Abstract
Noroviruses are members of the family Caliciviridae. Norovirus infections are a global health burden that impacts >20 million individuals annually in the U.S. alone. Noroviruses are associated with high morbidity among vulnerable populations, particularly immunocompromised patients. This perspective highlights recent developments related to the discovery and development of norovirus-specific small-molecule therapeutics as well as recent advances in our understanding of norovirus biology and pathogenesis. Most of the work in this area is at the early discovery stage and has been primarily focused on inhibitors of norovirus 3C-like protease and RNA dependent RNA polymerase. However, recent discoveries emanating from basic studies in norovirus research have resulted in the identification of new host-related drug targets that can be exploited. A repurposed compound has been advanced to human clinical studies.
Graphical abstract
INTRODUCTION
The family Caliciviridae is comprised of the genera Norovirus, Sapovirus, Vesivirus, Lagovirus and Nebovirus.1 The viruses in the Norovirus and Sapovirus genera include enteric caliciviruses which cause acute gastroenteritis in humans and animals. Noroviruses are the leading cause of acute gastroenteritis, accounting for >20 million cases of gastroenteritis annually in the U.S., resulting in 56000–70000 hospitalizations and nearly 800 deaths.2–4 The vomiting and diarrhea associated with norovirus infection are incapacitating but self-limiting among healthy adults, however, morbidity is high in immunocompromised patients, young children, and the elderly.5,6 In developing countries, mortality among young children may exceed 200000/year.7,8 Gastroenteritis outbreaks occur frequently in enclosed settings, such as navy and cruise ships, hospitals, nursing homes, and schools, and they are difficult to control because of the highly contagious and genetically diverse nature of noroviruses as well as their prolonged shedding and high stability in the environment.8,9 The most common routes of virus transmission are fecal–oral, food- or waterborne, and person-to-person.1,8,10 Despite the significant impact of noroviruses on public health,11 there are currently no effective vaccines or norovirus-specific small-molecule therapeutics in the clinic for the treatment and prophylaxis of norovirus infection. Progress in this area has been primarily hindered by the lack of an animal model that recapitulates all aspects of the human disease and the fact that human noroviruses cannot be cultivated in cell culture. However, pioneering studies in this area have established norovirus replicon harboring cells and have demonstrated the feasibility and utilization of the cell-based system for high throughput screening and antiviral drug development (vide infra).12 Furthermore, the seminal discovery that murine noroviruses (MNV) replicate in cell culture and share many of the biological properties of human noroviruses13 has made possible the availability of a small animal model of the human norovirus infection14 and has also illuminated many fundamental aspects of norovirus biology (vide infra).4,15,16 We review herein the state-of-the art in norovirus research and attempt to provide a balanced assessment of ongoing research and future directions in this area, with special emphasis on the discovery of small-molecule norovirus therapeutics.17,18
CALICIVIRUS CLASSIFICATION AND GENETIC DIVERSITY
Phylogenetic analysis of the major viral capsid (VP1) gene has served as the basis for classifying noroviruses into six genogroups (GI–VI). Human noroviruses causing gastroenteritis belong to three distinct genogroups (GI, GII, and GIV), which are further subdivided into 26 or more genotypes. Viruses in GII genogroup are more prevalent, and GII.4 strains are primarily responsible for most infections and outbreaks of acute gastroenteritis. Mutations and recombination account for the high degree of genetic and antigenic diversity found in noroviruses and, as a consequence, the emergence of new strains results in sporadic outbreaks and epidemics worldwide.19–21
CALICIVIRUS GENOMIC ORGANIZATION, POLYPROTEIN PROCESSING, AND FUNCTIONS OF VIRAL GENES
Caliciviruses are small, nonenveloped viruses that possess a single-stranded, (+) sense genomic RNA (7–8 kb) that is covalently linked to a viral protein (VPg, virion protein, genome-linked) at the 5′ end and polyadenylated at the 3′ end (Figure 1).1,15 The genome consists of three open reading frames (ORF1–3). ORF1 and 2 encode a 200 kDa polyprotein (ORF1) and a major capsid protein VP1 (ORF2) which contains antigenic and cell binding determinants,22,23 respectively. VP1 is comprised of a shell (S) domain and a protruding (P) domain, which is further subdivided into two subdomains (P1 and P2).1,15,24 The many functions associated with the hypervariable region in P2 include interactions with individual oligosaccharide residues of the histo-blood group antigen (HBGA) receptors25–28 and sialic acid-containing glycosphingolipids.29 ORF3 encodes a small basic protein VP2,1,24,30,31 which is believed to enhance the stability and structural integrity of VP1.32 The mature polyprotein is processed by a virus-encoded 3C-like cysteine protease (3CLpro) to generate six nonstructural proteins: p48 (NS1/2), NTPase/RNA helicase (NS3), p22 (NS4), VPg (NS5), a protease (NS6), and an RNA-dependent RNA polymerase (RdRp) (NS7) (Figure 1).1,15,24,30,31 Co- and post-translational processing of the polyprotein by norovirus 3CLpro is essential for virus replication. The functions of p48 and p22 have not been fully elucidated, however, the 15 kDa VPg protein is covalently linked to genomic and subgenomic mRNAs and its covalent linkage to the 5′ end of norovirus RNA is essential for virus infectivity.15,16,33 Norovirus 3CLpro is a chymotrypsin-like cysteine protease with an active site comprised of a prototypical catalytic triad (Cys139, His30, and Glu54) that is located at the interface of a β-sheet domain and a β-barrel domain. Norovirus 3CLpro is an induced fit enzyme with an extended binding cleft. Like all (+) strand RNA viruses, replication of the viral genome is effected by the virally encoded RdRp.34–38 Genomic and subgenomic RNA synthesis by the viral RdRp entails two different mechanisms: a de novo-initiated mechanism and a VPg-dependent mechanism.1,15,16,39 The central roles played by 3CLpro and RdRp in virus replication render them attractive targets for the development of norovirus-specific antivirals.
Figure 1.
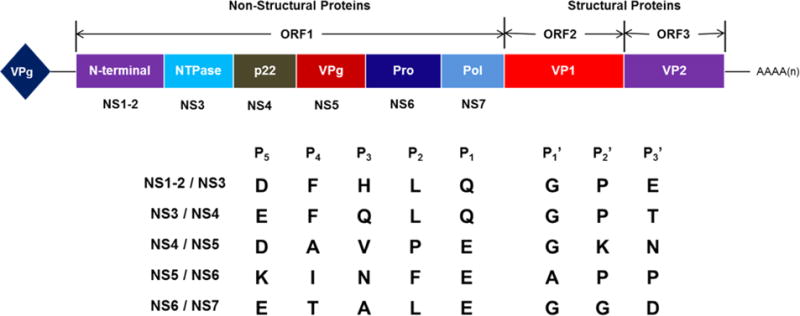
Human norovirus genome organization and cleavage sites by viral 3CLpro (NS6) to generate nonstructural proteins. The genome consists of a positive-sense RNA (~7.6 kb) that is covalently linked to VPg at the 5′ end and polyadenylated at the 3′ end. Open reading frame one (ORF1) encodes a polyprotein that is cleaved under the action of the viral cysteine protease (NS6) to generate six nonstructural proteins. The structural proteins VP1 (capsid protein) and VP2 (minor structural protein) are encoded by ORF2 and ORF3, respectively.
NOROVIRUS BIOLOGY AND PATHOGENESIS4,40
Human noroviruses are enteric pathogens that are etiologically associated with gastroenteritis in humans of all age groups. Norovirus infection is a complex disease that involves the interplay of multiple host and viral factors and mediators, including a variance in genetic susceptibility to norovirus infections arising from different binding affinities of norovirus strains and blood antigens.41 In experimental conditions, human noroviruses can infect primates, pigs, and cattle.42–45 In pigs and cattle infected with human noroviruses, replicating viruses are found in the enterocytes and they develop mild to moderate diarrhea.42,43 In primates, human virus infection leads to fecal virus shedding without clear clinical symptoms.44,45 Early biopsy results from norovirus-infected humans suggested that norovirus infections may induce histopathological changes such as villus stunts, which may result in malabsorption and diarrhea.1 However, the limited histopathological changes in the intestinal tract observed do not exclude the possibility that noroviruses may infect cells other than enterocytes and contribute to noroviral diarrhea and vomiting via less known mechanisms. In immunocompromised patients, norovirus infections lead to persistent gastroenteritis with diarrhea.5,21,46 Recent findings related to norovirus infection, including the disruption of human or mouse gut microbiota,47,48 the persistence of infection associated with commensal microbes and interferon-λ,49 infection of dendritic cells, macrophages, and B cells,50–52 evasion of the immune system via continuous viral evolution,53 and the role of host cell factors,54,55 have illuminated many aspects of the disease. These studies have laid the groundwork for formulating a model of how noroviruses infect the intestine which, briefly, involves the binding of noroviruses to carbohydrate receptors expressed on enterocytes, followed by transcytosis across the intestinal epithelium and infection of macrophages, dendritic cells, and B cells.56 However, while significant advances have been made in this area of research, there remains a need for a better definition of the molecular mechanisms which underlie norovirus-induced diarrhea.
