Abstract
Spinal cord injury (SCI) typically manifests with a loss of sensorimotor control of the lower limbs. In order to overcome some of the disadvantages of chronic wheelchair use by such patients, robotic exoskeletons are an emerging technology that has the potential to transform the lives of patients. However, there are a number of points of contact between the robot and the user, which lead to interaction forces. In a recent study, the authors have shown that peak interaction forces are particularly prominent at the anterior aspect of the right leg. This study uses a similar experimental protocol with additional electromyography (EMG) analysis to examine whether such interaction forces are due to the muscular activity of the participant or the movement of the exoskeleton itself. Interestingly, the authors found that peak forces preceded peak EMG activity. This study did not find a significant correlation between EMG activity and force data, which would indicate that the interaction forces can largely be attributed to the movement of the exoskeleton itself. However, we also report significantly higher correlation coefficients in muscle/force pairs located at the anterior aspect of the right leg. In their previous research, the authors have shown peak interaction forces at the same locations, which suggests that muscular activity of the participant makes a more significant contribution to the interaction forces at these locations. The findings of this study are of significance for incomplete SCI patients, for whom EMG activity may provide an important input to an intuitive control schema.
Keywords: artificial limbs, electromyography, injuries, wheelchairs
Keywords: muscular activity, physical interaction forces, lower limb exoskeleton use, spinal cord injury, SCI, sensorimotor control, lower limbs, chronic wheelchair use, robotic exoskeletons, peak interaction forces, anterior aspect, right leg, electromyography analysis, EMG, intuitive control schema
1. Introduction
As a result of spinal cord injury (SCI), many people typically experience loss of sensorimotor control of the lower limbs. Therefore, in order to regain a degree of autonomy, patients often use wheelchairs. However, prolonged wheelchair use is known to elicit an array of complications, including shoulder pain, the profound loss of bone mineral density as well as pressure ulcer development [1, 2]. Moreover, wheelchair use is completely impractical for achieving many activities of daily living that we take for granted, such as reaching shelves and climbing stairs. Therefore, robotic exoskeletons are beginning to emerge as a technology that has the potential to transform the lives of patients physically, psychologically and socially [3].
When using any type of exoskeleton device, there is both a cognitive and a physical coupling with the user. The latter often involves multiple points of human-robot contact, at which a net flux of power generated by the exoskeleton is transferred to the viscoelastic soft tissues of the patient [3]. In most lower limb exoskeletons, this transfer of power is distributed through one or two types of interface: connection cuffs (soft belts) and orthoses (plastic braces against which the leg is supported) [4]. Furthermore, the complexity of human joint kinematics is almost impossible to emulate exactly in robotic design and it varies wildly from person to person; joint movement is influenced by a variety of internal structures (i.e. ligaments and tendons) and the inherent migration of the joint centre during movement [5]. As a consequence, the axes of joint rotation between the user and the exoskeleton are micro-misaligned, which generates potentially harmful interaction forces, such as shear forces at the physical human-robot interface (pHRI) [5].
Studies investigating the changes in physiological metrics during exoskeleton operation and on the safety of such devices need to be pursued [3]. For example, trained therapists currently use protocols that have a high dependence upon heuristic personal experience when strapping users into the exoskeleton apparatus. This issue is of particular importance for SCI patients (since they are an extremely heterogeneous population) who are susceptible to skin lesions and pressure ulcer development. This can be due to impaired sensation as well as physiological changes in denervated skin, which antagonises efficient wound healing and typically manifest in this patient population.
Until now, there has only been a handful of studies that begin to investigate the interaction forces at the pHRI [6–8]. Such studies have measured interaction forces using mathematical modelling [6], direct measurement using load cells [7] and opto-electronic sensors [8]. However, due to the complex interaction dynamics presented by the viscoelastic soft tissues and elastic cuffs, the accuracy of the mathematical model is difficult to measure. Moreover, these studies used single point sensors, which are able to record data from only one specific point of the interface. In this sense, the use of such sensors are limited when used to provide data on interaction forces at the pHRI since they do not provide information on the distribution of force across the human-exoskeleton interface (cuffs and orthoses). It is worth acknowledging that this issue can be countered by constructing an array of load cells to measure a multiple number of contact points.
