Abstract
Aims
Serum carbohydrate-deficient transferrin (CDT) is a validated test for chronic heavy alcohol drinking, but CDT abnormalities have been associated with liver disease, limiting its use in these patients. We report here on the association between poor chromatographic resolution of disialotransferrin from trisialotransferrin (the so-called ‘di–tri bridging’) and liver disease severity and etiology.
Methods
Subjects were patients in whom detailed clinical data, including histology results, were available on their existing liver diseases (n=139). Percent disialo-CDT (%dCDT) was measured by high-performance liquid chromatography, and the risks for di–tri bridging associated with cirrhosis, with and without adjustment for alcohol use and alcohol-related liver disease, were estimated.
Results
Di–tri bridging was present in 22/73 (30%) cirrhotic subjects and 7/66 (11%) non-cirrhotic subjects. The unadjusted risk for di–tri bridging in cirrhotics relative to non-cirrhotics was 3.6 (95% confidence interval 1.4–9.2). Adjustment for alcohol-related liver disease and current drinking had little effect on this estimate (adjusted odds ratio 3.4), and neither alcohol-related liver disease nor current drinking were independently associated with di–tri bridging after accounting for the effect of cirrhosis.
Conclusions
The presence of di–tri bridging was associated with cirrhosis in individuals with both alcohol-related and non-alcoholic liver disease, although most cirrhotic subjects did not exhibit di–tri bridging. When di–tri bridging is seen in individuals being tested for chronic heavy drinking, investigation for cirrhosis should be considered.
Short summary
There are known liver-disease-associated abnormalities in CDT. In this study, we found that such abnormalities were strongly associated with cirrhosis rather than less-advanced disease, but were only clinically evident in 30% of cirrhotics. Abnormalities also occurred in severe hepatitis without cirrhosis and were not specific for liver disease etiology.
INTRODUCTION
Transferrin is an abundant liver-derived serum glycoprotein for which several common isoforms exist that include the presence of two N-glycosylation sites and up to six terminal sialic acids. The dominant isoform is tetrasialo-transferrin involving two N-glycans, each capped by two terminal sialic acid moieties (de Jong et al., 1990). ‘Carbohydrate-deficient’ transferrin (CDT), resulting from an increase in isoforms with relatively fewer sialic acid moieties, was first reported as a biomarker of heavy alcohol consumption in 1978 (Stibler et al., 1978). In the ensuing decades, CDT became the most heavily validated biomarker in the alcohol field (Salaspuro, 1999; Bortolotti et al., 2006). Further refinements have identified a specific isoform, disialotransferrin with a single N-glycan chain (Landberg et al., 1995; Peter et al., 1998; Flahaut et al., 2003), as the more specific marker for chronic heavy alcohol drinking. This disialo-isoform can be quantified by high-performance liquid chromatography (HPLC) (Helander et al., 2003, 2010) and expressed as a percentage of total transferrin, which is referred to in this manuscript as %dCDT. Values of 1.7% or higher provide approximately 60% sensitivity and 95% specificity for chronic heavy alcohol use (e.g. exceeding 60 g of ethanol on most days) (Bergstrom and Helander, 2008). Despite its utility in identifying chronic heavy drinking, CDT assay methods have diminished specificity for heavy alcohol use in patients with liver disease and particularly cirrhosis (Heinemann et al., 1998; DiMartini et al., 2001). Case series using the relatively newer HPLC or capillary electrophoresis assays for %dCDT have shown that liver disease can be associated with a diminished chromatographic resolution of disialotransferrin from trisialotransferrin (Arndt et al., 2008; Stewart et al., 2010; Gonzalo et al., 2012), which is the likely cause of this diminished specificity for heavy drinking. This abnormal pattern is due to the presence of higher mass disialotransferrin isoforms resulting from liver-disease-associated changes in transferrin glycosylation (Landberg et al., 2012). Because liver diseases, particularly alcoholic liver disease and chronic Hepatitis C, are common in heavily drinking individuals who may undergo %dCDT testing, it is important that those who perform and order this test understand the relationship between this abnormal pattern and liver disease severity. We report here on the relationship of abnormal disialo–trisialo transferrin resolution (referred to as ‘di–tri bridging’ and illustrated in Fig. 1) with liver disease severity and etiology, in patients with well-characterized chronic liver disease.