DISCOVERY OF NOROVIRUS DRUGS. GENERAL STRATEGY
Besides supportive fluid therapy,8 treatment options available to individuals suffering from acute gastroenteritis are currently limited because no norovirus-specific antiviral agent has yet been approved by the FDA. Thus, the availability of an efficacious and safe norovirus therapeutic or prophylactic antiviral would have a major impact on the control of norovirus infection. In principle, both viral and host factors can serve as potential targets for antiviral drug discovery and, furthermore, any step in the viral replication cycle can be targeted (Figure 2). The advent of a robust and highly sensitive FRET-based assay for norovirus 3CLpro and a cell-based replicon system has greatly facilitated the identification and evaluation of antinorovirus hits.57 As is generally true for other viruses, antivirals aimed at targeting viral components (attachment, entry, and uncoating inhibitors, as well as protease and polymerase inhibitors) of norovirus may result in the selection of resistant mutants, while unwanted side effects from inhibiting host factors involved in bodily functions are a concern for antivirals targeting host factors. In either case, a nontoxic norovirus therapeutic that rapidly reduces viral load and is effective as a prophylactic would constitute a major advance in the field.
Figure 2.
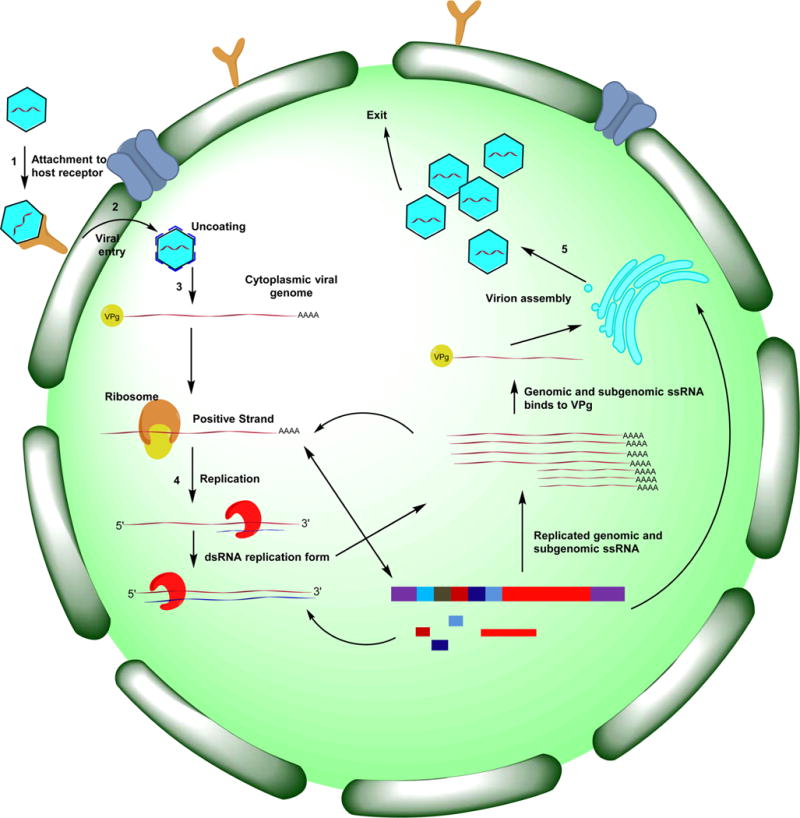
Human norovirus life cycle and potential therapeutic intervention sites. Sites 1–3: attachment, entry, and uncoating, respectively. Sites 4–5: 3CL protease and RdRp inhibitors, respectively. Site 6: virion assembly inhibitors.
ENZYME INHIBITORS
3CL Protease Inhibitors
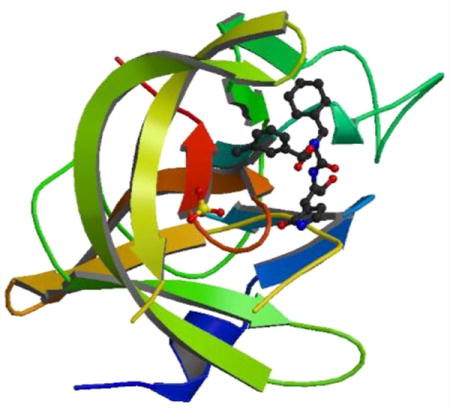
Norovirus 3CLpro is an attractive target for the discovery and development of norovirus-specific therapeutics because of the vital role the protease plays in the viral life cycle (Figure 2).1,15 Efforts focused on 3CLpro have been abetted by the availability of several high-resolution X-ray crystal structures of the enzyme with or without bound ligands (Figure 3).58–64 The X-ray crystal structures of 3CLpro in complex with inhibitors provide a detailed map of the active site which can be exploited for the structure-based design of potent inhibitors. The structure of the protease in solution has been determined using high-field NMR (Figure 3).65 The enzyme is predominantly a monomer in solution, while X-ray crystal structures of norovirus 3CLpro show the enzyme exists as a dimer.63,65 Interestingly, the flexible backbone of the T123–G133 loop appears to play an important role in substrate recognition. The substrate specificity and relevant determinants of substrate specificity of the enzyme have been ascertained using an array of tools, including in vitro transcription/translation, structural studies, and the use of chromogenic and fluorogenic substrates (Table 1).60–62,66–68 For comparative purposes, the substrate specificity of the 3C protease (3Cpro) or 3CLpro of some related viruses are also included in Table 1. The similarity in the active site topography of the norovirus, picornavirus, and coronavirus 3C and 3CL proteases suggests that fashioning a broad-spectrum antiviral agent is, in principle, feasible.58
Figure 3.
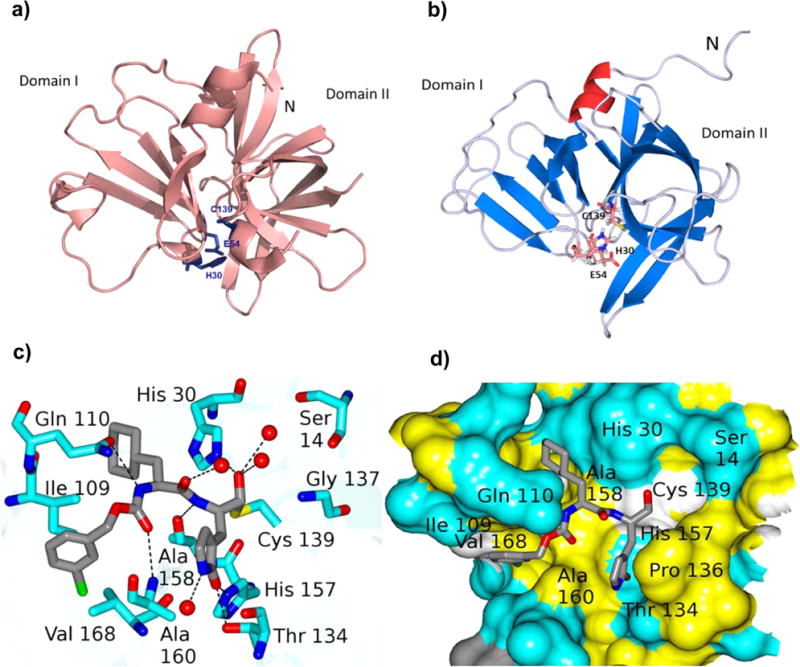
(a) Norwalk virus 3CLpro (2FYQ)63 showing the two domains. The active site catalytic residues (Cys139, His30, and Glu54) are located at the interface of the two domains. (b) Ribbon representation of the solution structure of NV 3CLpro (2LNC)65 with the catalytic residues shown as sticks and the very short strand shown in green color. (c) Connolly surface of precursor aldehyde 1 of inhibitor 2 bound to NV 3CL protease.59 Neighboring residues are colored yellow (nonpolar), white (weakly polar), and cyan (polar). The P2 residue cyclohexyl alanine side chain is nestled into the hydrophobic S2 pocket. (d) Hydrogen bond interactions between NV 3CLpro and precursor aldehyde derived from compound 2 (under the crystallization conditions used, bisulfite adduct 2 reverts back to aldehyde inhibitor 1, which then interacts with Cys139 to form the enzyme–inhibitor complex).59 Backbone hydrogen bonds with Ala158, Ala168, and Gln110 facilitate proper binding and positioning of the inhibitor at the active site. The carbonyl oxygen of the glutamine surrogate forms two hydrogen bonds with His157 and Thr134.
Table 1.
Substrate Specificity of Viral 3C and 3CL Proteases
| viral 3Cpro or 3CLpro | P5 | P4 | P3 | P2 | P1 | P1′ | P2′ |
|---|---|---|---|---|---|---|---|
| NV | D/E | F/Y | H/Q/E | L | Q | G | P |
| EV71 | E | A | V/L/T | L/F | Q | G | P |
| CVA16 | E | A | L | F | Q | G | P |
| SARS-CoV | S | A | V/T/K | L | Q | A/S | G |
The mechanism of action of 3CLpro is similar to that established for related cysteine proteases where Cys139 acts as a nucleophile, His30 functions as a general acid–base, and Glu54 facilitates the alignment of His30 and promotes deprotonation of Cys139.69,70 The oxyanion of the tetrahedral intermediate is stabilized by the presence of an oxyanion hole adjacent to the 3CLpro active site. The aforementioned structural and biochemical studies have placed the design and optimization of 3CLpro inhibitors on a secure structural and biochemical footing and have made possible the use of structure-based approaches in the discovery of antinorovirus therapeutics.