However, this is both complex to calibrate and costly. Similarly, pressure pads have been developed in one study that were used to record pressure maps for one healthy participant and one SCI patient [9]. This approach may be flawed since the integration of force sensing resistors (FSRs) into a flexible pressure-distributing pad may cause undue bending of the FSRs when a load is applied, which is likely to modulate the voltage output and hence affect the accuracy of their results.
To address this, Rathore et al. (2016) present an alternative real-time force-monitoring apparatus using FSRs installed at the pHRI along with experimental protocols to quantify the pHRI forces during a range of exoskeleton movement primitives (Fig. 1) [10]. 16 key locations were identified to monitor and obtain force data from ten healthy participants. Notably, the study identified peak forces that were particularly prominent at the anterior aspect of the leg, an area particularly prone to the development of pressure ulcers. However, it is largely unknown whether the interaction forces recorded at the pHRIs are due to the muscular activity of the participant (e.g. due to co-contraction, anticipation or hesitance) or due to the movement of the exoskeleton itself. Thus, this study sets out to explore the phenomenon further by measuring the activity of selected muscles in the lower limb, via electromyography (EMG), when using a similar experimental protocol to that proposed in [10].
Fig. 1.

Testing interaction force data acquisition at the pHRI
The acquisition and subsequent analysis of EMG data from exoskeleton participants is of great significance. For incomplete SCI patients, who still have some voluntary (albeit perhaps not functional) muscular activity, it is thought that the EMG activity could provide an important input for the development of a user intention based algorithm to replace the cumbersome joystick interface currently used in a range of exoskeleton models. Moreover, there has been limited research investigating EMG activity when using a robotic lower limb exoskeleton device, most of which has been acquired using a small subset of exoskeleton movement primitives such as stand to sit or vice versa [11, 12]. Such studies have investigated EMG activity with the view of developing a user intention based algorithm for the control of the exoskeleton [11, 12]. However, this knowledge has been largely developed away from the complex physical human-robot interactions that have been highlighted in a number of studies [9, 10]. Hence, this study presents suitable experimental protocols and data to extend the findings of [10].
In this Letter we extend the previous findings by incorporating EMG analysis into the experimental protocol to identify whether the interaction forces recorded here are due to the movement of the exoskeleton itself or the muscular activity of the participant. Thus, we address two key research questions in the present Letter to analyse this further:
Is there a significant correlation between the force profile and the EMG activity through the gait cycle?
Are there any muscle groups that show a statistically significant difference in correlation coefficient across different tasks?
2. Materials and methods
A mechanically stable lower limb robotic exoskeleton, REX Personal (Rex Bionics, New Zealand), which is controlled by a joystick interface, was used for the experiments. This device did not require the users to balance, by compensating with their upper body and e.g. crutches or parallel bars. This is important since it reduced the confounding factors when analysing the interaction forces with the lower limbs.
The Interlink Electronics FSR (FSR 400) were selected to record force data at the physical interface due to their low cost, reliability and versatility. Furthermore, they have a large and appropriate sensitivity range for our application (0.1N-100N) and a small sensing area (5.1 mm diameter). The latter property permits force measurements over small contact points and reduces bending effects of the cuff/strap.
We created a force data acquisition module, based on an Arduino microcontroller (Arduino Mega 2560, Italy) and built a graphical user interface in Matlab (MathWorks, USA). Each sensor was interfaced with a plastic Velcro backing and individually calibrated. The latter step was taken to minimise any systematic error in the measurements. A mechanical testing machine (Zwick Roell Z005, Germany), equipped with a 5kN load cell was used to calibrate the sensors. A flat and rigid indenter constructed from stainless steel whose diameter matched the area of the FSR sensing area, was applied over the Velcro at the location corresponding to the sensing area of the underlying FSR. A ramped load between 1 and 100N was then administered at a speed of 1 mm/minute (three repeats) for each sensor. Simultaneously, a force-deformation profile was recorded with the voltage response for each sensor. Using Matlab, the force vs voltage output data was then plotted and fitted with a first-order exponential equation.
Four force sensors were placed at each of the four c-shaped braces/orthosis (two thigh braces and two leg braces): medially, laterally and posteriorly on the brace, and one anteriorly by interfacing with the strap (16 FSRs in total). This study extended the methodology presented in [10] to include EMG analysis such that muscle/force pairs could be identified for further investigation of the peak interaction forces found at the pHRI.
