventilator, time on intensive care unit, extent of bleeding, incidence of atrial fibrillation, time on ECC, immunological parameters, etc.
If assumptions for parametric analysis are clearly violated, data transformations or a non-parametric approach will be applied.
Organisation and time line
The study team involves at the moment 10 investigators and 2 research nurses. It is a single-centre study performed in a tertiary hospital (Department of Cardiothoracic Surgery, Anaesthesia and Intensive Care, Skane University Hospital, Lund, Sweden). The first patient was included on 6 April 2015. The last patient is expected to be included during 2016.
Ethics and dissemination
Ethics
The trial is conducted in compliance with the current version of the Declaration of Helsinki and the ICH Good Clinical Practice guidelines E6 (R1). The trial has been registered under NCT02397213 and EudraCT: 2014-004610-29, Sponsor's Protocol Code Number 2014.001.
Safety considerations
The reporting of adverse events (AE) will begin after the start of study medication and last until the follow-up phone call is made 1 month after day of operation. AEs also include SAE and SUSARs reporting. This is performed according to the Swedish Medical Products Agency’s (MPA) regulations.
AEs, safety blood chemistry (table 1) and clinical data are collected daily including day 4. A telephone call on day 30 will assess and register possible SAE and signs of infection.
Drug Safety Monitoring Board
An independent Drug Safety Monitoring Board (DSMB) including three experts in clinical testing and/or renal function and one biostatistician will assess safety, including acute renal failure, when the study drug has been administered to 50 and 100 patients. The DSMB will recommend the study team to continue the study or not based on these safety data. A first meeting after 50 patients, in October 2015, and a second meeting in March 2016 after 100 patients were enrolled, resulted in the recommendation to continue the study without any changes in the protocol.
Specific safety considerations
The risks associated with a single 2.5 mg/kg intravenous dose of ciclosporin are considered small. Clinically relevant ciclosporin-associated side effects according to the Investigator Brochure (IB) for CicloMulsion are adverse allergic responses (anaphylactoid reactions) that may lead to anaphylactic shock, acute renal failure, hypertension or infections. These last three AEs are dose-dependent and usually occur during repeated administration.
Anaphylactoid reactions
Importantly, anaphylactoid reaction observed with the commercially available form of ciclosporin (Sandimmun) has been attributed to the presence of Kolliphor EL (Cremophor EL) in the product. In this study, a cremophor-free ciclosporin (CicloMulsion) and placebo will be used. However, this does not fully exclude the risk for allergic reactions to the study drug.
Renal failure
Calcineurin inhibitors such as ciclosporin are associated with induction of renal failure. The proposed mechanism for this is induction of vasoconstriction of the afferent arteriole by causing an imbalance between vasoconstricting47–49 and vasodilating50 51 agents. This results in an acute reversible52 53 impairment of renal function with reduction in glomerular filtration rate54 and tubular dysfunction.55 Renal failure as an AE is associated with long-term use and higher doses of ciclosporin than will be used in our study.
Induction of acute reversible impairment of renal failure as described above cannot be excluded in our study. However, we consider it as unlikely. Three clinical trials in a similar population administered ciclosporin in the same dose, but different formulations, as in our study.45 56 57 No renal side effects were reported. A recent large trial administering CicloMulsion in the same dose and in a similar population as in our study did not find any renal side effects.58 These four studies excluded patients with severe renal impairment and the CicloMulsion IB reports known creatinine clearance <30 mL/min/1.73/m2 as a contraindication. Preoperative impairment of renal function is a strong risk factor for developing AKI after cardiac surgery.42 To investigate if patients with the lowest renal function might also benefit from ciclosporin preoperatively, we include patients with eGFR 15–90 mL/min/1.73/m2, that is, with worse renal function. In addition, we also obtain safety data on these patients.
Hypertension
Blood pressure is included as a safety parameter.
Infections
We follow infection parameters such as CRP, leucocytes and body temperature daily. The leg wound infection rate is assessed on D4 using a standardised method.59 Also, we study immunological parameters as secondary variables on an exploratory base.
