Abstract
Aims
Neither the predictive value of early continuous abstinence in alcohol use disorder (AUD) or the point at which this effect may emerge has been evaluated. This analysis of the Combined Pharmacotherapies and Behavioral Interventions (COMBINE) clinical trial evaluated whether abstinence early in treatment was a predictor of longer term abstinence.
Methods
Participants who stated a goal of total abstinence (N = 954) were dichotomized into Early Abstainer vs. Nonabstainers and were compared on a variety of drinking outcome measures that are frequently used in clinical trial evaluations of alcohol treatment strategies, as a function of duration of early continuous abstinence.
Results
Significant differences existed for every outcome. Early Abstinence was significantly associated with fewer drinks per drinking day, number of drinking and number of heavy drinking days, and longer time to first drinking and first heavy drinking day. Effects were evident within the first week. The magnitude of all effects increased as the duration of early abstinence (1–4 weeks) increased, though the size of increase varied across the outcomes.
Conclusions
These data provide evidence that drinking at the beginning of alcohol treatment is significantly and robustly associated with drinking throughout and at the end of a clinical trial treatment for AUD. Early drinking may be a useful early index to identify whether patients are responding positively to a treatment strategy, and provides a useful method for tailoring treatment to patients that is consistent with a personalized medicine approach.
INTRODUCTION
In 2013, >66% of adults in the USA used alcohol and >18 million adults were believed to require treatment for an alcohol-related problem (Substance Abuse and Mental Health Services Administration (SAMHSA), 2014). Excessive alcohol consumption is currently the fourth leading cause of preventable death in the USA, resulting in 88,000 deaths and 2.5 million years of potential life lost (Mokdad et al., 2004; Gonzales et al., 2014), and costs society an estimated $223.5 billion, annually (Bouchery et al. 2011).
In 2013, 1.4 million people sought treatment for alcohol use disorder (AUD) (SAMHSA, 2014). Several strategies have been developed for the treatment of AUD, ranging from behavioral treatments to pharmacotherapies (Morgenstern and Longabaugh, 2000; Anton et al. 2014). Despite the diversity of options, no single approach has emerged as the gold standard treatment. Rather, research suggests that different treatments are generally only effective for a limited subgroup of individuals. Though numerous AUD treatment mediators and moderators (e.g. genetic polymorphisms, personality and psychiatric functioning) have been identified, these variables are often challenging to assess in real-world clinical settings and can make it difficult for practitioners to easily predict and/or readily identify individuals who may respond optimally to a specific treatment strategy.
Given the unpredictable individual variability that exists regarding response to different AUD treatments, it would be advantageous to identify an easily observable marker that can be used to determine whether an individual has a high likelihood for responding positively to any particular strategy. This would be consistent with a recent report advocating for advancements in the use of personalized medicine, or tailored intervention approaches, to guide individualized AUD treatment (Litten et al., 2012). A clearly identifiable marker of treatment success could guide provision of resources toward patients who may not be responding optimally to a particular intervention or identify those who many need to switch to another treatment strategy. Tailoring treatments could result in significant cost savings and prevent patients from needing to undergo treatment for an extended period of time before determining it is not effective; it may also reduce the potential for a patient to experience adverse medication-related side effects in the absence of any benefit. Ultimately, an empirically supported method for early detection of whether an AUD treatment may be working effectively for a patient could improve the provider's ability to match patients with successful treatments and reduce patient frustration and risk of attrition due to lack of treatment response.
Abstinence that occurs early in treatment has been repeatedly identified as a robust and reliable predictor of longer term abstinence (Cochran et al., 2014) among patients in treatment for cigarette smoking (Higgins et al., 2000; Ferguson et al., 2009), marijuana (Moore and Budney 2002, 2003), opioids (Strain et al., 1998; Morral et al., 1999) and stimulants (Alterman et al., 1997; Ehrman et al., 2001; Sofuoglu et al., 2003; Stitzer et al., 2007; Plebani et al., 2009). Despite this robust association, the predictive utility of early abstinence has yet to be evaluated within the context of AUD treatment. Early abstinence is also an easily observed behavior, which makes it highly clinically relevant and something that could be relatively easy to assess within primary care settings.
The present analyses evaluated whether abstinence that occurs during the first 4 weeks of AUD treatment successfully predicts abstinence during and at the end of treatment, and whether there is a particular early abstinence threshold (1, 2, 3 or 4 weeks) that is most predictive of outcomes. This latter evaluation of threshold is a novel contribution to this literature; though studies have reliably determined that abstinence from other substances during the first 2 weeks of treatment is a strong predictor of treatment outcome, it is not clear that this time period is the most appropriate evaluation period. It remains possible that this effect is already evident during Week 1, or that there may be additional predictive value for defining early abstinence as occurring within the first 3 or 4 weeks of treatment. This evaluation was conducted in the context of the Combined Pharmacotherapies and Behavioral Interventions (COMBINE) study, a multisite trial that compared acamprosate and/or naltrexone (vs. placebo), in combination with medical monitoring or a more intensive behavioral treatment, as treatments for AUDs. To demonstrate generality between this analysis and the broader substance abuse treatment field, analyses were restricted to patients who explicitly identified abstinence from alcohol as their treatment goal (vs. a nonabstinence goal), since endorsing the latter is predictive of continuing to drink at the end of treatment in the COMBINE study sample (Dunn and Strain, 2013; Gueorguieva et al., 2014). Participants were dichotomized into Early Abstainer (no self-reported alcohol during the weeks of interest) vs. Early Nonabstainers (≥1 day with alcohol consumption during the weeks of interest) and compared on a variety of drinking outcome measures that are frequently used in clinical trial evaluations of alcohol treatment strategies. The results may help AUD providers to identify early in treatment whether a particular strategy is likely to be effective for a given patient.
