Abstract
An adhesive yet easily removable burn wound dressing represents a breakthrough in second-degree burn wound care. Current second-degree burn wound dressings absorb wound exudate, reduce bacterial infections, and maintain a moist environment for healing, but are surgically or mechanically debrided from the wound, causing additional trauma to the newly formed tissues. We have developed an on-demand dissolvable dendritic thioester hydrogel burn dressing for second-degree burn care. The hydrogel is composed of a lysine-based dendron and a PEG-based crosslinker, which are synthesized in high yields. The hydrogel burn dressing covers the wound and acts as a barrier to bacterial infection in an in vivo second-degree burn wound model. A unique feature of the hydrogel is its capability to be dissolved on-demand, via a thiol-thioester exchange reaction, allowing for a facile burn dressing removal.
Keywords: dendrimers, dissolution, hydrogel, polymers, thiol-thioester
Graphical abstract
A hydrogel-based dressing for second-degree burn wounds has been synthesized and tested in vivo. It is composed of a dendritic macromonomer and a PEG crosslinker that form a hydrogel upon mixing. An on-demand and atraumatic hydrogel dissolution proceeds via thiol-thioester exchange reaction in presence of a cysteine methyl ester solution.
Polymeric materials used to treat second-degree burn wounds provide transient physiologic wound closure by absorbing wound exudate, preventing wound desiccation, and isolating the wound from the environment.[1] Selection of a dressing for a second-degree burn is based on healing effect, ease of application and removal, dressing change requirements, cost, and patient comfort. Various commercially available dressings such as hydrocolloids, polyurethane films, silicon coated nylons, biosynthetic skin substitutes, antimicrobial (silver and iodine) dressings, fibers, hydrogels and wound dressing pads, along with secondary adhesive dressings, are already used for the treatment of burns.[1–2] However, currently available dressings adhere to the wound surface, requiring cutting and mechanical debridement for a dressing change, leading to traumatization of newly epithelialized tissues, delayed healing, and personal suffering in the injured patient.[3] The average duration of a burn dressing change is reported to be 57.6 ± 34.4 min.[3] Burn specialists estimate that it takes three people 138 min to dress a 10–30% burn, 105 min to dress a facial burn and 66 min to change a hand dressing.[4] The need for anesthesia further increases time and complexity of the procedure – and is routinely done for pediatric patients.[5] Consequently, new approaches and/or materials that enable facile dressing change, while minimizing procedurally induced tissue damage, are needed in the clinic.
This is especially evident, given that each year, more than 300,000 people die from fire-related burn injuries and millions suffer from burn-related disabilities with significant psychological, emotional, and economic consequences on the survivors and their families.[6] Burn injuries (e.g., caused by fire, electricity, chemicals, radiation) are among the most challenging to manage: significant fluid loss and extensive tissue damage resulting from deep wounds impair multiple vital functions performed by the skin.[7] Repeated painful dressing changes and wound infection, which further increase local tissue damage, pose common complications, while systemic inflammatory and immunological responses lead to a higher predisposition to life-threatening sepsis and multi-organ failure. Immediate and effective clinical treatments are critical to improve patient outcomes and reduce burn mortality rates.
An ideal second-degree burn dressing should: 1) conform to the irregular shapes of a wound and be easily applied to areas difficult to access with conventional pre-shaped dressings, 2) possess elasticity to accommodate movement, 3) absorb wound exudate and maintain a moist environment of the wound bed, 4) lessen trauma to the wound during dressing changes, 5) be biocompatible, and 6) protect the wound from infections. Based on these criteria, we designed and synthesized an in situ gelling hydrogel-based dressing possessing thioester linkages. We hypothesized that it will seal the burn wound, act as a physical barrier to bacterial migration, and dissolve on-demand via cleavage of the thioester linkages upon addition of a cysteine methyl ester (CME) solution to the hydrogel.
