Abstract
Pancreatic cancer, the fourth leading cause of cancer death in the United States, has a negative prognosis because metastasis occurs before symptoms manifest. Leiodermatolide, a polyketide macrolide with antimitotic activity isolated from a deep water sponge of the genus Leiodermatium, exhibits potent and selective cytotoxicity towards the pancreatic cancer cell lines AsPC-1, PANC-1, BxPC-3, and MIA PaCa-2, and potent cytotoxicity against skin, breast and colon cancer cell lines. Induction of apoptosis by leiodermatolide was confirmed in the AsPC-1, BxPC-3 and MIA PaCa-2 cells. Leiodermatolide induces cell cycle arrest but has no effects on in vitro polymerization or depolymerization of tubulin alone, while it enhances polymerization of tubulin containing microtubule associated proteins (MAPs). Observations through confocal microscopy show that leiodermatolide, at low concentrations, causes minimal effects on polymerization or depolymerization of the microtubule network in interphase cells, but disruption of spindle formation in mitotic cells. At higher concentrations, depolymerization of the microtubule network is observed. Visualization of the growing microtubule in HeLa cells expressing GFP-tagged plus end binding protein EB-1 showed that leiodermatolide stopped the polymerization of tubulin. These results suggest that leiodermatolide may affect tubulin dynamics without directly interacting with tubulin and hint at a unique mechanism of action. In a mouse model of metastatic pancreatic cancer, leiodermatolide exhibited significant tumor reduction when compared to gemcitabine and controls. The anti-tumor activities of leiodermatolide, as well as the proven utility of anti-mitotic compounds against cancer, make leiodermatolide an interesting compound with potential chemotherapeutic effects that may merit further research.
Keywords: Marine Natural Products, Pancreatic Cancer, Microtubule Associated Proteins, Tubulin dynamics
Introduction
Every 15 minutes, one person is diagnosed with pancreatic cancer in North America1. Of those, only 28% of patients will survive one year post diagnosis and only 7% will survive 5 years after diagnosis2. Pancreatic cancer is an extremely aggressive disease that ranks 4th in the US for cancer-induced deaths2. Current treatments do little to prolong life or ameliorate symptoms and there is an urgent need for new treatments2.
Anti-mitotic agents of natural origin have shown useful anti-cancer properties3, 4. The vinca alkaloids (such as vincristine) and the taxanes (such as paclitaxel) are currently used to treat many types of cancer5. These compounds induce cell cycle arrest5 by affecting the microtubules required for spindle formation and chromosome segregation4. While these compounds are clinically effective, the development of drug resistance creates the need for the discovery and development of other tubulin interacting compounds. The tubulin depolymerizing agents combretastatin A-4 and its synthetic analogues show promise for both their anti-tumor and anti-vascular properties6. The prodrug has recently entered clinical trials6. Other compounds such as 2-methoxyestradiol-bis-sulfamate (STX140), a tubulin depolymerizing agent, show in vitro and in vivo efficacy against tumors while presenting reduced toxicity compared to current taxane compounds7. 2-methoxyestradiol and many of its analogues are currently in clinical trials for solid tumors, breast cancer, ovarian cancer, and refractory prostate cancers8.
Although historically tubulin interacting compounds have not been used to treat pancreatic cancer, many tubulin interacting compounds are currently in clinical trials for pancreatic cancer. Albumin-bound paclitaxel (nab-paclitaxel Abraxane®) has recently been shown to improve survival in metastatic pancreatic cancer when given in combination with gemcitabine and was approved for use against pancreatic cancer by the FDA9. Ixabepilone, a tubulin stabilizing compound that binds at a site different than paclitaxel, was in a phase II trial both as a single agent and in combination with the epidermal growth factor blocker cetuximab. While its use in combination therapy failed to show improvement10, as a single agent it showed greater efficacy11. Future trials might define whether ixabepilone has a future as a treatment of pancreatic cancer. Eribulin, a synthetic macrocyclic ketone analogue of the marine sponge natural product halichondrin B, is a tubulin depolymerizing agent recently approved by the FDA to be used in recurrent metastatic breast cancer and has progressed to at least phase II trials for pancreatic cancer12. Leiodermatolide is a polyketide derived macrolide isolated from a deep-water lithistid sponge of the genus Leiodermatium13. It was originally purified following activity in the phospho-nucleolin assay14 which detects compounds with anti-mitotic activity. This paper describes the effects of leiodermatolide on pancreatic cancer cells as well as initial studies towards understanding its mechanism of action.
Materials and Methods
Reagents
Leiodermatolide was obtained from the Harbor Branch Oceanographic Institute pure compound library. The material was isolated from a sponge of the genus Leiodermatium as described previously13. The leiodermatolide stock solution was at a concentration of 1 mg/mL in methanol. Methanol and isopropanol used in the experiments were purchased from Fisher Scientific, Fair Lawn, NJ. The 3-[4,5-Dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide (MTT) used for cell viability assays was purchased from Sigma Chemical Co., St. Louis, MO.
Cell Culture
The human pancreatic cancer cell lines PANC-1 (CRL-1469), AsPC-1 (CRL-1682), BxPC-3 (CRL-1687) and MIA PaCa-2 (CRL-1420) cell lines were obtained from ATCC, grown, aliquotted and maintained in liquid nitrogen. Aliquots of AsPC-1, PANC-1, and BxPC-3 were thawed and grown in RPMI-1640 supplemented with 10% Fetal Bovine Serum, 0.11 mg/mL Sodium Pyruvate, 4.5 g/L D-glucose, 18 mM HEPES Buffer, 100 U/mL penicillin G sodium, 100 μg/mL streptomycin sulfate, 0.25 μg/mL amphotericin B, 2 mM L-glutamine and 50 μg/mL gentamicin (Complete RPMI). The MIA PaCa-2 cell line was grown in RPMI-1640 supplemented with 10% Fetal Bovine Serum, 2.5% horse sera, 100 U/mL penicillin G sodium, 100 μg/mL streptomycin sulfate, 0.25 μg/mL amphotericin B, and 50 μg/mL gentamicin. The MDA-MB-435 (HBT-129) metastatic melanoma, HT-29 (HTB-39) colon cancer, and U-937 (CRL-1593.2) lymphoma cell lines were obtained from ATCC. The SF295 glioblastoma cell line was obtained from DCTD Tumor Repository (Fredrick, MD) and the U251 glioblastoma cell line was obtained from Sigma Aldrich (St. Louis, MO). All these cell lines were cultured in media recommended by suppliers. Cells were maintained in a humidified incubator at 37°C and 5% CO2. Cells were kept in culture for 10 weeks (20 passages) when a new aliquot was thawed.
