Abstract
Aim:
The purpose of this study is to establish a normative database of subfoveal choroidal thickness (CT) in healthy young Indians using spectral domain optical coherence tomography (SD OCT). Evaluation and comparison of CT of central serous chorioretinopathy (CSC) and fellow eyes were also performed.
Materials and Methods:
This was a prospective, cross-sectional, and observational study. It included 112 normal eyes of 112 healthy volunteers who had no evidence of ocular or systemic disease, 84 CSC eyes with acute, treatment-naïve CSC, and 69 fellow eyes with no evidence of neurosensory detachment or pigment epithelium detachment on SD OCT. Complete history, examination, and SD OCT were performed in all eyes.
Results:
The mean age of 81 patients (84 eyes) with CSC was 35.04 ± 8.86 years, 69 fellow eyes was 34.61 ± 8.71 years, and 112 healthy volunteers (112 eyes) was 33.16 ± 9.4 years (P < 0.05). The mean subfoveal CT of CSC eyes was 429 ± 74.18 μ, fellow eyes was 360 ± 57.99 μ, and normal eyes was 301.80 ± 46.59 μ (P < 0.001).
Conclusion:
CT varies not only with age, axial length, and refractive error but also with races. Therefore, it is important to establish a normative database in a particular population before carrying out further research in diseased states. CT in CSC eyes is significantly thicker than fellow eyes, and CT of fellow eyes is significantly thicker than normal eyes. This reinforces the fact that choroidal permeability is increased in both eyes of patients with CSC.
Keywords: Central serous chorioretinopathy, choroidal imaging, choroidal thickness, normative database
Central serous chorioretinopathy (CSC) is a disorder characterized by serous retinal detachment and/or retinal pigment epithelial (RPE) detachment, changes most often confined to the macula, and associated with leakage of fluid through the RPE into the subretinal space. It is classically seen in young male patients with no associated systemic illness. Earlier theory of pathophysiology believed that diffuse RPE abnormality was the cause of this disorder, but with advanced imaging techniques such as optical coherence tomography (OCT) and indocyanine green (ICG) angiography, choroidal hyperpermeability is believed to be the prime culprit.[1,2,3,4] Accurate measurement of choroidal thickness (CT) in vivo is an important step in monitoring disease process. However, CT varies with age, ethnicity, refractive error, axial length, and type of OCT machine.[5,6,7]
The purpose of this study was to establish a normative database of subfoveal CT in healthy young Indians and to evaluate and compare subfoveal CT in eyes with CSC, in fellow eyes and in normal eyes on NIDEK spectral domain OCT (SD OCT).
Materials and Methods
This prospective study was carried out at a tertiary care eye hospital in North India. It was cross-sectional, observational, and comparative in nature on 112 normal eyes of 112 healthy volunteers, 84 CSC eyes, and 69 fellow eyes. Written and informed consent was obtained from each participant before enrolment. The study was performed in accordance with the tenets of the Declaration of Helsinki. A detailed history and complete ophthalmological examination of participants were noted. Measurement of uncorrected visual acuity (UCVA), refraction, best-corrected visual acuity (BCVA), axial length, intraocular pressure (IOP), assessment of ocular motility, slit-lamp examination of anterior chamber and lens, and fundus examination with 90 D noncontact lens was performed. Spherical equivalent was measured by autorefractometry, axial length by partial coherence interferometry, and IOP with Goldman applanation tonometry. All patients clinically diagnosed as having CSC underwent fundus fluorescein angiography. ICG angiography (ICGA) was applied when necessary to exclude polypoidal choroidal vasculopathy and coexisting choroidal neovascularization.
Inclusion criteria for normal eyes
Inclusion criteria for normal eyes was patients aged 20–50 years, male gender, UCVA better than 20/40 correctable with refraction to a BCVA of 20/20, normal anterior chamber and fundus examination.
Inclusion criteria for central serous chorioretinopathy eyes
Inclusion criteria for CSC eyes was duration of complaints 1–4 months, single angiographic leak at subfoveal or juxtafoveal location with a neurosensory detachment, treatment-naïve eyes.