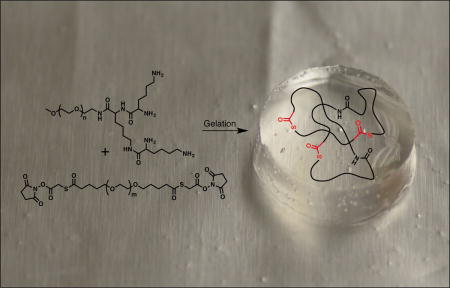
The hydrogel dressing is composed of two components: a dendron and a crosslinker (Figure 1). The use of a dendritic macromonomer enables fine control over the composition, structure and molecular weight while providing a multivalent effect. Dendritic macromonomers are finding increased uses as carriers for drugs, scaffolds for tissue engineering, and precursors for tissue adhesives.[8] We previously reported the synthesis of a dissolvable PEG-LysSH hydrogel using a thiol-terminated dendron and an N-hydroxysuccinimide (NHS) -activated poly(ethylene glycol) (PEG) -based crosslinker.[8m] In the current study, we have altered the hydrogel system to meet the additional criteria of ease of use, enhanced stability, slower hydrogel formation, and faster hydrogel dissolution. These advantages will facilitate adoption and translation of this polymeric medical device to the clinic. The previous thiol-capped dendron oxidized in air to disulfides, requiring special precautions and handling, and had a limited storage life. Once oxidized, the thiol moieties are unable to participate in the crosslinking reaction to form the hydrogel. To overcome this challenge, the thioester linkage is not formed during hydrogel gelation, but is a part of the PEG crosslinker. The lysine-based dendron used in the current hydrogel system is capped with nucleophilic amines. This shortens the dendron synthesis by two steps by bypassing the introduction and subsequent deprotection of the thiol moieties. Additionally, the rate of hydrogel formation is more controllable than the previous thiol-based system, which gelled instantaneously, and allows the matrix to easily fill the complex geometry of the burned areas.
Figure 1.
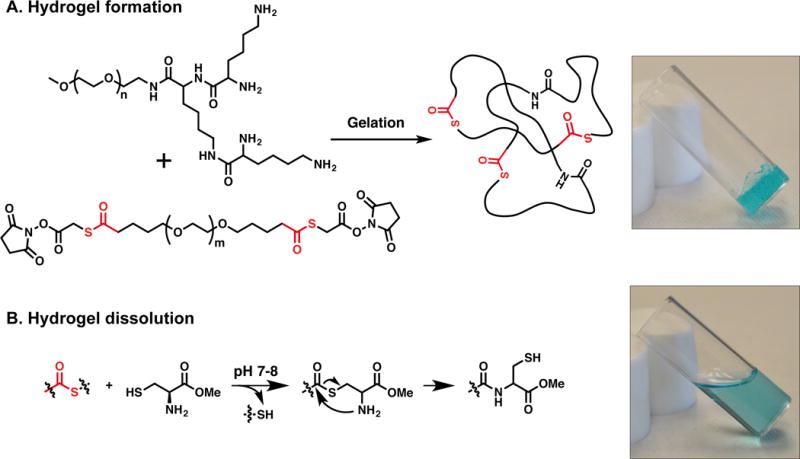
A. An idealized crosslinked hydrogel formed by the reaction between the dendron and crosslinker. B. On-demand dissolution of the hydrogel relies on a thiol-thioester exchange reaction upon exposure to a CME solution.
