Abstract
Background and Aims:
Dexamethasone has a powerful anti-inflammatory action with significant analgesic benefits. The aim of this study was to compare the efficacy of dexamethasone administered through intravenous (IV) and caudal route on post-operative analgesia in paediatric inguinal herniotomy patients.
Methods:
One hundred and five paediatric patients undergoing inguinal herniotomy were included and divided into three groups. Each patient received a single caudal dose of ropivacaine 0.15%, 1.5 mL/kg combined with either corresponding volume of normal saline (Group 1) or caudal dexamethasone 0.1 mg/kg (Group 2) or IV dexamethasone 0.5 mg/kg (Group 3). Baseline, intra- and post-operative haemodynamic parameters, pain scores, time to rescue analgesia, total analgesic consumption and adverse effects were evaluated for 24 h after surgery. Unpaired Student's t-test and analysis of variance were applied for quantitative data and Chi-square test for qualitative data. Time to first analgesic administration was analysed by Kaplan–Meier survival analysis and log-rank test.
Results:
Duration of analgesia was significantly longer (P < 0.001), and total consumption of analgesics was significantly lower (P < 0.001) in Group II and III compared to Group I. The incidence of nausea and vomiting was higher in Group I (31.4%) compared to Group II and III (8.6%).
Conclusions:
Addition of dexamethasone both caudally or intravenously as an adjuvant to caudal 0.15% ropivacaine significantly reduced the intensity of post-operative pain and prolonged the duration of post-operative analgesia with the significant advantage of caudal over IV route.
Keywords: Caudal anaesthesia, dexamethasone, paediatric inguinal herniotomy, ropivacaine
INTRODUCTION
Paediatric anaesthesia has advanced enormously from the days when anaesthesiologists and surgeons adapted adult techniques and equipment to small children. Since then significant developments have occured including a better understanding regarding pain perception in neonates and advances in techniques of general anaesthesia, regional anaesthesia (RA) and perioperative pain management.[1]
By far the most commonly used RA technique in paediatric practice is the caudal epidural block. It is the easiest block to perform and to teach, with extensive safety record in children. However, it has the disadvantage of short duration of action after single injection.[2] Prolongation of single-shot caudal block has been achieved by the addition of various adjuvants such as opioids, ketamine, adrenaline and α2 agonists, but their utility has been challenged by unacceptable adverse effects in children.[3,4,5,6]
Dexamethasone is a corticosteroid hormone with powerful anti-inflammatory as well as analgesic property. Its administration through epidural route has been shown to reduce the incidence and severity of post-operative pain in adults[7] and improved morbidity such as nausea, vomiting, fever and delayed oral intake, in children.[8]
Systemic administration of steroids suppresses tissue levels of bradykinin and the release of neuropeptides from nerve endings; it also inhibits synthesis of cyclooxygenase-2 in peripheral tissues and in the central nervous system resulting in a reduction in prostaglandin production which contribute to enhanced nociception in inflamed tissue.[9,10,11] Dexamethasone administration through intravenous (IV) route along with caudal block has been shown to significantly reduce severity of pain and rescue analgesic requirement in the post-operative period.[12]
The present study was aimed at comparing the effects of dexamethasone on the duration of first rescue analgesia when administered through IV or caudal route.
METHODS
The present prospective randomised double-blinded study was conducted after getting approval from hospital's ethical committee and informed/written parental consent. A total of 105 American Society of Anesthesiologists physical status I and II children, aged between 4 and 10 years, undergoing inguinal herniotomy were enrolled. Exclusion criteria exercised were children with contraindications to the caudal block such as allergy to local anaesthetic (LA) agents, coagulation disorders, pre-existing neurological disease, spine abnormalities or any infection at the local site.
During pre-operative visit, patient's age, weight and baseline vital parameters were recorded. Detailed history, general physical and systemic examinations were done. Routine laboratory investigations including haemoglobin level were carried out for all patients. All patients were kept fasting as per institutional protocol (2 h for clear liquid and 6 h for semi-solid and solid). Parents were taught to perform their role in the study and the use of visual analogue scale (VAS) for pain assessment.[13]
Computer randomisation with allocation concealment (sequentially numbered, opaque, sealed envelopes method) was carried out and all children were evenly assigned into three groups of equal numbers. A person not participating in the study kept the table of random numbers and prepared all medications. According to the weight, the volume to be injected was prepared in 1 mL syringes marked as IV test and caudal test.