In principle, the general design strategy employed in the construction of a transition state (TS) inhibitor or TS mimic,71 for example, involves the linking of a peptidyl recognition element (X) that corresponds to the known substrate specificity of the target enzyme to an appropriate warhead, or a moiety that resembles the transition state of the enzymatic reaction in the case of a TS mimic (Figure 4). The recognition element typically spans two or more of the S subsites and serves to dock and position the inhibitor correctly at the active site via a network of hydrogen bonds between the backbone of the inhibitor and active site residues (Figure 4). Thus, formation of the initial enzyme–inhibitor noncovalent complex within the protease active site is followed by reaction of the active site cysteine with the warhead, leading to reversible formation of a stable tetrahedral adduct (Figure 5a). Common warheads employed include aldehyde, α-ketoamide, α-ketoester, nitrile, and α-ketoheterocycle moieties. Examples of TS mimics include α-hydroxyphosphonates and α-hydroxyesters. Unlike inhibitors that incorporate a warhead in their structure, the interaction of TS mimics with the enzyme involves noncovalent binding interactions only. Examples 1–5 (Chart 1) represent highly effective norovirus 3CLpro inhibitors that illustrate this approach.58,59,61,72–75 Importantly, compound 1 has shown in vivo efficacy in the MNV mouse model.59 Tripeptidyl inhibitors 6–8 are also effective, however, the increased polar surface area results in diminished cellular permeability.75 Rupintrivir 9 (Chart 1), an enterovirus protease inhibitor that incorporates in its structure a Michael acceptor that interacts with Cys139 via a Michael addition reaction, also displays antinorovirus activity.58,76
Figure 4.
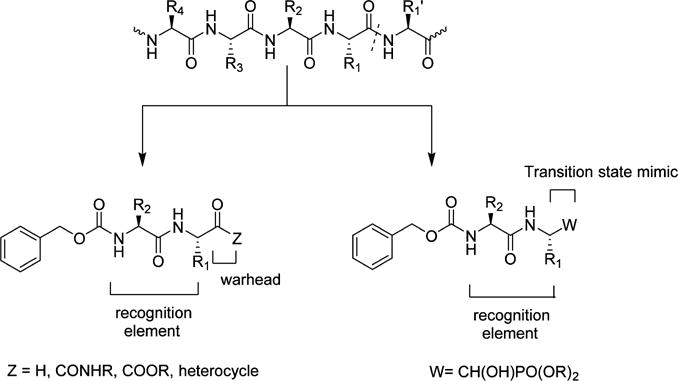
General strategy related to the design of peptidyl transition state inhibitors and transition state mimics.
Figure 5.
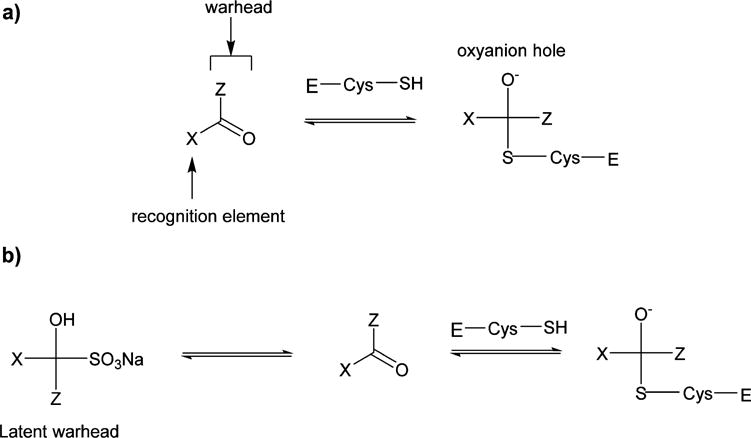
(a) General representation of the reversible interaction between a transition state inhibitor and a cysteine protease. The oxyanion of the tetrahedral intermediate is located in the oxyanion hole. (b) Depiction of the mechanism of inhibition of a cysteine protease by a bisulfite adduct derived from a transition state inhibitor (in this case, an aldehyde).77 The equilibrium between the bisulfite adduct and precursor aldehyde is pH-dependent.
Chart 1.
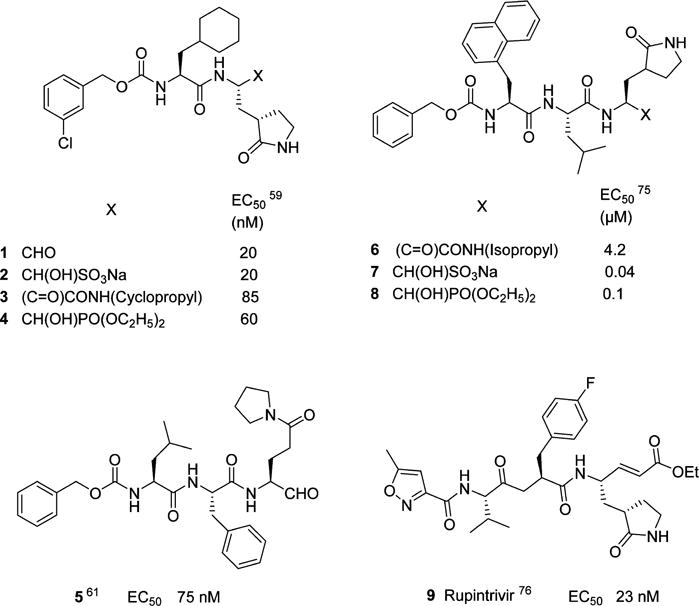
Peptidyl Inhibitors of Norovirus 3CL Protease
The chemical reactivity of the warheads employed in the design of TS inhibitors is occasionally associated with a lack of selectivity. As a consequence, off-target effects may result in unacceptable toxicity, necessitating further investigation until optimal selectivity is attained. The use of a masked warhead, such as, for example, a bisulfite adduct capable of reverting to the precursor carbonyl under physiological conditions, can be highly advantageous (Figure 5b).77 The pharmacological activity of the bisulfite adducts of peptidyl aldehydes has been shown to parallel that of the parent aldehydes.77 Compounds 2 and 7 (Chart 1) illustrate the use of this approach.
In general, the use of peptide-derived drugs is associated with several disadvantages, including high conformational flexibility, susceptibility to proteolytic degradation, poor membrane permeability, and low oral bioavailability.78,79 These shortcomings are frequently mitigated through depeptitization. An effective way of depeptitizing a linear peptide is through macrocyclization.80,81 The preorganization and structural rigidity that characterize macrocyclic peptides frequently enhances pharmacological activity by reducing the entropic penalty associated with binding and, furthermore, increases proteolytic stability.82 The effect of macrocyclization on cellular permeability and oral bioavailability is less predictable, however, these parameters are augmented when a macrocycle can engage in intramolecular hydrogen bonding.83,84
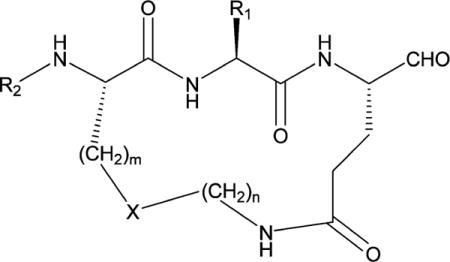
On the basis of the aforementioned considerations, peptidyl TS inhibitors of 3CLpro were depeptidized by tethering the P1 Gln residue with the P3 residue side chain in a linear peptide using an appropriate linker, yielding a macrocyclic scaffold (Figure 6). Additional design considerations related to macrocycles 10 and 11 included ensuring that the ring size of the macrocycle would be optimal in terms of allowing the macrocycle to assume a β-strand conformation, a structural motif recognized by proteases.85–87 This is of paramount importance because a β-strand conformation would allow proper docking/positioning of the inhibitor to the active site which, in turn, would orient correctly the side chains of the P1 and P2 residues, thereby maximizing hydrogen bonding and hydrophobic binding interactions. Indeed macrocyclic compounds 10 and 11 have been found to be potent and permeable inhibitors of 3CLpro (Table 2) (unpublished data).
Figure 6.
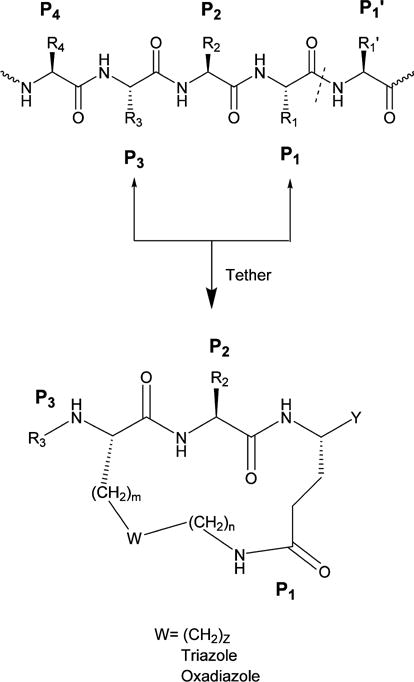
General design of macrocyclic inhibitors of 3CLpro by tethering the side chains of the P1 (Gln) and P3 residues with a suitable linker. The primary substrate specificity of NV 3CLpro requires an invariant Gln (or Glu) at P1.
Table 2.