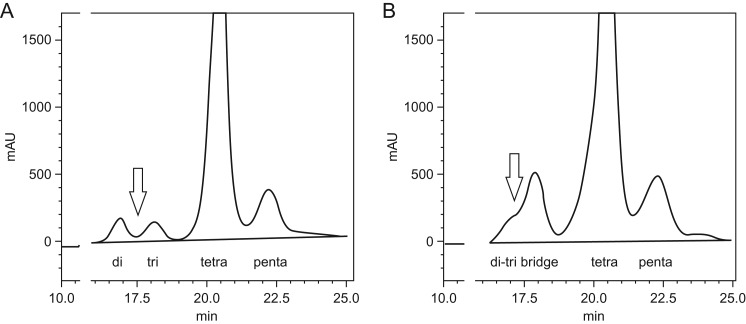
Fig. 1.
Chromatogram illustrating (A) normal resolution of disialo and trisialotransferrin (arrow) in a non-cirrhotic heavy drinker and (B) di–tri bridging (arrow) in a cirrhotic subject followed by tetra- and penta-sialo transferrin peaks. The y-axis represents absorbance of iron-saturated transferrin at 470 nm and the x-axis represents elution time in minutes. Di–tri bridging may be seen in cirrhotic or non-cirrhotic severe liver disease and when heavy alcohol use may or may not be present.
MATERIALS AND METHODS
The subjects included in this report were part of a previously published study on alcohol consumption biomarkers (Stewart et al., 2014). This current analysis included subjects (n=139) with liver disease of varying severity who had undergone liver biopsy in the past or at the time of study participation, who were recruited from the inpatient and outpatient services of a university Hepatology service. Following informed consent, serum was collected for %dCDT measurement, past-90-day alcohol use was estimated using timeline followback methods and the electronic medical record was reviewed to abstract pertinent details on liver disease. The study protocol was approved by the Medical University of South Carolina (MUSC) Institutional Review Board for the protection of human subjects, where the research was conducted.
Serum samples from each subject were brought to the Clinical Neurobiology Laboratory (CNL) at MUSC where the HPLC assay for %dCDT (often referred to as disialotransferrin [DST] in Europe) was performed within 48 h. In this method, pre-treatment of the serum sample includes iron-saturation and precipitation of lipoproteins, followed by chromatographic separation of the transferrin glycoforms with an anion-exchange column (SOURCE 15 Q, GE Healthcare) using salt gradient elution. Quantification of individual glycoforms is performed by monitoring the absorbance of the transferrin-iron complex at 470 nm (Shimadzu UV detector), with disialotransferrin quantified as the relative amount (% of total transferrin) based on peak areas. The International Federation of Clinical Chemistry (IFCC) recommends this HPLC assay as the current reference assay (Helander et al., 2016). The CNL is certified by the College of American Pathology and Clinical Laboratory Improvement Act, proficient with the HPLC assay and a member of the working group for standardization of CDT within the IFCC (Helander et al., 2010, 2016).
To examine the association between di–tri bridging and liver disease severity and etiology, we categorized the liver disease subjects as having biopsy-confirmed cirrhosis or non-cirrhotic liver disease, and alcohol or non-alcohol-related liver disease. Cirrhosis vs. lesser degrees of fibrosis was determined by the hospital pathologist in the course of clinical care, where Stage 4 fibrosis represents cirrhosis, Stage 0 the absence of fibrosis and Stages 1 through 3 increasing degrees of fibrosis (Batts and Ludwig, 1995). The alcohol-related liver disease group included subjects with alcoholic liver disease alone, or those with another primary liver disease in whom chronic heavy drinking was felt to represent an important comorbidity (e.g. an individual with Hepatitis C who drinks heavily). These diagnoses, which were extracted from the medical records, had been made clinically by the Hepatology service before study enrollment, based on a prolonged history of heavy drinking and results of investigations for other causes of liver disease, on a case-by-case basis. Percent dCDT results were categorized as either ‘interpretable’ (i.e. a %dCDT value could be provided by the laboratory) or ‘uninterpretable’ because of di–tri bridging.