Inclusion criteria for fellow eyes
Fellow eyes of patients with acute CSC were included if UCVA was better than 20/40 and correctable with refraction to a BCVA of 20/20, normal anterior chamber and fundus examination. No evidence of neurosensory detachment or pigment epithelium detachment on OCT.
Exclusion criteria for normal/central serous chorioretinopathy/fellow eyes
Axial length <23 mm or >24.5 mm, refractive error >2 D spherical equivalent, IOP >21 mm Hg, cataract that would hinder an image capture of high quality, history or evidence of any ocular disease such as uveitis, glaucoma, amblyopia, strabismus, retinal degeneration, proliferative retinopathies of any type, epiretinal membrane, tapetoretinal dystrophy, angioid streaks, choroidal neovascularization, geographic atrophy, drusen, ocular trauma, ocular tumor, or any systemic illness such as diabetes, hypertension, heart disease, or hyperlipidemia. Patients who had a history of taking systemic medications such as oral steroids or any other drug were excluded as were the patients with any previous ocular surgery or intravitreal medication.
Spectral domain optical coherence tomography examination
SD OCT scans were obtained using Nidek RS-3000 Advance (Nidek Co. Ltd., Gamagori, Japan). The NAVIS-EX Image Filing Software of Nidek RS-3000 Advance was used for analyzing the images. This SD OCT has a scanning speed of 53,000 A scans/second and a resolution of 4 µ. Macular line protocol with tracing high definition (HD) function allows accurate averaging of 120 images. The choroid was imaged in the macular line protocol by positioning the SD OCT close enough to the eye to obtain an inverted image (choroidal mode, choroidal intensity +5). This image is averaged for 120 scans using the automatic averaging and eye tracking features. Images were captured with ultrafine sensitivity (which ensures image capture at higher definition at the cost of scan speed) and after optimization. Software analysis segmentation of 6 + 1 retinal layers enables to manually draw a line and form one additional layer besides automatically formed 6 layers in an HD OCT image.
To account for the effects of diurnal variation of CT, all scans were performed within a 2-h period from 12 PM to 2 PM. Each subject's refractive error was corrected and all the eyes in this study underwent pupillary dilatation with tropicamide 1%. HD OCT was performed by a single trained technician in macular line protocol. Four sections (each comprised of 120 averaged scans) were captured for each eye in four different directions: horizontal, vertical, 45°, and 135°. Between scan acquisitions, there was a time delay, and subject position and focus was randomly disrupted, meaning that alignment parameters had to be newly adjusted at the start of each image acquisition. The quality of the scans was assessed before the analysis. To be included in this study, images had to be at least 6 out of 10 in intensity and taken as close to the fovea as possible, by choosing to image the thinnest point of the macula, with the understanding that slight differences in positioning could affect the measured thicknesses.
The manual line at chorioscleral interface was drawn by two independent observers, and CT recorded to determine interobserver reproducibility. If the difference in CT at any point between the two examiners was ≥20 µm, the senior author determined the level of the chorioscleral interface and CT was then recorded. CT was measured as the perpendicular distance between the automated RPE line and the manually drawn line at chorioscleral interface. In the software, perpendicular distance between any two lines (automated RPE line and manually drawn line at choroid scleral interface in this study) can be measured at any number of points by moving the cursor to that point. CT measurements were recorded at 9 points in each scan (center of the fovea, 300 µm temporal and nasal to the fovea; 600 µm temporal and nasal to the fovea; 900 µm temporal and nasal to fovea; 1200 µm temporal and nasal to fovea). Thus, for each eye, 36 measurements (9 points in each scan × 4 scans in different directions) were obtained.
An average of these 36 measurements was CTaverage for each eye. Subfoveal CT was measured 4 times in each eye: along the horizontal scan, vertical scan, and two oblique scans. An average of these four values was Csubfoveal of each eye. Along the vertical scan, an average of CT at 900 µ and 1200 µ away from fovea superiorly was S1, 900 µ and 1200 µ away from fovea inferiorly was I1. Along the horizontal scan, an average of CT at 900 µ and 1200 µ away from fovea nasally was N1, 900 µ and 1200 µ away from fovea temporally was T1. Statistical analysis was done using SPSS software version 15 (IBM/SPSS Inc, Chicago, Illinois, USA) with unpaired t-test/Mann–Whitney test.