METHODS
Participants
To be eligible for the COMBINE study, participants were required to meet DSM-IV criteria for alcohol dependence and to self-report drinking ≥14 and ≥21 drinks/week, including ≥2 heavy drinking days (defined as ≥4 and ≥5 drinks/day), for women and men, respectively, in the 30 days prior to study enrollment. Participants were also required to have between 4 and 21 days of alcohol abstinence prior to randomization. The total COMBINE study sample was N = 1383. For the purpose of these analyses, participants who reported a nonabstinence treatment goal at study intake were excluded from the analyses, resulting in a final study sample of N = 954.
COMBINE study procedures
Only the procedures from the primary clinical trial that are relevant to the current analyses are presented below; full descriptions of the study procedures are available elsewhere (Anton et al., 2006). The COMBINE study was an outpatient, randomized and controlled clinical trial that was conducted across 11 sites between January 2001 and January 2004. Participants were assigned to one of nine treatment groups for 16 weeks. Medications were provided in a double-blind, double-dummy format and consisted of placebo/placebo, naltrexone/placebo, placebo/acamprosate and naltrexone/acamprosate. Eight treatment groups received medication management therapy to assist with medication compliance, and four groups received an additional combined behavioral intervention (CBI) to enhance alcohol abstinence. One final group received no medication and CBI only to assess for a potential placebo effect on treatment outcomes. The primary COMBINE study outcome measures were percent drinking days during the study and time to first heavy drinking day. The primary study reported no main effect of study medication or CBI on percent drinking days, though a main effect of naltrexone was evident on time to first heavy drinking day. Naltrexone + CBI was also associated with a significantly greater percent abstinent days, fewer drinks per day and fewer heavy drinking days per month (Anton et al., 2006).
Measures
Measure of alcohol consumption
A time-line follow back procedure was used to assess self-reported drinking during the 16-week study (Sobell et al., 1988; Sobell and Sobell, 1992). Drinks were converted into standard drink units and were categorized as drinking (yes/no), drinks per drinking day and heavy drinking (yes/no; ≥4 or ≥5 drinks a day for women and men, respectively) for each participant for each study day.
Abstinence goals
During the screening procedure, participants were asked to select one of seven potential intentions that were categorized post hoc as representing abstinence and nonabstinence treatment goals. Abstinence goals consisted of “I want to be totally abstinent from all alcohol for a period of time, after which I will make a decision about whether or not I will use alcohol again anyway”, “I want to quit using alcohol once and for all, even though I realize that I may slip up and use alcohol again once in awhile” and “I want to quit alcohol once and for all, to be totally abstinent and never use alcohol again for the rest of my life”. Nonabstinence goals consisted of “I want to use alcohol in a controlled manner, to be in control of how often I use and how much I use” and “I don't want using alcohol to be a habit for me anymore but would occasionally like to use alcohol when I really have an urge”. Participants were required to endorse an abstinence-related goal to be included in the present study analyses.
Alcohol Dependence Scale
The Alcohol Dependence Scale (ADS) is a 25-item self-report measure that queries alcohol use in the past 12 months (Skinner and Allen, 1982). Answers are summed to produce a total severity score, and baseline ADS ratings were used for multiple imputation analyses.
Medication adherence
Medication adherence was assessed by calculating the percent of scheduled medication doses that were consumed by participants; participants in the CBI-only condition (who received no medication) were omitted from analyses of adherence.
University of Rhode Island Change Assessment Scale
The University of Rhode Island Change Assessment Scale (URICA) is a 24-item self-report measure that was administered at Screening to assess stages of change to stop drinking (McConnaughy et al., 1983). Stages of change have been previously associated with positive response to pharmacotherapy-based alcohol treatment trials (Hernandez-Avila et al., 1998), and the URICA has shown specific sensitivity to predicting alcohol treatment outcomes (Willoughby and Edens, 1996). Ratings from the URICA Readiness Subscale were included as a covariate in the analyses.
Data analysis
Four independent analyses were conducted to evaluate the hypothesis that early abstinence would predict better treatment outcomes, and to assess the degree to which this effect may be impacted by the duration of early abstinence (e.g. 1, 2, 3, or 4 weeks). For each set of analyses, participants were dichotomized into Early Abstainers (defined as no self-reported drinking) or Early Nonabstainers (defined as reporting >1 drink during the period under evaluation) and compared on a variety of drinking-related outcome measures. All analyses were restricted to the study weeks following the defined drinking period. Thus, the analysis for 1 week of abstinence was restricted to Weeks 2–16, for 2 weeks was restricted to Weeks 3–16, for 3 weeks was restricted to Weeks 4–16 and for 4 weeks was restricted to Weeks 5–16. Analyses focused only on the period during which the active COMBINE intervention occurred (e.g. 16 weeks). Both drinking day and heavy drinking day variables were evaluated.