Chi-square analysis and logistic regression were used to estimate the risks for di–tri bridging associated with cirrhosis with and without adjustment for alcohol-related diagnosis and current alcohol consumption (estimated daily grams of alcohol during the prior 90 days). In cirrhotic patients, we utilized the Model for End-Stage Liver Disease (MELD) (Kamath et al., 2001) and the Child-Turcotte-Pugh (CTP) scores (Pugh et al., 1973) to compare cirrhosis severity between subjects with and without di–tri bridging. In both cirrhotic and non-cirrhotic subjects, individual liver tests (i.e. aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, total bilirubin, albumin, International Normalized Ratios (INRs) and platelets) were compared between subjects with and without di–tri bridging. As the severity scores and laboratory values tended to be positively skewed, distributions were compared using the Kruskal–Wallis test. SAS v. 9.2 software (SAS Inc, Research Triangle, NC, USA) was used for all statistical analyses.
RESULTS
About 73 (52%) of the 139 subjects had cirrhosis, and 37 (27%) were clinically known or suspected to have alcohol-related liver disease. Subject characteristics are listed in Table 1 stratified by di–tri bridging, which was observed in 29 (21%) subjects, including men and women and each major ethnic group participating in the study. Di–tri bridging was present in both alcohol-related liver disease and non-alcoholic liver disease. Of the 29 subjects with di–tri bridging, 6 (21%) were diagnosed with alcoholic liver disease alone, and 6 (21%) had Hepatitis C with heavy drinking as a known or clinically suspected comorbidity. Other diagnoses included Hepatitis C without reported heavy drinking (n = 11, 38%), non-alcoholic fatty liver disease (n = 3, 10%) and 1 (3%) each with medication-induced cholestasis, primary biliary cholangitis (previously termed primary biliary cirrhosis) and cryptogenic liver disease. The proportion of subjects reporting alcohol use in the past 90 days was similar in the groups with and without di–tri bridging, but consistent with the higher percentage of alcohol-related liver disease in di–tri bridge subjects (41% vs. 23%), alcohol use among current drinkers was heavier in that group, albeit shy of statistical significance.
Table 1.
Characteristics of liver disease subjects
| Characteristic | Di–tri bridge | Di–tri bridge | P-valuea |
|---|---|---|---|
| Present (n = 29) | Absent (n = 110) | ||
| Male (%) | 17 (59) | 54 (49) | 0.361 |
| Mean age (SD) | 54 (6) | 51 (12) | 0.319 |
| Ethnicity (%) | |||
| Non-Hispanic white | 22 (76) | 82 (75) | 0.493 |
| Non-Hispanic black | 5 (17) | 27 (24) | |
| Other ethnicity | 2 (7) | 1 (1) | |
| Cirrhotic (%) | 22 (76) | 51 (46) | 0.005 |
| Alcohol-related disease (%) | 12 (41) | 25 (23) | 0.043 |
| Self-report any past-90-day drinking (%) | 17 (59) | 62 (56) | 0.827 |
| Median daily grams alcohol consumed in self-reported drinkers (interquartile range) | 53 (16–77) | 14 (2–45) | 0.056 |
aKruskal–Wallis for continuous variables and chi-square for categorical variables.
Di–tri bridging was present in 22/73 (30%) of the cirrhotic subjects, and 7/66 (11%) of the non-cirrhotic subjects, resulting in an odds ratio (OR) for di–tri bridging in cirrhosis of 3.6 (95% confidence interval (CI) 1.4–9.2). Because alcohol-related disease was more prevalent in the di–tri bridge group and alcohol use marginally heavier among reported drinkers, we estimated the OR for di–tri bridging after adjusting for these factors. The adjusted OR for di–tri bridging in cirrhosis was 3.4 (95% CI 1.3–8.8). In this analysis, alcohol-related diagnosis was neither independently associated with di–tri bridging (adjusted OR 1.5, 95% CI 0.5–4.3) nor was alcohol consumption (P = 0.418).