Cell Cycle Analysis
PANC-1 cells were trypsinized and split into a 6-well plate where they were allowed to adhere for 24 hours. Cells were then treated for 48 hours with 10 or 100 nM leiodermatolide, the vehicle control, media alone, or 100 nM paclitaxel as a positive control. At the end of treatment, cells were trypsinized, pelleted, fixed with ice cold ethanol and incubated at −20°C for an hour. Cells were washed and stained with propidium iodide in the presence of RNAse A for 30 minutes at 37°C. Cells were transferred to tubes or plates and analyzed in a BDFACSCanto flow cytometer acquiring 10,000 events. Cell cycle analysis was performed using ModFit Software.
Cell Viability Assay (MTT)
6,000 cells were plated per well in a 96-well tissue culture plate and allowed to adhere for 24 hours. At the end of this incubation, 100 μL of medium was removed from each test well and 100 μL of medium containing treatment was added. Treatment consisted of a range of concentrations from 0.00013 to 5 μg/mL of leiodermatolide or media with methanol. The cells were then incubated for 72 hours at 37°C and 5% CO2. After this incubation, 75 μL of a 5 mg/mL MTT solution was added to each well. The cells were then incubated for 3 hours at 37°C. The plates were centrifuged for 10 minutes at 800 rpm. The supernatant was removed and 200 μL acidified isopropyl alcohol (1:500 solution of hydrochloric acid to isopropanol) was added to each well. The plates were shaken for 15 minutes. The absorbencies of these solutions were measured at 570 nm with a plate reader (NOVOstar, BMG Labtech Inc., Durham, NC). The resulting absorbencies were normalized against methanol treated cells using Microsoft Excel. Determination of the dose at which 50% inhibition is found (IC50) was done using a non-linear regression curve fit with GraphPad Prism 5 software. For wash out experiments, only the PANC-1 cell line was used. The protocol above was followed using leiodermatolide at 10, 25 and 100 nM concentrations. Treatments were removed at 0.5, 1, 2, 4, 6, 12, 18, 24 and 48 hours by centrifugation of the plate, suctioning of the media containing treatment and replacing it with pre-warmed fresh media. Paclitaxel was used as a control at the concentration it will induce 50% cytotoxicity in this cell line (IC50 5 nM). Cytotoxicity was measured at the standard 72 hours using the protocol above.
Caspase 3/7 Cleavage
To determine induction of apoptosis, cleavage of caspase 3/7 was measured using a commercially available kit (Promega Caspase-Glo, G0890; Madison, WI) following the manufacturer’s protocol. Briefly, 10,000 cells were plated in a 96-well flat bottom white plate. Cells were allowed to adhere overnight then treated with 10 nM leiodermatolide or its vehicle control for 1, 3, 6 or 24 hours. At the end of treatment an equal volume of pro-luminescent caspase 3/7 substrate was added to the wells and incubated at room temperature with mild shaking for 30–60 minutes. In cells with active caspase 3/7, the tetrapeptide sequence DEVD gets cleaved to release aminoluciferin, and the resulting luminescence was read with a plate reader (NOVOstar, BMG Labtech Inc., Durham, NC). Luminescence results are presented as fold induction compared to the vehicle control treated sample.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
To determine induction of apoptosis, fragmented DNA was measured by incorporating fluorescein 12-dUTP at 3′-OH DNA ends using the terminal deoxynucleotidyl transferase enzyme found in a commercially available kit (Promega G3250; Madison, WI) following the manufacturer’s protocol. Briefly, 500,000 cells were plated in a 6-well plate. Cells were allowed to adhere overnight then treated with 10 nM leiodermatolide or its vehicle control for 24 hours. At the end of treatment, cells were trypsinized, fixed with 4% paraformaldehyde and permeabilized with ice cold ethanol. Cells were transferred to a 96 well U-bottom plate, equilibrated for five minutes and then treated with 25 μL of a reaction mix containing the nucleotide mix and the enzyme to label the fragmented DNA for 60 minutes at 37°C. The reaction was stopped by the addition of 20 mM EDTA. The resulting fluorescence was visualized via flow cytometry in a BD FACSCanto equipped with a plate adapter, acquiring 20,000 events.
Confocal Microscopy
70,000 PANC-1 cells were plated on a 6 well plate with a coverslip placed on the bottom, allowed to adhere overnight and then treated with either 10, 100 or 1000 nM leiodermatolide, 100 nM vinblastine, 100 nM nocodazole or their respective vehicle controls for 24 hours. Cells were labeled with antibodies against α tubulin (Sigma Aldrich, St. Louis, MO), phosphorylated Histone H3 (Cell Signaling Technology, Danvers, MA) or counterstained with propidium iodide to label the nuclei. After staining, the cover slip was dried and placed on a slide with 1 drop slowfade (Cat #536936, Invitrogen, Carlsbad, CA) and sealed with clear nail polish. Images were captured using an Olympus confocal microscope FVX (FV-200) system equipped with the Fluoview software.
Microtubule Dynamics Assay
HeLa cells stably expressing EB1-GFP 15, 16 were seeded in 35 mm glass-based dishes, allowed to adhere overnight, then changed to imaging medium and allowed to acclimate for 4 hours prior to initiating time lapse photography. At the 5 minute mark, treatments were added to the cells. Treatments consisted of 1, 10 or 30 times the IC50 for leiodermatolide (IC50 2.66 nM), 30× IC50 vincristine (IC50 4.26 nM) or DMSO. Treatments were prepared in conditioned imaging medium pre-warmed to 37°C obtained prior to initiating time lapse photography. Images of the cells were collected with Olympus IX81 (UPlanSApo 60x/1.35 oil immersion objective) every 15 seconds for ten minutes.
In Vitro tubulin polymerization Assay
Purified bovine tubulin (TL238) and bovine tubulin containing microtubule associated proteins (MAPS; ML113) were obtained from Cytoskeleton (Denver, CO) and the polymerization reaction was done according to their methods. Briefly, 99% pure tubulin or tubulin containing MAPS (70% tubulin, 30% MAPS) was diluted to a 3 mg/mL concentration in buffer containing 80 mM Pipes, 2 mM MgCl2, 0.5 mM EGTA, 1 mM GTP and 3.75 % glycerol and kept on ice. 1, 10, 50 or 100 μM leiodermatolide in methanol, 10 μM paclitaxel in DMSO, 10 μM nocodazole in DMSO, 10 μM vincristine in DMSO, vehicle controls methanol or DMSO or buffer alone were plated at a final volume of 10 microliters in a half-area clear 96 well plate. Compound solutions were warmed to 37°C in an incubator for 5–10 minutes prior to treatment. The plate reader (NOVOstar, BMG Labtech Inc., Durham, NC) was also set to 37°C. Once the temperature was reached, 90 μL tubulin (equal to a final concentration of 27 μM tubulin for tubulin alone and 19 μM tubulin in the tubulin with MAPS assay) was plated into the treatment wells simultaneously using a multipipetter. Absorbance at 340 nm was recorded every minute for one hour. The absorbance of buffer alone was subtracted from the results and the corrected values were plotted using Microsoft Excel.