Data were fit to both Poisson regression and negative binomial models. Poisson models were determined to have better fit to the data for both incomplete and imputed data and were then used to evaluate the effect of early abstinence on number of drinks per drinking day, number of drinking days and number of heavy drinking days within each abstinence duration category (e.g. Weeks 1–4). The models evaluated daily drinking values for all study days, as opposed to weekly averages. The rate of missing data varied from 0.2% to 6.7% across all analyses. Missing data were treated both as missing (missing = missing) and imputed (Enders et al., 2016), consistent with a recent report that outlined methods to deal with data missing from the COMBINE data set (Witkiewitz et al., 2014). Specifically, analyses used imputation by chained equations that were based upon each conditional density of a variable, given other variables. Missing data were imputed with 50 imputations using age, sex, site, adherence, ADS total score, number of pretreatment drinking and heavy drinking days, URICA Readiness Scale score and the three treatment groups (analyzed dichotomously as acamprosate (yes/no), naltrexone (yes/no) and CBI (yes/no)) as auxiliary variables for the imputation. These variables were selected because they were observed to differ statistically between the groups or were theoretically conceptualized to be associated with drinking during treatment. Potential moderating effects of treatment condition (acamprosate, naltrexone and CBI) on these outcomes were also evaluated by assessing for significant interactions. Cox proportional hazard models were then fit to evaluate the association between early abstinence and time to first drinking and first heavy drinking day for each abstinence duration category. Since all variables used in the time-to-event outcomes (Cox measures) are non-missing, imputation was deemed unnecessary for these analyses. Alpha (two-tailed) was set at <0.05 and data were analyzed using Stata12.0 (StataCorp, Texas, 2009).
RESULTS
Participants
The participants were 71.9% male, 75.2% Caucasian and a mean (SD) of 44.1 (10.0) years old. In each of the analyses, the Early Abstainer and Early Nonabstainer groups differed significantly by age, sex and medication adherence (Table 1). The percent of participants who were continuously abstinent from drinking for the first 1, 2, 3 and 4 weeks of treatment were 59.6%, 49.6%, 44.5% and 39.8%, respectively.
Table 1.
Participant characteristicsa
| Duration of early abstinence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Week | 2 Weeks | 3 Weeks | 4 Weeks | |||||||||
| Early abstainer (N = 569) | Early nonabstainer (N = 385) | P-value | Early abstainer (N = 474) | Early nonabstainer (N = 472) | P-value | Early abstainer (N = 425) | Early nonabstainer (N = 511) | P-value | Early abstainer (N = 380) | Early nonabstainer (N = 552) | P-value | |
| Age (years) | 44.7 (10.2) | 43.1 (9.6) | 0.02 | 44.9 (10.2) | 46.3 (9.8) | 0.02 | 44.9 (10.4) | 43.5 (9.6) | 0.04 | 45.1 (10.4) | 43.6 (9.6) | 0.03 |
| Male (%) | 74.9 | 67.5 | 0.02 | 75.1 | 68.6 | 0.03 | 75.3 | 69.3 | 0.05 | 75.5 | 69.6 | 0.05 |
| Caucasian (%) | 75.4 | 74.8 | 0.89 | 75.1 | 75 | 0.99 | 74.6 | 75.3 | 0.82 | 74.5 | 75.5 | 0.76 |
| Naltrexone (%) | 46.0 | 43.6 | 0.47 | 46.6 | 43.4 | 0.33 | 47.3 | 42.9 | 0.19 | 48.2 | 42.6 | 0.09 |
| Acamprosate (%) | 46.9 | 44.4 | 0.47 | 47.9 | 44.1 | 0.24 | 47.8 | 44.2 | 0.29 | 46.8 | 45.3 | 0.64 |
| CBI (%) | 49.4 | 49.7 | 0.32 | 49.5 | 43.0 | 0.23 | 51.1 | 47.4 | 0.03 | 51.0 | 47.8 | 0.06 |
| URICA Readiness Scale score (range 0–15) | 11.0 (1.6) | 10.7 (1.4) | <0.01 | 11.0 (1.6) | 10.7 (1.4) | 0.02 | 11.0 (1.6) | 10.8 (1.4) | 0.03 | 11.0 (1.6) | 10.8 (1.4) | 0.02 |
| Pretreatment drinking days (#; range 0–120) | 71.9 (24.0) | 76.4 (22.6) | 0.20 | 77.8 (24.0) | 75.7 (23.0) | 0.01 | 71.8 (24.0) | 75.5 (23.2) | 0.02 | 71.1 (23.8) | 75.7 (23.0) | <0.01 |
| Pretreatment heavy drinking days (#; range 0–120) | 66.4 (26.0) | 68.6 (26.0) | 0.20 | 66.2 (26.2) | 68.4 (25.8) | 0.19 | 66.3 (26.0) | 68.3 (26.0) | 0.24 | 65.7 (26.0) | 68.5 (25.8) | 0.11 |
| Medication adherenceb | 92.0 (13.4) | 86.1 (19.4) | <0.01 | 92.3 (13.6) | 87.2 (18.0) | <0.01 | 92.5 (13.2) | 87.6 (17.8) | <0.01 | 93.0 (12.6) | 87.6 (17.8) | <0.01 |
aAll analyses represent missing–missing analyses; values represent mean (SD) unless otherwise indicated.
bNot assessed in CBI-only group.