Specifically, the tri-lysine dendron possesses four reactive amines for rapid gelation with an electrophilic bifunctional crosslinker. For increased aqueous solubility, PEG, Mw = 2 kDa, is attached to the focal point of the dendritic structure. The dendron is synthesized following a previously reported procedure.[8m] The new crosslinker is based on PEG with two internal thioester linkages and is end-capped with NHS groups. It is synthesized by reacting SVA-PEG-SVA, Mw = 3.4 kDa, with thioglycolic acid in the presence of N,N′-diisopropylethylamine (DIPEA) to introduce two thioester moieties. Next, the macromolecule is capped with NHS groups using N,N′-dicyclohexylcarbodiimide (DCC) as the coupling agent (see SI). To prepare the hydrogel dressing, a solution of the dendron in borate buffer at pH 8.6 is mixed with a solution of the crosslinker in phosphate buffered saline (PBS) at pH 6.5. The molar ratio of amine to NHS is 1:1, and the total concentration of the polymer in solution is 40 wt%. A hydrophilic hydrogel dressing forms spontaneously within seconds upon mixing the two aqueous solutions in which the amines of the tri-lysine dendron react with the NHS-esters of the crosslinker, resulting in the formation of amide bonds, giving a crosslinked network (Figure 1). Due to its in situ gelation, the hydrogel can be easily applied as a solution to areas difficult to access with conventional pre-formed dressings and forms a gel that conforms to irregular shapes of a wound. This in situ sol-gel transition allows a complete wound coverage and contact across the burn area. Importantly, the hydrogel dressing is stable to hydrolysis for several days in PBS at pH 7.4.
In order to determine the viscoelastic properties and mechanical strength of the hydrogel, rheological studies were performed. First, the oscillatory stress sweep was evaluated in order to determine the linear viscoelastic region of the material, i.e. region where the properties observed are independent of imposed stress or strain levels. Thus, the storage (G′) and loss (G″) moduli were plotted as a function of the oscillatory stress at a constant frequency of 1 Hz (Figure S1, SI). Then, the frequency sweep was run at oscillatory stress of 50 Pa (value chosen from the linear viscoelastic region). At frequencies between 0.1 and 10 Hz, the hydrogel exhibited gel character as G′ > G″. At frequency of 1 Hz, the G′ and G″ values for the hydrogel were ~13,000 and 500 Pa, respectively (Figure S2, SI). Physically, the hydrogel exhibits elasticity, is soft to the touch and is transparent.
The ability of the hydrogel to hold water translates to its capacity to absorb wound exudate and maintain a moist environment of the wound bed. After exposure to an excess of PBS at pH 7.4, the hydrogel swells to 174% in 1 h and 650% in 18 h, reaching equilibrium, while maintaining its integrity (Figures S3 and S4, SI).
Next, the adhesive properties of the hydrogel were measured using an established lap-shear by tension loading test (American Society for Testing and Materials, F2255-05). Two portions of murine skin were adhered together using the hydrogel and upon increasing shear stress, rupture of the hydrogel occurred within the bulk of the material, rather than at the tissue-hydrogel interface. Control experiments performed with only the dendron or crosslinker solutions did not afford a seal, and the tissue portions remained separated (see SI). This suggests the hydrogel is not covalently bound to but is mechanically interlocked with the surrounding tissue.
Unlike other types of trauma, in which pain diminishes over time, the interventions, i.e. burn dressing changes necessary to prevent infection and promote healing, worsen the pain of burn injury. As documented in the literature, procedural pain is more severe than background pain and can be excruciating without adequate analgesia. Hence, our goal is to design a hydrogel dressing which dissolves on-demand, eliminating the need for mechanical and/or surgical debridement. The dissolution mechanism proceeds via thiol-thioester exchange between the thioester present in the hydrogel network and an exogenous thiolate solution.[8l, 9] Using quantitative rheological measurements (Figure 2), a time sweep was run in which the thioester-containing hydrogel was exposed to CME (0.3 M, pH 8.6). After 30 min of exposure, the hydrogel was dissolved (G′ < 200 Pa). As expected, when in contact with lysine methyl ester (LME, 0.3 M, pH 8.5) or air, the mechanical properties of the hydrogel remained unchanged and no hydrogel dissolution was observed. In addition, a control hydrogel sample was prepared with a commercially available crosslinker, SVA-PEG-SVA, Mw = 3.4 kDa, which does not possess thioester bonds, and subjected to CME (0.3 M, pH 8.6). After 1 h of exposure, the control hydrogel did not dissolve confirming that the thiol-thioester exchange is responsible for the hydrogel dissolution.