Differential Gene Expression Analysis
MDA-MB-435 cells were treated with leiodermatolide for 12hr at 30× IC50 (IC50 of leiodermatolide in MDA-MB-435 is 1.9 nM) or its vehicle control. Total RNA was extracted and subjected to microarray analysis using Affymetrix HU133A 2.0 chips. Data was analyzed using the Broad Institute Connectivity Map Resource.
Orthotopic Xenograft Mouse Model for Pancreatic Tumors
Nude mice were injected with 1 million L3.6pl cells into the pancreas to induce tumors. Seven days later when tumors were well established, 10 mice were treated with 10 mg/kg leiodermatolide one time per week for three weeks. 10 mice were treated with 100 mg/kg gemcitabine two times per week for 5 weeks, and 10 mice were treated with saline to serve as controls. All treatments were delivered by intraperitoneal injection. Body weight was measured on the day of treatment. Tumor weight was determined on day of termination. Experiment was conducted for 9 weeks. For this experiment, leiodermatolide was dried and reconstituted at 10 mg/mL in DMSO, and then diluted 1:4 in saline prior to injection. The maximum tolerated dose (MTD) for leiodermatolide is not known.
Statistics
Statistical analysis of the data sets to determine mean, standard deviation, and standard error of the mean was performed using Microsoft Excel. Data sets were compared using the Student’s T Test. A p value ≤ 0.05 was considered significant. Outliers were detected through the Grubbs Test.
Results
In an effort to find small molecule inhibitors of mitosis in the HBOI library of marine natural products, the HBOI extract library was screened in a cytoblot assay using antibodies against phosphorylated nucleolin, as described previously14. This screen identified a crude extract of the sponge Leiodermatium with potent activity in the cytoblot assay (849 fold more active than control at 10 μg/mL and 218 fold more active than control at 0.16 μg/mL) which also had potent cytotoxicity against the PANC-1 tumor cell line. Bioassay-guided fractionation using both assays led to the isolation of leiodermatolide (Figure 1a). This initial isolation was described in patent application PCT/US2007/01750017 and the structure elucidation is the subject of a recent publication13.
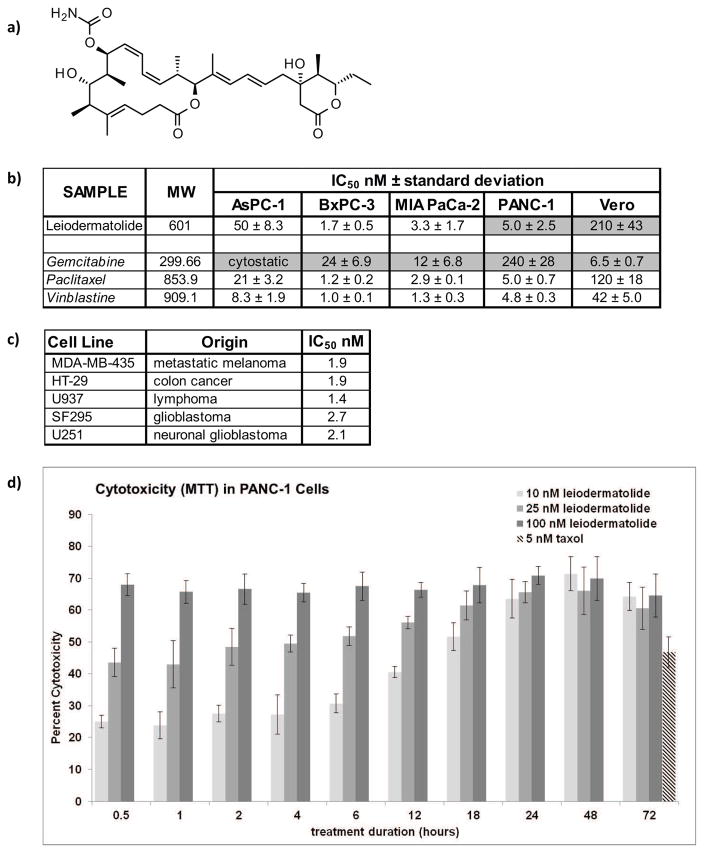
Figure 1. Structure of Leiodermatolide and Induction of Cytotoxicity in Cancer Cells.
a) Structure of Leiodermatolide. b) Four different pancreatic cancer cell lines were treated for 72 hours in the presence of vehicle controls, media alone, and a range of concentrations of leiodermatolide, paclitaxel and vinblastine. Changes in color due to metabolization of MTT by live cells were measured, normalized against vehicle control and the IC50 determined using a non-linear regression curve fit. The resulting average of 3 experiments ± standard deviation is shown. The IC50 of leiodermatolide for the PANC-1 and Vero cell lines13, and the IC50 for Gemcitabine31 in these pancreatic cancer cell lines (shaded) have been previously reported and are provided here for ease of comparison. Leiodermatolide caused cytotoxicity to pancreatic cancer cells in low nanomolar concentrations. A much higher concentration was needed to cause similar cytotoxicity in the non-carcinogenic Vero cell line. Leiodermatolide exhibits similar potency to paclitaxel and vincristine with better selectivity, and more potency and selectivity than gemcitabine. c) Leiodermatolide induces potent cytotoxicity in other cancer cell lines of different origin. d) Washout experiments were conducted using 10, 25, 100 nM leiodermatolide in PANC-1 cells. Treatments were removed by aspiration at the times listed and replaced with pre-warmed media. Cytotoxicity was measured at 72 hours. Paclitaxel was used as a control at its IC50 as a positive control. Only treatments with 10 nM leiodermatolide were reversible if the compound was removed within 12 hours. The average of 3 experiments ± standard deviation is shown.
The cytotoxicity of leiodermatolide was determined in four pancreatic cancer cell lines using an MTT assay for 72 hours. The results are shown in Figure 1b. Leiodermatolide induces cytotoxicity in pancreatic cancer cells at very low nanomolar concentrations. As expected, the aggressive AsPC-1 cell line required the highest concentration. However, all concentrations needed to induce cytotoxicity in pancreatic cancer cells were much lower than concentrations needed to induce cytotoxicity in the non-carcinogenic monkey kidney Vero cell line. Leiodermatolide exhibits similar potency to paclitaxel and vincristine, and more potency and selectivity than gemcitabine. Studies done in cell lines of different origin (colon cancer, melanoma, lymphoma and glioblastoma) showed that leiodermatolide also induced potent cytotoxicity against these cell lines (Figure 1c). A recent publication has further expanded the cytotoxic effects of leiodermatolide to leukemia and gastric, breast, and colon cancer cell lines18. This publication also reported the preparation and assay of a series of synthetic analogues but the natural compound remained the most potent18.
