Abstract
Aims
Monitors of transdermal alcohol concentration (TAC) provide an objective measurement of alcohol consumption that is less invasive than measurements in blood, breath or urine; however, there is a substantial time delay in the onset of TAC compared to blood or breath alcohol concentrations (BrACs). The current study examined the characteristics of the delay between peak TAC and peak BrAC.
Methods
Data was aggregated from three experimental laboratory studies (N = 61; 32 men, 29 women) in which participants wore a TAC monitor and BrAC was monitored while drinking one, two, three, four and five beers in the laboratory. Analyses examined the sex- and dose-related differences in peak BrAC and TAC, the time-to-peak BrAC and TAC, and time lag between the peak BrAC and TAC values.
Results
The times-to-peak were an increasing function of the number of beers consumed. At each level of beer consumption the peak TAC averaged lower than peak BrAC and times-to-peak TAC were longer than for BrAC. The time-to-peak BrAC and TAC was longer for women than men. The congruence between peak TAC and BrAC increased as a function of the beers consumed. No sex difference in the time lag between peak BrAC and TAC was detected.
Conclusions
The congruence between TAC and BrAC and time lags between TAC and BrAC are related to the number of beers consumed. Peak values of TAC and BrAC became more congruent with higher doses but the time lag increased as a function of the amount of alcohol consumed.
Short summary
The time delay (or lag) and congruence between transdermal vs. BrACs increases as the number of beers increases. Though sex differences are evident in peak transdermal and BrACs, no sex differences were evident in the time lag and the congruence between transdermal and breath alcohol concentrations.
INTRODUCTION
Objective means of monitoring alcohol consumption are useful in clinical research, therapeutic and judicial settings. Traditionally, objective measures of alcohol consumption have relied on blood, breath or urine measurements of biomarkers, which are either not specific for alcohol use, limited in their window of detection, or require frequent visits to obtain samples. Wearable devices for electronic monitoring of transdermal alcohol concentrations (TACs) allow for less obtrusive, objective and frequent measurements of alcohol consumption. TAC readings are a measurement of the small amount of ethanol that is excreted through sweat (Swift, 2000). The most commonly used transdermal alcohol monitor is the Secure Continuous Remote Alcohol Monitor (SCRAM) produced by Alcohol Monitoring Systems (AMS) Inc. (Highlands Ranch, CO). The SCRAM monitor records TAC every 30 min, 24 h/d, 7 days a week and used as an estimate of blood alcohol concentrations (BACs) (Swift et al., 1992; Sakai et al., 2006; Dougherty et al., 2012). The SCRAM has been widely adopted in judicial settings for monitoring drinking in driving while intoxicated offenders (Voas et al., 2011) and more recently has been increasingly used within clinical research (Barnett et al., 2011; Dougherty et al., 2014, 2015a).
In controlled laboratory alcohol drinking studies where men and women consumed equal amounts of beer, we previously reported alcohol dose-related effects in peak BrAC and TAC (Dougherty et al., 2012; Hill-Kapturczak et al., 2014; Dougherty et al., 2015b; Hill-Kapturczak et al., 2015). Using this information, we developed an equation to estimate BrAC from TAC to quantify drinking levels from TAC data. Those studies demonstrated that sex, time-to-peak TAC and peak TAC are important factors influencing estimation of BrAC. Other investigators (Marques and McKnight, 2007, 2009) have suggested that TAC levels underestimate BAC levels to a greater extent in women than in men. In one of our studies (Hill-Kapturczak et al., 2015), we observed significant sex differences in BrAC but not in TAC, evidence that there may be sex differences in the BrAC to TAC relationship. However, additional research is required to more precisely define the role of sex and other pharmacokinetic variables such as peak and time-to-peak to modulate the relationship between BrAC and TAC.
One of the major issues in using TAC levels to estimate BrAC or BAC levels is the time delay, or ‘time lag’, in the time-to-peak TAC relative to the time-to-peak BrAC or BAC levels (Anderson and Hlastala, 2006; Marques and McKnight, 2009). However, a wide range of time lags have been reported and range from 1 to 2 h (Marques and McKnight, 2009), an average of 1 h and 27 min (Swift et al., 1992), 2–3 h (Sakai et al., 2006), an average of 2 h and 9 min (Hill-Kapturczak et al., 2015), and as great as 4.5 h (Marques and McKnight, 2007). This variation in estimates of the time lag could be due to a number of different variables related to sex, dose or other procedural variables in addition to the fact that several of the early studies (Sakai et al., 2006; Marques and McKnight, 2007, 2009) used an older version of the SCRAM monitor with some known issues such as water getting into the monitor. Furthermore, the sex and dose relationship of important pharmacokinetic parameters such as peak and time to peak are well known for BrAC or BAC levels, but are less well-characterized for TAC or the TAC-to-BrAC relationships. Thus, further research is required to characterize how these TAC-to-BrAC relationships may vary as a function of dose and sex.