The results for cirrhosis severity scores and laboratory tests are shown in Table 2 for cirrhotic subjects, stratified by di–tri bridging. When comparing cirrhotic subjects with di–tri bridging (n = 22) to cirrhotic subjects without di–tri bridging (n = 51), the median CTP scores (7 vs. 6, P = 0.377) and MELD scores (10.5 vs. 8.7, P = 0.305) were slightly higher in di–tri bridge subjects but did not differ statistically. However, di–tri bridge cirrhotics had significantly higher AST, ALT, total bilirubin and significantly lower platelet counts.
Table 2.
Severity scores and median laboratory values (interquartile range) in cirrhotic subjects
| Laboratory test [reference range] | Di–tri bridge | Di–tri bridge | Kruskal–Wallis test P-value |
|---|---|---|---|
| Present (n = 22) | Absent (n = 51) | ||
| MELD score | 10.5 (6.5–15.9) | 8.7 (5.0–14.1) | 0.305 |
| CTP score | 7 (6–9) | 6 (6–8) | 0.377 |
| Albumin (g/dL) [3.5–5] | 3.1 (2.6–3.5) | 3.2 (2.8–3.6) | 0.451 |
| ALT (IU/L) [5–45] | 53 (32–90) | 31 (24–45) | 0.001 |
| AST (IU/L) [5–34] | 89 (74–151) | 52 (35–69) | <0.001 |
| Alkaline phosphatase (IU/L) [35–150] | 119 (106–152) | 109 (85–172) | 0.317 |
| Total bilirubin (mg/dL) [0.2–1.2] | 1.9 (1.5–3.1) | 1.1 (0.8–2.1) | 0.006 |
| INRa [≤1.1] | 1.3 (1.2–1.5) | 1.3 (1.1–1.5) | 0.235 |
| Platelets (1000/µL) [140–440] | 73 (60–111) | 122 (95–180) | <0.001 |
aA measure of blood coagulation that is often impaired with advanced liver disease.
Table 3 includes laboratory comparisons between non-cirrhotic subjects with (n = 7) and without (n = 59) di–tri bridging, with statistical comparisons limited by the small number of di–tri bridge subjects. Nevertheless, di–tri bridge subjects had significantly lower albumin, and significantly higher AST, total bilirubin and INR. MELD and CTP scores are valid only in cirrhosis and were thus not calculated for this group. The biopsy findings from the non-cirrhotic subjects without di–tri bridging included all degrees of fibrosis short of cirrhosis. About 2 (3%) had hepatocellular cancer without a description of non-cancerous tissue, 4 (7%) had no fibrosis (Stage 0), 17 (29%) had Stage 1 fibrosis, 20 (34%) had Stage 2 fibrosis and 14 (24%) had Stage 3 fibrosis. The remaining two reports were not considered specific enough to gauge the extent of fibrosis (as only non-classified ‘fibrosis’ and ‘primary biliary cirrhosis’ were stated explicitly, with the latter being considered an etiologic rather than a histologic diagnosis). In the non-cirrhotic subjects with di–tri bridging, 3/7 had known esophageal varices and imaging suggestive of cirrhosis, with biopsies that had occurred at least 3 years prior to study participation and were interpreted as at least Stage 2 fibrosis. Given the varices and imaging results, these subjects may have progressed to cirrhosis. A fourth subject had Stage 3 fibrosis 2 years prior and had continued to drink heavily, and could also have progressed to cirrhosis. However, the final three subjects were definitely not cirrhotic at the time of study participation. Two were hospitalized with acute alcoholic hepatitis at the time of the study and had severe inflammation on biopsy. The third had sub-acute medication-related cholestasis with a recent biopsy from another facility reported only as ‘cholestatic inflammation’.
Table 3.