To ascertain the reversibility of the cytotoxic activity, cytotoxicity was evaluated in PANC-1 cells after short exposures to leiodermatolide followed by wash-out. As shown in Figure 1d, exposure to 100 nM leiodermatolide for as little as half an hour was sufficient to induce cytotoxicity in this cell line. While the cytotoxicity was not at its maximum, a similar effect was observed when the cells were exposed to 25 nM leiodermatolide. At 10 nM leiodermatolide cytotoxicity was avoided if leiodermatolide was removed within 12 hours of exposure. This concentration, however, was sufficient to induce maximum cytotoxicity at the standard 72 hours if the exposure to treatment lasted at least 24 hours.
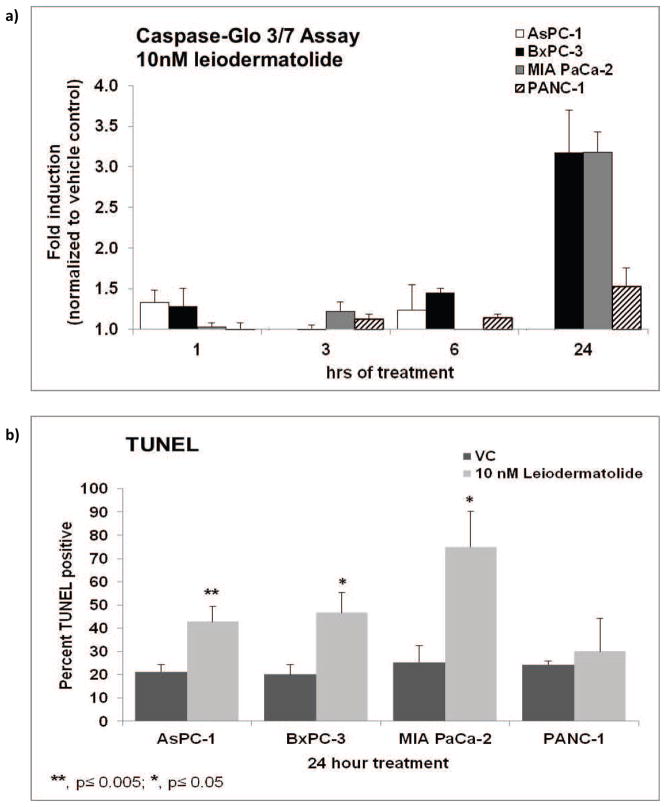
While leiodermatolide clearly induces potent cytotoxicity against cancer cells, it was of importance to ascertain if the observed cytotoxicity happened through apoptosis. Four pancreatic cancer cell lines were used for these studies. Significant cleavage of caspase 3 was seen in the BxPC-3 and MiaPaCa-2 cells treated with 10 nM leiodermatolide for 24 hours (Figure 2a). No significant caspase cleavage was observed in AsPC-1 cells, but this could be because these cells have strong levels of basal cleaved caspase 319 and the concentration used was one fifth the IC50 of leiodermatolide for this cell line. While an increase in caspase 3 cleavage was seen in PANC-1 cells between 6 and 24 hours of treatment, it was very modest. By following DNA fragmentation through TUNEL, apoptosis was detected in the AsPC-1, BxPC-3 and MiaPaCa-2 cells treated with 10 nM leiodermatolide for 24 hours (Figure 2b). No significant DNA fragmentation was seen as a result of treatment with leiodermatolide in the PANC-1 cells. It is possible that either a different exposure time is required to visualize leiodermatolide induced apoptosis in PANC-1 cells or that an alternative pathway for programmed cell death occurs in this cell line after treatment with leiodermatolide. Nevertheless, taken together the results of caspase 3 cleavage and TUNEL show that leiodermatolide induces apoptosis in pancreatic cancer cells.
Figure 2. Leiodermatolide Induces Apoptosis in Pancreatic Cancer Cells.
a) The pancreatic cancer cell lines AsPC-1, PANC-1, BxPC3 and MIA PaCa2 were treated with 10 nM leiodermatolide for 1, 3, 6, and 24 hours followed by measurement of caspase 3 cleavage with the CaspaseGlo assay. Cleavage of caspase 3 was significant in the BxPC-3 and MIA PaCa2 cells after 24 hours of treatment with 10 nM leiodermatolide. The average of 3 experiments ± standard error of the mean is shown. b) The pancreatic cancer cell lines AsPC-1, PANC-1, BxPC3 and MIA PaCa2 were treated with 10 nM leiodermatolide for 24hours. Induction of apoptosis was determined by monitoring DNA fragmentation (TUNEL). Treatment with leiodermatolide induced DNA fragmentation in the AsPC-1, BxPC3 and MIA PaCa2 cells. The average of 3 experiments ± standard deviation is shown.
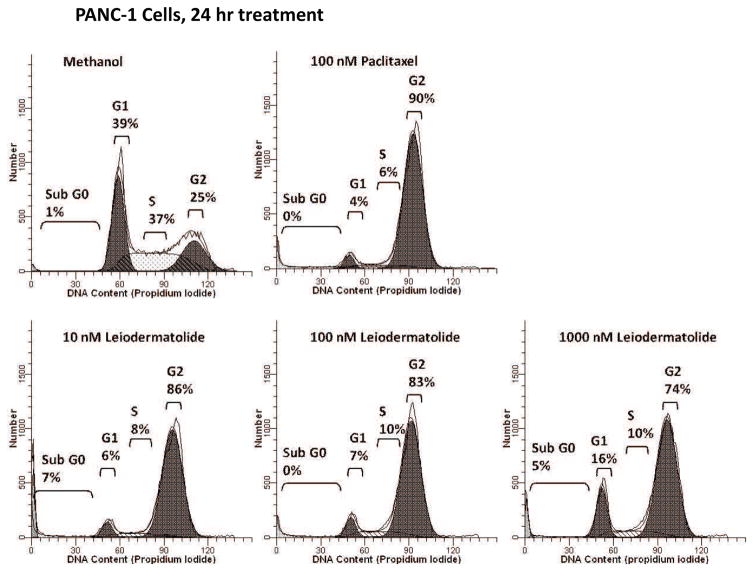
To confirm the results of the phosphorylated nucleolin screening, cell cycle analysis was done on PANC-1 cells treated with different concentrations of leiodermatolide. At concentrations of 10, 100 or 1000 nM, Leiodermatolide induced a strong G2/M block after 24 hours of treatment in PANC-1 cells (Figure 3), similar to that induced by the positive control paclitaxel. Similar to the cytotoxicity results, cell cycle arrest at G2/M was observed in PANC-1 cells treated with 100 nM leiodermatolide even if the compound was washed out within half an hour of treatment. With 10 nM leiodermatolide treatment, G2/M arrest could be reversed if the treatment was removed within 18 hours of treatment (Data not shown). However, 10 nM leiodermatolide was sufficient to induce G2/M arrest in PANC-1 cells at 24 hours of treatment. Leiodermatolide also induces G2/M cell cycle arrest in other cell lines such as the lung carcinoma A54913, 17 and the lymphoma line U937 (data not shown).