The muscles investigated on each leg were the Rectus Femoris, Tibialis Anterior and the Soleus. These muscles were chosen based on both their functional significance in the gait cycle and on the locations of the peak forces observed in the previous study [10].
Bipolar pairs of surface EMG electrodes were used to record the activity of the rectus femoirs, tibialis anterior and soleus muscles on each leg. The position of each electrode to measure each respective muscle group was determined by the recommendations of SENIAM [13]. The TMSi Porti system was used for the purposes of EMG data acquisition. The force and EMG data was synchronised by sending a hardware trigger to the TMSi amplifier whenever the arduino began sampling and recording the force data.
The gait cycle of REX does not emulate the natural gait of humans [14]. Therefore, a series of triggers were also developed in Matlab so that the force and EMG data could be easily related to the kinematics of the exoskeleton [10].
Once data acquisition was complete, the EMG data was first normalised by measuring muscle activation as a percentage of the maximal observed EMG activity during the movement of the exoskeleton. This method was used to normalise the EMG data as opposed to maximal voluntary contraction since the muscle groups under investigation were too difficult to isolate. The EMG data was then processed further using full wave rectification with a low pass filter of 2 Hz.
3. Experiment protocol
This study received ethical approval from the University College London Research Ethics Committee (6859/001). Participants were able-bodied adults, aged between 18 and 65 and were both physically and cognitively healthy. Volunteers were excluded if they had any pathology that affected their gait.
Data was recorded from ten participants and Table 1 describes the participant demographics.
Table 1.
Participant demographics
| Variables | Range |
|---|---|
| gender | male = 8, female = 2 |
| age | 18–43 years |
| dominant foot | right = 10 Left = 0 |
Before each participant mounted the exoskeleton, the area of skin to which the electrodes were to be attached was treated to remove dead skin and cells. The electrodes were then attached to the participant. The participant was then asked to wear the exoskeleton and the experimenter helped them to fasten the cuffs to a tension that the participant deemed comfortable. A period of five minutes was given to each participant to experience the movement primitives of the exoskeleton. After this, data was acquired whilst the participants performed two steps forward (a full gait cycle). This protocol was repeated again but this time taking two steps backward and then finally taking two left sidesteps. The participant repeated these protocols three times.
4. Results
Results were determined statistically insignificant where p > 0.05 unless stated otherwise. Asterisks displayed on graphs represent where a statistically significant difference (p < 0.05) has been reported.
To measure the relationship between muscle activity and interaction forces, we calculated the Pearsons correlation coefficient between the force and EMG variables using the following equation
where x = force and y = EMG.
All statistical analysis was performed using the t-test to assess whether there was a statistically significant difference between the different muscle groups across the same movement primitive and/or whether there are any muscle groups that show a statistically significant difference in correlation coefficient across different tasks.
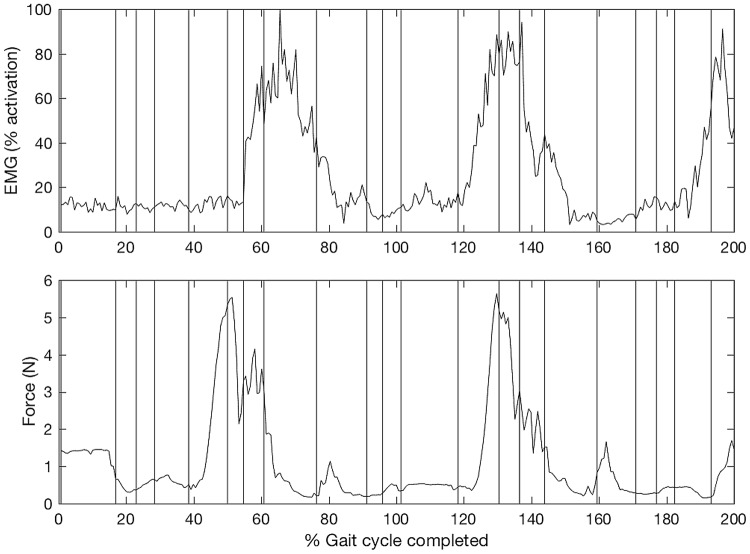
Fig. 2 illustrates that significant increases in force precede the corresponding increases in the EMG signals by around 20 samples. Indeed, across all the data collected in this study, the increases in force precede the corresponding increases in EMG activity by an average of 18 ± 6 samples (p < 0.001). The force data was sampled at a frequency of 100 Hz. This indicates that on average there is a time interval of around 180 ms between the increase in force and a corresponding contraction of the muscle, suggesting the muscular contraction is in response to the exoskeleton movement.