Macrocyclic Inhibitors of Norovirus 3CL Protease
| ||||||
|---|---|---|---|---|---|---|
| R1 | R2 | X | m | n | EC50 (μM) | |
| 10 | isobutyl | Cbz | triazole | 1 | 5 | 6.1 |
| 11 | isobutyl | Boc | oxadiazole | 2 | 3 | 4.5 |
RdRp Inhibitors
The inhibition of viral RNA dependent RNA polymerases has been a highly successful endeavor that has resulted in the introduction of several RdRp inhibitors in the clinic. The central role played by norovirus RdRp in virus replication and the lack of human homologues renders RdRp an attractive target for antiviral drug discovery. Several X-ray crystal structures of human and murine norovirus RdRp that are well-suited to serving as a launching pad for the design of RdRp inhibitors have been reported, including complexes with 5-fluorouracil,88 2-thiouridine and ribavirin,89 and suramin-related compounds (Figure 7).90 RdRp inhibitors that have been reported thus far are listed in Chart 2. High throughput screening has led to the identification of a low micromolar nonnucleoside inhibitor of RdRp (compound 18, Chart 2).91 Importantly, it has been shown that the RdRp inhibitor 2′-C-methylcytidine 14 is effective against norovirus-induced diarrhea and mortality in the murine model of norovirus infection.92,93 Favipiravir 15 has also been shown to inhibit norovirus replication in vitro.94 Future developments related to RdRp inhibitors are likely to identify additional promising nucleoside and nonnucleoside direct acting antivirals.
Figure 7.
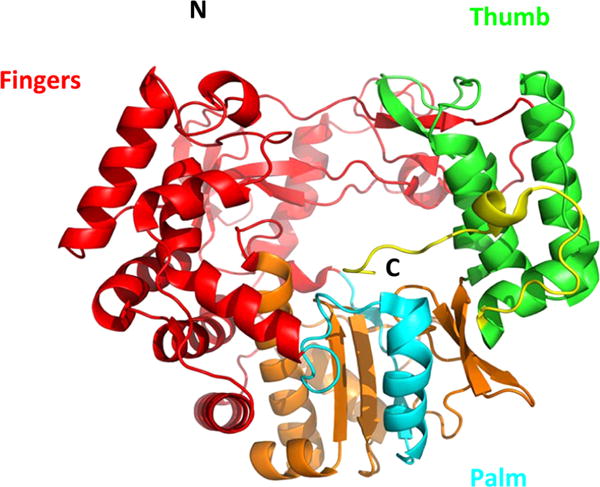
Norovirus RNA dependent RNA polymerase (NS7) (PDB code 1SH2) structure comprised of the palm (blue), thumb (green), and fingers (red) domains.119
Chart 2.
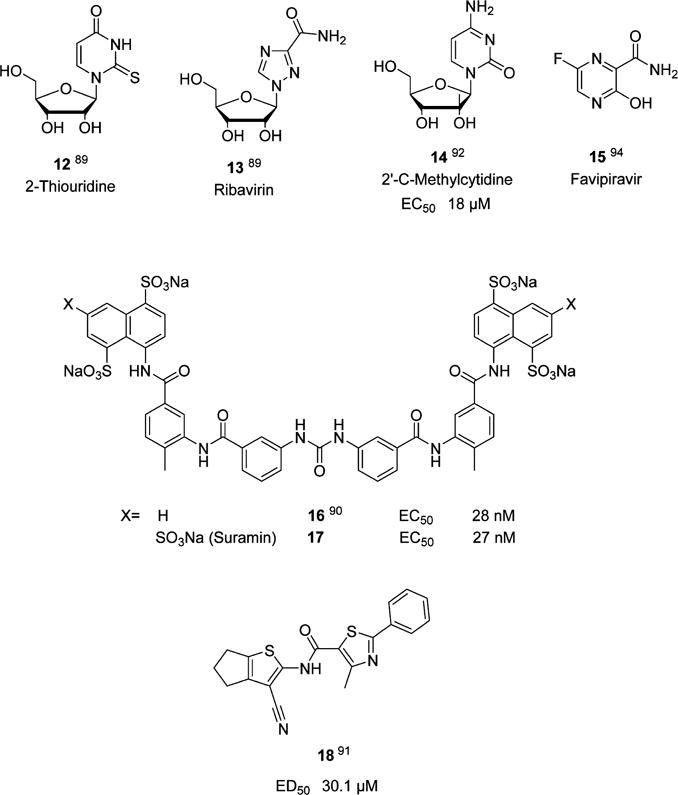
Norovirus RNA Dependent RNA Polymerase Inhibitors
Entry Blockers
There has been a flurry of activity related to the design and discovery of inhibitors of norovirus attachment, entry, and/or uncoating. Because HBGA-binding sites are conserved in human noroviruses, molecules that block the HBGA binding site are of potential therapeutic value.95–97 Screening of carbohydrate libraries and small molecule libraries has resulted in the identification of multiple binding blockers.98–100 The recent demonstration that the endosomal cysteine protease cathepsin L is required for calicivirus replication offers another promising avenue of investigation for identifying molecules of potential therapeutic value.101
Host Factors
Despite impressive progress in our understanding of the norovirus life cycle,4,15,16,40,102 there is a need for a sharper definition of how the virus exploits host factors for survival, propagation, and infectivity. As mentioned earlier, multiple host cell factors, including a network of host proteins that interact with the 5′ and 3′ ends of the human and murine norovirus genome, play critical roles in viral RNA replication.15,16,102,103 Moreover, studies have shown that the cholesterol pathway, as well as the molecular chaperone Hsp90, a component of the ribonucleoprotein complex, could also serve as potential targets for novel therapeutic options.104,105 Hsp90 inhibitor 19 (Chart 3), as well as repurposed Hsp50 inhibitors and others,106 provide intriguing possibilities for norovirus drug discovery. Lastly, deubiquitinase and elF4F inhibitors 20107 and 21,108 respectively, provide another promising avenue for developing norovirus therapeutics (Chart 3).
Chart 3.
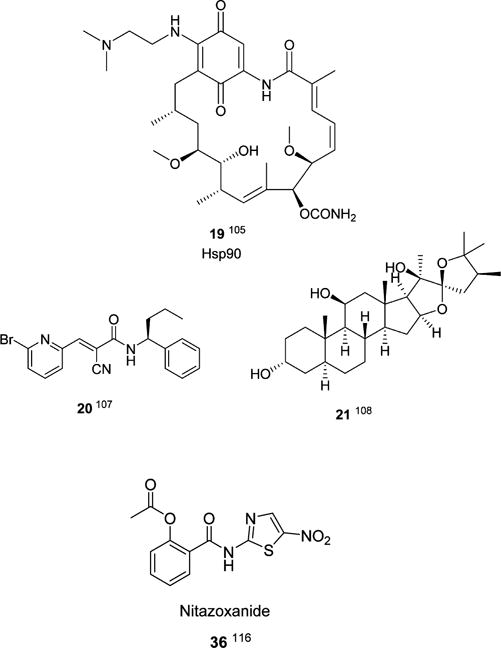
Inhibitors of Host Factors
Miscellaneous
An array of molecules with antinorovirus activity, including cyclic and acyclic sulfamides109–111 22–23, 33, piperazine112 24–25, 27, 31–32, and pyranobenzopyrone 34113 derivatives, as well as related compounds discovered by scaffold hopping114 26, 28–30, have been reported, however, their mechanism of action remains to be elucidated (Chart 4). In the case of the cyclic and acyclic sulfamides, they were found to moderately inhibit MNV. Furthermore, no resistant virus was selected after serial passages (unpublished data), suggesting that these classes of compounds may target cellular factors. Lastly, (E)-2-styryl chromone derivatives 35 have also been shown to possess antinorovirus activity (Chart 4).115
Chart 4.
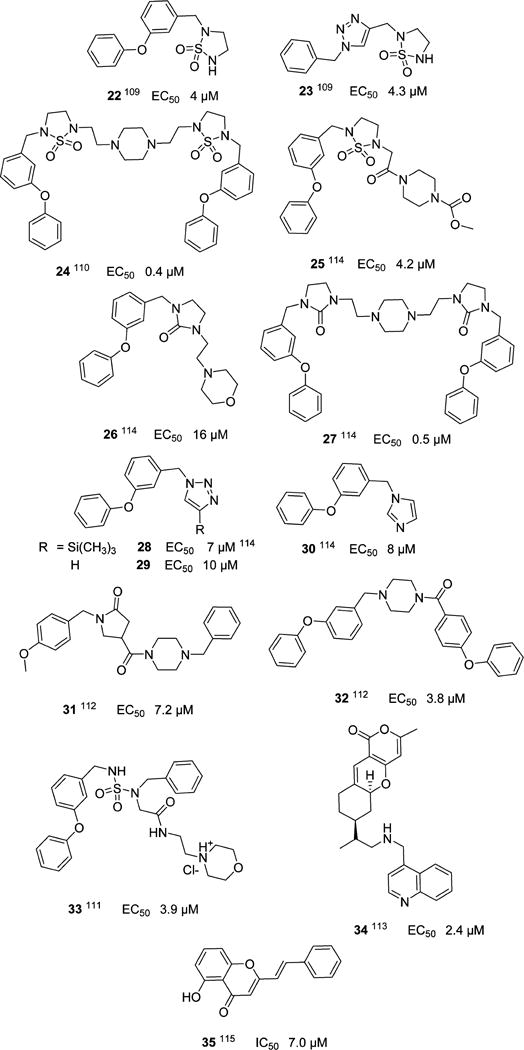
Miscellaneous Compounds with Anti-Norovirus Activity and an Unknown Mechanism of Action
NOROVIRUS DRUGS IN CLINICAL DEVELOPMENT
Nitazoxanide 36, an antiprotozoal prodrug used in the treatment of infectious diarrhea caused by Cryptospiridium parvum and Giardia lamblia has demonstrated efficacy against norovirus infection in clinical trials.116 There is limited information on its antinoroviral effects and mechanism of action.117 Nitazoxanide provides strong validation of drug repurposing105,106 as a means of identifying compounds that inhibit norovirus.