On arrival of the patient to the operating room, all standard monitors were attached, and baseline heart rate [HR], mean arterial pressure (MAP) and oxygen saturation [SpO2] were recorded by an investigator who was unaware of the group allocation. All patients were induced with sevoflurane 8% in oxygen and air (50/50) with spontaneous ventilation and IV line was secured with appropriate size cannula (22 and 24 gauge) and Ringer's lactate was started at the rate of 4 ml/kg/h. All the patients were premedicated with IV atropine 0.01 mg/Kg, IV midazolam 0.05 mg/Kg and IV fentanyl 1.5 μg/kg. An appropriate sized classic Laryngeal mask airway (cLMA) was inserted, and after insertion, sevoflurane concentration was reduced to 3% with fresh gas flow of 3–4 L/min. All the patients were placed in left lateral decubitus position, and under aseptic precautions, single-dose caudal block was performed using a 23-gauge short-bevelled hypodermic needle according to the group assigned.
Patients in Group I received caudal ropivacaine 0.15% (1.5 ml/kg) (ropivacaine hydrochloride ampoule, 0.75%, one ml diluted in 5 ml distilled water) along with caudal normal saline (NS) 0.025 ml/kg and NS 0.125 ml/kg, IV. Patients in group II received caudal ropivacaine 0.15% (1.5 ml/kg) with caudal dexamethasone 0.1 mg/Kg (0.025 ml/kg) and IV NS 0.125 ml/kg. Patients in Group III received caudal ropivacaine 0.15% (1.5 mL/kg) along with caudal NS 0.025 ml/kg and IV dexamethasone 0.5 mg/kg (maximum 10mg) (dexamethasone sodium phosphate vial 4mg/ml).
All the blocks were performed by the same anaesthesiologist throughout the study. The time of caudal block was recorded, and the surgery was allowed to start 10 min after caudal injection. Anaesthesia was maintained with sevoflurane in oxygen and air (50:50), and its concentration was adjusted to achieve haemodynamic changes within 20% of baseline value. No other, sedatives, and anti-emetics were used intraoperatively. Sevoflurane was discontinued at the beginning of skin closure, and during emergence from anaesthesia, LMA was removed, and the patient was transported to the post-anaesthesia care unit.
Vital parameters were recorded every 5 min till 30 min and every 15 min till the end of surgery. The maximum concentration of sevoflurane used was also recorded. Post-operative monitoring including vitals and pain (using VAS score) was recorded every hour till 3 h, every 3 h till 12 h and thereafter every 6 h till 24 h.
The primary outcome of this study i.e., the meantime to first rescue analgesic (time interval between a caudal block to the recording of VAS score ≥4) was recorded[14]. Rescue analgesia was provided with IV paracetamol (15 mg/kg). Secondary outcomes such as total doses of rescue analgesic and adverse effects such as post-operative nausea and vomiting, respiratory depression (fall in SpO2 of <92% requiring supplementary O2), urinary retention, hypotension (fall in the blood pressure >20% of the baseline value) and bradycardia (HR below 60 beats/min) were looked for and recorded. Incidence of bladder catheterisation, the initiation of clear liquid and time to discharge from hospital were also recorded.
We calculated that 35 patients in each group would be needed to detect an intergroup difference in the mean time to first rescue analgesic of at least 20% (α = 0.05, ί = 0.2) based on previous study[15] demonstrating mean (standard deviation [SD]) time of 554.5 (114.6) min to first rescue analgesia in children who received caudal analgesia using 1.5 ml/kg of ropivacaine 0.15%. Based on the above study we assumed a prolongation of duration of analgesia by 20% using ropivacaine 0.15% 1.5ml/kg along with dexamethasone as an adjuvant, whose effect is to be assessed separately for caudal and IV route Summary data were tabulated and analysed using SPSS IBM software version 21 (IBM SPSS Advanced Statistics; Chicago, IL, USA). Results of the quantitative variables were represented as median (95% of confidence interval) or mean (SD), and results of categorical measurements were presented in numbers or ratio. Unpaired Student's t-test and analysis of variance using Bonferroni multiple comparisons’ test were applied for comparing quantitative data, and Chi-square test was applied for qualitative data. Time to first analgesic administration was analysed by Kaplan–Meier survival analysis and log-rank test. The difference was considered significant if P < 0.05 was obtained.