Figure 2.
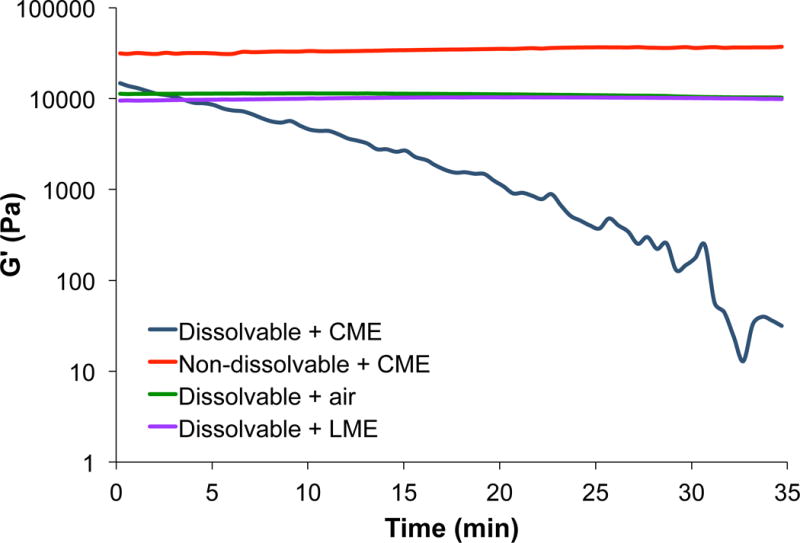
Time sweep of hydrogel dissolution after exposure of the hydrogel to CME, LME, or air.
We were also able to observe the on-demand dissolution of the hydrogel (Figure 3). The hydrogel was applied to a second-degree burn wound on a rat and left to gel for 1 h (3a), after which a CME-soaked gauze was administered to half of the hydrogel (3b). As time elapsed (3c–e), new CME-soaked gauzes were introduced until complete hydrogel dissolution occurred (30 min, (3f)). Together, these results show that the ability of the hydrogel to be dissolved on-demand provides a desirable alternative to debridement of the dressing. In the clinic, we envision the hydrogel dressing to be applied onto the second-degree burn wound without a secondary dressing to hold it in place due to its adhesive properties. In order to dissolve the hydrogel, the CME solution would be applied onto the hydrogel with irrigation and proceed as presented in Figure 3.
Figure 3.
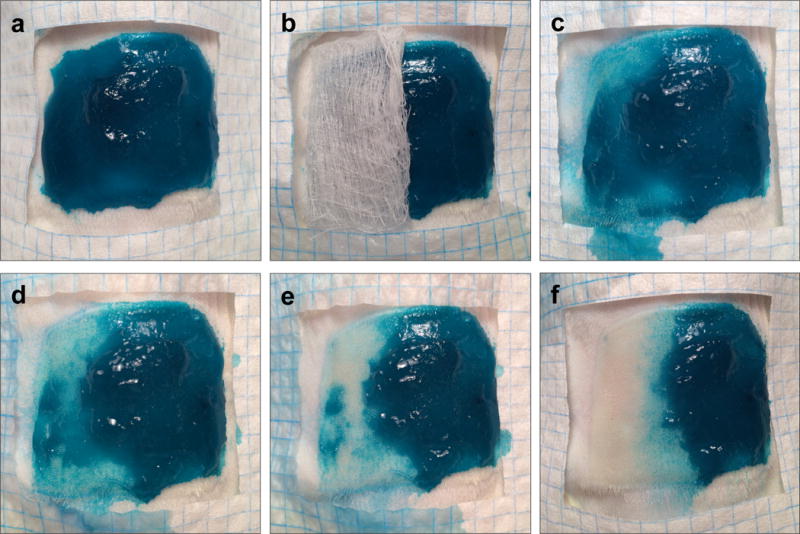
Photographs of the dissolution of the hydrogel as a function of time after treatment with an aqueous CME solution (0.3 M, pH 8.6). Fast Green FCF was added to the hydrogel for visualization.