The current study pooled the results of three studies to better characterize the relationships between peak BrAC and peak TAC with respect to their magnitude and relative times by examining characteristics of the curves in terms of the number of drinks consumed. Data were from three controlled laboratory studies where participants consumed one, two, three, four and five beers on separate days under slightly different conditions (previously published in Dougherty et al., 2012; Hill-Kapturczak et al., 2014; Dougherty et al., 2015b; Hill-Kapturczak et al., 2015; Roache et al., 2015). These data were used to model the BrAC and TAC time-course curves and to examine characteristics of the time-course curves such as sex and dose differences in the peak and times to peak.
METHOD
Participants
Data (n = 61) for this analysis came from three studies (Study 1 (11 women, 10 men), Study 2 (10 women, 11 men), Study 3 (8 women, 11 men)), in which one, two, three, four and five beers were consumed under controlled laboratory conditions as previously described (Dougherty et al., 2012; Hill-Kapturczak et al., 2014; Dougherty et al., 2015b; Hill-Kapturczak et al., 2015). Participants for these studies were recruited through community advertisements seeking healthy men and women aged 21–47 years who regularly consume alcohol. Participants self-reported at least one heavy drinking episode (i.e. five standard drinks) in the past month. Participants were excluded for: a body mass index < 18 or > 30 kg/m2, DSM-IV diagnosis other than alcohol abuse, pregnancy, a current medical condition, or a positive urine–drug test for drugs of abuse. The University institutional review board reviewed and approved the studies and participants provided informed consent.
Study designs
Participants fasted after midnight and provided negative drug and pregnancy urine tests on each study day for five days (mostly Monday–Friday). Breath alcohol samples ensured sobriety at the start of each study day and participants were given a meal at approximately 6 h post-alcohol consumption.
Alcohol administration
On five separate study days, men consumed one, two, three, four and five 12 oz Corona beers (Grupo Modelo S.A.B. de C.V., Mexico City, Mexico), equivalent to 0.92 standard units each (NIAAA, 2010), in all three studies. In Studies 1 and 2, the drinking rate was controlled by giving one beer every 24 min, but in Study 3, participants could drink at their own pace over a 3 h period, as long as the beers for that day (e.g. four beers on a 4-beer day) were consumed in 3 h (time to complete all five beers ranged from 47 to 166 min, with a 3-fold range in drinking rates observed among men and women, though drinking patterns were similar for men and women; see Hill-Kapturczak et al., 2014 for more details regarding pace of drinking). To control for sex-related differences in alcohol metabolism, in Study 1, women consumed only one, two, three, and four beers at the rate of one beer every 30 min (up to four beers); in Studies 2 and 3, women and men were treated the same. All beers were consumed in the morning, usually beginning at 9:00 am, and participants consumed the beers in a temperature-controlled room while mostly sitting, with freedom to move around within the laboratory.
Transdermal alcohol monitoring
TACs were automatically recorded by the SCRAM device approximately every 30 min. The TAC data were analysed as follows: (a) the curve-fitted time-course of rising and falling TAC levels; (b) peak TAC, the highest recorded TAC value per drinking episode; and (c) time-to-peak TAC, the time in minutes to peak TAC since beginning drinking.
Breath alcohol concentrations
A portable breathalyzer (Dräger Alcotest 6810, Irving, TX) was used to measure breath alcohol concentrations (BrACs) expressed as percent blood alcohol concentrations (% BAC). BrACs were measured every 15 min after drinking began for the first 2 h and every 30 min thereafter for the next 4 h. Participants were allowed to leave the laboratory after their BrAC readings were ≤0.02% BAC post-alcohol consumption. The BrAC data were analysed as follows: (a) curve-fitted time-course of rising and falling BrAC levels; (b) peak BrAC, the highest recorded BrAC value per drinking episode; and (c) time-to-peak BrAC, the time in minutes to peak BrAC since beginning drinking.
Data analysis
TAC data are auto-sampled by the SCRAM transdermal alcohol monitors at approximately 30 min intervals, but it is not always exactly 30 min and the time of start of drinking was not exactly aligned with the approximate 30 min intervals. These facts coupled with individual differences in the absorption and elimination time-courses for positive TAC and BrAC measures required the use of a generalized additive models (GAMs) approach to produce overall smoothed curves of TAC or BrAC as curve-fitted functions of time based on penalized regression splines (Wood, 2003, 2006). The R (version 3.1.1) package mgcv was used to fit the GAM models and plot the overall smoothed curves.
However, for inferential analyses of the observed data, a data reduction approach derived several key pharmacokinetic parameters of the time-course curves: peak TAC, peak BrAC, time-to-peak TAC and time-to-peak BrAC. Additionally, the time lag and the peak TAC-to-BrAC ratio of the observed data was used to further examine the parameters of the time-course curves. The ‘time lag’ between the peak TAC and BrAC levels was defined as the difference (delay) in time between the time-to-peak TAC minus the time-to-peak BrAC. The peak TAC-to-BrAC ratio was the ratio of the peak TAC level compared to the peak BrAC level. There were 24 observations after one beer and three observations after two beers that had TAC levels of zero and were consequently removed from analyses of time-to-peak TAC and time lag.
Mixed effect models with unstructured variance-covariance structure were used to examine the main effects and interactions of sex and the number of beers consumed on peak TAC, peak BrAC, time-to-peak TAC and time-to-peak BrAC. Due to the known individual differences and sex differences in the relationship between the number of beers consumed and actual BrAC levels achieved, we also used the peak BrAC value as a measure of alcohol exposure in mixed effect models. Those results were similar to the mixed effect models results using the number of beers consumed so only analyses using beers consumed are reported herein.