CONCLUSIONS
No specific antiviral therapy or prophylaxis currently exists for norovirus infection. The increasing realization that norovirus infection represents a significant health burden worldwide and exacts a heavy toll among the elderly, young, and immunocompromised populations has provided the impetus behind efforts related to the discovery of norovirus therapeutics, prophylactics, and vaccines. These efforts have been abetted by advances in the basic science underlying the biology and pathophysiology of the disease. Although target-based approaches to drug discovery in this area have focused on viral targets, primarily norovirus 3CLpro and RNA dependent RNA polymerase, the identification of an increasing number of host factors as potential targets is likely to continue. An integral part of the drug discovery process is drug target validation and drug target selection based on relatively well-established criteria,118 consequently, new molecular targets will need to be validated. Recent studies with norovirus 3CLpro have resulted in the identification of lead compounds which are highly effective against multiple human norovirus genotypes as well as murine norovirus, and demonstration of proof of concept in the MNV animal model.59 These inhibitors are well-suited to conducting further preclinical studies, ultimately leading to the identification of a clinical candidate. Much of the ongoing work in this area, irrespective of molecular target, is at the early stage of drug discovery. Nevertheless, prospects for the eventual introduction of norovirus therapeutics in the clinic would appear to be excellent.
Acknowledgments
The generous financial support of this work by the National Institutes of Health (R01AI109039) is gratefully acknowledged.
ABBREVIATIONS USED
- 3CLpro
3C like protease
- MNV
murine norovirus
- VPg
virion protein, genome-linked
- ORF
open reading frame
- RdRp
RNA dependent RNA polymerase
- FRET
fluorescence resonance energy transfer
- elF4F
eukaryotic initiation factor 4F
Biographies
Yunjeong Kim received her DVM (1993) from Seoul National University, Seoul, Korea, and her Ph.D. (2001) in Veterinary Preventive Medicine from The Ohio State University, Ohio, under the supervision of Professor Linda Saif in the field of vaccinology and viral pathogenicity of bovine enteric viruses. During her postdoctoral study at the National Cancer Institute, she studied the molecular pathogenesis of HTLV-1 and HIV. Since 2010, she has been a research assistant professor in the Department of Diagnostic Medicine and Pathobiology in the College of Veterinary Medicine, Kansas State University, Kansas. Her research interests include the development of antiviral screening platforms, animal models, and viral pathogenicity of human and animal viruses.
Anushka C. Galasiti Kankanamalage received his B.S. (honors) degree in Chemistry from the University of Colombo, Sri Lanka, in 2010. He did his undergraduate research under the supervision of Professor E. Dilip de Silva on the investigation of biologically active secondary metabolites from plants and endophytes. Since August 2012, he has been working towards his Ph.D. in Organic Medicinal Chemistry under the supervision of Professor William C. Groutas in the Department of Chemistry at Wichita State University. His current research is mainly focused on drug discovery, lead optimization, and structure-based design of antiviral agents, with particular emphasis on small molecule NV 3CL protease inhibitors.
Kyeong-Ok Chang received his D.V.M. (1989) from Seoul National University, Seoul, Korea, and Ph.D. (1999) in Veterinary Preventive Medicine from The Ohio State University, Ohio, under the supervision of Professor Linda Saif, where he studied viral pathogenicity of enteric viruses. He was a postdoctoral fellow at Kim Green’s laboratory at the National Institutes of Health, studying molecular virology focusing on calicivirus (including norovirus). In 2005, he was appointed to the faculty at the College of Veterinary Medicine, Kansas State University, Kansas, where he is currently Professor of Virology. His research focuses on viral pathogenicity and antiviral drug development. Using cell- and enzyme-based assays and animal models, his group is working with medicinal chemists in preclinical drug development of antivirals against various viruses, including norovirus.
William C. Groutas received his B.S. (honors) degree in Chemistry from the American University of Beirut and his Ph.D. in Organic Chemistry from the University of Kentucky. Following completion of a postdoctoral fellowship in Bioorganic Chemistry at Cornell University, he joined the University of Wisconsin—Eau Claire before moving to Wichita State University, where he is currently the Endowment Association Distinguished Professor of Chemistry. His research interests lie in the general areas of medicinal chemistry, drug discovery and development, and mechanistic enzymology, with primary focus on the structure-based design of inhibitors of human and viral proteases of medical relevance.
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Green KY. Caliciviridae: The Noroviruses. In: Knipe DM, Howley PM, editors. Green’s Virology. Vol. 1. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 949–979. [Google Scholar]
- 2.(a) Hall AJ, Lopman BA, Payne DC, Patel MM, Gastanaduy PA, Vinje J, Parashar UD. Norovirus Disease in the United States. Emerging Infect Dis. 2013;19:1198–1205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Norovirus. Centers for Disease Control and Prevention; Atlanta, GA: 2013. www.cdc.gov/norovirus (accessed January 2015) [Google Scholar]
- 3.Pringle K, Lopman B, Vega E, Vinje J, Parashar UD, Hall AJ. Noroviruses: Epidemiology, Immunity and Prospects for Prevention. Future Microbiol. 2015;10:53–67. doi: 10.2217/fmb.14.102. [DOI] [PubMed] [Google Scholar]
- 4.Karst SM, Zhu S, Goodfellow IG. The Molecular Pathology of Noroviruses. J Pathol. 2015;235:206–216. doi: 10.1002/path.4463. [DOI] [PubMed] [Google Scholar]
- 5.Bok K, Green KY. Norovirus Gastroenteritis in Immunocompromised Patients. N Engl J Med. 2012;367:2126–2132. doi: 10.1056/NEJMra1207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DuPont HL. Acute Infectious Diarrhea in Immunocompetent Adults. N Engl J Med. 2014;370:1532–1540. doi: 10.1056/NEJMra1301069. [DOI] [PubMed] [Google Scholar]
- 7.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. Systematic Literature Review of Role of Noroviruses in Sporadic Gastroenteritis. Emerging Infect Dis. 2008;14:1224–1231. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robilotti E, Deresinski S, Pinsky BA. Norovirus. Clin Microbiol Rev. 2015;28:134–164. doi: 10.1128/CMR.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall AJ. Noroviruses: the Perfect Human Pathogen? J Infect Dis. 2012;205:1622–1624. doi: 10.1093/infdis/jis251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore MD, Goulter RM, Jaykus LA. Human Norovirus as a Foodborne Pathogen: Challenges and Developments. Annu Rev Food Sci Technol. 2015;6:411–433. doi: 10.1146/annurev-food-022814-015643. [DOI] [PubMed] [Google Scholar]
- 11.Belliot G, Lopman BA, Ambert-Balay K, Pothier P. The Burden of Norovirus Gastroenteritis: An Important Foodborne and Healthcare-related. Clin Microbiol Infect. 2014;20:724–730. doi: 10.1111/1469-0691.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang KO, Sosnovtsev SV, Belliot G, King AD, Green YK. Stable Expression of a Norwalk Virus RNA Replicon in a Human Hepatoma Cell Line. Virology. 2006;353:463–473. doi: 10.1016/j.virol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Wobus CE, Thackray LB, Virgin HW. Murine Norovirus: A Model System to Study Norovirus Biology and Pathogenesis. J Virol. 2006;80:5104–5112. doi: 10.1128/JVI.02346-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taube S, Kolawole AO, Hohne M, Wilkinson JE, Handley SA, Perry JW, Thackray LB, Akkina R, Wobus CE. A Mouse Model for Human Norovirus. mBio. 2013;4:e00450–13. doi: 10.1128/mBio.00450-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorne LG, Goodfellow IG. Norovirus Gene Expression and Replication. J Gen Virol. 2014;95:278–291. doi: 10.1099/vir.0.059634-0. [DOI] [PubMed] [Google Scholar]