Dissemination plan
Results are going to be presented in a peer-reviewed medical journal. The study is investigator initiated and the protocol is written without any external influence. The study group will have the freedom to publish results regardless of the outcome. A clinical study report including study results will also be sent to the Swedish MPA and to the Regional Ethical Review Board. Archived study documents and source data will be filed at least 10 years after the study report has been finalised and submitted to the MPA. All processed data will be stored on the hospitals data servers with the same level of security as patient electronical records.
Discussion
Ciclosporin to Protect Renal function In Cardiac Surgery (CiPRICS) is designed to investigate if ciclosporin has renoprotective effects if administered as a single intravenous injection before start of ECC in CABG surgery. It is, to the best of our knowledge, the first study to test this hypothesis and therefore a proof-of-concept study.
We consider the experimental studies where ciclosporin is demonstrated to inhibit a cyclophilin D-associated opening of the mPTP and thus protect the mitochondria from deterioration after ischaemia–reperfusion injury as a good scientific rationale to raise and test our hypothesis.
Earlier clinical trials administering ciclosporin in the same way, to a similar population, have not reported a difference in creatinine between active study drug and placebo. However, renal function was only evaluated through P-creatinine and only as a safety variable. Although it is by far the most used biomarker for AKI, we consider P-creatinine to have limitations. Consensus does not exist in the literature on how renal function could best be evaluated. In our opinion, cystatin C has a higher precision and less confounding factors than creatinine,60 61 especially in the milder degrees of renal impairment. Other interesting biomarkers are U-TIMP-2 and U-IGFBP762 and also U-albumin/creatinine. We believe that the probability for finding a true renal effect is higher with these biomarkers as compared with fluctuations in P-creatinine only.
Since existing impairment in renal function already preoperatively is a considerable risk factor for developing AKI after cardiac surgery, we only include patients with eGFR 15–90 mL/min/1.73/m2. Thus, we believe that we study the population most prone to postoperative AKI. Since we do not know if it is the patients with the worst or the best renal function who might benefit the most from pretreatment with ciclosporin, if the hypothesis should be confirmed, we will stratify the patients into groups with milder (eGFR 60–90 mL/min/1.73/m2) and more severe (eGFR 15–59 mL/min/1.73/m2) impairment of renal function preoperatively. Based on the prevalence in patients with CABG, it can be difficult to enrol a sufficient number of patients in the lower eGFR range. We therefore predefined that this group should enclose ∼50 patients. Importantly, we also needed to evaluate the eGFR 15–59 mL/min/1.73/m2 group from a safety perspective, which also motivated a stratification. The primary objective, however, is to examine the whole study population with eGFR 15–90 mL/min/1.73/m2. In summary, we consider our design to reflect a good balance between safety and efficacy where the proof-of-concept can be tested.
As presented above, the cellular mechanisms for our hypothesis concerning ciclosporin's renoprotective effect and its documented nephrotoxic effects are different. Approximately 580 patients have been exposed to ciclosporin in an identical dose as in our study.45 56–58 The safety data collected in these four clinical studies in cardiac patients is of high quality and renal side effects are reported equally in placebo and ciclosporin-treated patients. However, CicloMulsion was used only in the CIRCUS study, the largest of the studies.58 Other formulations were used in the other three. Taken together, the safety profile in our study is, in our view, reasonable.
Possible weaknesses derive from the fact that we cannot include all patients suitable for enrolment because of an uneven distribution in the operation programme over the weekdays. Thus, on some days when several patients are available for enrolment, there could be a bias introduced in the selection of patients. Also, the eGFR stratification might infer a bias in the sense that a larger proportion of the study population might be shifted towards higher stages of chronic kidney disease compared with the general CABG population. Moreover, if the hypothesis is true, we do not know the optimal dose for ciclosporin to exert its renoprotective effect. The choice of 2.5 mg/kg is considered to give the best efficacy combined with good safety. However, the measurement of ciclosporin concentration in blood during the day of operation will help us to evaluate this issue.
In summary, the CiPRICS study investigates the possibility of ciclosporin as a novel preventive pharmacological treatment to attenuate the AKI associated with CABG surgery. The study aims to be completed during the second half of 2016.