Figure 3. Leiodermatolide Induces Cell Cycle Arrest in PANC-1 Cells.
PANC-1 cells were treated for 24 hours in the presence of methanol, 10, 100, 1000 nM leiodermatolide or 100 nM paclitaxel and subjected to cell cycle analysis by flow cytometry. Experiment was repeated 3 times and one representative experiment is shown. At all concentrations tested, leiodermatolide caused a G2/M cell cycle arrest similar to that caused by paclitaxel.
Many anti-mitotic compounds function by affecting cytoskeletal organization, either by affecting the microtubules required for spindle formation and chromosome segregation or by affecting actin which is necessary for cytokinesis4. Therefore the effects of leiodermatolide on both actin and tubulin were investigated. Imaging of cells labeled with actin antibody after leiodermatolide treatment did not show any differences from cells treated with controls (Data not shown). The effects of leiodermatolide on the microtubule network at 10 nM were observed by confocal microscopy. As previously reported, while leiodermatolide markedly disrupted spindle formation, no effects on the microtubule structure indicative of either tubulin polymerization or depolymerization were observed13.
These unexpected results led to the study of the differential gene expression caused by leiodermatolide in MDA-MB-435 cells. This cell line was chosen for ease of comparison with data from other compounds already available at the Broad Institute. Cells were treated with leiodermatolide for 12hr at 30× IC50 (IC50 of leiodermatolide in MDA-MB-435 cells is 1.9 nM). Total RNA was extracted and subjected to microarray analysis using Affymetrix HU133A 2.0 chips. Leiodermatolide caused significant changes in 98 genes (more than 2 fold change with P value less than 0.05). The list of the differential gene expression is included as supplementary data. The gene expression pattern was analyzed using the Broad Institute Connectivity Map Resource that matches the effects of small molecules on gene expression with known compounds that share a similar mechanism of action20, 21. The connectivity map showed that leiodermatolide matched to four known tubulin interacting compounds: celastrol, nocodazole, paclitaxel and demecolcine (Supplemental Table 1). Further analysis of the genes using the Molecular Signals Database sets of biological processes with the Gene Set Enrichment Analysis software of the Broad Institute22 showed that the genes differentially expressed by leiodermatolide overlap with gene sets of microtubule functions, mitotic arrest and apoptosis (Supplemental Figure 1).
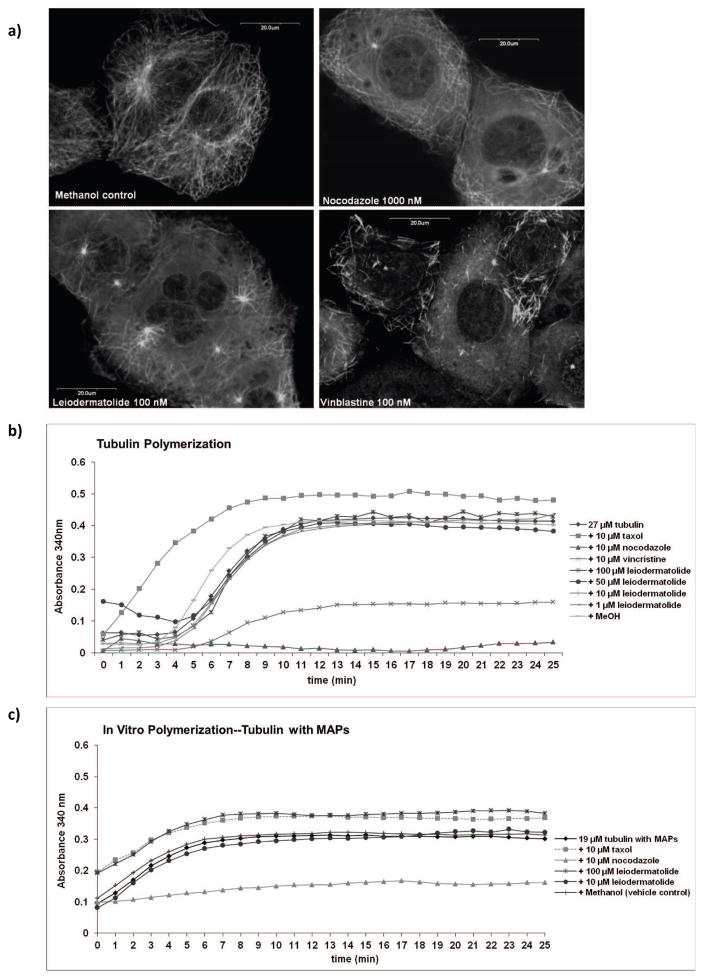
These results prompted the renewal of efforts to understand the effects of leiodermatolide on tubulin. Thus, to expand the studies done, confocal microscopy of tubulin staining was done on PANC-1 cells treated with 100 nM leiodermatolide (20× IC50). As shown in Figure 4a, leiodermatolide did not cause tubulin polymerization. At this higher concentration, the effects of leiodermatolide on tubulin resemble those caused by high concentrations of depolymerizing agents such as nocodazole and vinblastine.
Figure 4. Effects of Leiodermatolide on Polymerization/Depolymerization of Tubulin.
a) PANC-1 cells were treated for 24 hours with methanol (vehicle control), 100 nM leiodermatolide, 100 nM vinblastine or 1000 nM nocodazole. Cells were fixed and stained with an antibody against β-tubulin and counterstained with propidium iodide and subjected to confocal microscopy. Experiment was repeated 3 times and one representative experiment is shown. Leiodermatolide appears to induce depolymerization at this high 100 nM concentration. b) Purified bovine tubulin and c) bovine tubulin containing microtubule associated proteins (MAPS) were induced to polymerize by warming the tubulin to 37°C in the presence of 1, 10, 50 or 100 μM Leiodermatolide, 10 μM paclitaxel, 10 μM nocodazole, 10 μM vincristine, methanol or DMSO (vehicle controls) or buffer alone. Polymerization was enhanced by the presence of glycerol in the buffer. Polymerization was measured by recording turbidity every minute for 1 hour at 340 nm with a plate reader. Experiment was repeated 3 times and one representative experiment is shown. Leiodermatolide had no effect on tubulin polymerization or depolymerization on tubulin alone at any of the concentrations tested. Leiodermatolide had no effect on tubulin polymerization or depolymerization on tubulin with MAPS at 10 μM, but appeared to enhance polymerization at 100 μM when MAPS are present.