- 16.Alhatlani B, Vashist S, Goodfellow I. Functions of the 5′ and 3′ Ends of Calicivirus Genomes. Virus Res. 2015;206:134–143. doi: 10.1016/j.virusres.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufman SS, Green KY, Korba BE. Treatment of Norovirus Infection: Moving Antivirals from the Bench to the Bedside. Antiviral Res. 2014;105:80–91. doi: 10.1016/j.antiviral.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocha-Pereira J, Neyts J, Jochmans D. Norovirus: Targets and Tools in Antiviral Drug Discovery. Biochem Pharmacol. 2014;91:1–11. doi: 10.1016/j.bcp.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee BE, Pang X-L. New Strains of Norovirus and the Mystery of Viral Gastroenteritis Epidemics. Can Med Assoc J. 2013;185:1381–1382. doi: 10.1503/cmaj.130426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parra GI, Green KY. Genome of Emerging Norovirus GII.17, United States, 2014. Emerging Infect Dis. 2015;21:1477–1479. doi: 10.3201/eid2108.150652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karst SM, Baric RS. What is the Reservoir of Emergent Human Norovirus Strains? J Virol. 2015;89:5756–5759. doi: 10.1128/JVI.03063-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strong DW, Thackray LB, Smith TJ, Virgin HW. Protruding Domain of Capsid Protein is Necessary and Sufficient to Determine Murine Norovirus Replication and Pathogenesis in Vitro. J Virol. 2012;86:2950–2958. doi: 10.1128/JVI.07038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subba-Reddy CV, Yunus MA, Goodfellow IG, Kao CC. Norovirus RNA Synthesis is Modulated by an Interaction Between the Viral RNA-Dependent RNA Polymerase and the Major Capsid Protein, VP1. J Virol. 2012;86:10138–10149. doi: 10.1128/JVI.01208-12. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Hardy ME. Norovirus Protein Structure and Function. FEMS Microbiol Lett. 2005;253:1–8. doi: 10.1016/j.femsle.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Tan M, Jiang X. Norovirus gastroenteritis, carbohydrate receptors and animal models. PLoS Pathog. 2010;6(8):e1000983. doi: 10.1371/journal.ppat.1000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Chen Y, Jiang X, Xia M, Yang Y, Tan M, Li X, Rao Z. A Unique Human Norovirus Lineage with a Distinct HBGA Binding Interface. PLoS Pathog. 2015;11(7):e1005025. doi: 10.1371/journal.ppat.1005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan M, Jiang X. The P domain of Norovirus Capsid Protein Forms a Subviral Particle that Binds to Histo-Blood Group Antigen Receptors. J Virol. 2005;79:14017–14030. doi: 10.1128/JVI.79.22.14017-14030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han L, Kitova EN, Tan M, Jiang X, Pluvinage B, Boraston AB, Klassen JS. Affinities of Histo-blood Group Antigens for Norovirus Capsid Protein Complexes. Glycobiology. 2015;25:170–180. doi: 10.1093/glycob/cwu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han L, Tan M, Xia M, Kitova EN, Jiang X, Klassen JS. Gangliosides are Ligands for Human Noroviruses. J Am Chem Soc. 2014;136:12631–12637. doi: 10.1021/ja505272n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke IN, Lambden PR. Organization and Expression of Calicivirus Genes. J Infect Dis. 2000;181:S309–S316. doi: 10.1086/315575. [DOI] [PubMed] [Google Scholar]
- 31.Xi JN, Graham DY, Wang KN, Estes MK. Norwalk Virus Genome Cloning and Characterization. Science. 1990;250:1580–1583. doi: 10.1126/science.2177224. [DOI] [PubMed] [Google Scholar]
- 32.Bertolotti-Ciarlet A, Crawford SE, Hutson AM, Estes MK. The 3′ End of Norwalk Virus m-RNA Contains Determinants that Regulate the Expression and Stability of the Viral Capsid Protein VP1: a Novel Function for the VP2 Protein. J Virol. 2003;77:11603–11615. doi: 10.1128/JVI.77.21.11603-11615.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subba-Reddy CV, Yunus MA, Goodfellow IG, Kao CC. Norovirus RNA Synthesis is Modulated by an Interaction Between the Viral RNA-dependent RNA Polymerase and the major Capsid Protein, VP1. J Virol. 2012;86:10138–10149. doi: 10.1128/JVI.01208-12. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Hogbom M, Jager K, Robel Y, Unge T, Rohayem J. The Active Form of the Norovirus RNA Polymerase is a Homodimer with Cooperative Activity. J Gen Virol. 2009;90:281–291. doi: 10.1099/vir.0.005629-0. [DOI] [PubMed] [Google Scholar]
- 35.Han KR, Choi Y, Min BS, Jeong H, Cheon D, Kim J, Jee Y, Shin S, Yang JM. Murine Norovirus-1 3Dpol Exhibits RNA-Dependent RNA Polymerase Activity and Nucleotidylylates on Tyr of the VPg. J Gen Virol. 2010;91:1713–1722. doi: 10.1099/vir.0.020461-0. [DOI] [PubMed] [Google Scholar]
- 36.Belliot G, Sosnovtsev SV, Chang KO, Babu V, Uche U, Arnold JJ, Cameron CE, Green KY. Norovirus Proteinase-Polymerase and Polymerase are Both Active Forms of RNA-Dependent RNA Polymerase. J Virol. 2005;79:2393–2403. doi: 10.1128/JVI.79.4.2393-2403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eden J-S, Sharpe LJ, White PA, Brown AJ. Norovirus RNA-Dependent RNA Polymerase is Phosphorylated by an Important Survival Kinase, Akt. J Virol. 2011;85:10894–10898. doi: 10.1128/JVI.05562-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bull RA, Hyde J, Mackenzie JM, Hansman GS, Oka T, Takeda N, White PA. Comparison of the Replication Properties of Murine and Human Calicivirus RNA-Dependent RNA Polymerase. Virus Genes. 2011;42:16–27. doi: 10.1007/s11262-010-0535-y. [DOI] [PubMed] [Google Scholar]
- 39.Lin X, Thorne L, Jin Z, Hammad LA, Li S, Deval J, Goodfellow IG, Kao CC. Subgenmic Promoter Recognition by the Norovirus RNA-dependent RNA-polymerases. Nucleic Acids Res. 2015;43:446–460. doi: 10.1093/nar/gku1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karst SM, Wobus CE, Goodfellow IG, Green YK, Virgin HW. Advances in Norovirus Biology. Cell Host Microbe. 2014;15:668–680. doi: 10.1016/j.chom.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taube S, Perry JW, McGreevy E, Yetming K, Perkins C, Henderson K, Wobus CE. Murine Noroviruses Bind Glycolipid and Glycoprotein Attachment Receptors in a Strain-Dependent Manner. J Virol. 2012;86:5584–5593. doi: 10.1128/JVI.06854-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheetham S, Souza M, Meulia T, Grimes S, Han MG, Saif LJ. Pathogenesis of a Genogroup II Human Norovirus in Gnotobiotic Pigs. J Virol. 2006;80:10372–10381. doi: 10.1128/JVI.00809-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Souza M, Azevedo MS, Jung K, Cheetham S, Saif LJ. Pathogenesis and Immune Responses in Gnotobiotic Calves After Infection with the Genogroup II.4-HS66 Strain of Human Norovirus. J Virol. 2008;82:1777–1786. doi: 10.1128/JVI.01347-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bok K, Parra GI, Mitra T, Abente E, Shaver CK, Boon D, Engle R, Yu C, Kapikian AZ, Sosnovtsev SV, Purcell RH, Green KY. Chimpanzees as an Animal Model for Human Norovirus Infection and Vaccine Development. Proc Natl Acad Sci U S A. 2011;108:325–330. doi: 10.1073/pnas.1014577107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rockx BH, Bogers WM, Heeney JL, van Amerongen G, Koopmans MP. Experimental Norovirus Infections in Non-human Primates. J Med Virol. 2005;75:313–320. doi: 10.1002/jmv.20273. [DOI] [PubMed] [Google Scholar]
- 46.Green KY. Norovirus Infection in Immunocompromised Hosts. Clin Microbiol Infect. 2014;20:717–723. doi: 10.1111/1469-0691.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson AM, Walk ST, Taube S, Taniuchi M, Houpt ER, Wobus CE, Young VB. Disruption of the Human Gut Microbiota Following Norovirus Infection. PLoS One. 2012;7:e48224. doi: 10.1371/journal.pone.0048224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. Intestinal Microbiota Promote Enteric Virus Replication and Systemic Pathogenesis. Science. 2011;334:249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, Virgin HW. Commensal Microbes and Interferon-λ Determine Persistence of Enteric Murine Norovirus Infection. Science. 2015;347:266–269. doi: 10.1126/science.1258025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wobus CE, Karst SM, Thackray LB, Chang K-O, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. Replication of Norovirus in Cell Culture Reveals a Tropism for Dendritic Cells and Macrophages. PLoS Biol. 2004;2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinje J, Tibbetts SA, Wallet SM, Karst SM. Enteric Bacteria Promote Human and Murine Norovirus Infection of B Cells. Science. 2014;346:755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karst SM. Identification of a Novel Cellular Target and a Co-factor for Norovirus Infection–B Cells and Commensal Bacteria. Gut Microbes. 2015;6:266–271. doi: 10.1080/19490976.2015.1052211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donaldson EF, Lindesmith LC, LoBue AD, Baric RS. Viral Shape-Shifting: Norovirus Evasion of the Human Immune System. Nat Rev Microbiol. 