Because drinking rates varied somewhat between the three studies, we examined how our general findings might differ between-studies by examining each study separately. The model-based least squares mean sex differences (corrected for multiple comparisons) and corresponding 95% confidence intervals at each level of beer consumption were used to estimate effect size for time lag and the peak TAC-to-peak BrAC ratios for the combined sample as well as for each study separately. In addition, we conducted analyses with ‘study’ as a co-varying variable in the aggregated data. However, because these results did not differ from the presented results, we did not report them here to reduce the abundance of results. All analyses were conducted using Stata/SE version 13.
RESULTS
Participants
Of the 61 participants, 32 were men and 29 were women with an average age of 27.72 years (range: 21–47). The sample was 61.5% Hispanic white, and 38.5% non-Hispanic white, consistent with demographics in San Antonio, TX. On average, participants reported drinking 19.1 drinks per week, with participants in Study 2 (M = 26.8) reporting more drinks per week than those in Study 1 (M = 12.7) or Study 3 (M = 17.5). There were no other differences between the three studies in participant demographic characteristics (Dougherty et al., 2012; Hill-Kapturczak et al., 2014; Dougherty et al., 2015b; Hill-Kapturczak et al., 2015 for further demographic characteristics).
Time-course of TAC and BrAC
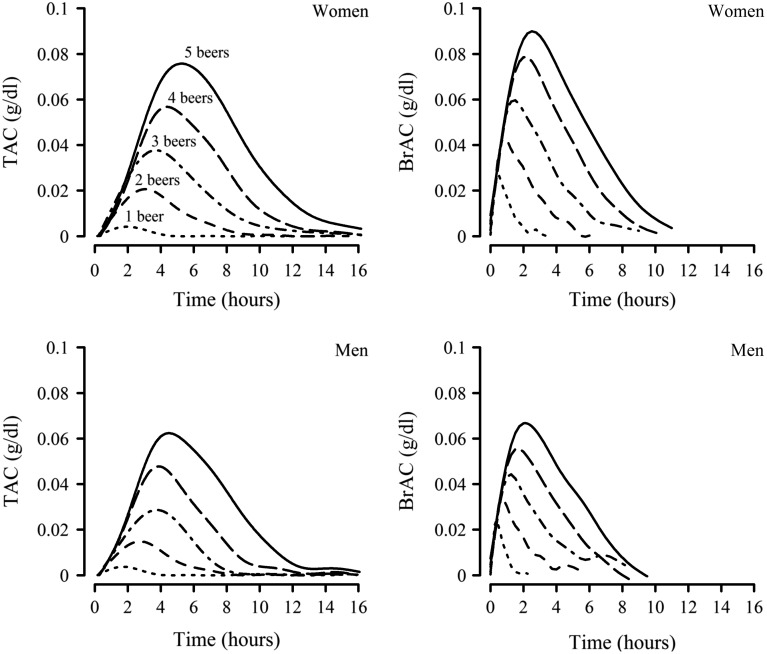
Figure 1 displays the curve-fitted TAC and BrAC time-course functions for men and women consuming one to five beers. Clearly visible in these curves is the fact that the peak levels, time to peak and the duration of time course of TAC and BrAC each were an increasing function of the number of beers consumed. There also were tendencies for TAC to show lower peaks, with longer times to peak and longer duration than the comparable BrAC levels. Generally, the peaks (both TAC and BrAC) are higher in women than in men. These observations are statistically examined in detail below using the observed data.
Fig. 1.
Time course of TAC and BrAC for men and women. The curve-fitted TAC and BrAC time-course functions for men and women consuming one to five beers. Generalized additive models (GAM) were used to produce overall smoothed curves of TAC or BrAC as curve-fitted functions of time.
Peak BrAC and peak TAC
Both peak TAC and peak BrAC levels increased as an orderly effect of the number of beers consumed (Table 1). Overall, women had higher peak BrACs and peak TACs than men. Both peak BrAC and TAC were significantly related to the number of beers consumed. There also was a significant interaction between sex and the number of beers consumed for peak BrAC and a marginal one for peak TAC, reflecting a growing sex difference with increasing number of beers consumed.
Table 1.