Median laboratory values (interquartile range) in non-cirrhotic subjects
| Median laboratory result [reference range] | Di–tri bridge | Di–tri bridge | Kruskal–Wallis test P-value |
|---|---|---|---|
| Present (n = 7) | Absent (n = 59) | ||
| Albumin (g/dL) [3.5–5] | 2.1 (1.4–3.9) | 3.8 (3.5–4.1) | 0.004 |
| ALT (IU/L) [5–45] | 56 (26–111) | 61 (37–109) | 0.625 |
| AST (IU/L) [5–34] | 89 (76–121) | 56 (43–84) | 0.029 |
| Alkaline phosphatase (IU/L) [35–150] | 120 (103–151) | 80 (60–106) | 0.054 |
| Total bilirubin (mg/dL) [0.2–1.2] | 2.8 (1.2–16.2) | 0.7 (0.6–1.0) | 0.006 |
| INRa [≤1.1] | 1.5 (1.0–1.9) | 1.0 (0.9–1.1) | 0.009 |
| Platelets (1000/µL) [140–440] | 155 (107–222) | 207 (182–290) | 0.146 |
aA measure of blood coagulation that is often impaired with advanced liver disease.
DISCUSSION
The measurement of disialotransferrin (%dCDT or DST) by HPLC is a well-validated test for chronic heavy alcohol consumption, and is the recommended reference assay (Helander et al, 2016). However, a proportion of subjects with severe liver disease will have uninterpretable HPLC results due to the presence of di–tri bridging. While absent in most cirrhotics, di–tri bridging is highly associated with cirrhosis as opposed to lesser degrees of fibrosis, with the caveat that patients presenting with acute hepatitis may also demonstrate di–tri bridging.
Di–tri bridging was originally observed in the laboratory without detailed clinical data, and was hypothesized to represent a genetic variant (Helander et al., 2001). Although genetic variation could contribute to this pattern, in subsequent case reports it has been associated with significant liver disease (Arndt et al., 2008; Stewart et al., 2010; Gonzalo et al., 2012). The current study confirmed and extended these observations. In our liver disease patient cohort, we were also able to evaluate the association of di–tri bridging with liver disease severity. Our results suggest that the presence of di–tri bridging increases the probability of cirrhosis, and may indicate a need for evaluating complications of portal hypertension and screening for hepatocellular carcinoma. However, most cirrhotic patients will not exhibit di–tri bridging, and severe but non-cirrhotic inflammatory conditions can also result in di–tri bridging in some cases. Our results did not show a significant association of di–tri bridging with alcohol-related diagnoses or current alcohol use independent of cirrhosis. However, while di–tri bridging clearly occurs in alcohol-related and non-alcohol-related disease, we cannot rule out a greater probability for di–tri bridging with alcohol-related disease, as we did not have sufficient power to test a cirrhosis-by-etiology interaction.
Our results conflict with those in a recent report, where di–tri bridging was not found in a sample of 254 liver disease patients, 48 of whom had biopsy-confirmed cirrhosis (Fagan et al., 2013). These authors speculated that di–tri bridging might only occur in patients with decompensated cirrhosis, but among cirrhotic subjects we did not find a strong link to cirrhosis severity as estimated by MELD and CTP scores. However, AST, ALT and total bilirubin were higher and platelets lower in di–tri bridge subjects with cirrhosis, and laboratory findings were less favorable in non-cirrhotic subjects with di–tri bridging relative to non-cirrhotic subjects without di–tri bridging. Thus there may be a link between di–tri bridging and disease severity and activity in patients with advanced fibrosis. Hypothetically, since fibrotic tissue (representing replacement of functioning liver cells by scar tissue) will not secrete transferrin, the presence of bridging may reflect the proportion of functioning liver cells that are under stress from active disease, and as a result produce and secrete abnormal glycoforms. This could also explain the occurrence of di–tri bridging in some subjects with fulminant hepatitis in the absence of cirrhosis. As disease activity (estimated by liver inflammation) can vary over relatively short periods of time, more definitive evidence for this would require liver biopsy concurrent with %dCDT testing, which was not routine in our sample. In any event, despite mixed results, a causal link to cirrhosis is highly probable given the epidemiologic association and biological plausibility. In this regard, increased fucosylation of some liver-secreted serum proteins (including transferrin) has been identified in cirrhotic patients (Mehta and Block, 2008). Providing even stronger evidence, other research has demonstrated that di–tri bridging itself is due to an increase in fucosylated and highly branched glycans (Landberg et al., 2012). These abnormal disialotransferrin glycoforms would elute between the lower mass normal disialo and higher mass trisialo fractions in the CDT HPLC reference assay and cause di–tri bridging.
