Abstract
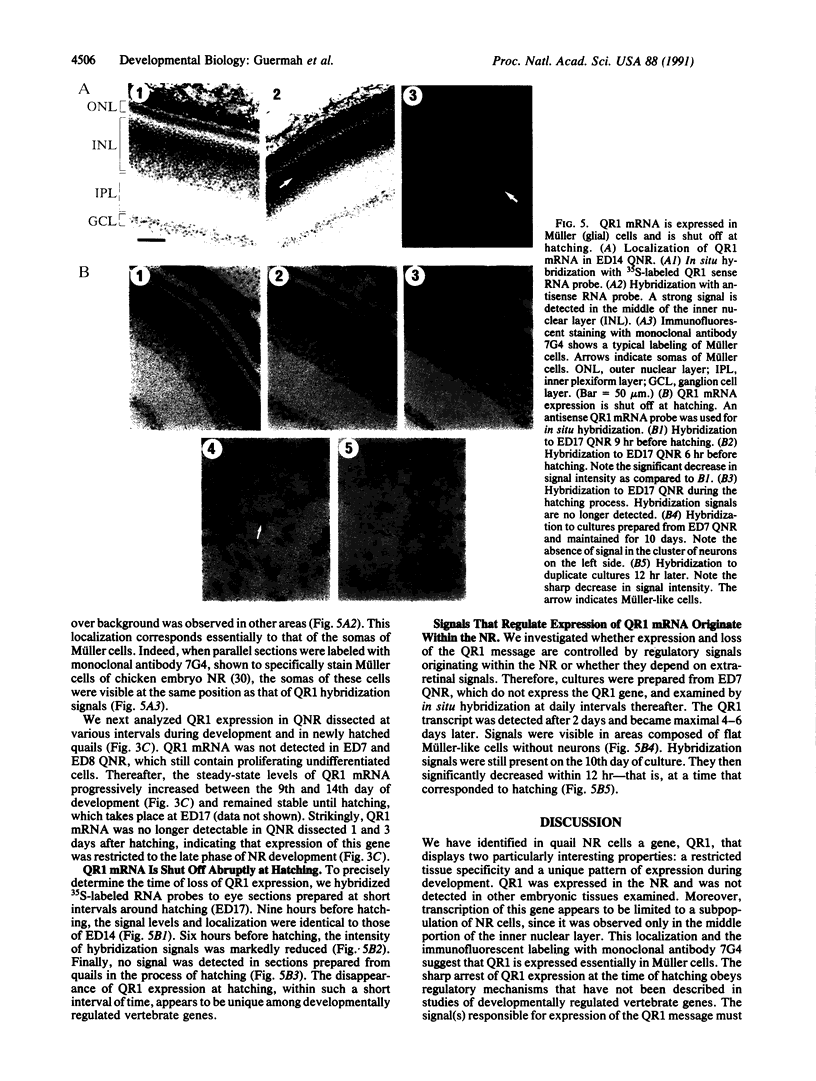
The avian neuroretina (NR) is part of the central nervous system and is composed of photoreceptors, neuronal cells, and Müller (glial) cells. These cells are derived from proliferating neuroectodermal precursors that differentiate after terminal mitosis and become organized in cell strata. Genes that are specifically expressed at the various stages of retinal development are presently unknown. We have isolated a quail (Coturnix coturnix japonica) cDNA clone, named QR1, encoding a 676-amino acid protein whose carboxyl-terminal portion shows significant similarity to those of the extracellular glycoprotein osteonectin/SPARC/BM40 and of the recently described SC1 protein. The QR1 cDNA identifies a mRNA detected in NR but not in other embryonic tissues examined. The levels of this mRNA are markedly reduced when nondividing NR cells are induced to proliferate by the v-src oncogene. QR1 expression in NR is limited to the middle portion of the inner nuclear layer, a localization that essentially corresponds to that of Müller cells. Transcription of QR1 takes place only during the late phase of retinal development and is shut off sharply at hatching. Signals that regulate this unique pattern of expression appear to originate within the NR, since the QR1 mRNA is transcribed in cultured NR cells and is shut off also in vitro at a time coinciding with hatching.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bechade C., Calothy G., Pessac B., Martin P., Coll J., Denhez F., Saule S., Ghysdael J., Stéhelin D. Induction of proliferation or transformation of neuroretina cells by the mil and myc viral oncogenes. Nature. 1985 Aug 8;316(6028):559–562. doi: 10.1038/316559a0. [DOI] [PubMed] [Google Scholar]
- Bolander M. E., Young M. F., Fisher L. W., Yamada Y., Termine J. D. Osteonectin cDNA sequence reveals potential binding regions for calcium and hydroxyapatite and shows homologies with both a basement membrane protein (SPARC) and a serine proteinase inhibitor (ovomucoid). Proc Natl Acad Sci U S A. 1988 May;85(9):2919–2923. doi: 10.1073/pnas.85.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calothy G., Laugier D., Cross F. R., Jove R., Hanafusa T., Hanafusa H. The membrane-binding domain and myristylation of p60v-src are not essential for stimulation of cell proliferation. J Virol. 1987 May;61(5):1678–1681. doi: 10.1128/jvi.61.5.1678-1681.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calothy G., Poirier F., Dambrine G., Mignatti P., Combes P., Pessac B. Expression of viral oncogenes in differentiating chick embryo neuroretinal cells infected with avian tumor viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):983–990. doi: 10.1101/sqb.1980.044.01.106. [DOI] [PubMed] [Google Scholar]
- Calothy G., Poirier F., Dambrine G., Pessac B. A transformation defective mutant of Rous sarcoma virus inducing chick embryo neuroretinal cell proliferation. Virology. 1978 Aug;89(1):75–84. doi: 10.1016/0042-6822(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Combes P. C., Privat A., Pessac B., Calothy G. Differentiation of chick embryo neuroretina cells in monolayer cultures. An ultrastructural study. I. Seven-day retina. Cell Tissue Res. 1977 Dec 13;185(2):159–173. doi: 10.1007/BF00220661. [DOI] [PubMed] [Google Scholar]