Mixed effects models to examine the effects and interactions of sex and the numbers of beers consumed on the peak TAC, peak BrAC, time-to-peak TAC, time-to-peak BrAC, TAC-to-BrAC ratio and time lag in combined data and Studies 1, 2 and 3 individually
| Overall | Study 1 | Study 2 | Study 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wald χ2 | df | P | Wald χ2 | df | P | Wald χ2 | df | P | Wald χ2 | df | P | |
| Peak TAC | ||||||||||||
| Sex | 5.52 | 1 | 0.02 | 0.05 | 1 | 0.83 | 3.03 | 1 | 0.08 | 4.09 | 1 | 0.04 |
| Beers consumed | 833.19 | 4 | <0.001 | 171.19 | 4 | <0.001 | 346.32 | 4 | <0.001 | 407.48 | 4 | <0.001 |
| Sex * beers consumed | 8.87 | 4 | 0.07 | 0.95 | 4 | 0.81 | 4.27 | 4 | 0.37 | 13.49 | 4 | 0.01 |
| Peak BrAC | ||||||||||||
| Sex | 37.94 | 1 | <0.001 | 13.63 | 1 | <0.001 | 34.18 | 1 | <0.001 | 5.25 | 1 | 0.02 |
| Beers consumed | 1888.05 | 4 | <0.001 | 771.36 | 4 | <0.001 | 741.21 | 4 | <0.001 | 672.71 | 4 | <0.001 |
| Sex * beers consumed | 72.27 | 4 | <0.001 | 32.29 | 4 | <0.001 | 37.32 | 4 | <0.001 | 23.45 | 4 | <0.001 |
| Time-to-peak TAC | ||||||||||||
| Sex | 5.35 | 1 | 0.02 | 0.31 | 1 | 0.58 | 6.38 | 1 | 0.01 | 2.15 | 1 | 0.14 |
| Beers consumed | 410.26 | 4 | <0.001 | 96.88 | 4 | <0.001 | 194.05 | 4 | <0.001 | 150.19 | 4 | <0.001 |
| Sex * beers consumed | 1.41 | 4 | 0.84 | 0.51 | 4 | 0.92 | 4.29 | 4 | 0.37 | 2.89 | 4 | 0.58 |
| Time-to-peak BrAC | ||||||||||||
| Sex | 8.18 | 1 | 0.004 | 10.38 | 1 | 0.001 | 0.88 | 1 | 0.35 | 3.91 | 1 | 0.05 |
| Beers consumed | 772.05 | 4 | <0.001 | 1075.01 | 4 | <0.001 | 276.98 | 4 | <0.001 | 142.25 | 4 | <0.001 |
| Sex * beers consumed | 3.35 | 4 | 0.50 | 0.84 | 4 | 0.84 | 1.76 | 4 | 0.78 | 3.72 | 4 | 0.45 |
| Time lag | ||||||||||||
| Sex | 0.94 | 1 | 0.33 | 0.03 | 1 | 0.87 | 5.26 | 1 | 0.02 | 0.25 | 1 | 0.62 |
| Beers consumed | 64.19 | 4 | <0.001 | 8.07 | 4 | 0.09 | 39.80 | 4 | <0.001 | 38.34 | 4 | <0.001 |
| Sex * beers consumed | 0.29 | 4 | 0.99 | 0.38 | 4 | 0.94 | 2.32 | 4 | 0.69 | 2.67 | 4 | 0.62 |
| Peak TAC-to-BrAC ratio | ||||||||||||
| Sex | 0.08 | 1 | 0.78 | 1.84 | 1 | 0.18 | 0.17 | 1 | 0.68 | 0.67 | 1 | 0.41 |
| Beers consumed | 338.13 | 4 | <0.001 | 54.43 | 4 | <0.001 | 175.88 | 4 | <0.001 | 221.37 | 4 | <0.001 |
| Sex * beers consumed | 5.04 | 4 | 0.28 | 4.94 | 4 | 0.18 | 8.41 | 4 | 0.08 | 4.99 | 4 | 0.29 |
Differences related to study. An analysis of each study individually showed that both peak TAC and BrAC increased significantly with the number of beers consumed (Table 1). In addition, sex differences were identified in peak BrAC in all studies, such that women had higher peak BrAC levels than men. However, for peak TAC, women had significantly higher peak TACs only in Study 3 where drinking rates were uncontrolled and subjects were free to drink at a pace comfortable to them.