Limitations to the current study include the absence of concurrent or very recent liver biopsies for some of our subjects, as older results may underestimate the current severity of liver fibrosis if there had been disease progression in the interim. This may have been the case for some but not all of our subjects with di–tri bridging who were classified as non-cirrhotic, and therefore we may have underestimated the risk for cirrhosis in individuals with di–tri bridging. This study evaluated %dCDT at a single point in time, and additional study is needed to assess how the di–tri bridging pattern may evolve with deterioration or improvement in liver function. Our sample size is also modest and does not include subjects without liver disease, and causes of di–tri bridging other than cirrhosis or acute hepatitis, such as genetic variants, may exist. Finally, although Fig. 1B illustrates clear di–tri bridging with no valley between the disialo and trisialo glycoforms, in practice, there can be partial resolution of disialo from trisialotransferrin. A value for %dCDT as a heavy drinking test can be estimated with partial resolution using baseline integration, but the relationship of this chromatographic finding to liver pathology remains to be determined.
CONCLUSION
In conclusion, this study shows that the HPLC assay for %dCDT will be interpretable in most patients with liver disease, but will not be useful to detect and monitor heavy drinking in a substantial minority of patients with severe liver disease, due to the presence of di–tri bridging. When di–tri bridging is present in an individual who has had HPLC testing for %dCDT to evaluate heavy drinking, strong consideration should be given to a thorough evaluation for advanced liver disease, which entails a number of medical risks including development of hepatocellular cancer. While concurrent abnormalities may exist in traditional liver tests, these are not necessarily specific for cirrhosis, whereas the di–tri bridging signature could suggest the presence of cirrhosis.
ACKNOWLEDGEMENTS
Thanks to Ms. Ashley Green for her work in study coordination and data collection. Emily Leonard and Lorie Blakeley performed the %dCDT assays for this study.
FUNDING
This work was supported by the National Institute on Alcohol Abuse and Alcoholism [R01 AA017911 to S.H.S and K05 AA017435 to R.F.A.].
CONFLICT OF INTEREST STATEMENT
Dr Anton reports he is a member of the International Federation of Clinical Chemistry workgroup on standardization of CDT measurement. He has received support in the past to travel to meetings where the work of the group was discussed. He also reports that he receives salary support for Directing the Clinical Laboratory within the Academic Center where the CDT measurements were done.
REFERENCES
- Arndt T, van der Meijden BB, Wielders JP (2008) Atypical serum transferrin isoform distribution in liver cirrhosis studied by HPLC, capillary electrophoresis and transferrin genotyping. Clin Chim Acta 394:42–6. [DOI] [PubMed] [Google Scholar]
- Batts KP, Ludwig J (1995) Chronic hepatitis: an update on terminology and reporting. Am J Surg Pathol 19:1409–17. [DOI] [PubMed] [Google Scholar]
- Bergstrom JP, Helander A (2008) Clinical characteristics of carbohydrate-deficient transferrin (%disialotransferrin) measured by HPLC: sensitivity, specificity, gender effects, and relationship with other alcohol biomarkers. Alcohol Alcohol 43:436–41. [DOI] [PubMed] [Google Scholar]
- Bortolotti F, De Paoli G, Tagliaro F (2006) Carbohydrate-deficient transferrin (CDT) as a marker of alcohol abuse: a critical review of the literature 2001–2005. J Chromatogr B Analyt Technol Biomed Life Sci 841:96–109. [DOI] [PubMed] [Google Scholar]
- de Jong G, van Dijk JP, van Eijk HG (1990) The biology of transferrin. Clin Chim Acta 190:1–46. [DOI] [PubMed] [Google Scholar]