Treatment outcomes
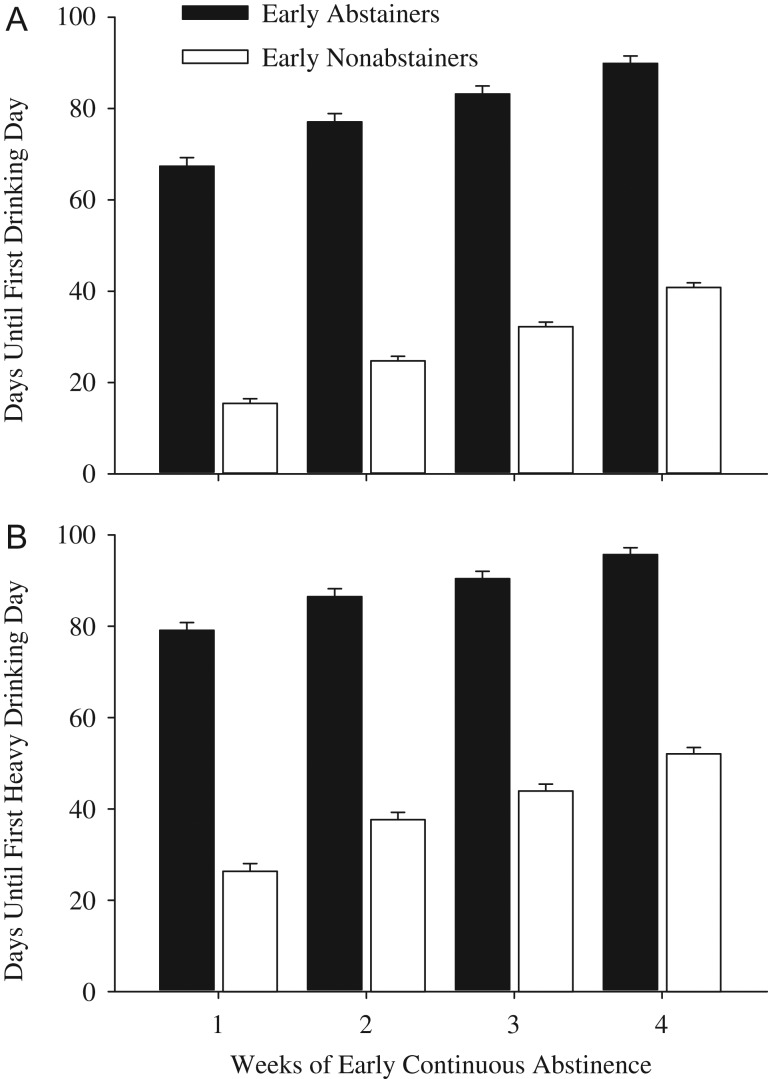
As shown in Table 2, mean drinks per drinking day, number of drinking days and number of heavy drinking days differed significantly between the Early Abstainer and Early Nonabstainer groups. For instance, examination of the heavy drinking days variable in the missing = missing analyses for the 1 week analysis in Table 2 reveal that Early Nonabstainers would be expected to have a 3.4-fold increase (95% CI: 2.63–4.39) in number of heavy drinking days relative to Early Abstainers. The association between early abstinence and fewer drinks per drinking day and number of drinking and heavy drinking days was significant for each length of continuous abstinence that was evaluated (1–4 weeks), and when missing data were treated as missing or imputed (Table 2). Early abstinence was also associated with an increased time to first drinking and first heavy drinking day (Fig. 1). For instance, examination of the time to first drinking day in the Abstinent 1 week analysis indicates Early Nonabstainers were 5.78 (95% CI 4.84–6.94) times more likely to fail (have a first drinking day, given they had not yet drank during the at-risk period) relative to Early Abstainers. For all analyses, the beneficial effects of early abstinence on positive treatment outcomes were evident among participants who were continuously abstinent during Week 1 of treatment, and the magnitude of the effect grew stronger with the addition of each subsequent week of abstinence, up to the 4 weeks evaluated (Table 2).
Table 2.