Footnotes
Contributors: The authors are exclusively responsible for the design and conduct of this study. All study analyses, the drafting and editing of the final manuscript, and its final contents are planned to include all authors. PE is the co-principal investigator and was involved in writing of study protocol, writing and review of study protocol article, conducting of study including enrolment of patients, collection of study data. EG is the investigator and was involved in writing of study protocol, writing and review of study protocol article, conducting of study including enrolment of patients, collection of study data, performing analysis for immunological exploratory parameters. AD, BB, CM, AE, SN and AM are investigators and were involved in writing of study protocol, writing and review of study protocol article, conducting of study including enrolment of patients, collection of study data. MJH, EE and LA are investigators and were involved in writing of study protocol, writing and review of study protocol article. SJ was involved in providing laboratory and performing analysis for immunological exploratory parameters, writing of study protocol, writing and review of study protocol article. HB is the sponsor and primary investigator and was involved in writing of study protocol, writing and review of study protocol article, conducting of study including enrolment of patients, collection of study data.
Funding: This work was supported by the Swedish Heart-Lung foundation grant number 86601. NeuroVive Pharmaceutical AB, Lund, Sweden, provided the study treatment CicloMulsion and its placebo together with unrestricted research grants covering costs for the research nurses.
Competing interests: PE has received lecture fees from Orion Pharma AB. MJH received employment by and shareholder of NeuroVive Pharmaceutical AB, Lund, Sweden. EE received employment by and shareholder of NeuroVive Pharmaceutical AB, Lund, Sweden.
Ethics approval: The research project was approved by the Regional Ethical Review Board, Lund (18–12–2014) and the Swedish Medical Products Agency (19–02–2015).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Brown JR, Cochran RP, Dacey LJ et al. . Perioperative increases in serum creatinine are predictive of increased 90-day mortality after coronary artery bypass graft surgery. Circulation 2006;114:I409–13. 10.1161/CIRCULATIONAHA.105.000596 [DOI] [PubMed] [Google Scholar]
- 2.Brown JR, Cochran RP, MacKenzie TA et al. . Long-term survival after cardiac surgery is predicted by estimated glomerular filtration rate. Ann Thorac Surg 2008;86:4–11. 10.1016/j.athoracsur.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 3.Dardashti A, Ederoth P, Algotsson L et al. . Incidence, dynamics, and prognostic value of acute kidney injury for death after cardiac surgery. J Thorac Cardiovasc Surg 2014;147:800–7. 10.1016/j.jtcvs.2013.07.073 [DOI] [PubMed] [Google Scholar]
- 4.Hobson CE, Yavas S, Segal MS et al. . Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009;119:2444–53. 10.1161/CIRCULATIONAHA.108.800011 [DOI] [PubMed] [Google Scholar]
- 5.Lassnigg A, Schmid ER, Hiesmayr M et al. . Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med 2008;36:1129–37. 10.1097/CCM.0b013e318169181a [DOI] [PubMed] [Google Scholar]
- 6.Bragadottir G, Redfors B, Ricksten SE. Effects of levosimendan on glomerular filtration rate, renal blood flow, and renal oxygenation after cardiac surgery with cardiopulmonary bypass: a randomized placebo-controlled study. Crit Care Med 2013;41:2328–35. 10.1097/CCM.0b013e31828e946a [DOI] [PubMed] [Google Scholar]
- 7.Dardashti A, Ederoth P, Algotsson L et al. . Erythropoietin and Protection of Renal Function in Cardiac Surgery (the EPRICS Trial). Anesthesiology 2014;121:582–90. 10.1097/ALN.0000000000000321 [DOI] [PubMed] [Google Scholar]