Fig. 2.
Top graph represents EMG activity of the rectus femoris while the bottom graph represents force data from the corresponding anterior thigh force sensor when performing the forward movement primitive. The vertical lines indicate the recorded trigger points used in the data analysis
Moreover, Fig. 2 demonstrates that in some participants across some muscle/force pairs, a good correlation was observed between EMG and force data for certain movement primitives. Indeed, in four of the participants, we found a good correlation between the right rectus femoris/anterior force sensor (defined as r > 0.7) when performing the forwards movement primitive. This was observed in the first of the three repeats of this movement primitive. Upon subsequent repeats, the correlation coefficient decreased. The average correlation coefficient across the next two trials was r = 0.34.
4.1. Analysis of interaction forces
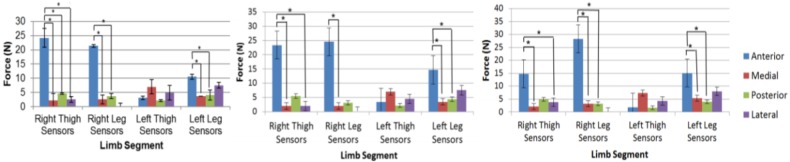
In comparison with force sensors that had been interfaced with the exoskeleton orthosis, forces acting across the exoskeleton strap anteriorly (across the exoskeleton strap) are higher in magnitude. This is demonstrated in Fig. 3. Furthermore, Fig. 3 shows that a median peak force of 22.58N was found at the anterior sensor for the right thigh when taking a step forward. This is significantly higher than the median peak force of the medial (1.91N), posterior (4.83N) and lateral (1.46N) sensors of the right thigh. In order to compute statistical significance a (non-parametric) Friedman test (χ2 = 19.56, p < 0.001) followed by pairwise comparisons with a Bonferroni correction for multiple comparisons (p < 0.001, p = 0.034 and p = 0.002, respectively) was used. An exception to this was the anterior sensor of the left thigh where peak force is not significantly different compared with the medial, posterior and lateral sensors of the left thigh when walking forward, backward or to the side.
Fig. 3.
Average peak force for sensors across various limb segments when walking forward (leftmost plot), backward (centre plot) and to the side (rightmost plot). The asterisks represent statistically significant differences (p < 0.005). Adapted from [10]
Significant differences between the peak forces at the pHRI (p < 0.005) were reported for only five of the 16 sensors that were interfaced with the exoskeleton for all three movement primitives. Of particular interest, the peak forces observed at the anterior aspect of the right and left legs showed a significant difference for the three different movement primitives according to a Friedman test; χ2(2) = 10.4, p = 0.006 and χ2(2) = 15.2, p < 0.002, respectively (Fig. 3).
4.2. Analysis of correlation between muscle activity and interaction forces
Here we address our research question (1): Is there a statistically significant difference between the correlation coefficients for the muscle groups across the same movement primitive?
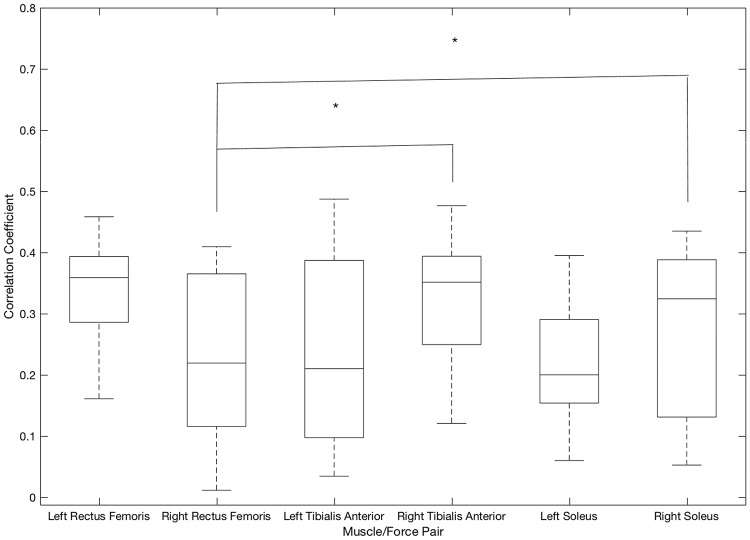
Forwards movement primitive (Fig. 4): The average correlation coefficient for the right soleus/force pair (r = 0.34) was significantly higher (p = 0.003) than that for the right rectus femoris force pair (r = 0.21). However, for the same muscle/force pairs in the left leg the difference between the correlation coefficients were not statistically significant.