Results
The mean age of 81 patients (84 eyes) with CSC (three patients had bilateral CSC) was 35.04 ± 8.86 years, 69 patients with fellow eyes was 34.61 ± 8.71 years (nine patients with CSC in one eye did not satisfy the inclusion criteria in the fellow eyes, and three patients had bilateral CSC), and 112 healthy volunteers (112 eyes) was 33.16 ± 9.4 years. Mean refractive error (spherical equivalent) in CSC eyes was 0.49 ± 0.70 D, fellow eyes was 0.45 ± 0.73 D, and normal eyes was 0.40 ± 0.73 D. Mean axial length of CSC, fellow, and normal eyes was 23.77 ± 0.45 mm, 23.80 ± 0.45 mm, and 23.70 ± 0.48 mm, respectively. Mean IOP was 15 ± 2.21 mm Hg, 15.09 ± 2.28 mmHg, and 15.45 ± 2.0 mm Hg in CSC, fellow, and normal eyes, respectively. There was no statistically significant difference between the three groups in terms of age, refractive error, axial length, and IOP (P < 0.05).
The mean CTaverage of CSC eyes was 425.72 ± 72.30 µ, fellow eyes was 357.25 ± 53.20 µ, and normal eyes was 297.83 ± 45.30 µ. Mean Csubfoveal of CSC eyes was 429 ± 74.18 µ, fellow eyes was 360 ± 57.99 µ, and normal eyes was 301.80 ± 46.59 µ. Mean S1 of CSC eyes was 428.38 ± 80.76 µ, fellow eyes was 362.0 ± 64.25 µ, and normal eyes was 315.14 ± 56.14 µ. Mean I1 was 422.96 ± 78.72 µ, 354.59 ± 65.53 µ, and 287.98 ± 54.96 µ for CSC, fellow, and normal eyes, respectively. Mean N1 was 414.05 ± 79.46 µ for CSC eyes, 357.61 ± 62.64 µ for fellow eyes, and 288.74 ± 51.78 µ for normal eyes. Mean T1 was 411.88 ± 73.01 µ, 350.15 ± 46.09 µ, and 288.96 ± 47.75 µ for CSC, fellow, and normal eyes, respectively. When comparison was made between CSC eyes, fellow eyes, and normal eyes, each of these values (Csubfoveal, CTaverage, S1, I1, N1, T1) was significantly different from each other (P < 0.001 for CSC versus fellow eyes, CSC versus normal eyes, and fellow versus normal eyes).
Of the 84 eyes with acute CSC, 34 eyes followed up with us for 4 months (for observation) while others were either treated or were lost to follow-up. At the baseline, CTaverage of these 34 eyes was 416.73 ± 64.1 µ. It decreased significantly to 389.15 ± 56.34 µ (P < 0.001) at 2 weeks, 368.31 ± 54.25 µ (P < 0.001) at 4 weeks, 353.18 ± 44.74 µ (P < 0.001) at 8 weeks, and 361.87 ± 46.4 µ (P < 0.001) at 16 weeks. At 16 weeks, CTaverage (361.84 ± 46.4 µ) and Csubfoveal (358.46 ± 51.3 µ) of 34 self-resolved CSC eyes were not significantly different from CTaverage (357.25 ± 53.20 µ) and Csubfoveal (360 ± 57.99 µ) of 69 fellow eyes (P = 0.667 and 0.896, respectively).
Discussion
The role of choroid has already been established in the pathophysiology of CSC.[7,8] However, choroid is difficult to image in vivo because the pigments in the RPE and choroid impede image capture by ophthalmoscopy, fundus photography, fluorescein angiography, and conventional OCT. Imaging of choroid has been done with ultrasound, magnetic resonance imaging (MRI), and ICG. MRI and ultrasonography have a resolution of about 100 µ and 150 µ, respectively, and are not reliable for measurement of CT accurately.[5,9] ICGA has been the best tool for studying choroidal vasculature; however, it does not provide a quantitative evaluation of the layer. ICGA also is relatively invasive and inconvenient to perform repeatedly during follow-up. Recent advancements in OCT now enable in vivo and noninvasive characterization of more refined details of the choroid with high accuracy using SD OCT and swept source OCT.