- 8.McGuinness SP, Parke RL, Bellomo R et al. . Sodium bicarbonate infusion to reduce cardiac surgery-associated acute kidney injury: a phase II multicenter double-blind randomized controlled trial. Crit Care Med 2013;41:1599–607. 10.1097/CCM.0b013e31828a3f99 [DOI] [PubMed] [Google Scholar]
- 9.Sisillo E, Marenzi G. N-acetylcysteine for the prevention of acute kidney injury after cardiac surgery. J Clin Pharmacol 2011;51:1603–10. 10.1177/0091270010384117 [DOI] [PubMed] [Google Scholar]
- 10.Kumar AB, Suneja M. Cardiopulmonary bypass-associated acute kidney injury. Anesthesiology 2011;114:964–70. 10.1097/ALN.0b013e318210f86a [DOI] [PubMed] [Google Scholar]
- 11.Bishopric NH, Andreka P, Slepak T et al. . Molecular mechanisms of apoptosis in the cardiac myocyte. Curr Opin Pharmacol 2001;1:141–50. 10.1016/S1471-4892(01)00032-7 [DOI] [PubMed] [Google Scholar]
- 12.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J 1999;341(Pt 2):233–49. 10.1042/bj3410233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Lisa F, Menabo R, Canton M et al. . Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem 2001;276:2571–5. 10.1074/jbc.M006825200 [DOI] [PubMed] [Google Scholar]
- 14.Hajnoczky G, Csordas G, Madesh M et al. . Control of apoptosis by IP(3) and ryanodine receptor driven calcium signals. Cell Calcium 2000;28:349–63. 10.1054/ceca.2000.0169 [DOI] [PubMed] [Google Scholar]
- 15.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc Res 2004;61:372–85. 10.1016/S0008-6363(03)00533-9 [DOI] [PubMed] [Google Scholar]
- 16.Pacher P, Csordas G, Hajnoczky G. Mitochondrial ca(2+) signaling and cardiac apoptosis. Biol Signals Recept 2001;10:200–23. doi:46888 [DOI] [PubMed] [Google Scholar]
- 17.Pacher P, Hajnoczky G. Propagation of the apoptotic signal by mitochondrial waves. EMBO J 2001;20:4107–21. 10.1093/emboj/20.15.4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernardi P, Krauskopf A, Basso E et al. . The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J 2006;273:2077–99. 10.1111/j.1742-4658.2006.05213.x [DOI] [PubMed] [Google Scholar]
- 19.Halestrap AP, Brenner C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem 2003;10:1507–25. 10.2174/0929867033457278 [DOI] [PubMed] [Google Scholar]
- 20.Baines CP, Kaiser RA, Purcell NH et al. . Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005;434:658–62. 10.1038/nature03434 [DOI] [PubMed] [Google Scholar]
- 21.Basso E, Fante L, Fowlkes J et al. . Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem 2005;280:18558–61. 10.1074/jbc.C500089200 [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa T, Shimizu S, Watanabe T et al. . Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005;434:652–8. 10.1038/nature03317 [DOI] [PubMed] [Google Scholar]
- 23.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 1988;255:357–60. [PMC free article] [PubMed] [Google Scholar]
- 24.Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J 1990;268:153–60. 10.1042/bj2680153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devalaraja-Narashimha K, Diener AM, Padanilam BJ. Cyclophilin D gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol 2009;297:F749–59. 10.1152/ajprenal.00239.2009 [DOI] [PubMed] [Google Scholar]
- 26.Gill RS, Manouchehri N, Lee TF et al. . Cyclosporine treatment improves mesenteric perfusion and attenuates necrotizing enterocolitis (NEC)-like intestinal injury in asphyxiated newborn piglets during reoxygenation. Intensive Care Med 2012;38:482–90. 10.1007/s00134-011-2436-5 [DOI] [PubMed] [Google Scholar]
- 27.Hu W, Chen Z, Ye Z et al. . Knockdown of cyclophilin D gene by RNAi protects rat from ischemia/ reperfusion-induced renal injury. Kidney Blood Press Res 2010;33:193–9. 10.1159/000316704 [DOI] [PubMed] [Google Scholar]