Evaluation between early abstinence and outcomesa
| Duration of early abstinence | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 Week | 2 Weeks | 3 Weeks | 4 Weeks | |||||
| IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | |
| Missing = missingb | ||||||||
| Drinks per drinking day | 3.40 | 2.63–4.39 | 3.43 | 2.67–4.41 | 3.60 | 2.79–4.65 | 4.69 | 3.60–6.10 |
| Number of drinking days | 3.56 | 2.86–4.44 | 3.77 | 3.03–4.67 | 4.08 | 3.28–5.07 | 5.15 | 4.13–6.43 |
| Number of heavy drinking days | 3.81 | 2.92–4.97 | 3.77 | 2.89–4.91 | 3.87 | 2.96–5.04 | 5.27 | 4.01–6.94 |
| Missing = imputedb , c | ||||||||
| Drinks per drinking day | 3.02 | 2.36–3.88 | 3.13 | 2.45–3.99 | 3.33 | 2.60–4.27 | 4.2 | 3.25–5.43 |
| Number of drinking days | 3.29 | 2.65–4.07 | 3.60 | 2.92–4.43 | 3.94 | 3.19–4.87 | 4.87 | 3.93–6.04 |
| Number of heavy drinking days | 3.40 | 2.63–4.39 | 3.42 | 2.65–4.40 | 3.63 | 2.81–4.69 | 4.87 | 3.74–6.35 |
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Time to first drinking dayd | 5.78 | 4.84–6.94 | 6.09 | 5.06–7.33 | 5.80 | 4.80–7.01 | 6.05 | 4.94–7.41 |
| Time to first heavy drinking dayd | 4.97 | 4.13–5.99 | 4.88 | 4.02–5.93 | 4.78 | 3.90–5.85 | 5.19 | 4.16–6.47 |
aAll analyses included interactions between time and treatment assignment, and adjusted for site, sex, age, percent medication adherence, being assigned to receive acamprosate, naltrexone, CBI, pretreatment drinking and heavy drinking days and scores on the URICA Readiness Scale. Variables are comparing Early Abstainer and Early Nonabstainer groups. All comparisons are statistically significant at the P < 0.001 level; referrant group = Early Abstainer during period of interest; IRR = incidence-rate ratio; HR = hazard ratio; CI = confidence interval.
bPoisson regression models.
cMultiple imputation for missing data.
dCox proportional hazard model.
Fig. 1.
Values show mean days before first drinking (A) and first heavy drinking (B) day for the Early Abstainer (filled bars) and Early Nonabstainer (open bars) groups. Error bars represent standard error of the mean. X-axis represents duration of early abstinence; the Early Abstainer group contains all participants who were continuously abstinent for the duration of interest (1–4 weeks). All comparisons are statistically significant at P < 0.001 (Table 2).
Interactions between time and treatment assignment are presented in Table 3. Analyses revealed that acamprosate significantly moderated the effects of drinks per drinking day and number of drinking days for each of the durations examined (1, 2, 3 and 4 weeks), as well as the number of heavy drinking days at the 1, 2 and 3 week durations. All associations were positive, suggesting that acamprosate was associated with increased drinking over time, relative to the placebo group. Naltrexone significantly moderated the effects of drinks per drinking day in the 2, 3 and 4-week analyses, the number of drinking days at the 2-week analyses and the number of heavy drinking days for each of the durations examined (1, 2, 3 and 4 weeks). All associations were negative, suggesting that naltrexone was associated with decreased drinking. Finally, CBI significantly moderated the effects of drinks per drinking day for the 3- and 4-week durations; these associations were negative and suggested that CBI was associated with decreased drinking. Significance of results did not vary between the missing = missing and imputed analyses for any of the moderation analyses. Despite significant associations, the coefficients for each of the moderations were in the hundredths (Table 3), which suggests that these interactions would have minor clinical implications. For instance, closer evaluation of the 1 week, nonimputed effect of acamprosate on drinks per drinking day revealed that participants receiving placebo showed an increase in drinks per drinking day by a factor of 1.03 (3%) per week (coefficient = 0.026); whereas participants receiving acamprosate showed an increase in drinks per drinking day by a factor of 1.05 (5%) per week (coefficient = 0.023). This additional 2% increase is minor and of small clinical importance when compared to the 339% increase in drinks per drinking days associated with using alcohol during the first week of the study.
Table 3.
Moderation analysesa
| Duration of early abstinence | ||||
|---|---|---|---|---|
| 1 Week | 2 Weeks | 3 Weeks | 4 Weeks | |
| Coef. (SE), P | Coef. (SE), P | Coef. (SE), P | Coef. (SE), P | |
| Acamprosate | ||||
| Missing = missingb | ||||
| Drinks per drinking day | 0.023 (0.003), P < 0.001 | 0.019 (0.003), P < 0.001 | 0.015 (0.003), P < 0.001 | 0.012 (0.003), P < 0.01 |
| Number of drinking days | 0.017 (0.003), P < 0.001 | 0.014 (0.003), P < 0.001 | 0.010 (0.004), P < 0.01 | 0.01 (0.040), P = 0.02 |
| Number of heavy drinking days | 0.022 (0.004), P < 0.001 | 0.016 (0.004), P < 0.001 | 0.011 (0.004), P < 0.01 | ns |
| Missing = imputedb , c | ||||
| Drinks per drinking day | 0.025 (0.003), P < 0.001 | 0.019 (0.003), P < 0.001 | 0.016 (0.003), P < 0.001 | 0.012 (0.003), P = 0.001 |
| Number of drinking days | 0.017 (0.003), P < 0.001 | 0.014 (0.003), P < 0.001 | 0.010 (0.004), P < 0.01 | 0.01 (0.040), P = 0.02 |
| Number of heavy drinking days | 0.023 (0.004), P < 0.001 | 0.047 (0.004), P < 0.001 | 0.012 (0.004), P < 0.01 | ns |
| Naltrexone | ||||
| Missing = missingb | ||||
| Drinks per drinking day | ns | −0.008 (0.003), P = 0.02 | −0.012 (0.004), P < 0.01 | −0.014 (0.004), P < 0.001 |
| Number of drinking days | ns | ns | ns | |
| Number of heavy drinking days | −0.009 (0.004), P = 0.03 | −0.009 (0.004), P = 0.04 | −0.011 (0.005), P = 0.02 | −0.012 (0.005), P = 0.03 |
| Missing = imputedb , c | ||||
| Drinks per drinking day | ns | −0.009 (0.003), P = 0.01 | −0.013 (0.004), P < 0.001 | −0.014 (0.004), P = 0.001 |
| Number of drinking days | ns | ns | ns | |
| Number of heavy drinking days | −0.009 (0.004), P = 0.03 | −0.01 (0.004), P = 0.03 | −0.012 (0.005), P = 0.01 | −0.013 (0.006), P = 0.02 |
| CBI | ||||
| Missing = missingb | ||||
| Drinks per drinking day | ns | ns | −0.01 (0.004), P < 0.01 | −0.013 (0.004), P = 0.001 |
| Number of drinking days | ns | ns | ns | ns |
| Number of heavy drinking days | ns | ns | ns | ns |
| Missing = imputedb , c | ||||
| Drinks per drinking day | ns | ns | −0.008 (0.004), P = 0.03 | −0.012 (0.004), P < 0.01 |
| Number of drinking days | ns | ns | ns | ns |
| Number of heavy drinking days | ns | ns | ns | ns |
aAnalyses represent interaction between time and treatment assignment. Only significant associations shown. Coef. = coefficient, SE = standard error, ns = nonsignificant.