Fig. 4.
Correlation coefficients between muscle activity and force profile when performing the forwards movement primitive
Similarly, the right tibialis anterior/anterior leg force sensor (r = 0.33) had a significantly higher average correlation coefficient (p = 0.0129) than the right rectus femoris/anterior thigh force sensor (r = 0.20). Yet, for the same muscle/force pairings in the left leg, there was no statistically significant difference between the correlation coefficients for any of the other muscle/force pairs.
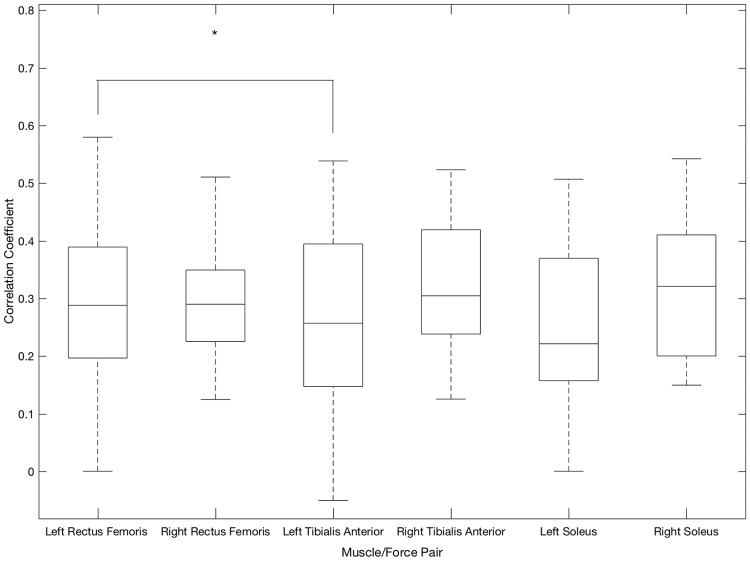
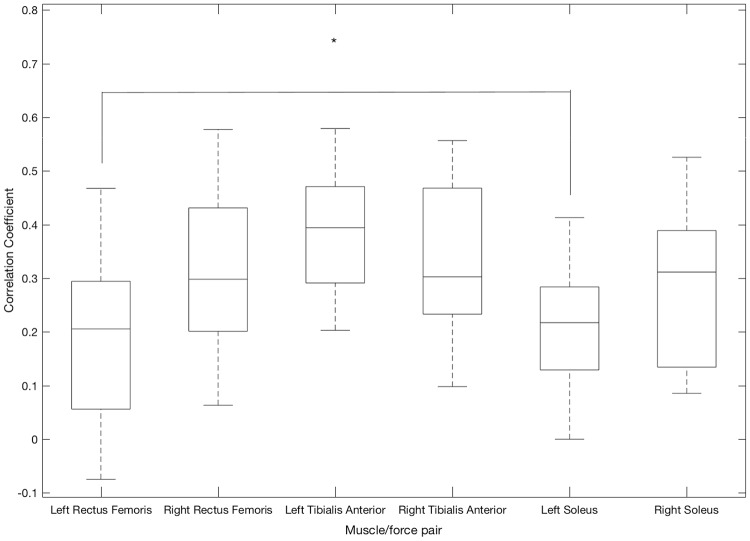
Backwards movement primitive (Fig. 5): The average correlation coefficient for the left rectus femoris/anterior thigh sensor (r = 0.35) was significantly higher (p = 0.0258) than that for the left tibialis anterior muscle/force pair (r = 0.23). None of the other muscle/force pairs yielded statistically significant differences between correlation coefficients when performing the backwards movement primitive.
Fig. 5.