In this study, SD OCT was used to establish a normative database of CT in young healthy males of Indian origin and also demonstrate that subfoveal CT in symptomatic eyes in CSC is significantly thicker than fellow eyes and normal eyes. Subfoveal CT in fellow eyes is also significantly thicker than normal eyes, thereby reinforcing the fact that choroidal permeability is increased in both eyes of patients with CSC.
It is important to establish a normative database in a particular population before carrying out further research. Various studies have been conducted to establish a normative database in different populations using different OCT machines [Table 1]. The reason for the difference in subfoveal CT in different studies could be due to different OCT machines (different software and difference in light source [1060 vs. 840 nm]), racial differences, and patient characteristics.[5] While some investigators use the scleral rim as the boundary, others have used the lamina fusca as the outer boundary leading to significant variations.[19] In this study, the outer choroidal boundary was used for measurements. Patients in our study were younger (mean age 33.16 ± 9.4 years) compared to the above-mentioned studies and it has been shown that CT decreases with age.[5,11] The cause of the racial difference in CT is still unknown.
Table 1.
Studies measuring choroidal thickness in normal eyes/healthy volunteers
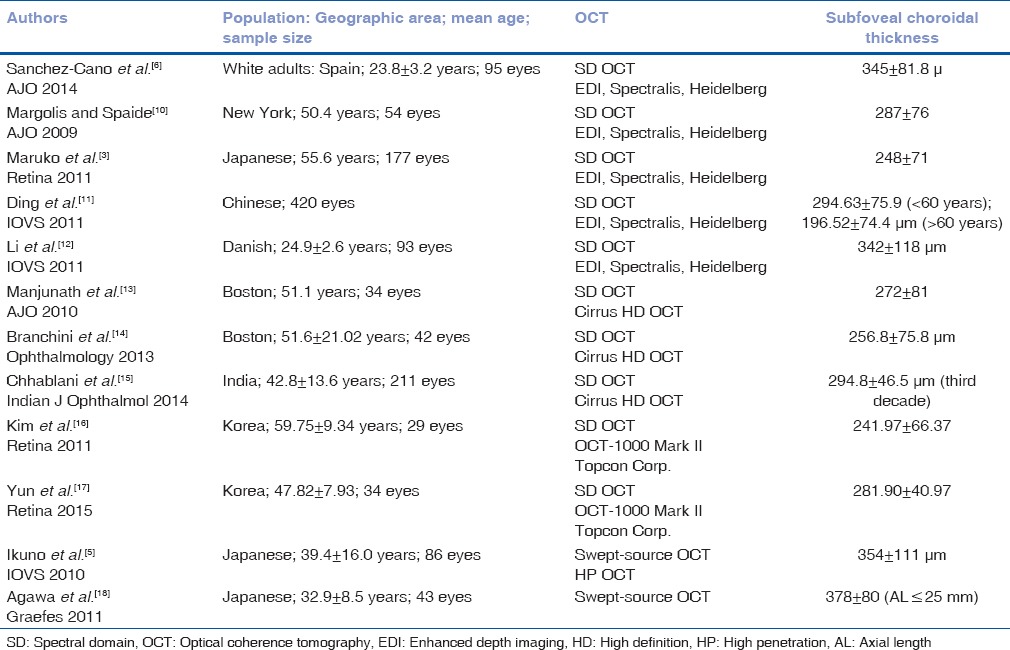
Various studies have demonstrated increased CT in CSC [Table 2] and that choroid is diffusely thickened[4] irrespective of the site of leakage. It has also been shown that CT is increased in both eyes of patients with CSC even if the subretinal fluid is present only in one eye.[3,21] However, Iida et al.[22] also reported that the rate of choroidal vascular hyperpermeability was 62% in the fellow eyes of Japanese patients with unilateral CSC in an ICGA study, suggesting that the choroid in the fellow eyes of those with CSC is not always thickened. Our study on Indian eyes is in agreement with those of Imamura et al.[21] and Maruko et al.[3] to prove that CT is not only significantly increased in symptomatic eyes with CSC compared to fellow eyes and normal eyes but also CT of fellow eyes is significantly higher than normal eyes. Thus, fellow eyes of patients with CSC should also be followed up carefully as they have a high chance of developing CSC over a period. The CT might be the reference index for evaluating choroidal vascular hyperpermeability.[3]
Table 2.