To determine if higher concentrations of leiodermatolide affected tubulin assembly, an in vitro tubulin polymerization assay was performed. Purified bovine tubulin was added to different known tubulin interacting agents as well as to different concentrations of leiodermatolide and heated to 37°C to induce polymerization of tubulin. As shown in Figure 4b, tubulin by itself polymerizes at the concentration used when warmed to 37°C except in the presence of nocodazole and vinblastine, which are known tubulin depolymerizering agents. Paclitaxel, a known polymerizing and hyperstabilizing agent, enhanced the polymerization. Leiodermatolide in concentrations of 1, 10, 50 and 100 μM, did not cause any changes in the polymerization of tubulin alone. The use of tubulin containing MAPS in similar experiments showed that at 10 μM leiodermatolide had no effect on tubulin polymerization, while at 100 μM leiodermatolide appears to facilitate polymerization (Figure 4c).
While the results obtained through confocal microscopy and the in vitro tubulin polymerization assay in the presence of MAPS conflicted regarding whether leiodermatolide induces polymerization or depolymerization of tubulin, they indicated an effect on tubulin. Moreover, the discrepancy suggested that leiodermatolide might affect microtubule dynamics rather than tubulin itself. Studies were conducted to determine if leiodermatolide binds directly to tubulin, but the results have been inconclusive (Data not shown). If leiodermatolide binds tubulin, it does it at a different stoichiometry than the observed 1:1 for compounds such as paclitaxel23. A recent publication has confirmed our observation that leiodermatolide does not directly bind tubulin18.
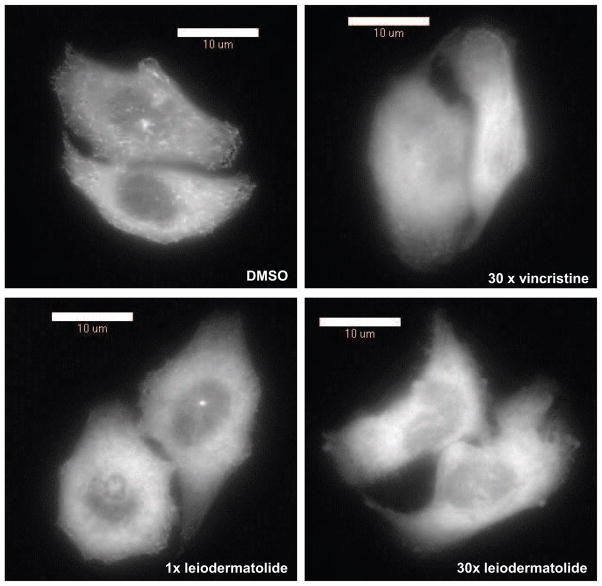
To confirm that leiodermatolide affects tubulin dynamics, time lapse photography was taken of HeLa cells with stably expressed GFP- End Binding Protein 1 (HeLa GFP-EB1) every 15 seconds for ten minutes to observe movement of the EB1-GFP comets as the GFP-tagged EB1 binds to the plus ends of the growing microtubules. Treatment with leiodermatolide, vincristine or respective controls was done at the halfway point. As seen in Figure 5 (movies available as supplementary data) leiodermatolide inhibited microtubule elongation in these cells. The inhibition caused by leiodermatolide occurred nearly instantaneously with treatment versus a slower response in the tubulin depolymerizing agent vincristine, which was used as a positive control. The effect of leiodermatolide on microtubule elongation was also confirmed in the sea urchin embryo assay where leiodermatolide causes spinning of the embryos as expected from a compound that affects tubulin polymerization24 (movie available as supplementary data).
Figure 5. Leiodermatolide Affects Tubulin Dynamics.
Time lapse photography of HeLa EB1-GFP cells was taken every 15 seconds for ten minutes to observe the addition of the GFP-tagged EB1 microtubule associated protein to the plus ends of the microtubules. At five minutes, treatments were added and photography continued. Treatments consisted of 1×, 10× or 30× the IC50 for leiodermatolide, 30× IC50 vincristine or DMSO. Treatment with leiodermatolide immediately caused cessation of GFP-EB-1 comets similar to, but faster than, that observed for the depolymerizing agent vincristine.
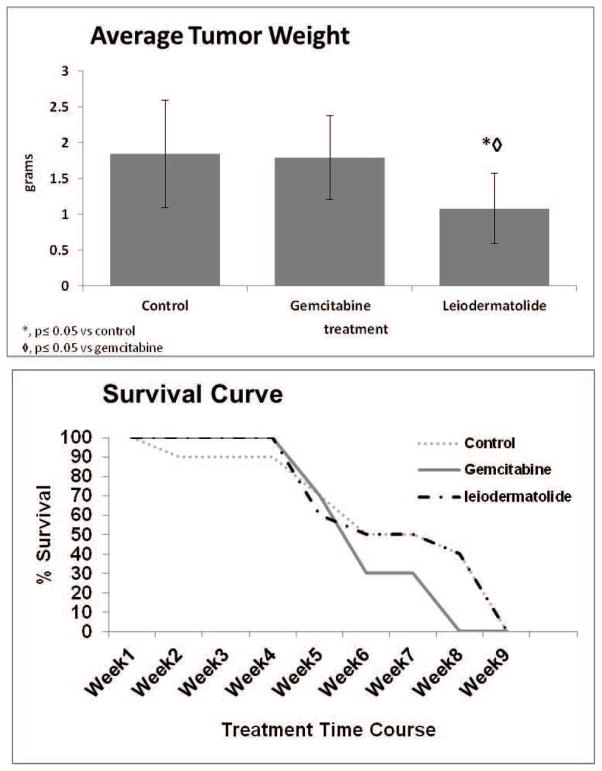
While the exact mechanism of action of leiodermatolide remains elusive, the data suggests that leiodermatolide interacts with tubulin in a novel way compared to other anti-mitotic natural products. This novel mechanism, as well as the potency exhibited in vitro, led to study the effects of leiodermatolide in vivo. As shown in Figure 6, leiodermatolide treatment resulted in a statistically significant reduction in tumor weight in an orthotopic xenograft mouse model of pancreatic cancer compared to both controls and gemcitabine, with a similar survival curve as the control treated mice. While leiodermatolide significantly reduced tumor weight, it failed to improve survival. There is the possibility of toxicity although no significant body weight differences were seen between leiodermatolide treated mice and saline treated mice and no other indication of toxicity was seen. Therefore, these results warrant additional studies whereby leiodermatolide is administered at lower doses. We anticipate that lower doses of leiodermatolide would increase survival and lead to more accurate tumor efficacy data. The dosing regimen chosen for leiodermatolide (1× per week for three weeks) was similar to those used for tubulin active agents such as 6-epidictyostatin, paclitaxel and discodermolide. It is likely that continued dosing over the nine week period of the study might improve survival. Further studies are needed to determine the optimal dosing regimen that would reduce tumor size but also confer improved survival versus control. This initial study demonstrates that leiodermatolide is able to reduce tumor weight in vivo.