Time-to-peak BrAC and peak TAC
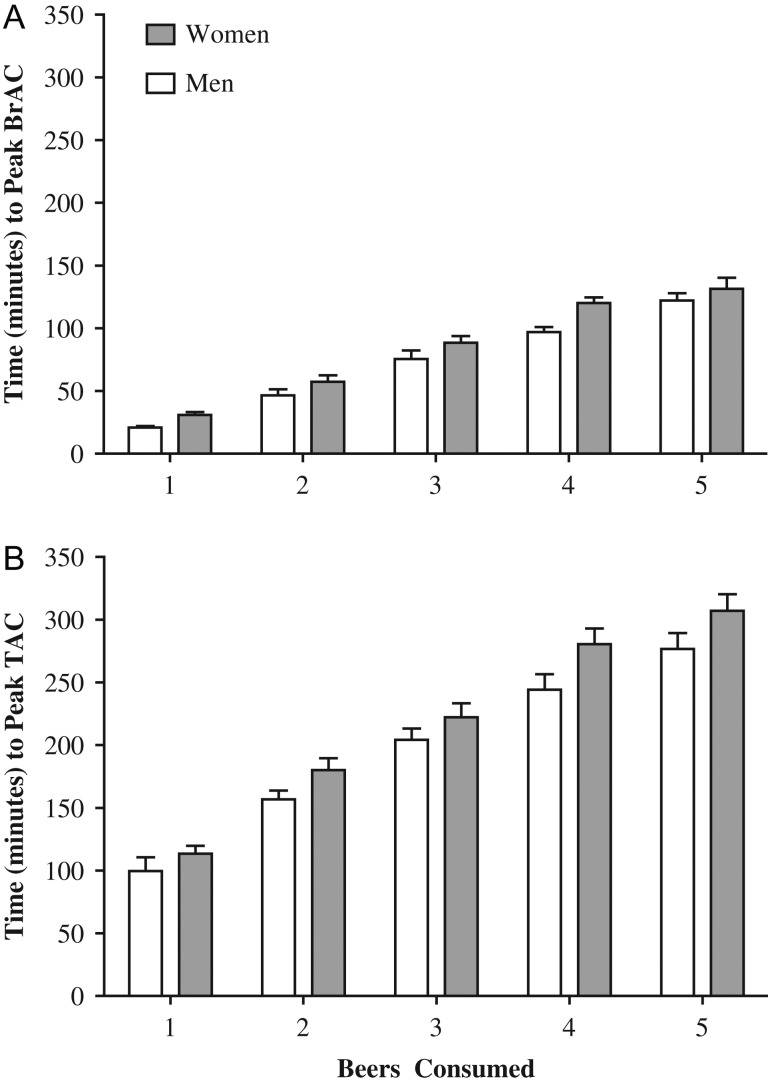
Figure 2A and B shows the times-to-peak BrAC and TAC, respectively, as a function of beers consumed for each of the sexes. Clearly, the times-to-peak TAC were much greater than the times-to-peak BrAC for both men and women at each level of beer consumed. As expected, times to peak increased as a significant function of beers consumed for both BrAC and TAC (Table 1). There was a significant tendency for time-to-peak BrAC and time-to-peak TAC to be longer for women than men. No significant interaction between sex and the number of beers consumed was detected for either measure.
Fig. 2.
Time-to-peak BrAC and time-to-peak TAC as a function of the number of beers consumed. The average time in minutes to (A) peak BrAC and (B) peak TAC for women and men consuming one to five beers. A total of 24 TAC observations from the one beer condition and three from the two beer condition were removed prior to analyses due to having peak TAC = 0. All participants had a measureable BrAC; and no other observations were removed
Differences related to study. In an analysis of each study individually, the times-to-peak TAC and times-to-peak BrAC increased significantly with the number of beers consumed (see Table 1). Additionally, significant sex differences were identified in the time-to-peak TAC in Study 2 and moderate sex differences were identified in time-to-peak BrAC in Study 3, such that women had longer times-to-peak TAC and BrAC than men.
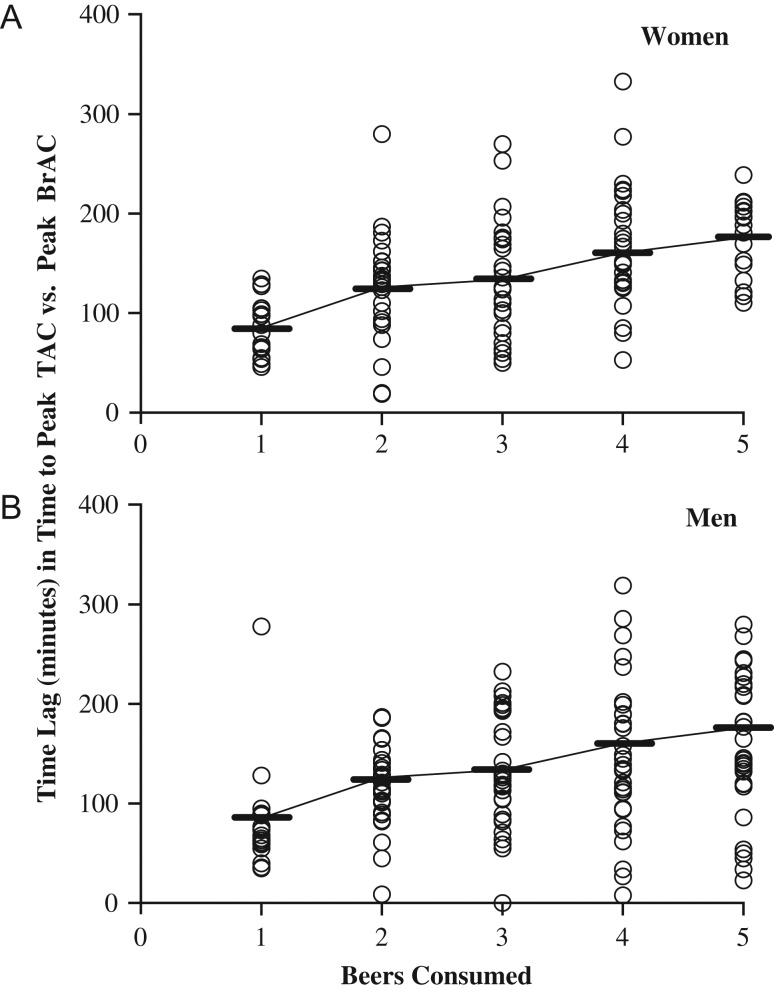
Time lag
Figure 3 presents the time lags separately for women (Fig. 3A) and men (Fig. 3B) expressed as a function of the number of beers consumed. The time lag between the times-to-peak TAC and the times-to-peak BrAC was a significant positive function of the numbers of beers consumed (Table 1). The time lag between the times-to-peak TAC and the times-to-peak BrAC averaged (in minutes; M (SD)) for one to five beers, respectively, as follows: 82.5 (41.2), 119.8 (46.0), 131.2 (56.6), 153.6 (71.0) and 162.0 (60.7). There was no significant sex difference in average time lag at the various numbers of beers consumed. The magnitude of the sex difference in average time lag (men relative to women) is very small for each beer consumed (see Table S1 for the model-based least squares mean estimates of differences).