Studies measuring choroidal thickness in central serous chorioretinopathy
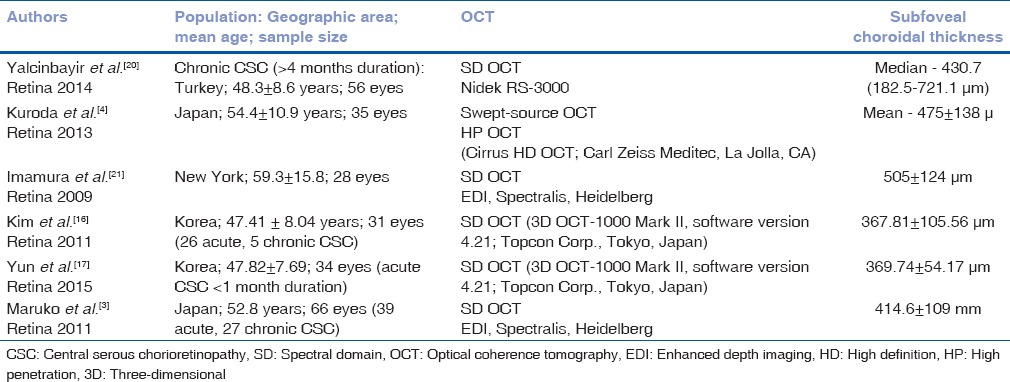
It is also interesting to note that as the eyes with acute CSC were observed over time, their CT decreased steadily (as CSC self-resolved) to match the CT of the fellow eyes. Since CT in eyes with resolved CSC is still thicker compared to normal eyes, they are prone to recurrence due to high choroidal permeability.
To the best of our knowledge, there has been only one study[20] in Indian eyes to establish the normative database of CT in healthy adults and none on NIDEK SDOCT. We have already emphasized the need of normative database in a particular ethnic group on a particular OCT machine. The strength of our study is our stringent methodology. The inclusion of only young (20–50 years of age) healthy volunteers in the study removed the confounding factor of age. Our inclusion criteria for axial length and refractive error were also more stringent as compared to other studies. To account for diurnal variation of CT, we performed OCT at a fixed time of the day. CT was measured at a large number of points (36 points) in each eye, and then CTaverage was calculated. Csubfoveal was also an average of four measurements along different axis. Limitations of this study include its cross-sectional nature and ICGA was not performed in all cases. An interesting area of further research would be to monitor CT in fellow eyes periodically. It has been shown that on longitudinal follow-up, certain unilateral cases turn into bilateral cases. It would be interesting to note CT in fellow eyes just before developing subretinal fluid and becoming symptomatic and compare it with those fellow eyes which remain stable and do not become symptomatic and correlate it with ICGA findings.