- DiMartini A, Day N, Lane T, et al. (2001) Carbohydrate deficient transferrin in abstaining patients with end-stage liver disease. Alcohol Clin Exp Res 25:1729–33. [PMC free article] [PubMed] [Google Scholar]
- Fagan KJ, Irvine KM, McWhinney BC, et al. (2013) BMI but not stage or etiology of nonalcoholic liver disease affects the diagnostic utility of carbohydrate-deficient transferrin. Alcohol Clin Exp Res 37:1771–8. [DOI] [PubMed] [Google Scholar]
- Flahaut C, Michalski JC, Danel T, et al. (2003) The effects of ethanol on the glycosylation of human transferrin. Glycobiology 13:191–8. [DOI] [PubMed] [Google Scholar]
- Gonzalo P, Pecquet M, Bon C, et al. (2012) Clinical performance of the carbohydrate-deficient transferrin (CDT) assay by the Sebia Capillary2 system in case of cirrhosis. Interest of the Bio-Rad %CDT by HPLC test and Siemens N-Latex CDT kit as putative confirmatory methods. Clin Chim Acta 413:712–8. [DOI] [PubMed] [Google Scholar]
- Heinemann A, Sterneck M, Kuhlencordt R, et al. (1998) Carbohydrate-deficient transferrin: diagnostic efficiency among patients with end-stage liver disease before and after liver transplantation. Alcohol Clin Exp Res 22:1806–12. [DOI] [PubMed] [Google Scholar]
- Helander A, Eriksson G, Stibler H, et al. (2001) Interference of transferrin isoform types with carbohydrate-deficient transferrin quantification in the identification of alcohol abuse. Clin Chem 47:1225–33. [PubMed] [Google Scholar]
- Helander A, Husa A, Jeppsson JO (2003) Improved HPLC method for carbohydrate-deficient transferrin in serum. Clin Chem 49:1881–90. [DOI] [PubMed] [Google Scholar]
- Helander A, Wielders JP, Jeppsson JO, et al. (2010) Toward standardization of carbohydrate-deficient transferrin (CDT) measurements: II. Performance of a laboratory network running the HPLC candidate reference measurement procedure and evaluation of a candidate reference material. Clin Chem Lab Med 48:1585–92. [DOI] [PubMed] [Google Scholar]
- Helander A, Wielders J, Anton R, et al. (2016) Standardisation and use of the alcohol biomarker carbohydrate-deficient transferrin (CDT). Clin Chim Acta 459:19–24. [DOI] [PubMed] [Google Scholar]
- Kamath PS, Math PS, Wiesner RH, et al. (2001) A model to predict survival in patients with end-stage liver disease. Hepatology 33:464–70. [DOI] [PubMed] [Google Scholar]
- Landberg E, Astrom E, Kagedal B, et al. (2012) Disialo-trisialo bridging of transferrin is due to increased branching and fucosylation of the carbohydrate moiety. Clin Chim Acta 414:58–64. [DOI] [PubMed] [Google Scholar]
- Landberg E, Pahlsson P, Lundblad A, et al. (1995) Carbohydrate composition of serum transferrin isoforms from patients with high alcohol consumption. Biochem Biophys Res Commun 210:267–74. [DOI] [PubMed] [Google Scholar]
- Mehta A, Block TM (2008) Fucosylated glycoproteins as markers of liver disease. Dis Markers 25:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J, Unverzagt C, Engel WD, et al. (1998) Identification of carbohydrate deficient transferrin forms by MALDI-TOF mass spectrometry and lectin ELISA. Biochim Biophys Acta 1380:93–101. [DOI] [PubMed] [Google Scholar]
- Pugh RNH, Murray-Lyon IM, Dawson JL, et al. (1973) Transection of the esophagus for bleeding esophageal varices. Br J Surg 60:646–9. [DOI] [PubMed] [Google Scholar]
- Salaspuro M. (1999) Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol 19:261–71. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Bowen E, Comte-Walters S, et al. (2010) Liver disease and HPLC-quantification of disialotransferrin for heavy alcohol use: a case series Alcohol Clin Exp Res 34:1956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SH, Koch DG, Willner IR, et al. (2014) Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res 38:1706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibler H, Allgulander C, Borg S, et al. (1978) Abnormal microheterogeneity of transferrin in serum and cerebrospinal fluid in alcoholism. Acta Med Scand 204:49–56. [DOI] [PubMed] [Google Scholar]