- 28.Oka N, Wang L, Mi W et al. . Cyclosporine A prevents apoptosis-related mitochondrial dysfunction after neonatal cardioplegic arrest. J Thorac Cardiovasc Surg 2008;135:123–30, 30 e1–2 10.1016/j.jtcvs.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 29.Okonkwo DO, Melon DE, Pellicane AJ et al. . Dose-response of cyclosporin A in attenuating traumatic axonal injury in rat. Neuroreport 2003;14:463–6. 10.1097/01.wnr.0000058958.85541.d3 [DOI] [PubMed] [Google Scholar]
- 30.Hansson MJ, Morota S, Chen L et al. . Cyclophilin D-sensitive mitochondrial permeability transition in adult human brain and liver mitochondria. J Neurotrauma 2011;28:143–53. 10.1089/neu.2010.1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morota S, Manolopoulos T, Eyjolfsson A et al. . Functional and pharmacological characteristics of permeability transition in isolated human heart mitochondria. PLoS ONE 2013;8:e67747 10.1371/journal.pone.0067747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider A, Ad N, Izhar U et al. . Protection of myocardium by cyclosporin A and insulin: in vitro simulated ischemia study in human myocardium. Ann Thorac Surg 2003;76: 1240–5. 10.1016/S0003-4975(03)00830-0 [DOI] [PubMed] [Google Scholar]
- 33.Shanmuganathan S, Hausenloy DJ, Duchen MR et al. . Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol 2005;289:H237–42. 10.1152/ajpheart.01192.2004 [DOI] [PubMed] [Google Scholar]
- 34.Cologna AJ, Lima LV, Tucci S Jr et al. . Cyclosporine action on kidneys of rats submitted to normothermic ischaemia and reperfusion. Acta Cir Bras 2008;23(Suppl 1):36–41; discussion 41 10.1590/S0102-86502008000700007 [DOI] [PubMed] [Google Scholar]
- 35.Shihab FS, Bennett WM, Andoh TF. Donor preconditioning with a calcineurin inhibitor improves outcome in rat syngeneic kidney transplantation. Transplantation 2009;87:326–9. 10.1097/TP.0b013e3181945332 [DOI] [PubMed] [Google Scholar]
- 36.Singh D, Chander V, Chopra K. Cyclosporine protects against ischemia/reperfusion injury in rat kidneys. Toxicology 2005;207:339–47. 10.1016/j.tox.2004.09.018 [DOI] [PubMed] [Google Scholar]
- 37.Yang CW, Ahn HJ, Han HJ et al. . Pharmacological preconditioning with low-dose cyclosporine or FK506 reduces subsequent ischemia/reperfusion injury in rat kidney. Transplantation 2001;72:1753–9. 10.1097/00007890-200112150-00008 [DOI] [PubMed] [Google Scholar]
- 38.Zhu T, Au-Yeung KK, Siow YL et al. . Cyclosporine A protects against apoptosis in ischaemic/reperfused rat kidneys. Clin Exp Pharmacol Physiol 2002;29:852–4. 10.1046/j.1440-1681.2002.03736.x [DOI] [PubMed] [Google Scholar]
- 39.Levey AS, Coresh J, Greene T et al. . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–54. 10.7326/0003-4819-145-4-200608150-00004 [DOI] [PubMed] [Google Scholar]
- 40.Inker LA, Schmid CH, Tighiouart H et al. . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–9. 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Servi S, Mariani M, Mariani G et al. . C-reactive protein increase in unstable coronary disease cause or effect? J Am Coll Cardiol 2005;46:1496–502. 10.1016/j.jacc.2005.05.083 [DOI] [PubMed] [Google Scholar]
- 42.Mariscalco G, Lorusso R, Dominici C et al. . Acute kidney injury: a relevant complication after cardiac surgery. Ann Thorac Surg 2011;92:1539–47. 10.1016/j.athoracsur.2011.04.123 [DOI] [PubMed] [Google Scholar]
- 43.Kashani K, Al-Khafaji A, Ardiles T et al. . Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013;17:R25 doi:10.1186/cc12503doi: 10.1186/cc12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Englberger L, Suri RM, Li Z et al. . Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care 2011;15:R16 10.1186/cc9960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piot C, Croisille P, Staat P et al. . Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 2008;359:473–81. 10.1056/NEJMoa071142 [DOI] [PubMed] [Google Scholar]