In vitro cytotoxicity and immunogenicity of the hydrogel were evaluated to determine its biocompatibility. The viability of hydrogel-exposed NIH3T3 murine fibroblasts did not differ from untreated controls after a 24 h incubation period (91 ± 1% v. 100 ± 6%, respectively; P = 0.109). The expression of IL-6 among RAW 264.7 macrophages exposed to the hydrogel did not differ from media-only controls (6 ± 4 v. −1 ± 2 pg/mL, respectively; P = 0.20) and was significantly lower than positive controls exposed to lipopolysaccharide (LPS) (6 ± 4 v. 2610 ± 21 pg/mL, respectively; P = 3.14×10−11) (see SI). In vitro cytotoxicity of CME and CME + hydrogel dissolution products decreased viability of NIH3T3 fibroblasts to 65 ± 5% (P = 0.0010) and 72 ± 4% (P = 0.0024), respectively, as compared to the media-only control. There was no significant difference between the viability of the CME and CME + hydrogel dissolution products treatment groups. The decrease in cell viability may reflect the limitations of this in vitro assay where cysteine and its analogs, including CME, act as metal chelators, leading to cytotoxic effects. CME also affords a hypertonic shock due to high osmolarity as the commercially available hydrochloride salt. The oral LD50 (mouse) of CME is 2,300 mg/kg and intraperitoneal LD50 (mouse) is 1,340 mg/kg, as reported in the MSDS. Finally, CME itself is a pharmaceutical product and used to treat humans for symptomatic relief of cough with sputum (see SI).
A major cause of death of severely burned patients is infection. The scarcity of blood vessels in the burn wound prevents the delivery of immune cells, cytokines, and antibiotics. In addition, the presence of coagulated proteins and other microbial nutrients provide an optimal environment for bacterial growth and the development of infection. Thus, the efficacy of the hydrogel in preventing wound infection and sepsis was evaluated in an animal model of second-degree burn.[10] Animal experiments in this study were approved by the Animal Care and Use Committee (IACUC) at Beth Israel Deaconess Medical Center and Boston University. Second-degree burns, covering approximately 5% of total body surface area, were induced under general anesthesia on 30 adult female Sprague-Dawley rats. Immediately after burn induction, the animals were divided into three groups: 1) burn only (negative controls, n = 10), 2) burn + bacterial contamination (positive controls, n = 10), or 3) burn + hydrogel + bacterial contamination (hydrogel-treated group, n = 10). Bacterial contamination in the positive controls and hydrogel-treated group was achieved by covering the burn and the burn + hydrogel wounds with a gauze containing 2×108 CFU (colony forming units) of log-phase Pseudomonas aeruginosa (Strain PAO1, ATCC 47085), respectively.[11] P. aeruginosa is the most common source of burn infections.[12] The rats were euthanized 72 h later, and bacterial counts were taken from the burn wound (as a measure of local proliferation) and from the spleen (as a measure of systemic dissemination).[13]
The hydrogel prevented the occurrence of detectable local infections (defined as those with >100 CFU/g of tissue) as their prevalence did not differ from the negative controls (20 ± 17% v. 0 ± %; P = 0.29) but was significantly decreased compared to positive controls (20 ± 17% v. 100 ± 0%; P = 0.001) (Figure 4). Additionally, the total bacterial burden of the wound in the positive controls was significantly higher than in the hydrogel group and the negative controls (1.39×108 ± 8.30×107 CFU/g v. 4.04×103 ± 3.99×103 CFU/g v. 6.88×102 ± 6.38×102 respectively; P = 0.009). The hydrogel also prevented detectable systemic infections (sepsis) when compared to positive controls (0 ± 0% v. 60 ± 21%; P = 0.038). The total systemic bacterial burden in the positive controls was significantly higher than the hydrogel group and the negative controls (9×102 ± 7.76×107 CFU/g v. 5×101 ± 0 CFU/g v. 5×101 ± 0 CFU/g, respectively; P = 0.031).