RESULTS
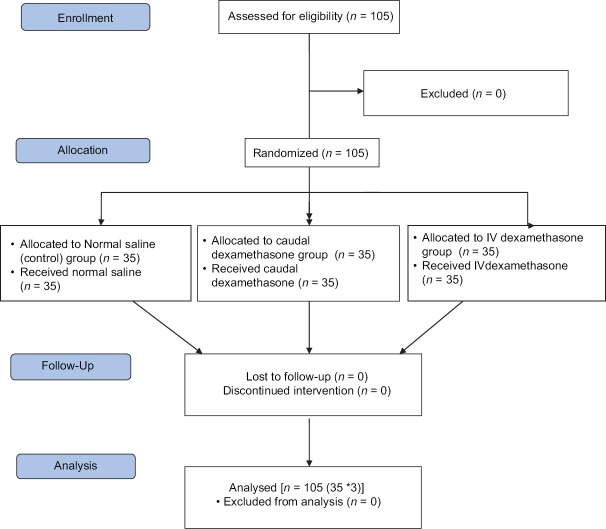
All the patients screened were enrolled in the study [Figure 1]. All the groups of patients undergoing inguinal herniotomy were comparable regarding demographic profile, end-tidal sevoflurane concentration and duration of surgery (P > 0.05) [Table 1]. Failure of caudal block was not reported in any patient. The magnitude of haemodynamic (HR and MAP) changes between the groups was comparable [Figure 2], and therapeutic interventions were not required.
Figure 1.
Consort flow chart of participants
Table 1.
Comparison of demographic parameter, end-tidal sevoflurane and duration of surgery among three groups
Figure 2.
Comparison of haemodynamic parameters (heart rate in bpm and mean arterial pressure in mmHg in Y axis) among three groups over time (X axis)
There was a significant difference between the groups in the VAS score measured 6 h after discharge from the PACU [Figure 3]. Group I patients achieved significantly higher VAS score compared with Groups II and III, where 54.29% of patients achieved a VAS score of >4 compared with 0% in Groups II and III [Figure 3]. The time to first rescue analgesia/duration of analgesia recorded as median (95% CI) was significantly longer in Group II 720.0 min (709.5–729.8 min) and Group III 620.0 (612.1–625.6 min) compared to Group I 220.0 min (210.4–239.2 min) (P < 0.001) [Table 2 and Figure 4]. Total rescue analgesic doses recorded as median (95% CI) were significantly more in Group I (2.0 [2.0–2.3]) compared to Group II (1.0 [0.9–1.1]) and Group III (1.0 [1.0–1.3]) (P < 0.001) [Table 2].
Figure 3.
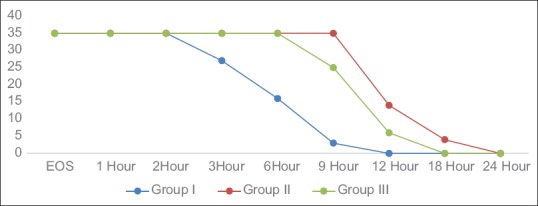
Number of patients having adequate caudal analgesia at different time interval in the post-operative period among three groups
Table 2.
Comparison of time to first rescue analgesia, total number of rescue analgesic consumption, initiation of clear liquid and time to discharge among three group

Figure 4.
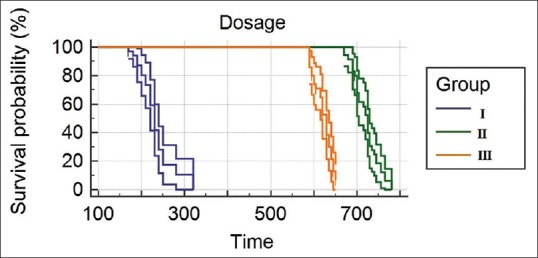
Kaplan–Meier survival curve for time to first rescue analgesic administration
The incidence of nausea and vomiting was higher in group I (31.4%) compared to Group II and III (8.6%). There was no incidence of other complications such as hypotension, bradycardia and urinary retention between the groups. Clear fluids were initiated at early stage in Group II (5.94 ± 0.76 h) and Group III (6.17 ± 0.56 h) compared to Group I (7.11 ± 1.937 h) (P < 0.001) [Table 2]. The times of discharge of the patients were comparable between the groups [Table 2].
DISCUSSION
Several studies suggested that the addition of dexamethasone through caudal route[16] or IV route[12,17,18,19] as an adjuvant to RA significantly reduced the post-operative pain by prolonging the duration of analgesia with significantly lesser analgesic consumption. Our study results are in line with this, in which we demonstrated that the addition of dexamethasone through caudal or IV route could significantly prolong the mean time to first rescue analgesic with the significant advantage of caudal over IV route in children undergoing inguinal hernia repair.