bPoisson regression models.
cMultiple imputation for missing data.
DISCUSSION
Drinking that occurred early in treatment for AUD was significantly and robustly associated with drinking that occurred throughout and at the end of treatment. This association was evident for several different and diverse drinking outcome measures that may be employed by alcohol treatment trials, and did not appear to be better explained by the moderating effects of COMBINE treatment (e.g. acamprosate, naltrexone and CBI) assignment. Participants in this study who were completely abstinent from alcohol during the first 1–4 weeks of treatment were significantly and uniformly more likely to remain abstinent throughout the study, and had a longer period of abstinence before resuming heavy drinking, relative to participants who drank during that early period. This study adds to a growing body of literature suggesting that abstinence that occurs early in treatment for drugs or alcohol is predictive of positive treatment outcomes and long-term abstinence. While the notion that early abstinence predicts later abstinence may conform to clinical practice, this is the first formal demonstration of this effect in the context of alcohol treatment, the largest sample size from which this effect has been demonstrated, and the first of these studies to independently assess different durations of continuous abstinence. These data extend existing studies by suggesting that early abstinence may be a uniform phenomenon that is not restricted to drug class, which strengthens its value as a metric for gauging response to treatment in personalized medicine designs.
The predictive relationship between early continuous abstinence and positive treatment outcomes was evident as early as 1 week into treatment, and continued to remain significant up to 4 weeks after beginning treatment, and the magnitude of the effect increased in value with each subsequent week of continuous abstinence. The change in magnitude of this effect was smaller when the time to first drinking and first heavy drinking day variables were evaluated. These results suggest that, while the association between early continuous abstinence and subsequent drinking was significant for all variables and time periods examined, the magnitude of the effect may be outcome specific. This is an important distinction for clinical trial treatment design, and it will be important to evaluate the degree to which this association is evident with other clinically relevant outcome measures, such as percent of subjects with no heavy drinking days and drinks per day. These results also suggest that the effect of early abstinence is fairly well established as early as 1 week into treatment, but that providers have at least a 4-week window during which they may identify whether changes in drinking may be predictive of a positive long-term response. The effects did not appear to be better explained by the moderating effects of the COMBINE study treatment conditions (e.g. acamprosate, naltrexone and CBI). Moderation analyses revealed that naltrexone and CBI were associated with decreased drinking over time, relative to placebo, which is consistent with the primary COMBINE study outcomes (Anton et al., 2006), while acamprosate was associated with increased drinking over time relative to placebo. Yet in all cases, the coefficient levels were extremely low, which suggests that any potential clinical impact the COMBINE treatment condition exerted on early abstinence and subsequent drinking outcomes in these analyses would be minor, and it remains possible that results are of such high significance because of the large sample size in the COMBINE trial. Altogether, these data provide strong support for the positive association between early continuous abstinence and positive alcohol treatment outcomes up to 16 weeks later.
Previous efforts to identify predictors of treatment outcome in the COMBINE data set have focused largely on participant characteristics, and have reported that craving serves as both a mediator and moderator of percent days abstinent (Subbaraman et al., 2013), that pain can predict lapse to heavy drinking (Witkiewitz et al., 2015), that consecutive days of abstinence prior to treatment and abstinence goal can predict heavy drinking during treatment (Gueorguieva et al., 2014) and that genetic status can predict response to naltrexone (Anton, 2008). While these findings are informative and advance our general understanding of AUD treatment, these variables may be difficult to assess in a primary care setting for use in making clinical decisions. The results of this study suggest that assessing self-reported drinking, which is an easily observed variable, may be a powerful method for anticipating treatment outcome. Additional research is needed to evaluate the degree to which making decisions based upon early drinking may lead to larger treatment effects, consistent with the goals of personalized medicine.