- 46.Mazzeo AT, Alves OL, Gilman CB et al. . Brain metabolic and hemodynamic effects of cyclosporin A after human severe traumatic brain injury: a microdialysis study. Acta Neurochir (Wien) 2008;150:1019–31; discussion 31 10.1007/s00701-008-0021-7 [DOI] [PubMed] [Google Scholar]
- 47.Hocherl K, Dreher F, Vitzthum H et al. . Cyclosporine A suppresses cyclooxygenase-2 expression in the rat kidney. J Am Soc Nephrol 2002;13:2427–36. 10.1097/01.ASN.0000031702.86799.B9 [DOI] [PubMed] [Google Scholar]
- 48.Lassila M. Interaction of cyclosporine A and the renin-angiotensin system; new perspectives. Curr Drug Metab 2002;3:61–71. 10.2174/1389200023337964 [DOI] [PubMed] [Google Scholar]
- 49.Textor SC, Burnett JC Jr, Romero JC et al. . Urinary endothelin and renal vasoconstriction with cyclosporine or FK506 after liver transplantation. Kidney Int 1995;47:1426–33. 10.1038/ki.1995.200 [DOI] [PubMed] [Google Scholar]
- 50.Kou R, Greif D, Michel T. Dephosphorylation of endothelial nitric-oxide synthase by vascular endothelial growth factor. Implications for the vascular responses to cyclosporin A. J Biol Chem 2002;277:29669–73. 10.1074/jbc.M204519200 [DOI] [PubMed] [Google Scholar]
- 51.Roullet JB, Xue H, McCarron DA et al. . Vascular mechanisms of cyclosporin-induced hypertension in the rat. J Clin Invest 1994;93:2244–50. 10.1172/JCI117222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klintmalm GB, Iwatsuki S, Starzl TE. Nephrotoxicity of cyclosporin A in liver and kidney transplant patients. Lancet 1981;1:470–1. 10.1016/S0140-6736(81)91851-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris PJ, French ME, Dunnill MS et al. . A controlled trial of cyclosporine in renal transplantation with conversion to azathioprine and prednisolone after three months. Transplantation 1983;36:273–7. 10.1097/00007890-198309000-00009 [DOI] [PubMed] [Google Scholar]
- 54.Issa N, Kukla A, Ibrahim HN. Calcineurin inhibitor nephrotoxicity: a review and perspective of the evidence. Am J Nephrol 2013;37:602–12. 10.1159/000351648 [DOI] [PubMed] [Google Scholar]
- 55.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 2009;4:481–508. 10.2215/CJN.04800908 [DOI] [PubMed] [Google Scholar]
- 56.Chiari P, Angoulvant D, Mewton N et al. . Cyclosporine protects the heart during aortic valve surgery. Anesthesiology 2014;121:232–8. 10.1097/ALN.0000000000000331 [DOI] [PubMed] [Google Scholar]
- 57.Hausenloy D, Kunst G, Boston-Griffiths E et al. . The effect of cyclosporin-A on peri-operative myocardial injury in adult patients undergoing coronary artery bypass graft surgery: a randomised controlled clinical trial. Heart 2014;100:544–9. 10.1136/heartjnl-2013-304845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cung TT, Morel O, Cayla G et al. . Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 2015;373:1021–31. 10.1056/NEJMoa1505489 [DOI] [PubMed] [Google Scholar]
- 59.Elahi MM, Haesey AM, Graham KC et al. . Leg wound infections following cardiac surgery: a scoring system for assessment and management. J Wound Care 2005;14:337–40. 10.12968/jowc.2005.14.7.26808 [DOI] [PubMed] [Google Scholar]
- 60.Dardashti A, Nozohoor S, Algotsson L et al. . The predictive value of s-cystatin C for mortality after coronary artery bypass surgery. J Thorac Cardiovasc Surg 2016;152:139–46. 10.1016/j.jtcvs.2016.02.070 [DOI] [PubMed] [Google Scholar]
- 61.Lee SH, Youn YN, Choo HC et al. . Cystatin C as a predictive marker of renal dysfunction and mid-term outcomes following off-pump coronary artery bypass grafting. Heart 2015;101:1562–8. 10.1136/heartjnl-2015-307986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meersch M, Schmidt C, Van Aken H et al. . Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS ONE 2014;9:e93460 10.1371/journal.pone.0093460 [DOI] [PMC free article] [PubMed] [Google Scholar]