Various literatures support the analgesic effects of dexamethasone when administered as an adjuvant to RA for post-operative pain in adults and children.[16,20] Even the systemic administration of steroids has to be effective for reducing post-operative pain, but the results are inconclusive.[8,21,22,23]
As for all additives in RA, the true question is to compare the potential local effects to a systemic administration, particularly when additives were used off-label. Lack of control group with IV dexamethasone did not allow researcher of previous studies to comment on the potential local effect of dexamethasone. Hence, to study the local effects of dexamethasone as a caudal adjuvant in detail, we included group with IV dexamethasone.
Hong JY et al.[12,15] demonstrated that a single dose of i.v. dexamethasone (0.5 mg/kg) in combination with a larger volume (1.5 ml kg) of diluted ropivacaine 0.15% prolongs analgesic duration, reduces postoperative pain, decreases rescue analgesic requirements compared with a caudal block alone. Similarly Yousef GT et al.[16] found that addition of either magnesium sulphate (50mg) or dexamethasone (0.1mg/kg) to 0.15% caudal ropivacaine significantly prolonged the duration of analgesia with reduced analgesic consumption. Based on the above studies we used 0.15% ropivacaine along with dexamethasone IV (0.5mg/kg) or caudal route (0.1mg/kg) according to group allocation.
The mechanism of the analgesia induced by corticosteroids is not fully understood. However, this effect is suspected to be mediated by their anti-inflammatory or immunosuppressive effects.[24,25] Adjuvants such as epinephrine produce prolongation of RA by its vasoconstrictive property, but this is not responsible for block prolongation by dexamethasone because of its relatively rapid effect.[26] Corticosteroids may have a local effect on the nerve, and the dexamethasone effect may be related to this action. Some authors believe that analgesic properties of corticosteroids are the result of their systemic effect.[27]
The antiemetic property of dexamethasone is well known through direct central action at the tract nucleus, interaction with the neurotransmitter serotonin and receptor proteins tachykinin NK1 and NK2, alpha-adrenaline, etc.[28] Results of our studies are in line with this, we found that the incidence of nausea/vomiting was comparatively low in group receiving dexamethasone 8.6% (3/35) than control group 31.4% (11/35). It is advisable to use, therefore, prophylactic antiemetics to all patients administered caudal epidural with local anaesthetics alone. Other studies[12,16] found no difference in the incidence of nausea and vomiting when dexamethasone was used as an adjuvant to caudal block.
Adverse effects with a single dose of dexamethasone are probably extremely rare and minor in nature, and previous studies[12,16] have demonstrated that short-term use of dexamethasone was safe.[29] Similarly, in our studies, there were no incidences of systemic complications such as hypotension, bradycardia and urinary retention or local complications such as neurotoxicity, respiratory depression and pruritus, postoperatively when dexamethasone was given intravenously or caudally along with ropivacaine.
In children with limited understanding and communication skills, reliable assessment of pain is challenging and assessments made by their parents are often used as a proxy measure. The paediatric pain assessment tools are based on either self-report or observation of behaviour. Self-report is the only truly direct measure of pain, and hence it is considered the ‘gold standard’ of measurement. We used visual analogue scale (VAS) for assessing the post-operative pain because of its ease to perform and simplicity to teach parents and nurses; in addition, all observer-rated scales, except the simple VAS, are likely to require raters to be trained in their use. Although pain cannot be necessarily quantified based on parents’ report as VAS is subject to bias, there is a lack of sufficient literature to prove this pitfall and hence VAS was preferred.
The main limitation of our study is that motor block could not be evaluated as it is unlikely at the 0.15% concentration of ropivacaine we used. Although we used VAS as a pain assessment tool, the use of Faces Pain Scale-Revised would have been more appropriate. Moreover, we looked for immediate local complications of dexamethasone, but the delayed local complications could not be ruled out.
Several studies tried to investigate the analgesic effect of dexamethasone as an adjuvant to RA.[12,18,19] In line with this, our study is able to demonstrate the use of dexamethasone either caudally or intravenously as an adjuvant to caudal block could be a superior alternative to LA alone. Although dexamethasone is a safe drug with minimum side effects,[16,17] there is a need for further multicentric RCT to confirm the findings of our study. So that dexamethasone can become adjuvant of choice in paediatric caudal block.