- Engel J., Taylor W., Paulsson M., Sage H., Hogan B. Calcium binding domains and calcium-induced conformational transition of SPARC/BM-40/osteonectin, an extracellular glycoprotein expressed in mineralized and nonmineralized tissues. Biochemistry. 1987 Nov 3;26(22):6958–6965. doi: 10.1021/bi00396a015. [DOI] [PubMed] [Google Scholar]
- FUJITA S., HORII M. ANALYSIS OF CYTOGENESIS IN CHICK RETINA BY TRITIATED THYMIDINE AUTORADIOGRAPHY. Arch Histol Jpn. 1963 May;23:359–366. doi: 10.1679/aohc1950.23.359. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guermah M., Gillet G., Michel D., Laugier D., Brun G., Calothy G. Down regulation by p60v-src of genes specifically expressed and developmentally regulated in postmitotic quail neuroretina cells. Mol Cell Biol. 1990 Jul;10(7):3584–3590. doi: 10.1128/mcb.10.7.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W. Antisera to basal lamina and glial endfeet disturb the normal extension of axons on retina and pigment epithelium basal laminae. Development. 1989 Oct;107(2):281–297. doi: 10.1242/dev.107.2.281. [DOI] [PubMed] [Google Scholar]
- Holland P. W., Harper S. J., McVey J. H., Hogan B. L. In vivo expression of mRNA for the Ca++-binding protein SPARC (osteonectin) revealed by in situ hybridization. J Cell Biol. 1987 Jul;105(1):473–482. doi: 10.1083/jcb.105.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston I. G., Paladino T., Gurd J. W., Brown I. R. Molecular cloning of SC1: a putative brain extracellular matrix glycoprotein showing partial similarity to osteonectin/BM40/SPARC. Neuron. 1990 Jan;4(1):165–176. doi: 10.1016/0896-6273(90)90452-l. [DOI] [PubMed] [Google Scholar]
- Jove R., Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- Kahn A. J. An autoradiographic analysis of the time of appearance of neurons in the developing chick neural retina. Dev Biol. 1974 May;38(1):30–40. doi: 10.1016/0012-1606(74)90256-5. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Lankat-Buttgereit B., Mann K., Deutzmann R., Timpl R., Krieg T. Cloning and complete amino acid sequences of human and murine basement membrane protein BM-40 (SPARC, osteonectin). FEBS Lett. 1988 Aug 29;236(2):352–356. doi: 10.1016/0014-5793(88)80054-1. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lemmon V. Monoclonal antibodies specific for glia in the chick nervous system. Brain Res. 1985 Nov;355(1):111–120. doi: 10.1016/0165-3806(85)90010-0. [DOI] [PubMed] [Google Scholar]
- MacLeod A. R. Expression of the mRNA coding for glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1981 Oct;119(2):353–358. doi: 10.1111/j.1432-1033.1981.tb05615.x. [DOI] [PubMed] [Google Scholar]