Correlation coefficients between muscle activity and force profile when performing the backwards movement primitive
Sideways movement primitive (Fig. 6): The average correlation coefficient for the left soleus/force pair (r = 0.38) was significantly (p = 0.0022) higher than that for the left rectus femoris (r = 0.22). No statistically significant difference between correlation coefficients for any of the other muscle/force pairs was recorded.
Fig. 6.
Correlation coefficients between muscle activity and force profile when performing the sidestep movement primitive
4.3. Analysis of correlation between muscle activity and interaction forces across different movement primitives
Here we address our research question (2): Are there any muscle groups that show a statistically significant difference in correlation coefficient across different tasks?
Left rectus femoris muscle: The correlation coefficient for the left rectus femoris muscle/force pair was significantly (p = 0.0255) higher when performing the backwards movement primitive (r = 0.35) compared with the same muscle/force pair when carrying out the sideways movement primitive (r = 0.22). However, there was no significant difference between the correlation coefficients for the same muscle/force pair for the other movement primitives.
Right rectus femoris muscle: The average correlation coefficient for this muscle/force pair when performing the backwards movement primitive (r = 0.34) was significantly (p = 0.0018) higher than when performing the forwards movement primitive (r = 0.20). Moreover, the correlation coefficient for the sideways movement primitive (r = 0.32) was also significantly (p = 0.0174) higher than for the forwards movement primitive (r = 0.20). No significant difference between correlation coefficients for this muscle/force pair between the backwards and sideways movement primitives was recorded.
Left soleus muscle: No statistically significant difference was found between the forwards and backwards primitives for the correlation coefficients. This was also true for between the forwards and sideways movement primitives.
The average correlation coefficient when performing the sideways movement primitive (r = 0.38) was significantly (p = 0.0409) higher than the average correlation coefficient recorded for the same muscle/force pair when performing the backwards movement primitive (r = 0.28).
Right soleus muscle: No statistically significant difference was found between the correlation coefficients for any movement primitives for this muscle/force pair.
Left tibialis anterior muscle: No statistically significant difference was found between the correlation coefficients for any movement primitives for this muscle/force pair.
Right tibialis anterior muscle: No statistically significant difference was found between the correlation coefficients for any movement primitives for this muscle/force pair.
5. Discussion
In previous work, we have demonstrated that peak forces in the anterior aspect of the right leg were significantly higher compared with other sensor locations [10]. It is known that the risk of pressure ulcer development is dependent upon the position of contact between the user and the environment [15, 16]. This contact is enhanced at the anterior aspect of the leg, especially at areas of bony prominence, such as at the anterior tibial aspect of the leg where the soft tissues are more easily compressed between the exoskeleton cuff and the rigid underlying bone [16]. Furthermore, the use of cuffs/straps as the physical interface between the user and exoskeleton have been shown to constrict the soft tissues and underlying capillaries and thus creating frictional forces as they slide across the user's skin and/or clothes and can fold the user's skin contributing to a greater risk of skin lesion and pressure ulcer development compared with the use of orthoses/braces [17, 18].
This study used a similar experimental protocol to determine whether the interaction forces reported in our previous study [10] were due to the muscular activity of the participant or the movement of the exoskeleton itself. In order to address this, we utilised the same methodology with the addition of EMG surface electrodes to measure muscular activity when performing the movement primitives in the exoskeleton. Through this approach, one is able to compute correlation coefficients for different muscle/force pairs and compare which signal led the other. If the data has a high correlation, one can postulate that the interaction forces can be attributed to the muscular activity of the participant. On the other hand, muscle/force pairs that have little correlation one can perhaps attribute the interaction forces at such positions to the movement of the exoskeleton. Furthermore, for those muscle/force pairs that do have a high correlation, if the increase in force precedes the increase in muscular activity, then the muscular activity is in response to the movement of the exoskeleton. Conversely, if the increase in muscular activity precedes the corresponding increase in interaction force, then the muscular activity is in anticipatory of the action, with the users effectively leading the exoskeleton.
On initial inspection of the data presented in this study, one may conclude that since all of the muscle/force pairs investigated in this study were poorly correlated, the interaction forces are predominantly due to the movement of the exoskeleton and not the voluntary muscular activity of the participant. However, upon closer analysis, one can begin to understand some of the reasons behind significantly higher interaction forces at various pHRIs.