The results of this study are not intended to suggest that Early Nonabstainers should be conceptualized as treatment reticent or treatment failures. Rather, failure to achieve early abstinence may be a useful behavioral index to quickly identify individuals who may need more supportive resources early in treatment, or for whom their current course of treatment is not an ideal approach. Similarly, these data are not intended to suggest that Early Abstainers do not need continued support during their treatment, though it is possible that it may not need to be as intensive as for the Early Nonabstainer group.
It is not yet clear how evaluating early drinking as a predictor of treatment response may interact with the use of grace periods to assess drinking outcomes in clinical trial assessments of AUD treatment. Grace periods, or a priori-defined periods of time during which any drinking is not included into statistical analyses of treatment outcomes, is supported by a recent study from the COMBINE data set that reported heavy drinking during the final 8 weeks of treatment was predictive of drinking at post-treatment follow-up visits (Gueorguieva et al., 2015). Employing grace periods to refine primary outcome analyses in alcohol treatment trials has been endorsed by NIAAA (Falk et al., 2010) and the Food and Drug Administration (FDA) (FDA, 2015), and represents an acknowledgment that it may take time for a medication to begin exerting an effect on drinking patterns or for patients to become stable in their treatment regimen. Additional research that assesses the degree to which early drinking behavior may relate to drinking that occurs after a prespecified grace period would be of great value.
This study has some limitations. First, participants in COMBINE were required to achieve some abstinence from alcohol between intake and study enrollment. Therefore, Early Nonabstainers could also be conceptualized as having recently relapsed to alcohol. However, the predictive value of early drinking observed here is consistent with a robust literature examining this effect within other drugs of abuse, which suggests this is a more universal phenomenon and not just an artifact of the COMBINE study procedures. Second, all outcomes were based upon self-report ratings of alcohol use; though this is a common and accepted outcome measure in alcohol treatment trials, it is less rigorous than biochemical testing and could have resulted in some participants being improperly classified as Early Abstainer vs. Nonabstainers. That said, self-report is an easier metric to assess relative to biochemical testing and is the one most likely to be used by treatment providers, which increases the relevance of these results to treatment settings. Third, the COMBINE study was an outpatient trial so it is not clear the degree to which these results may generalize into residential treatment settings. Fourth, to remain consistent with previous studies examining the prognostic value of early abstinence in the context of substance use disorder treatment, study analyses were restricted to participants who identified abstinence as their AUD treatment goal. The degree to which this association may be true in individuals who endorse a nonabstinence treatment goal remains unknown and warrants examination. Finally, analyses all pertained to the weeks following the abstinence interval under examination, vs. having each analysis refer to the same time period (e.g. Weeks 5–16). This approach was selected to ensure the entire potential duration of treatment was included in the analyses, whereas analyzing only Weeks 5–16 would require several weeks of data to be omitted from the evaluation of 1, 2 and 3 weeks of early abstinence. Omitting weeks could also result in a participant who was abstinent during Week 1, but relapsed by Week 2, as being improperly classified as an early abstainer for all analyses.
In conclusion, abstinence from alcohol that occurred early in AUD treatment was a robust predictor of several clinically relevant outcomes. Early abstinence is a quick and easily observable variable that can be assessed without specific measures or expertise, and may therefore represent a useful index to determine early in treatment whether a patient is responding adequately and/or whether additional resources or changes to medications may be needed to better optimize treatment outcomes. To the best of our knowledge, the notion of customizing AUD treatment based upon early response has not yet been evaluated, and these data suggest that early drinking may provide a way to advance a personalized medicine approach to AUD treatment (Litten et al., 2012). These data also add to the growing recognition that early response to treatment is a strong prognostic indicator of long-term treatment outcomes.
FUNDING
These data were collected as part of a multisite clinical trial of alcoholism treatments supported by a series of grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute of Health and DHSS. This publication has not been reviewed or endorsed by NIAAA or the COMBINE research group, and does not necessarily reflect the opinions of its members or NIAAA, who are not responsible for its contents. This work was supported by the National Institute on Drug Abuse at the National Institute of Health grants K24 DA023186 (to E.C.S.), R21 DA035327 (to K.E.D.) and R01 DA035246 (to K.E.D.)
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENTS
The authors acknowledge that the reported results are, in whole or in part, based on analyses of the COMBINE data set.