Figure 4.
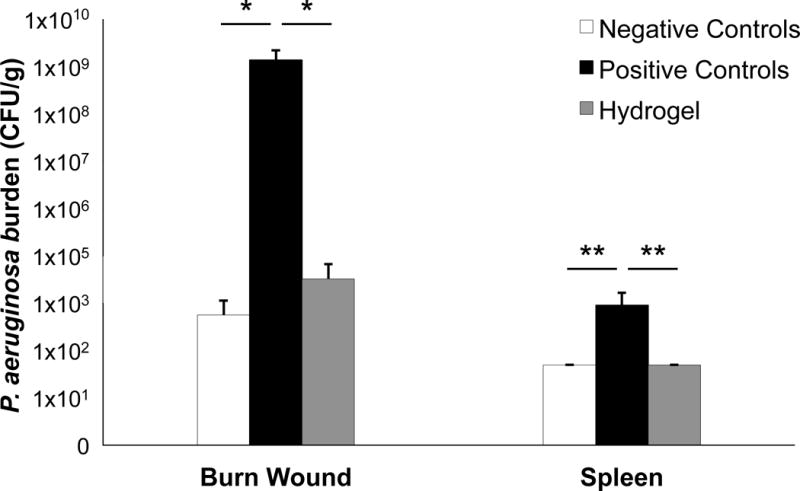
Local and systemic Pseudomonal burden by group. * P < 0.001; ** P = 0.013.
From a clinical perspective, designing a hydrogel dressing that seals the wound, prevents bacterial infection, and dissolves on-demand for atraumatic removal offers significant promise for a more effective treatment for second-degree burn patients. From a chemistry perspective, the use of a chemoselective transformation, i.e. thiol-thioester exchange, to site-specifically cleave the polymer network provides a unique approach to controlled dissolution of a material as opposed to the more mainstream activities of material formation.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support from The Wallace H. Coulter Foundation at BU and the NIH (R01EB021308). We thank Dr. Daniela Vecchio for help with the animal model. M.D.K. acknowledges Dr. John E. Sheets of Rider University, NJ, for mentorship. We thank Mr. Benjy Cooper for technical assistance with Instron.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.Wasiak J, Cleland H, Campbell F, Spinks A. Cochrane Database Syst Rev. 2013;3:CD002106. doi: 10.1002/14651858.CD002106.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Queen D, Evans JH, Gaylor JDS, Courtney JM, Reid WH. Burns. 1987;13:218–228. doi: 10.1016/0305-4179(87)90170-7. [DOI] [PubMed] [Google Scholar]; b) Madaghiele M, Demitri C, Sannino A, Ambrosio L. Burns Trauma. 2015;2:153–161. doi: 10.4103/2321-3868.143616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atchison NE, Osgood PF, Carr DB, Szyfelbein SK. Pain. 1991;47:41–45. doi: 10.1016/0304-3959(91)90009-M. [DOI] [PubMed] [Google Scholar]
- 4.Latarjet J, Choinere M. Burns. 1995;21:344–348. doi: 10.1016/0305-4179(95)00003-8. [DOI] [PubMed] [Google Scholar]
- 5.Beushausen T, Mucke K. Pediatr Surg Int. 1997;12:327–333. doi: 10.1007/BF01076931. [DOI] [PubMed] [Google Scholar]
- 6.a) Peck M, Molnar J, Swart D. Bull World Health Organ. 2009;87:802–803. doi: 10.2471/BLT.08.059733. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Judkins K. Pain Rev. 1998;5:133–146. [Google Scholar]
- 7.Gandhi M, Thomson C, Lord D, Enoch S. Int J Pediatr. 2010;2010 doi: 10.1155/2010/825657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Hawker CJ, Wooley KL. Science. 2005;309:1200–1205. doi: 10.1126/science.1109778. [DOI] [PubMed] [Google Scholar]; b) Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, Romero R, Kannan RM. Sci Transl Med. 2012;4:130ra146. doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Grayson SM, Frechet JM. Chem Rev. 2001;101:3819–3868. doi: 10.1021/cr990116h. [DOI] [PubMed] [Google Scholar]; d) Lee KY, Mooney DJ. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]; e) Wathier M, Jung PJ, Carnahan MA, Kim T, Grinstaff MW. J Am Chem Soc. 2004;126:12744–12745. doi: 10.1021/ja045870l. [DOI] [PubMed] [Google Scholar]; f) Kannan RM, Nance E, Kannan S, Tomalia DA. J Intern Med. 2014;276:579–617. doi: 10.1111/joim.12280. [DOI] [PubMed] [Google Scholar]; g) Altin H, Kosif I, Sanyal R. Macromolecules. 2010;43:3801–3808. [Google Scholar]; h) Newkome GR, Baker GR, Arai S, Saunders MJ, Russo PS, Theriot KJ, Moorefield CN, Rogers LE, Miller JE. J Am Chem Soc. 1990;112:8458–8465. [Google Scholar]; i) Carnahan MA, Middleton C, Kim J, Kim T, Grinstaff MW. J Am Chem Soc. 2002;124:5291–5293. doi: 10.1021/ja025576y. [DOI] [PubMed] [Google Scholar]; j) Degoricija L, Bansal PN, Söntjens SHM, Joshi NS, Takahashi M, Snyder B, Grinstaff MW. Biomacromolecules. 2008;9:2863–2872. doi: 10.1021/bm800658x. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Antoni P, Hed Y, Nordberg A, Nyström D, von Holst H, Hult A, Malkoch M. Angew Chem Int Edit. 2009;48:2126–2130. doi: 10.1002/anie.200804987. [DOI] [PubMed] [Google Scholar]; l) Ghobril C, Grinstaff MW. Chem Soc Rev. 2015;44:1820–1835. doi: 10.1039/c4cs00332b. [DOI] [PubMed] [Google Scholar]; m) Ghobril C, Charoen K, Rodriguez EK, Nazarian A, Grinstaff MW. Angew Chem Int Edit. 2013;52:14070–14074. doi: 10.1002/anie.201308007. [DOI] [PMC free article] [PubMed] [Google Scholar]; n) Lee C, Lo ST, Lim J, da Costa VC, Ramezani S, Oz OK, Pavan GM, Annunziata O, Sun X, Simanek EE. Mol Pharm. 2013;10:4452–4461. doi: 10.1021/mp400290u. [DOI] [PMC free article] [PubMed] [Google Scholar]; o) Mignani S, El Kazzouli S, Bousmina M, Majoral JP. Adv Drug Deliv Rev. 2013;65:1316–1330. doi: 10.1016/j.addr.2013.01.001. [DOI] [PubMed] [Google Scholar]; p) Mintzer MA, Grinstaff MW. Chem Soc Rev. 2011;40:173–190. doi: 10.1039/b901839p. [DOI] [PubMed] [Google Scholar]
- 9.Johnson ECB, Kent SBH. J Am Chem Soc. 2006;128:6640–6646. doi: 10.1021/ja058344i. [DOI] [PubMed] [Google Scholar]
- 10.Borman H, Maral T, Demirhan B, Haberal M. Ann Plas Surg. 1999;43:513–518. doi: 10.1097/00000637-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Steinstraesser L, Burkhard O, Fan MH, Jacobsen F, Lehnhardt M, Su G, Daigeler A, Steinau HU, Remick D, Wang SC. BMC Surg. 2005;5:19. doi: 10.1186/1471-2482-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Clin Microbiol Rev. 2006;19:403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnea Y, Carmeli Y, Kuzmenko B, Gur E, Hammer-Munz O, Navon-Venezia S. Ann Plas Surg. 2006;56:674–679. doi: 10.1097/01.sap.0000203984.62284.7a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