The finding of significantly higher interaction forces at the anterior aspect of the right leg reported by Rathore et al. can perhaps be explained by the results presented in this study. While the data recorded at this position is generally poorly correlated (average r = 0.33), this study finds that it is nevertheless significantly higher than that found for other muscle/force pairs. This suggests that while the movement of the exoskeleton may be predominantly responsible for the interaction forces at this location, it appears that the muscular activity of the participant also makes a more significant contribution to the interaction forces compared with other locations. In short, the peak interaction forces at this location appear to be due to the movement of the exoskeleton which is further antagonised by the muscular activity of the participant more so than at other muscle/force pair locations resulting in the significantly higher interaction forces recorded at this location.
The finding that a good correlation exists for four participants for the right rectus femoris/anterior thigh force sensor upon the first trial perhaps suggests that muscular factors such as hesitance or co-contraction are initially responsible for the interaction forces upon the first trial. The decline in correlation could perhaps be attributed to the differing abilities of participants to acclimatise to the un-natural statically balanced cycle exhibited by the exoskeleton used in this study [14]. As participants become more comfortable with the operation of the exoskeleton, the interaction forces become less dependent upon the involuntary/voluntary muscular movements of the participants and more dependent upon the movement of the exoskeleton itself. This is supported by the average correlation coefficient recorded in the subsequent two trials (average r = 0.31). This should be investigated further as it would appear from the data recorded in this study that the interaction forces upon initial use of the exoskeleton could be due to, at least in part, to the muscular activity of the participant, whereas data from subsequent trials may be more representative of users with paraplegia.
The correlation, coupled with the time delay, suggests that users are contracting their muscles in response to perturbations inflicted by the exoskeleton, rather than trying to lead the exoskeleton. It also indicates that the increases in physical interaction forces are likely due to the physical movement of the exoskeleton rather than changes in limb volume due to contraction of the users muscles. It is also of interest that this pattern was observed in the right lower limb of participants and not the left. Table 1 demonstrates the demographics of the participants in this study and reveals that all of our participants were right footed. Given that this pattern reported in these participants was persistently seen in the right lower limb, perhaps the fact that participants were right footed were responsible for this pattern not being present in the left lower limb.
6. Conclusions
The interaction forces at the pHRI appear to be largely attributable to the movement of the exoskeleton itself as opposed to the muscular activity of the participant. However, at locations where significantly higher interaction forces are reported (at the anterior aspect of the leg or thigh for example), muscular activity appears to be of more relevance on than at points where lower interaction forces are found. In short, as the magnitude of the interaction forces increase, muscular activity of the participant appears to be of greater importance.
In a clinical setting, this would suggest that therapists should have an awareness that patients with partial SCI which have some muscular function remaining are subject to higher interaction forces at the pHRI locations especially during their initial training on the exoskeleton. This would appear to be particularly prominent at the anterior aspects of the right leg based on the findings of this Letter and our previous research [10].
7. Acknowledgments
We are very grateful to REX Bionics for the provision of equipment and technical support throughout the project. We would also like to thank everyone who kindly volunteered to participate in the experiments.
8. Funding and Declaration of Interests
REX Bionics PLC provided Dr Carlson and his team with a REX Exoskeleton unit to be used for the duration of the study as well as full training necessary to operate the REX safely. REX Bionics PLC did not review the results or manuscript prior to submission and had no influence on the study design/execution/data processing/data analysis/reporting. Neither REX Bionics nor Dr Carlson will benefit financially from the publication of this scientific study.