- Mason I. J., Murphy D., Münke M., Francke U., Elliott R. W., Hogan B. L. Developmental and transformation-sensitive expression of the Sparc gene on mouse chromosome 11. EMBO J. 1986 Aug;5(8):1831–1837. doi: 10.1002/j.1460-2075.1986.tb04434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason I. J., Taylor A., Williams J. G., Sage H., Hogan B. L. Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell 'culture shock' glycoprotein of Mr 43,000. EMBO J. 1986 Jul;5(7):1465–1472. doi: 10.1002/j.1460-2075.1986.tb04383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. J., Jove R., Krane J. F., Poirier F., Calothy G., Hanafusa H. Genetic lesions involved in temperature sensitivity of the src gene products of four Rous sarcoma virus mutants. J Virol. 1986 Dec;60(3):858–867. doi: 10.1128/jvi.60.3.858-867.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Heimann B., Beimling P., Rapp U. R., Sander T. Serine- and threonine-specific protein kinase activities of purified gag-mil and gag-raf proteins. Nature. 1984 Dec 6;312(5994):558–561. doi: 10.1038/312558a0. [DOI] [PubMed] [Google Scholar]
- Pessac B., Calothy G. Transformation of chick embryo neuroretinal cells by Rous sarcoma virus in vitro: induction of cell proliferation. Science. 1974 Aug;185(4152):709–710. doi: 10.1126/science.185.4152.709. [DOI] [PubMed] [Google Scholar]
- Pessac B., Girard A., Romey G., Crisanti P., Lorinet A. M., Calothy G. A neuronal clone derived from a Rous sarcoma virus-transformed quail embryo neuroretina established culture. Nature. 1983 Apr 14;302(5909):616–618. doi: 10.1038/302616a0. [DOI] [PubMed] [Google Scholar]
- Poirier F., Calothy G., Karess R. E., Erikson E., Hanafusa H. Role of p60src kinase activity in the induction of neuroretinal cell proliferation by rous sarcoma virus. J Virol. 1982 Jun;42(3):780–789. doi: 10.1128/jvi.42.3.780-789.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg R. W., Werness P. G., Lollar P., Riggs B. L., Mann K. G. Isolation and characterization of native adult osteonectin. J Biol Chem. 1985 Mar 10;260(5):2728–2736. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H. Nucleotide sequence of Fujinami sarcoma virus: evolutionary relationship of its transforming gene with transforming genes of other sarcoma viruses. Cell. 1982 Oct;30(3):787–795. doi: 10.1016/0092-8674(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman J. E., Morabito M. A., Barnstable C. J. Opsin expression in the rat retina is developmentally regulated by transcriptional activation. Mol Cell Biol. 1988 Apr;8(4):1570–1579. doi: 10.1128/mcb.8.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufty R. M., Kretsinger R. H. Troponin and parvalbumin calcium binding regions predicted in myosin light chain and T4 lysozyme. Science. 1975 Jan 17;187(4172):167–169. doi: 10.1126/science.1111094. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., McMahon A. P. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987 Jul 3;50(1):79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- Young M. F., Bolander M. E., Day A. A., Ramis C. I., Robey P. G., Yamada Y., Termine J. D. Osteonectin mRNA: distribution in normal and transformed cells. Nucleic Acids Res. 1986 Jun 11;14(11):4483–4497. doi: 10.1093/nar/14.11.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]