Conclusion
CT varies not only with age, axial length and refractive error but also with races and with the type of OCT machine. Therefore, it is important to establish a normative database in a particular population on a particular OCT machine before carrying out further research in diseased states. CT in CSC eyes is significantly thicker than fellow eyes, and CT of fellow eyes is significantly thicker than normal eyes. This reinforces the fact that choroidal permeability is increased in both eyes of patients with CSC. Also, as resolution of CSC occurs, its CT approaches that of fellow eyes. This suggests that even after resolution, choroidal permeability does not become absolutely normal and therefore these patients are prone to recurrences.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Ho A, Orlock D. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112:1057–62. doi: 10.1001/archopht.1994.01090200063023. [DOI] [PubMed] [Google Scholar]
- 2.Spaide RF, Goldbaum M, Wong DW, Tang KC, Iida T. Serous detachment of the retina. Retina. 2003;23:820–46. doi: 10.1097/00006982-200312000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Maruko I, Iida T, Sugano Y, Ojima A, Sekiryu T. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina. 2011;31:1603–8. doi: 10.1097/IAE.0b013e31820f4b39. [DOI] [PubMed] [Google Scholar]
- 4.Kuroda S, Ikuno Y, Yasuno Y, Nakai K, Usui S, Sawa M, et al. Choroidal thickness in central serous chorioretinopathy. Retina. 2013;33:302–8. doi: 10.1097/IAE.0b013e318263d11f. [DOI] [PubMed] [Google Scholar]
- 5.Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010;51:2173–6. doi: 10.1167/iovs.09-4383. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Cano A, Orduna E, Segura F, Lopez C, Cuenca N, Abecia E, et al. Choroidal thickness and volume in healthy young white adults and the relationships between them and axial length, ammetropy and sex. Am J Ophthalmol. 2014;158:574–83.e1. doi: 10.1016/j.ajo.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Kim YT, Kang SW, Bai KH. Choroidal thickness in both eyes of patients with unilaterally active central serous chorioretinopathy. Eye (Lond) 2011;25:1635–40. doi: 10.1038/eye.2011.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63(Suppl):1–139. [PubMed] [Google Scholar]
- 9.Cheng H, Nair G, Walker TA, Kim MK, Pardue MT, Thulé PM, et al. Structural and functional MRI reveals multiple retinal layers. Proc Natl Acad Sci U S A. 2006;103:17525–30. doi: 10.1073/pnas.0605790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–5. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Ding X, Li J, Zeng J, Ma W, Liu R, Li T, et al. Choroidal thickness in healthy Chinese subjects. Invest Ophthalmol Vis Sci. 2011;52:9555–60. doi: 10.1167/iovs.11-8076. [DOI] [PubMed] [Google Scholar]
- 12.Li XQ, Larsen M, Munch IC. Subfoveal choroidal thickness in relation to sex and axial length in 93 Danish university students. Invest Ophthalmol Vis Sci. 2011;52:8438–41. doi: 10.1167/iovs.11-8108. [DOI] [PubMed] [Google Scholar]
- 13.Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150:325–9.e1. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branchini LA, Adhi M, Regatieri CV, Nandakumar N, Liu JJ, Laver N, et al. Analysis of choroidal morphologic features and vasculature in healthy eyes using spectral-domain optical coherence tomography. Ophthalmology. 2013;120:1901–8. doi: 10.1016/j.ophtha.2013.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhablani J, Rao PS, Venkata A, Rao HL, Rao BS, Kumar U, et al. Choroidal thickness profile in healthy Indian subjects. Indian J Ophthalmol. 2014;62:1060–3. doi: 10.4103/0301-4738.146711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SW, Oh J, Kwon SS, Yoo J, Huh K. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011;31:1904–11. doi: 10.1097/IAE.0b013e31821801c5. [DOI] [PubMed] [Google Scholar]
- 17.Yun C, Oh J, Han JY, Hwang SY, Moon SW, Huh K. Peripapillary choroidal thickness in central serous chorioretinopathy: Is choroid outside the macula also thick? Retina. 2015;35:1860–6. doi: 10.1097/IAE.0000000000000539. [DOI] [PubMed] [Google Scholar]
- 18.Agawa T, Miura M, Ikuno Y, Makita S, Fabritius T, Iwasaki T, et al. Choroidal thickness measurement in healthy Japanese subjects by three-dimensional high-penetration optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2011;249:1485–92. doi: 10.1007/s00417-011-1708-7. [DOI] [PubMed] [Google Scholar]
- 19.Bafiq R, Mathew R, Pearce E, Abdel-Hey A, Richardson M, Bailey T, et al. Age, sex, and ethnic variations in inner and outer retinal and choroidal thickness on spectral-domain optical coherence tomography. Am J Ophthalmol. 2015;160:1034–43.e1. doi: 10.1016/j.ajo.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Yalcinbayir O, Gelisken O, Akova-Budak B, Ozkaya G, Gorkem Cevik S, Yucel AA. Correlation of spectral domain optical coherence tomography findings and visual acuity in central serous chorioretinopathy. Retina. 2014;34:705–12. doi: 10.1097/IAE.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 21.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469–73. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 22.Iida T, Kishi S, Hagimura N, Shimizu K. Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina. 1999;19:508–12. doi: 10.1097/00006982-199911000-00005. [DOI] [PubMed] [Google Scholar]