9 References
- 1.Carver J., Ganus A., Ivey J.M., et al. : ‘The impact of mobility assistive technology devices on participation for individuals with disabilities’, Disabil. Rehabil. Assist. Technol., 2015, 11, pp. 1–10 (doi: 10.3109/17483107.2015.1027295) [DOI] [PubMed] [Google Scholar]
- 2.Samuelsson K.A.M., Tropp H., Gerdle B.: ‘Shoulder pain and its consequences in paraplegic spinal cord-injured, wheelchair users’, Spinal Cord, 2004, 42, (1), pp. 41–46 (doi: 10.1038/sj.sc.3101490) [DOI] [PubMed] [Google Scholar]
- 3.Dollar A.M., Herr H.: ‘Lower extremity exoskeletons and active orthoses: Challenges and state-of-the-art’, IEEE Trans. Robot., 2008, 24, (1), pp. 144–158 (doi: 10.1109/TRO.2008.915453) [Google Scholar]
- 4.de Rossi S.M.M., Vitiello N., Lenzi T., et al. : ‘Sensing pressure distribution on a lower-limb exoskeleton physical human-machine interface’, Sensors, 2011, 11, (1), pp. 207–227 (doi: 10.3390/s110100207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pons J.L.: ‘Wearable robots: biomechatronic exoskeletons’ 2008
- 6.Schiele A.: ‘An explicit model to predict and interpret constraint force creation in phri with exoskeletons’. IEEE Int. Conf. on Robotics and Automation, 2008. ICRA 2008. 2008, pp. 1324–1330 [Google Scholar]
- 7.Hidler J.M., Wall A.E.: ‘Alterations in muscle activation patterns during robotic-assisted walking’, Clin. Biomech., 2005, 20, (2), pp. 184–193 (doi: 10.1016/j.clinbiomech.2004.09.016) [DOI] [PubMed] [Google Scholar]
- 8.Donati M., Vitiello N., de Rossi S.M.M., et al. : ‘A flexible sensor technology for the distributed measurement of interaction pressure’, Sensors (Switzerland), 2013, 13, (1), pp. 1021–1045 (doi: 10.3390/s130101021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamez-Duque J., Cobian-Ugalde R., Kilicarslan A., et al. : ‘Real-time strap pressure sensor system for powered exoskeletons’, Sensors (Switzerland), 2015, 15, (2), pp. 4550–4563 (doi: 10.3390/s150204550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rathore T.A., Wilcox M., Morgado Ramirez D.Z., et al. ‘Quantifying the human-robot interaction forces between a lower limb exoskeleton and healthy users’. Proc. Annual Int. Conf. IEEE Engineering in Medicine Biology Society, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Reza S.M.T., Ahmad N., Choudhury I.A., et al. : ‘A fuzzy controller for lower limb exoskeletons during sit-to-stand and stand-to-sit movement using wearable sensors’, Sensors (Basel), 2014, 14, (3), pp. 4342–4363 (doi: 10.3390/s140304342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reza S.M.T., Ahmad N., Choudhury I.A., et al. : ‘A study on muscle activities through surface EMG for lower limb exoskeleton controller’. 2013 IEEE Conf. on Systems, Process and Control (ICSPC), 2013, pp. 159–163 [Google Scholar]
- 13.Sacco I.C.N., Gomes A.A., Otuzi M.E., et al. : ‘A method for better positioning bipolar electrodes for lower limb EMG recordings during dynamic contractions.’, J. Neurosci. Methods, 2009, 180, (1), pp. 133–137 (doi: 10.1016/j.jneumeth.2009.02.017) [DOI] [PubMed] [Google Scholar]
- 14.Barbareschi G., Richards R., Thornton M., et al. : ‘Statically vs dynamically balanced gait: Analysis of a robotic exoskeleton compared with a human’. Proc. of the Annual Int. Conf. of the IEEE Engineering in Medicine and Biology Society, EMBS, November 2015, vol. 2015, pp. 6728–6731 [DOI] [PubMed] [Google Scholar]
- 15.Bluestein D., Javaheri A.: ‘Pressure ulcers: prevention, evaluation, and management’, Am. Fam. Physician, 2008, 78, (10), pp. 1186–1194 [PubMed] [Google Scholar]
- 16.Bass M.J., Phillips L.G.: ‘Pressure sores.’, Curr. Probl. Surg., 2007, 44, (2), pp. 101–143 (doi: 10.1067/j.cpsurg.2006.12.007) [DOI] [PubMed] [Google Scholar]
- 17.Cushing C.a., Phillips L.G.: ‘Evidence-based medicine: pressure sores.’, Plast. Reconstr. Surg., 2013, 132, (6), pp. 1720–1732 (doi: 10.1097/PRS.0b013e3182a808ba) [DOI] [PubMed] [Google Scholar]
- 18.Noble J., Munro C.A., Prasad V.S., et al. : ‘Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries.’, J. Trauma, 1998, 45, (1), pp. 116–122 (doi: 10.1097/00005373-199807000-00025) [DOI] [PubMed] [Google Scholar]