2010;8:231–241. doi: 10.1038/nrmicro2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung L, Bailey D, Leen EN, Emmott EP, Chaudhry Y, Roberts LO, Curry S, Locker N, Goodfellow IG. Norovirus Translation Requires Interaction Between the C Terminus of the Genome-Linked Viral Protein VPg and Eukaryotic Translation Initiation Factor 4G*. J Biol Chem. 2014;289:21738–21750. doi: 10.1074/jbc.M114.550657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Royall E, Doyle N, Abdul-Wahab A, Emmott E, Morley SJ, Goodfellow I, Roberts LO, Locker N. Murine Norovirus 1 (MNV1) Replication Induces Translational Control of the Host by Regulating elF4E Activity During Infection. J Biol Chem. 2015;290:4748–4758. doi: 10.1074/jbc.M114.602649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karst SM, Wobus CE. A Working Model for How Noroviruses Infect the Intestine. PLoS Pathogens. 2015;11:e1004626. doi: 10.1371/journal.ppat.1004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang KO, Takahashi D, Prakash O, Kim Y. Characterization and Inhibition of Norovirus Proteases of Genogroups I and II Using a Fluorescence Resonance Energy Transfer Assay. Virology. 2012;423:125–133. doi: 10.1016/j.virol.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim Y, Lovell S, Tiew KC, Mandadapu SR, Alliston KR, Battaile KP, Groutas WC, Chang KO. Broad-Spectrum Antivirals Against 3C or 3C-like Proteases of Picornaviruses, Noroviruses, and Coronaviruses. J Virol. 2012;86:11754–11762. doi: 10.1128/JVI.01348-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galasiti Kankanamalage AC, Kim Y, Weerawarna PM, Uy RAZ, Damalanka VC, Mandadapu SR, Alliston KR, Mehzabeen N, Battaile KP, Lovell S, Chang K-O, Groutas WC. Structure-Guided Design and Optimization of Dipeptidyl Inhibitors of Norovirus 3CL Protease. Structure-Activity Relationships and Biochemical, X-Ray Crystallographic, Cell-Based, and In Vivo Studies. J Med Chem. 2015;58:3144–3155. doi: 10.1021/jm5019934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muhaxhiri Z, Deng L, Shanker S, Sankaran B, Estes MK, Palzkill T, Song Y, Venkataraman Prasad BV. Structural Basis of Substrate Specificity and Protease Inhibition in Norwalk Virus. J Virol. 2013;87:4281–4292. doi: 10.1128/JVI.02869-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deng L, Muhaxhiri Z, Estes MK, Palzkill T, Venkataram Prasad BV, Song Y. Synthesis, Activity and Structure-Activity Relationship of Noroviral Protease Inhibitors. MedChemComm. 2013;4:1354–1359. doi: 10.1039/C3MD00219E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hussey RJ, Coates L, Gill RS, Erskine PT, Coker S-F, Mitchell E, Cooper JB, Wood S, Broadbridge R, Clarke IN, Lambden PR, Shoolingin-Jordan PM. Structural Study of Norovirus 3C Protease Specificity: Binding of a Designed Active Site-Directed Peptide Inhibitor. Biochemistry. 2011;50:240–249. doi: 10.1021/bi1008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeitler CE, Estes MK, Venkataram Prasad BV. X-Ray Crystallographic Structure of the Norwalk Virus Protease at 1.5 A Resolution. J Virol. 2006;80:5050–5058. doi: 10.1128/JVI.80.10.5050-5058.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakamura K, Someya Y, Kumasaka T, Ueno G, Yamamoto M, Sato T, Takeda N, Miyamura T, Tanaka N. A Norovirus Protease Structure Provides Insights into Active and Substrate Binding Site Integrity. J Virol. 2005;79:13685–13693. doi: 10.1128/JVI.79.21.13685-13693.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takahashi D, Hiromasa Y, Kim Y, Anbanandam A, Yao X, Chang K-O, Prakash O. Structural and Dynamics Characterization of Norovirus Protease. Protein Sci. 2013;22:347–357. doi: 10.1002/pro.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hardy ME, Crone TJ, Brower JE, Ettayebi K. Substrate Specificity of the Norwalk Virus 3C-Like Proteinase. Virus Res. 2002;89:29–39. doi: 10.1016/s0168-1702(02)00114-4. [DOI] [PubMed] [Google Scholar]
- 67.Someya Y, Takeda N, Miyamura T. Characterization of the Norovirus 3C-Like Protease. Virus Res. 2005;110:91–97. doi: 10.1016/j.virusres.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blakeney SJ, Cahill A, Reilly PA. Processing of Norwalk Virus Nonstructural Proteins by a 3C-like Cysteine Proteinase. Virology. 2003;308:216–224. doi: 10.1016/s0042-6822(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 69.Allaire M, Chernaia MM, Malcolm BA, James MN. Picornaviral 3C Cysteine Proteinases Have a Fold Similar to Chymotrypsin-like Serine Proteinases. Nature. 1994;369:72–76. doi: 10.1038/369072a0. [DOI] [PubMed] [Google Scholar]
- 70.Malcolm BA. The Picornaviral 3C Proteinases: Cysteine Nucleophiles in Serine Proteinase Folds. Protein Sci. 1995;4:1439–1445. doi: 10.1002/pro.5560040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schramm VL. Transition States, Analogues, and Drug Development. ACS Chem Biol. 2013;8:71–81. doi: 10.1021/cb300631k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tiew K-C, He G, Aravapalli S, Mandadapu SR, Gunnam MR, Alliston KR, Lushington GH, Kim Y, Chang K-O, Groutas WC. Design, Synthesis, and Evaluation of Inhibitors of Norwalk Virus 3C Protease. Bioorg Med Chem Lett. 2011;21:5315–5319. doi: 10.1016/j.bmcl.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mandadapu SR, Weerawarna PM, Gunnam MR, Alliston KR, Lushington GH, Kim Y, Chang K-O, Groutas WC. Potent Inhibition of Norovirus 3CL Protease by Peptidyl α-Ketoamides and α-Ketoheterocycles. Bioorg Med Chem Lett. 2012;22:4820–4826. doi: 10.1016/j.bmcl.2012.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mandadapu SR, Gunnam MR, Galasiti Kankanamalage AC, Uy RAZ, Alliston KR, Lushington GH, Kim Y, Chang K-O, Groutas WC. Potent Inhibition of Norovirus by Dipeptidyl α-Hydroxyphosphonate Transition State Mimics. Bioorg Med Chem Lett. 2013;23:5941–5944. doi: 10.1016/j.bmcl.2013.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prior AM, Kim Y, Weerasekara S, Moroze M, Alliston KR, Uy RAZ, Groutas WC, Chang K-O, Hua DH. Design, Synthesis, and Bioevaluation of Viral 3C and 3C-like Protease Inhibitors. Bioorg Med Chem Lett. 2013;23:6317–6320. doi: 10.1016/j.bmcl.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rocha-Pereira J, Nascimento MS, Ma Q, Hilgenfeld R, Neyts J, Jochmans D. The Enterovirus Protease Inhibitor Rupintrivir Exerts Cross-Genotypic Anti-Norovirus Activity and Clears Cells from the Norovirus Replicon. Antimicrob Agents Chemother. 2014;58:4675–4681. doi: 10.1128/AAC.02546-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mandadapu SR, Gunnam MR, Tiew K-C, Uy RAZ, Prior AM, Alliston KR, Hua DH, Kim Y, Chang K-O, Groutas WC. Inhibition of Norovirus 3CL Protease by Bisulfite Adducts of Transition State Inhibitors. Bioorg Med Chem Lett. 2013;23:62–65. doi: 10.1016/j.bmcl.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin JH. Pharmacokinetics of Biotech Drugs: Peptides, Proteins, and Monoclonal Antibodies. Curr Drug Metab. 2009;10:661–691. doi: 10.2174/138920009789895499. [DOI] [PubMed] [Google Scholar]
- 79.Craik DJ, Fairlie DP, Liras S, Price D. The Future of Peptide-Based Drugs. Chem Biol Drug Des. 2013;81:136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 80.Mallinson J, Collins I. Macrocycles in New Drug Discovery. Future Med Chem. 2012;4:1409–1438. doi: 10.4155/fmc.12.93. [DOI] [PubMed] [Google Scholar]
- 81.Marsault E, Peterson ML. Macrocyles are Great Cycles: Applications, Opportunities, and Challenges of Synthetic Macrocycles in Drug Discovery. J Med Chem. 2011;54:1961–2004. doi: 10.1021/jm1012374. [DOI] [PubMed] [Google Scholar]
- 82.Giordanetto F, Kihlberg J. Macrocyclic Drugs and Clinical Candidates: What Can Medicinal Chemists Learn from Their Properties? J Med Chem. 2014;57:278–295. doi: 10.1021/jm400887j. [DOI] [PubMed] [Google Scholar]
- 83.Mathiowetz AM, Leung SSF, Jacobson MP. Optimizing the Permeability and Oral Bioavailability of Macrocyles. In: Levin J, editor. Macrocycles in Drug Discovery. Royal Society of Chemistry; Cambridge, UK: 2015. pp. 367–397. [Google Scholar]
- 84.Alex A, Millan DS, Perez M, Wakenhut F, Whitlock GA. Intramolecular Hydrogen Bonding to Improve Membrane Permeability and Absorption in Beyond the Rule of Five Chemical Space. MedChemComm. 2011;2:669–674. [Google Scholar]
- 85.Tyndall JDA, Fairlie DP. Macrocycles Mimic The Extended Peptide Conformation Recognized By Aspartic, Serine, Cysteine and Metallo Proteases. Curr Med Chem. 2001;8:893–907. doi: 10.2174/0929867013372715. [DOI] [PubMed] [Google Scholar]