Fig. 3.
The time lag between TAC and BrAC as a function of the number of beers consumed. A scatterplot of the association between the number of beers consumed and time lag (i.e. time-to-peak TAC minus time-to-peak BrAC). Data are all values collected from all participants across all drinking days
Differences related to study. The time lag was significantly related to the number of beers consumed in Studies 2 and 3, but only marginally so in Study 1 (Table S2). Study 1 differed from the other studies by slowing the rate of consumption for women as compared to men, and by reducing the range of alcohol consumed (i.e. women did not consume five beers in this study). As with the overall analysis, there were no main effects of sex or interactions with beers consumed observed within any one study alone. The magnitude of the sex difference in average time lag (men relative to women) is very small for each beer consumed within each study (Table S2).
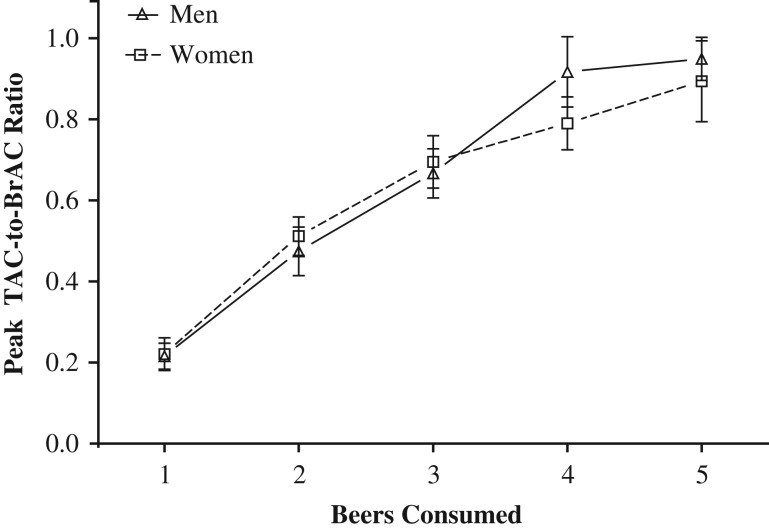
Peak TAC-to-BrAC ratio
Figure 4 shows that the peak TAC-to-peak BrAC ratios of men and women increased as a function of the number of beers consumed. Importantly, peak TAC substantially underestimated peak BrAC at lower numbers of beers consumed, and less underestimated peak BrAC (i.e. the peak TAC-to-BrAC ratio was < 1) at the one beer but approached unity (i.e. ratio = 1) as the number of beers consumed increased. For the peak TAC-to-BrAC ratio measure, there was no difference in sex on the slope of the relationship between the number of beers consumed and the peak TAC-to-peak BrAC ratio. Model-based least squares mean estimates of sex differences in the peak TAC-to-peak BrAC ratio showed that the magnitude of the sex differences (men relative to women) in average peak TAC-to-peak BrAC ratio at each beer consumed were very small, and not significant (Table S1).
Fig. 4.
Peak TAC-to-peak BrAC ratios of men and women as a function of the number of beers consumed. The average peak TAC-to-BrAC ratio of women and men as a function of the number of beers consumed. All observations are present including n = 27 participants who had peak TAC = 0 after drinking one or two beers.
Differences related to study. Each of the studies individually, also showed that peak TAC-to-BrAC ratios increased as a function of the number of beers consumed. In addition, there were no sex differences in the peak TAC-to-peak BrAC ratios by the beers consumed, and the magnitude of the sex differences (men relative to women) were very small (Table S2).
DISCUSSION
The purpose of the present study was to use a comprehensive set of laboratory-controlled drinking data to better characterize the relationships between peak BrAC and peak TAC and examine how these parameters vary as a function of the number of drinks consumed. We found amount consumed-related increases in the peak TAC and peak BrAC levels and found that peak TAC underestimated peak BrAC at lower levels of beers consumed but approached unity at higher levels beers consumed. We also found that the peak TAC-level lagged in time behind the time-to-peak BrAC-level and that this time lag increased with more beers consumed. These findings, for the most part, were replicated in each study separately, suggesting that minor differences in drinking rate across the three studies did not affect the basic findings of increasing time lag with increased number of beers consumed. Importantly, this study replicated the well-known sex differences in BrAC levels and demonstrated the same sex differences in TAC levels overall. There were no sex differences evident in the time lag between TAC and BrAC peaks and the peak TAC-to-peak BrAC ratios.