Figure 6. Leiodermatolide Exhibits Anti-tumor Effects.
Nude mice were injected with 1 million L3.6pl cells into the pancreas to induce tumors. Seven days later when tumors were well established, 10 mice were treated with 10 mg/kg leiodermatolide once per week for three weeks, 10 mice were treated with 100 mg/kg gemcitabine two times per week for five weeks and 10 mice received saline control. All treatments were delivered by intraperitoneal injection. Body weight was measured on the first day of treatment and each week thereafter. Tumor weight was determined on the day of termination. The experiment was conducted for 9 weeks. Leiodermatolide significantly reduced tumor weight when compared to gemcitabine treated or control mice, while exhibiting a similar survival curve as controls.
Discussion
Leiodermatolide is shown here to be a potent anti-mitotic agent with selectivity towards cancer cells. Its anti-mitotic activity appears to be effected through its interaction with tubulin, although it is unclear exactly how it interacts with tubulin. Leiodermatolide is unique because, at concentrations similar to its IC50 in PANC-1 cells, no effects on tubulin polymerization were observed using fluorescent microscopy; while at 20 times the IC50 some depolymerization-like effects were observed. No effects were observed in the in vitro polymerization assay regardless of the concentration of leiodermatolide used until tubulin containing MAPS was used, which surprisingly, enhanced polymerization. To our knowledge, the only known tubulin interacting compounds that do not show effects in the in vitro polymerization assay were the taccalonolides, plant natural products that induce tubulin polymerization25. However, the polymerization induced by the taccalonolides is easily perceived by fluorescent microscopy25. The conflicting results between the microscopy and the tubulin polymerization in the presence of MAPS remain puzzling. Both effects required much higher concentrations of leiodermatolide than those at which tubulin interacting compounds are usually tested in these assays. There is the possibility that the polymerization observed in the presence of MAPs could be an artifact as the assay measures optical density, and it is known that some depolymerizing agents have effects that could potentially increase turbidity when MAPS are present. For example, the known depolymerizing agent vincristine causes the formation of spirals in the presence of MAPS rather than the complete dissociation of microtubules that is observed when tubulin alone is used in the in vitro assay26, 27. The results of the in vitro polymerization of tubulin with MAPS were expected to resemble the effects seen on the cells. The contradictory results strongly suggest that leiodermatolide may not act upon tubulin itself, but may mediate its effects on tubulin by acting upon a microtubule associated protein or by affecting tubulin dynamics through a signaling molecule upstream in the signal transduction cascades that regulate tubulin dynamics. This belief is strengthened by the elapsed microscopy of GFP-EB1, which shows clearly that leiodermatolide affects tubulin dynamics. Studies to try to determine its mechanism of action and molecular target of leiodermatolide continue, but the data to date suggests that the mechanism of action of leiodermatolide may differ from other tubulin interacting compounds. A similar conclusion was reached by the Fürstner group who studied the effects of synthetic leiodermatolide on the U2OS osteosarcoma cell line18. They found that leiodermatolide does not directly bind tubulin, that its effects on cell cycle arrest and cytotoxicity are similar to other tubulin interacting compounds without in vitro effects on tubulin and they postulated centrosome declustering as a possible mechanism of action for leiodermatolide with further studies needed to confirm this potential mechanism of action18. Since cancer cells exhibit more centrosomes than normal cells, induction of centrosome declustering is considered an appealing anti-cancer strategy28. Whether the effects on microtubule dynamics reported here and the centrosome declustering observed by the Fürstner group are independent or connected remains to be defined.
One of the major obstacles in pursuing studies with marine natural products is their limited supply. However, several groups have reported total synthesis of leiodermatolide18, 29, 30. The synthesis done by the Fürstner group further showed that leiodermatolide produced through synthesis is more potent than a subset of non-natural analogues that were made at the same time18. We have previously reported that the IC50 of leiodermatolide is much higher in the multidrug resistant NCI/ADR ovarian cancer cell line13, suggesting that leiodermatolide may be a substrate for the PGP pump. However, the Fürstner group reported that they did not see loss of activity when testing leiodermatolide in a leukemia cell line that expresses the PGP pump18. Given that the NCI/ADR has other mechanisms to explain its resistance, it appears that leiodermatolide is not a substrate for the PGP pump. Given the uniqueness of its mechanism of action, its potency, its selectivity for cancer cells, and its in vivo efficacy, leiodermatolide is an extremely interesting compound that merits further studies to determine its therapeutic potential.
Brief Description.
Leiodermatolide, a polyketide macrolide isolated from a deep water sponge of the genus Leiodermatium, has anti-mitotic activity, and exhibits potent and selective cytotoxicity towards the pancreatic cancer cell lines AsPC-1, PANC-1, BxPC-3, and MIA PaCa-2. Leiodermatolide appears to affect microtubule dynamics through a unique mechanism of action compared to other microtubule interacting agents. In a mouse model of metastatic pancreatic cancer, leiodermatolide exhibited significant tumor reduction when compared to gemcitabine and controls.
Acknowledgments
This publication was made possible by grant 1R01CA093455 from the National Cancer Institute at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCI. Funding for this project was also provided by the State of Florida Center of Excellence in Biomedical & Marine Biotechnology (COE-HRE07), NOAA Cooperative Institute for Ocean Exploration, Research and Technology (NA09OAR4320073) and the Health Resources & Services Administration Center for Sustainable Use of Marine Resources (4C76HF00231-01-04). This is HBOI contribution 1997.
References
- 1.Pancreatic Cancer Canada. [Accessed on 06-09-2016];The Fight to End Pancreatic Cancer FACTS YOU NEED TO KNOW. 2015 Available at: http://www.pancreaticcancercanada.ca/site/DocServer/facts_you_need_to_know.pdf?docID=741.
- 2.American Cancer Society. [Accessed on 06-09-2016];Cancer Facts and Figures. 2015 Available at: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf.