- 86.Tyndall JD, Nall T, Fairlie DP. Proteases Universally Recognize β-Strands in Their Active Sites. Chem Rev. 2005;105:973–999. doi: 10.1021/cr040669e. [DOI] [PubMed] [Google Scholar]
- 87.Madala PK, Tyndall JD, Nall T, Fairlie DP. Update 1 of: Proteases Universally Recognize β-Strands in Their Active Sites. Chem Rev. 2010;110:PR1–PR31. doi: 10.1021/cr900368a. [DOI] [PubMed] [Google Scholar]
- 88.Lee J-H, Alam I, Han KR, Cho S, Shin S, Kang S, Yang JM, Kim KH. Crystal Structures of Murine Norovirus-1 RNA-dependent RNA Polymerase. J Gen Virol. 2011;92:1607–1616. doi: 10.1099/vir.0.031104-0. [DOI] [PubMed] [Google Scholar]
- 89.Alam I, Lee J-H, Cho KJ, Han KR, Yang JM, Chung MS, Kim KH. Crystal Structures of Murine Norovirus-1 RNA-dependent RNA Polymerase in Complex with 2-Thiouridine or Ribavirin. Virology. 2012;426:143–151. doi: 10.1016/j.virol.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 90.Croci R, Pezzullo M, Tarantino D, Milani M, Tsay S-C, Sureshbabu R, Tsai Y-J, Mastrangelo E, Rohayem J, Bolognesi M, Hwu JR. Structural Bases of Norovirus RNA Dependent RNA Polymerase InhibitIon by Novel Suramin-Related Compounds. PLoS One. 2014;9:e91765. doi: 10.1371/journal.pone.0091765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eltahla AA, Lim KL, Eden JS, Kelly AG, Mackenzie JM, White PA. Nonnucleoside Inhibitors of Norovirus RNA Polymerase: Scaffolds for Rational Drug Design. Antimicrob Agents Chemother. 2014;58:3115–3123. doi: 10.1128/AAC.02799-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rocha-Pereira J, Jochmans D, Debing Y, Verbeken E, Nascimento MSJ, Neyts J. The Viral Polymerase Inhibitor 2′-C-Methylcytidine Inhibits Norwalk Virus Replication and Protects Against Norovirus-Induced Diarrhea and Mortality in a Mouse Model. J Virol. 2013;87:11798–11805. doi: 10.1128/JVI.02064-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Costantini VP, Whitaker T, Barclay L, Lee D, McBrayer TR, Schinazi RF, Vinje J. Antiviral Activity of Nucleoside Analogues Against Norovirus. Antiviral Ther. 2012;17:981–991. doi: 10.3851/IMP2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rocha-Pereira J, Jochmans D, Dallmeier K, Leyssen P, Nascimento MS, Neyts J. Favipiravir (T-705) Inhibits In Vitro Norovirus Replication. Biochem Biophys Res Commun. 2012;424:777–780. doi: 10.1016/j.bbrc.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 95.Zhang X, Tan M, Chhabra M, Dai Y, Meller J, Jiang X. Inhibition of Blood Group Antigen Binding as a Novel Strategy to Block Norovirus Infections. PLoS One. 2013;8:e69379. doi: 10.1371/journal.pone.0069379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guiard J, Kitov PI, Fiege B, Peters T, Bundle DR. Double-Click” Protocol for Synthesis of Heterobifunctional Multivalent Ligands: Toward a Focused Library of Specific Norovirus Inhibitors. Chem – Eur J. 2011;17:7438–7441. doi: 10.1002/chem.201003414. [DOI] [PubMed] [Google Scholar]
- 97.Rademacher C, Guiard J, Kitov PI, Fiege B, Dalton KP, Parra F, Bundle DR, Peters T. Targeting Norovirus Infection–Multivalent Entry Inhibitor Design Based on NMR Experiments. Chem – Eur J. 2011;17:7442–7453. doi: 10.1002/chem.201003432. [DOI] [PubMed] [Google Scholar]
- 98.Zhang X-F, Tan M, Chhabra M, Dai Y-C, Meller J, Jiang X. Inhibition of Histo-blood Group Antigen Binding as a Novel Strategy to Block Norovirus Infections. PLoS One. 2013;8:e69379. doi: 10.1371/journal.pone.0069379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.El-Hawiet A, Shoemaker GK, Daneshfar R, Kitova EN, Klassen JS. Applications of a Catch and Release Electrospray Ionization Mass Spectrometry Assay for Carbohydrate Library Screening. Anal Chem. 2012;84:50–58. doi: 10.1021/ac202760e. [DOI] [PubMed] [Google Scholar]
- 100.Feng X, Jiang X. Library Screen for Inhibitors Targeting Norovirus Binding to Histo-blood Group Antigen Receptors. Antimicrob Agents Chemother. 2007;51:324–331. doi: 10.1128/AAC.00627-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shivanna V, Kim Y, Chang KO. Endosomal Acidification and Cathepsin L Activity is Required for Calicivirus Replication. Virology. 2014;464–465:287–295. doi: 10.1016/j.virol.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nagy PD, Pogany J. The Dependence of viral RNA Replication on Co-Opted Host Factors. Nat Rev Microbiol. 2012;10:137–149. doi: 10.1038/nrmicro2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vashist S, Urena L, Chaudhry Y, Goodfellow I. Identification of RNA-Protein Interaction Networks Involved in the Norovirus Life Cycle. J Virol. 2012;86:11977–11990. doi: 10.1128/JVI.00432-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chang KO. Role of Cholesterol Pathways in Norovirus Replication. J Virol. 2009;83:8587–8595. doi: 10.1128/JVI.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vashist S, Urena L, Gonzalez-Hernandez MB, Choi J, de Rougemont A, Rocha-Pereira J, Neyts J, Hwang S, Wobus CE, Goodfellow I. The Molecular Chaperone HSP90 is a Therapeutic Target for Noroviruses. J Virol. 2015;89:6352–6363. doi: 10.1128/JVI.00315-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheron N, Yu C, Kolawole AO, Shakhnovich EI, Wobus CE. Repurposing Rutin for the Inhibition of Norovirus Replication. Arch Virol. 2015 Jun 26; doi: 10.1007/s00705-015-2495-y. [DOI] [PubMed] [Google Scholar]
- 107.Gonzalez-Hernandez MJ, Pal A, Gyan KE, Charbonneau ME, Showalter HD, Donato NJ, O’Riordan M, Wobus CE. Chemical Derivatives of a Small Molecule Deubiquitinase Inhibitor Have Antiviral Activity Against Several RNA Viruses. PLoS One. 2014;9:e94491. doi: 10.1371/journal.pone.0094491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chaudhry Y, Nayak A, Bordeleau M-E, Tanaka J, Pelletier J, Belsham GJ, Roberts LO, Goodfellow IG. Caliciviruses Difffer in Their Functional Requirements for elF4F Components. J Biol Chem. 2006;281:25315–25325. doi: 10.1074/jbc.M602230200. [DOI] [PubMed] [Google Scholar]
- 109.Dou D, Tiew K-C, He G, Mandadapu SR, Aravapalli S, Alliston KR, Kim Y, Chang K-O, Groutas WC. Potent Inhibition of Norwalk Virus by Cyclic Sulfamide Derivatives. Bioorg Med Chem. 2011;19:5975–5983. doi: 10.1016/j.bmc.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dou D, Mandadapu SR, Alliston KR, Kim Y, Chang K-O, Groutas WC. Cyclosulfamide-based Derivatives as Inhibitors of Noroviruses. Eur J Med Chem. 2012;47:59–64. doi: 10.1016/j.ejmech.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dou D, Tiew K-C, Mandadapu SR, Gunnam MR, Alliston KR, Kim Y, Chang K-O, Groutas WC. Potent Norovirus Inhibitors Based on the Acyclic Sulfamide Scaffold. Bioorg Med Chem. 2012;20:2111–2118. doi: 10.1016/j.bmc.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dou D, He G, Mandadapu SR, Aravapalli S, Kim Y, Chang K-O, Groutas WC. Inhibition of Noroviruses by Piperazine Derivatives. Bioorg Med Chem Lett. 2012;22:377–379. doi: 10.1016/j.bmcl.2011.10.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pokhrel L, Kim Y, Nguyen TD, Prior AM, Lu J, Chang K-O, Hua DH. Synthesis and Anti-Norovirus Activity of Pyranobenzopyrone Compounds. Bioorg Med Chem Lett. 2012;22:3480–3484. doi: 10.1016/j.bmcl.2012.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dou D, Mandadapu SR, Alliston KR, Kim Y, Chang K-O, Groutas WC. Design and Synthesis of Potent Inhibitors of Noroviruses by Scaffold Hopping. Bioorg Med Chem. 2011;19:5749–5755. doi: 10.1016/j.bmc.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rocha-Pereira J, Cunha R, Pinto DC, Silva AM, Nascimento MS. E)-2-Styrylchromones as Potential Anti-Norovirus Agents. Bioorg Med Chem. 2010;18:4195–4201. doi: 10.1016/j.bmc.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 116.Rossignol JF, El-Gohary Y. Nitazoxanide in Treatment of Viral Gastroenteritis: a Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Aliment Pharmacol Ther. 2006;24:1423–1430. doi: 10.1111/j.1365-2036.2006.03128.x. [DOI] [PubMed] [Google Scholar]
- 117.Rossignol JF. Nitazoxanide: A First-in-Class Broad-Spectrum Antiviral Agent. Antiviral Res. 2014;110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wyatt PG, Gilbert IH, Read KD, Fairlamb AH. Target Validation: Linking Target and Chemical Properties to Desired Product Profile. Curr Top Med Chem. 2011;11:1275–1283. doi: 10.2174/156802611795429185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ng KK-S, Pendas-Franco N, Rojo J, Boga JA, Machin A, Martin Alonso JM, Parra F. Crystal Structure of Norwalk Virus Polymerase Reveals the Carboxyl Terminus in the Active Site Cleft. J Biol Chem. 2004;279:16638–16645. doi: 10.1074/jbc.M400584200. (PDB code 1SH2) [DOI] [PubMed] [Google Scholar]