The pharmacokinetics of blood alcohol are well known (Matsumoto and Fukui, 2002) and BrAC follows those same trends (Martin et al., 1984). The sex differences in blood alcohol and BrAC are due to differences in the distribution of alcohol throughout the body in men and women. For example, men have the tendency to have less body fat and more body water than women (Graham et al., 1998) and women tend to have lower gastric alcohol dehydrogenase activity (Baraona et al., 2001). Though the individual reports of our laboratory have observed that women had significantly higher BrACs than men (Hill-Kapturczak et al., 2015), our results on sex differences in peak TAC levels have been inconsistent (Dougherty et al., 2012; Hill-Kapturczak et al., 2014, 2015). Furthermore, Marques and McKnight (2007) have observed tendencies for women to have higher TAC relative to BAC values than men. With the larger sample size of this combined analysis we now can more confidently state that the well-known sex differences in BrAC levels are also reflected in higher peak TAC values for women as compared to men when drinking rates are uncontrolled (Study 3) or women drink at the same rate as men (Study 2) (Roache et al., 2015).
Understanding the parameters of peak TAC-level to peak BrAC-level ratios are important to the usefulness of TAC as an estimator of BrAC and BAC levels. The current finding that peak TAC levels underestimate peak BrAC at lower levels of beer consumption but approach unity at higher levels of beer consumption more fully characterizes the limitations of TAC to detect low-level drinking. Previously, we demonstrated in these same data (Roache et al., 2015) only 62.5% of men and 58.6% of women exhibited any positive TAC readings after drinking only one beer, though they had positive BrACs, and the sensitivity for TAC increased with the amount consumed. The current finding that the peak TAC-to-peak BrAC ratios approach unity at higher levels of beer consumption informs us that TAC levels more accurately estimate BrAC at higher levels of consumption (Dougherty et al., 2012; Hill-Kapturczak et al., 2014, 2015). Both Luczak and Rosen (2014), who developed an equation for estimating BrAC using TAC data, and Webster and Gabler (2007, 2008), who examined blood and skin ethanol concentrations, each note that it may be difficult to equate TAC to real-time BAC without information about the amount of alcohol consumed. The current study provides additional information regarding the time delays in peak TAC vs. peak BrAC, which should inform and may potentially improve equations for estimating peak BrAC or BAC using TAC data.
In the original report of Study 2 (Hill-Kapturczak et al., 2015) we suggested that there may be a sex difference in the peak TAC-to-peak BrAC ratio. Though there was no detectable sex-related trend in the current study, previous research has identified sex differences in the peak TAC readings relative to the peak BrAC readings. For example, Marques and McKnight (2009) suggested that drinking was less easily detected among women than men. However, the current study and our previous studies (Roache et al., 2015) suggest that alcohol is more likely to be detected in women by TAC when they drink at comparable levels to men.
The current study is the first to systematically investigate the time lag in time-to-peak TAC relative to the time-to-peak BrAC. In the current study, our time lags were linearly related to the amount consumed, ranging from 82 min after one beer to 162 min after five beers, on average, with an overall average time lag of 132 min. Previous research has observed time differences in time-to-peak TAC vs. time-to-peak BrAC ranging from 1 h (Marques and McKnight, 2009) to greater than 4.5 h (Marques and McKnight, 2007). We do not know for certain if the time lags would continue increasing with higher amounts of alcohol consumed; however, it is notable that the longest time lag of 4.5 h reported in the literature (Marques and McKnight, 2007) occurred after 31 men and women consumed only 0.64 g/kg (men) and 0.56 g/kg (women) in 30 min, resulting in BACs ≥ 0.08 g/dL.
We only detected a sex difference in the time lag between BrAC and TAC in Study 2 where men and women consumed the same amount of beers at the same rate. To our knowledge, Webster and Gabler (2008) is the only other study to specifically examine the time lag in TAC vs. BrAC. In that study, the BAC curve of one male participant was coupled to a skin diffusion model to simulate TAC and BrAC curves for varying amounts of alcohol consumed. Based upon simulated data, they concluded that sex did not affect the time lag of transdermal alcohol monitoring, consistent with the current study. However, this study was limited in that the data was simulated from one male participant, and all simulations used the same metabolic rate, stomach emptying and skin diffusion coefficient, which are all known to affect the rate of alcohol metabolism (Graham et al., 1998; Baraona et al., 2001).
Limitations and Future Directions
The current study is limited in generality by the fact that all observations were collected under controlled laboratory conditions with specific amounts and/or rates of alcohol consumption, and the rates of consumption differed amongst the three studies, though the results were mostly consistent across the studies.
In the future, studies should further examine how drinking larger amounts of alcohol over the course of longer time periods affects the time lags in peak TAC vs. peak BrAC, which more closely mimics ‘real world’ drinking. Furthermore, because TAC has become more commonplace in the criminal justice system and clinical research and BAC and BrAC are currently the most understood units of measurement of alcohol consumption, the findings in the current study should be used in future research examining how to predict BAC or BrAC using TAC.
CONCLUSION
Though other studies have suggested sex differences in peak TAC levels, this is the first study to definitively demonstrate that well-known sex differences in alcohol metabolism and BAC levels are also seen in peak TAC, to systematically demonstrate that TAC underestimates BrAC at low amounts of alcohol consumed, and to determine that peak TAC-to-peak BrAC ratios approximate unity at higher levels of peak BrAC. Our most important finding is the time delay of peak TAC vs. peak BrAC increases as the amount of alcohol consumed increases after acute intoxication within a 2–3 h time-period. Furthermore, the time lag in time-to-peak TAC vs. time-to-peak BrAC may increase as drinking is prolonged over longer episodes.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Alcohol and Alcoholism online.