CONCLUSION
We conclude that the addition of dexamethasone either caudally or intravenously as an adjuvant to caudal 0.15% ropivacaine may significantly prolong the mean time to first rescue analgesic with the significant advantage of caudal over IV route in children undergoing inguinal hernia repair. In addition to this, dexamethasone can be considered as a safe adjuvant to caudal block with no potential side effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Motoyama EK, Davis PJ, editors. Special characteristics of paediatric anaesthesia. In: Smith's Anaesthesia for Infants and Children. 7th ed. Philadelphia: Elsevier; 2005. [Google Scholar]
- 2.Bösenberg AT, Jöhr M, Wolf AR. Pro con debate: The use of regional vs systemic analgesia for neonatal surgery. Paediatr Anaesth. 2011;21:1247–58. doi: 10.1111/j.1460-9592.2011.03638.x. [DOI] [PubMed] [Google Scholar]
- 3.Constant I, Gall O, Gouyet L, Chauvin M, Murat I. Addition of clonidine or fentanyl to local anaesthetics prolongs the duration of surgical analgesia after single shot caudal block in children. Br J Anaesth. 1998;80:294–8. doi: 10.1093/bja/80.3.294. [DOI] [PubMed] [Google Scholar]
- 4.Semple D, Findlow D, Aldridge LM, Doyle E. The optimal dose of ketamine for caudal epidural blockade in children. Anaesthesia. 1996;51:1170–2. doi: 10.1111/j.1365-2044.1996.tb15063.x. [DOI] [PubMed] [Google Scholar]
- 5.Cook B, Grubb DJ, Aldridge LA, Doyle E. Comparison of the effects of adrenaline, clonidine and ketamine on the duration of caudal analgesia produced by bupivacaine in children. Br J Anaesth. 1995;75:698–701. doi: 10.1093/bja/75.6.698. [DOI] [PubMed] [Google Scholar]
- 6.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103:268–74. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 7.Khafagy HF, Refaat AI, El-Sabae HH, Youssif MA. Efficacy of epidural dexamethasone versus fentanyl on postoperative analgesia. J Anesth. 2010;24:531–6. doi: 10.1007/s00540-010-0949-7. [DOI] [PubMed] [Google Scholar]
- 8.Hanasono MM, Lalakea ML, Mikulec AA, Shepard KG, Wellis V, Messner AH. Perioperative steroids in tonsillectomy using electrocautery and sharp dissection techniques. Arch Otolaryngol Head Neck Surg. 2004;130:917–21. doi: 10.1001/archotol.130.8.917. [DOI] [PubMed] [Google Scholar]
- 9.Hargreaves KM, Costello A. Glucocorticoids suppress levels of immunoreactive bradykinin in inflamed tissue as evaluated by microdialysis probes. Clin Pharmacol Ther. 1990;48:168–78. doi: 10.1038/clpt.1990.132. [DOI] [PubMed] [Google Scholar]
- 10.Hong D, Byers MR, Oswald RJ. Dexamethasone treatment reduces sensory neuropeptides and nerve sprouting reactions in injured teeth. Pain. 1993;55:171–81. doi: 10.1016/0304-3959(93)90146-G. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira SH, Cunha FQ, Lorenzetti BB, Michelin MA, Perretti M, Flower RJ, et al. Role of lipocortin-1 in the anti-hyperalgesic actions of dexamethasone. Br J Pharmacol. 1997;121:883–8. doi: 10.1038/sj.bjp.0701211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong JY, Han SW, Kim WO, Kim EJ, Kil HK. Effect of dexamethasone in combination with caudal analgesia on postoperative pain control in day-case paediatric orchiopexy. Br J Anaesth. 2010;105:506–10. doi: 10.1093/bja/aeq187. [DOI] [PubMed] [Google Scholar]
- 13.Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37:378–81. doi: 10.1136/ard.37.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarbell SE, Cohen IT, Marsh JL. The Toddler-Preschooler Postoperative Pain Scale: An observational scale for measuring postoperative pain in children aged 1–5. Preliminary report. Pain. 1992;50:273–80. doi: 10.1016/0304-3959(92)90031-6. [DOI] [PubMed] [Google Scholar]