REFERENCES
- Alterman AI, Kampman K, Boardman CR, et al. (1997) A cocaine-positive baseline urine predicts outpatient treatment attrition and failure to attain initial abstinence. Drug Alcohol Depend 46:79–85. [DOI] [PubMed] [Google Scholar]
- Anton RF. (2008) Genetic basis for predicting response to naltrexone in the treatment of alcohol dependence. Pharmacogenomics 9:655–658. [DOI] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, et al. (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295:2003–17. [DOI] [PubMed] [Google Scholar]
- Anton RF, Schacht JP, Book SW (2014) Pharmacologic treatment of alcoholism. Handb Clin Neurol 125:527–42. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, et al. (2011) Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med 41:516–24. [DOI] [PubMed] [Google Scholar]
- Cochran G, Stitzer M, Nunes EV, et al. (2014) Clinically relevant characteristics associated with early treatment drug use versus abstinence. Addict Sci Clin Pract 9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Strain EC (2013) Pretreatment alcohol drinking goals are associated with treatment outcomes. Alcohol Clin Exp Res 37:1745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Cornish JW (2001) Results of a baseline urine test predict levels of cocaine use during treatment. Drug Alcohol Depend 62:1–7. [DOI] [PubMed] [Google Scholar]
- Enders CK, Mistler SA, Keller BT (2016) Multilevel multiple imputation: a review and evaluation of joint modeling and chained quations imputation. Psychol Methods 21:222–40. [DOI] [PubMed] [Google Scholar]
- Falk D, Wang XQ, Liu L, et al. (2010) Percentage of subjects with no heavy drinking days: evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol Clin Exp Res 34:2022–34. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Gitchell JG, Shiffman S, et al. (2009) Prediction of abstinence at 10 weeks based on smoking status at 2 weeks during a quit attempt: secondary analysis of two parallel, 10-week, randomized, double-blind, placebo-controlled clinical trials of 21-mg nicotine patch in adult smokers. Clin Ther 31:1957–65. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER) (February 2015) Alcoholism: Developing drugs for treatment. Guidance for Industry (Draft Guidance).
- Gonzales K, Roeber J, Kanny D, et al. (2014) Alcohol-attributable deaths and years of potential life lost--11 States, 2006-2010. MMWR 63:213–6. [PMC free article] [PubMed] [Google Scholar]
- Gueorguieva R, Wu R, Fucito LM, et al. (2015) Predictors of abstinence from heavy drinking during follow-up in COMBINE. J Stud Alcohol Drugs 76:935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueorguieva R, Wu R, O'Connor PG, et al. (2014) Predictors of abstinence from heavy drinking during treatment in COMBINE and external validation in PREDICT. Alcohol Clin Exp Res 38:2647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Burleson JA, Kranzler HR (1998) Stage of change as a predictor of abstinence among alcohol-dependent subjects in pharmacotherapy trials. Substance abuse 19:81–91. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ (2000) Initial abstinence and success in achieving longer term cocaine abstinence. Exp Clin Psychopharmacol 8:377–86. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, et al. (2012) Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol 17:513–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnaughy EN, Prochaska JO, Velicer WF (1983) Stages of change in psychotherapy: measurement and sample profiles. Psychother Theory Res Pract 20:368–75. [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, et al. (2004) Actual causes of death in the United States, 2000. JAMA 291:1238–45. [DOI] [PubMed] [Google Scholar]
- Moore BA, Budney AJ (2003) Relapse in outpatient treatment for marijuana dependence. J Subst Abuse Treat 25:85–89. [DOI] [PubMed] [Google Scholar]
- Moore BA, Budney AJ (2002) Abstinence at intake for marijuana dependence treatment predicts response. Drug Alcohol Depend 67:249–57. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, Longabaugh R (2000) Cognitive-behavioral treatment for alcohol dependence: a review of evidence for its hypothesized mechanisms of action. Addiction 95:1475–90. [DOI] [PubMed] [Google Scholar]
- Morral AR, Belding MA, Iguchi MY (1999) Identifying methadone maintenance clients at risk for poor treatment response: pretreatment and early progress indicators. Drug Alcohol Depend 55:25–33. [DOI] [PubMed] [Google Scholar]
- Plebani JG, Kampman KM, Lynch KG (2009) Early abstinence in cocaine pharmacotherapy trials predicts successful treatment outcomes. J Subst Abuse Treat 37:313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA (1982) Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol 91:199–209. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline follow-back: A technique for assessing self-reported alcohol consumption In Litten RZ, Allen JP (eds). Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press, 41. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, et al. (1988) The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. J Stud Alcohol 49:225–32. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Gonzalez G, Poling J, et al. (2003) Prediction of treatment outcome by baseline urine cocaine results and self-reported cocaine use for cocaine and opioid dependence. Am J Drug Alcohol Abuse 29:713–27. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Peirce J, Petry NM, et al. (2007) Abstinence-based incentives in methadone maintenance: interaction with intake stimulant test results. Exp Clin Psychopharmacol 15:344–50. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, et al. (1998) Useful predictors of outcome in methadone-treated patients: results from a controlled clinical trial with three doses of methadone. J Maint Addict 1:15–28. [Google Scholar]
- Subbaraman MS, Lendle S, van der Laan M, et al. (2013) Cravings as a mediator and moderator of drinking outcomes in the COMBINE study. Addiction 108:1737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) (2014) Results from the 2013 national survey on drug use and health: summary of national findings. NSDUH Series H-48, HHS publication No. (SMA) 14-4863.
- Willoughby FW, Edens JF (1996) Construct validity and predictive utility of the stages of change scale for alcoholics. J Subst Abuse 8:275–91. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Falk DE, Kranzler HR, et al. (2014) Methods to analyze treatment effects in the presence of missing data for a continuous heavy drinking outcome measure when participants drop out from treatment in alcohol clinical trials. Alcohol Clin Exp Res 38:2826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Vowles KE, McCallion E, et al. (2015) Pain as a predictor of heavy drinking and any drinking lapses in the COMBINE study and the UK Alcohol Treatment Trial. Addiction 110:1262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]