FUNDING
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism [R01AA14988] and the National Institute on Drug Abuse [T32DA031115] of the National Institutes of Health.
CONFLICT OF INTEREST STATEMENT
No conflicts exist for any of the authors of this article.
Supplementary Material
REFERENCES
- Anderson JC, Hlastala MP (2006) The kinetics of transdermal ethanol exchange. J Appl Physiol 100:649–55. [DOI] [PubMed] [Google Scholar]
- Baraona E, Abittan CS, Dohmen K, et al. (2001) Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res 25:502–7. [PubMed] [Google Scholar]
- Barnett NP, Tidey J, Murphy JG, et al. (2011) Contingency management for alcohol use reduction: a pilot study using a transdermal alcohol sensor. Drug Alcohol Depend 118:391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Charles NE, Acheson A, et al. (2012) Comparing the detection of transdermal and breath alcohol concentrations during periods of alcohol consumption ranging from moderate drinking to binge drinking. Exp Clin Psychopharm 20:373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Hill-Kapturczak N, Liang Y, et al. (2015. b) The potential clinical utility of transdermal alcohol monitoring data to estimate the number of alcohol drinks consumed. Addict Disord Their Treat 14:124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Hill-Kapturczak N, Liang Y, et al. (2014) Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug Alcohol Depend 142:301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Karns TE, Mullen J, et al. (2015. a) Transdermal alcohol concentration data collected during a contingency management program to reduce at-risk drinking. Drug Alcohol Depend 148:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K, Wilsnack R, Dawson D, et al. (1998) Should alcohol consumption measures be adjusted for gender differences. Addiction 93:1137–47. [DOI] [PubMed] [Google Scholar]
- Hill-Kapturczak N, Lake SL, Roache JD, et al. (2014) Do variable rates of alcohol drinking alter the ability to use transdermal alcohol monitors to estimate peak breath alcohol and total number of drinks. Alcohol Clin Exp Res 38:2517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Kapturczak N, Roache JD, Liang YY, et al. (2015) Accounting for sex-related differences in the estimation of breath alcohol levels using transdermal alcohol monitoring. Psychopharmacology (Berl) 232:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak S, Rosen IR (2014) Estimating BrAC from transdermal alcohol concentration data using the BrAC Estimator Software Program. Alcohol Clin Exp Res 38:2243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques PR, McKnight AS (2007) National Highway Traffic Safety Administration. Evaluating transdermal alcohol measuring devices (Report No. DOT HS 810 875). Washington, DC: U.S. Government.
- Marques PR, Mcknight AS (2009) Field and laboratory alcohol detection with 2 types of transdermal devices. Alcohol Clin Exp Res 33:703–11. [DOI] [PubMed] [Google Scholar]
- Martin E, Moll W, Schmid P, et al. (1984) The pharmacokinetics of alcohol in human breath, venous and arterial blood after oral ingestion. Eur J Clin Pharmacol 26:619–26. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Fukui Y (2002) Pharmacokinetics of ethanol: a review of the methodology. Addict Biol 7:5–14. [DOI] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism (NIAAA) (2010) Rethinking Drinking, in Series Rethinking Drinking.Washington, DC: National Institutes of Health. [Google Scholar]
- Roache JD, Karns TE, Hill-Kapturczak N, et al. (2015) Using transdermal alcohol monitoring to detect low-level drinking. Alcohol Clin Exp Res 39:1120–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai JT, Mikulich-Gilbertson SK, Long RJ, et al. (2006) Validity of transdermal alcohol monitoring: fixed and self-regulated dosing. Alcohol Clin Exp Res 30:26–33. [DOI] [PubMed] [Google Scholar]
- Swift RM. (2000) Transdermal alcohol measurement for estimation of blood alcohol concentration. Alcohol Clin Exp Res 24:422–423. [PubMed] [Google Scholar]
- Swift RM, Martin CS, Swette L, et al. (1992) Studies on a wearable, electronic, transdermal alcohol sensor. Alcohol Clin Exp Res 16:721–5. [DOI] [PubMed] [Google Scholar]
- Voas RB, Dupont RL, Talpins SK, et al. (2011) Towards a national model for managing impaired driving offenders. Addiction 106:1221–7. [DOI] [PubMed] [Google Scholar]
- Webster GD, Gabler HC (2007) Feasibility of transdermal ethanol sensing for the detection of intoxicated drivers. Annu Proc Assoc Adv Automot Med 51:449–64. [PMC free article] [PubMed] [Google Scholar]
- Webster GD, Gabler HC (2008) Modeling of transdermal transport of alcohol effect of body mass and gender. Biomed Sci Instrum 44:361–6. [PubMed] [Google Scholar]
- Wood SN. (2003) Thin plate regression splines. J R Stat Soc Series B Stat Methodol 65:95–114. [Google Scholar]
- Wood SN. (2006) On confidence intervals for generalized additive models based on penalized regression splines. Aust N Z J Stat 48:445–64. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.