- 15.Hong JY, Han SW, Kim WO, Cho JS, Kil HK. A comparison of high volume/low concentration and low volume/high concentration ropivacaine in caudal analgesia for pediatric orchiopexy. Anesth Analg. 2009;109:1073–8. doi: 10.1213/ane.0b013e3181b20c52. [DOI] [PubMed] [Google Scholar]
- 16.Yousef GT, Ibrahim TH, Khder A, Ibrahim M. Enhancement of ropivacaine caudal analgesia using dexamethasone or magnesium in children undergoing inguinal hernia repair. Anesth Essays Res. 2014;8:13–9. doi: 10.4103/0259-1162.128895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murni Sari Ahmad A, Azarinah I, Esa K, Khairulamir Z, Hamidah I, Norsidah Abdul M. Intravenous dexamethasone in combination with caudal block prolongs postoperative analgesia in pediatric daycare surgery. Middle East J Anaesthesiol. 2015;23:177–83. [PubMed] [Google Scholar]
- 18.Desmet M, Braems H, Reynvoet M, Plasschaert S, Van Cauwelaert J, Pottel H, et al. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: A prospective, randomized, placebo-controlled study. Br J Anaesth. 2013;111:445–52. doi: 10.1093/bja/aet109. [DOI] [PubMed] [Google Scholar]
- 19.Cummings KC, 3rd, Napierkowski DE, Parra-Sanchez I, Kurz A, Dalton JE, Brems JJ, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. 2011;107:446–53. doi: 10.1093/bja/aer159. [DOI] [PubMed] [Google Scholar]
- 20.Mohammed AA, Ibrahim WA, Safan TF. Dexamethasone as adjuvant to caudal ropivacaine as analgesic for labor. Ain Shams J Anesthesiol. 2012;5:33–41. [Google Scholar]
- 21.Mohamed SK, Ibraheem AS, Abdelraheem MG. Preoperative intravenous dexamethasone combined with glossopharyngeal nerve block: Role in paediatric postoperative analgesia following tonsillectomy. Eur Arch Otorhinolaryngol. 2009;266:1815–9. doi: 10.1007/s00405-009-0937-4. [DOI] [PubMed] [Google Scholar]
- 22.Vosdoganis F, Baines DB. The effect of single dose intravenous dexamethasone in tonsillectomy in children. Anaesth Intensive Care. 1999;27:489–92. doi: 10.1177/0310057X9902700509. [DOI] [PubMed] [Google Scholar]
- 23.Giannoni C, White S, Enneking FK. Does dexamethasone with preemptive analgesia improve pediatric tonsillectomy pain? Otolaryngol Head Neck Surg. 2002;126:307–15. doi: 10.1067/mhn.2002.122700. [DOI] [PubMed] [Google Scholar]
- 24.McCormack K. The spinal actions of nonsteroidal anti-inflammatory drugs and the dissociation between their anti-inflammatory and analgesic effects. Drugs. 1994;47(Suppl 5):28–45. doi: 10.2165/00003495-199400475-00006. [DOI] [PubMed] [Google Scholar]
- 25.Ahlgren SC, Wang JF, Levine JD. C-fiber mechanical stimulus-response functions are different in inflammatory versus neuropathic hyperalgesia in the rat. Neuroscience. 1997;76:285–90. doi: 10.1016/s0306-4522(96)00290-4. [DOI] [PubMed] [Google Scholar]
- 26.Taguchi H, Shingu K, Okuda H, Matsumoto H. Analgesia for pelvic and perineal cancer pain by intrathecal steroid injection. Acta Anaesthesiol Scand. 2002;46:190–3. doi: 10.1046/j.0001-5172.2001.00000.x-i1. [DOI] [PubMed] [Google Scholar]
- 27.Devor MD, Gorvin-Lippmann R, Raber P. Corticosteroids suppress ectopic neural discharge originating in experimental neuromas. Pain. 1985;22:127–37. doi: 10.1016/0304-3959(85)90173-3. [DOI] [PubMed] [Google Scholar]
- 28.Ho CM, Ho ST, Wang JJ, Tsai SK, Chai CY. Dexamethasone has a central antiemetic mechanism in decerebrated cats. Anesth Analg. 2004;99:734–9. doi: 10.1213/01.ANE.0000130003.68288.C7. [DOI] [PubMed] [Google Scholar]
- 29.Tan PH, Liu K, Peng CH, Yang LC, Lin CR, Lu CY. The effect of dexamethasone on postoperative pain and emesis after intrathecal neostigmine. Anesth Analg. 2001;92:228–32. doi: 10.1097/00000539-200101000-00044. [DOI] [PubMed] [Google Scholar]