- 3.Kingston DG. Tubulin-interactive natural products as anticancer agents. J Nat Prod. 2009;72:507–15. doi: 10.1021/np800568j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung DT, Jamison TF, Schreiber SL. Understanding and controlling the cell cycle with natural products. Chem Biol. 1996;3:623–39. doi: 10.1016/s1074-5521(96)90129-5. [DOI] [PubMed] [Google Scholar]
- 5.Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9:790–803. doi: 10.1038/nrd3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driowya M, Leclercq J, Verones V, Barczyk A, Lecoeur M, Renault N, Flouquet N, Ghinet A, Berthelot P, Lebegue N. Synthesis of triazoloquinazolinone based compounds as tubulin polymerization inhibitors and vascular disrupting agents. Eur J Med Chem. 2016;115:393–405. doi: 10.1016/j.ejmech.2016.03.056. [DOI] [PubMed] [Google Scholar]
- 7.Meyer-Losic F, Newman SP, Day JM, Reed MJ, Kasprzyk PG, Purohit A, Foster PA. STX140, but not paclitaxel, inhibits mammary tumour initiation and progression in C3(1)/SV40 T/t-antigen transgenic mice. PloS one. 2013;8:e80305. doi: 10.1371/journal.pone.0080305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar BS, Raghuvanshi DS, Hasanain M, Alam S, Sarkar J, Mitra K, Khan F, Negi AS. Recent Advances in chemistry and pharmacology of 2-methoxyestradiol: An anticancer investigational drug. Steroids. 2016;110:9–34. doi: 10.1016/j.steroids.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimou AT, Syrigos KN, Saif MW. Novel agents for the treatment of pancreatic adenocarcinoma: any light at the end of the tunnel? Highlights from the “2010 ASCO Annual Meeting”; Chicago, IL, USA. June 4–8, 2010; JOP; pp. 324–7. [PubMed] [Google Scholar]
- 11.Whitehead RP, McCoy S, Rivkin SE, Gross HM, Conrad ME, Doolittle GC, Wolff RA, Goodwin JW, Dakhil SR, Abbruzzese JL. A Phase II trial of epothilone B analogue BMS-247550 (NSC #710428) ixabepilone, in patients with advanced pancreas cancer: a Southwest Oncology Group study. Invest New Drugs. 2006;24:515–20. doi: 10.1007/s10637-006-8440-x. [DOI] [PubMed] [Google Scholar]
- 12.Newman S. Eribulin, a simplified ketone analog of the tubulin inhibitor halichondrin B, for the potential treatment of cancer. Curr Opin Investig Drugs. 2007;8:1057–66. [PubMed] [Google Scholar]
- 13.Paterson I, Dalby SM, Roberts JC, Naylor GJ, Guzman EA, Isbrucker R, Pitts TP, Linley P, Divlianska D, Reed JK, Wright AE. Leiodermatolide, a potent antimitotic macrolide from the marine sponge Leiodermatium sp. Angew Chem Int Ed Engl. 2011;50:3219–23. doi: 10.1002/anie.201007719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stockwell BR, Haggarty SJ, Schreiber SL. High-throughput screening of small molecules in miniaturized mammalian cell-based assays involving post-translational modifications. Chem Biol. 1999;6:71–83. doi: 10.1016/S1074-5521(99)80004-0. [DOI] [PubMed] [Google Scholar]
- 15.Mimori-Kiyosue Y, Grigoriev I, Lansbergen G, Sasaki H, Matsui C, Severin F, Galjart N, Grosveld F, Vorobjev I, Tsukita S, Akhmanova A. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J Cell Biol. 2005;168:141–53. doi: 10.1083/jcb.200405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mimori-Kiyosue Y, Shiina N, Tsukita S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol. 2000;10:865–8. doi: 10.1016/s0960-9822(00)00600-x. [DOI] [PubMed] [Google Scholar]
- 17.Wright AE, Reed JK, Roberts J, Longley RE. Antiproliferative Activity of the Leiodermatolide class of Macrolides. USA: 2007. PCT/US2007/017500. [Google Scholar]
- 18.Mailhol D, Willwacher J, Kausch-Busies N, Rubitski EE, Sobol Z, Schuler M, Lam MH, Musto S, Loganzo F, Maderna A, Furstner A. Synthesis, Molecular Editing, and Biological Assessment of the Potent Cytotoxin Leiodermatolide. J Am Chem Soc. 2014 doi: 10.1021/ja508846g. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Cao Z, Yan H, Wood WC. Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res. 2003;63:6815–24. [PubMed] [Google Scholar]
- 20.Lamb J. The Connectivity Map: a new tool for biomedical research. Nat Rev Cancer. 2007;7:54–60. doi: 10.1038/nrc2044. [DOI] [PubMed] [Google Scholar]
- 21.Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, Reich M, Hieronymus H, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–35. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 22.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parness J, Horwitz SB. Taxol binds to polymerized tubulin in vitro. J Cell Biol. 1981;91:479–87. doi: 10.1083/jcb.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semenova MN, Kiselyov A, Semenov VV. Sea urchin embryo as a model organism for the rapid functional screening of tubulin modulators. Biotechniques. 2006;40:765–74. doi: 10.2144/000112193. [DOI] [PubMed] [Google Scholar]
- 25.Risinger AL, Mooberry SL. Taccalonolides: Novel microtubule stabilizers with clinical potential. Cancer Lett. 2010;291:14–9. doi: 10.1016/j.canlet.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donoso JA, Haskins KM, Himes RH. Effect of microtubule-associated proteins on the interaction of vincristine with microtubules and tubulin. Cancer Res. 1979;39:1604–10. [PubMed] [Google Scholar]
- 27.Haskins KM, Donoso JA, Himes RH. Spirals and paracrystals induced by Vinca alkaloids: evidence that microtubule-associated proteins act as polycations. J Cell Sci. 1981;47:237–47. doi: 10.1242/jcs.47.1.237. [DOI] [PubMed] [Google Scholar]
- 28.Ogden A, Rida PC, Aneja R. Let’s huddle to prevent a muddle: centrosome declustering as an attractive anticancer strategy. Cell Death Differ. 2012;19:1255–67. doi: 10.1038/cdd.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paterson I, Ng KK, Williams S, Millican DC, Dalby SM. Total synthesis of the antimitotic marine macrolide (−)-leiodermatolide. Angew Chem Int Ed Engl. 2014;53:2692–5. doi: 10.1002/anie.201310164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willwacher J, Kausch-Busies N, Furstner A. Divergent total synthesis of the antimitotic agent leiodermatolide. Angew Chem Int Ed Engl. 2012;51:12041–6. doi: 10.1002/anie.201206670. [DOI] [PubMed] [Google Scholar]
- 31.Guzman EA, Johnson JD, Carrier MK, Meyer CI, Pitts TP, Gunasekera SP, Wright AE. Selective cytotoxic activity of the marine-derived batzelline compounds against pancreatic cancer cell lines. Anticancer drugs. 2009;20:149–55. doi: 10.1097/CAD.0b013e32831fa39e. [DOI] [PMC free article] [PubMed] [Google Scholar]