Abstract
Aims
Stress-induced anxiety is a risk factor for relapse to alcohol drinking. The aim of this study was to test the hypothesis that the central nervous system (CNS)-active α1-adrenergic receptor antagonist, prazosin, would block the stress-induced increase in anxiety that occurs during alcohol deprivations.
Methods
Selectively bred male alcohol-preferring (P) rats were given three cycles of 5 days of ad libitum voluntary alcohol drinking interrupted by 2 days of alcohol deprivation, with or without 1 h of restraint stress 4 h after the start of each of the first two alcohol deprivation cycles. Prazosin (1.0 or 1.5 mg/kg, IP) or vehicle was administered before each restraint stress. Anxiety-like behavior during alcohol deprivation following the third 5-day cycle of alcohol drinking (7 days after the most recent restraint stress ± prazosin treatment) was measured by performance in an elevated plus-maze and in social approach/avoidance testing.
Results
Rats that received constant alcohol access, or alcohol access and deprivations without stress or prazosin treatments in the first two alcohol deprivations did not exhibit augmented anxiety-like behavior during the third deprivation. In contrast, rats that had been stressed during the first two alcohol deprivations exhibited increased anxiety-like behavior (compared with control rats) in both anxiety tests during the third deprivation. Prazosin given before stresses in the first two cycles of alcohol withdrawal prevented increased anxiety-like behavior during the third alcohol deprivation.
Conclusion
Prazosin treatment before stresses experienced during alcohol deprivations may prevent the increased anxiety during subsequent deprivation/abstinence that is a risk factor for relapse to alcohol drinking.
Short summary
Administration of prazosin before stresses during repetitive alcohol deprivations in male alcohol-preferring (P) rats prevents increased anxiety during a subsequent deprivation without further prazosin treatment. Prazosin treatment during repeated alcohol deprivations may prevent the increased anxiety that is a risk factor for relapse to alcohol drinking.
INTRODUCTION
Alcoholism co-occurs at high rates with anxiety disorders (Kushner et al., 2000), anxious individuals report that they use alcohol primarily to control anxiety (Kushner et al., 2000), and the tendency to develop alcohol dependence exhibits co-morbidity with increased anxiety and with central nervous system (CNS) disinhibition/hyperexcitability (Merikangas et al., 1998; Sinha et al., 1998; Begleiter and Porjesz, 1999). Increased anxiety is a major risk factor for alcohol relapse (Koob and LeMoal, 1997). Individuals with alcoholism commonly exhibit increased anxiety during abstinence (Koob and LeMoal, 1997; Kushner et al., 2000) and report that relief of anxiety is a major reason for returning to alcohol drinking (Edwards et al., 1972). Like humans, rats exhibiting increased anxiety-like behavior consume more alcohol than do rats exhibiting low anxiety behavior (Spanagel et al., 1995) and rats selectively bred for high alcohol preference (e.g. P rats) exhibit higher levels of anxiety-like behavior than do alcohol non-preferring (NP) rats (Stewart et al., 1993). This evidence strongly suggests that anxiety can contribute to increased alcohol drinking and relapse to alcohol drinking (but see Langen and Fink, 2004, for evidence that anxiety is not a reliable predictor of alcohol preference in all rat strains and experimental models).
Repeated episodes of alcohol drinking and withdrawal increase abstinence-induced symptoms in subjects with alcoholism (Ballenger and Post, 1978). An increase in abstinence-induced symptoms is also evident in rats, in which repeated alcohol deprivations have been proposed to sensitize or ‘kindle’ subsequent deprivation-induced anxiety-like behavior (for review, see Breese et al., 2005a). Acute stress administered to rats during repetitive alcohol deprivations further increases this deprivation induction of anxiety behavior (Breese et al., 2005a), leading to the suggestion that stress during alcohol withdrawal and abstinence can produce a high anxiety state that persists across repetitive alcohol withdrawal cycles and may contribute to relapse to alcohol drinking and alcohol dependence (Breese et al., 2005a).
It has been reported that administration of corticotropin-releasing factor type 1 receptor (CRF1) antagonists at the time of stress application blocks the stress-induced increases in anxiety behavior during subsequent alcohol deprivation (Overstreet et al., 2005). Prazosin, an α1-adrenergic receptor antagonist, decreases brain CRF signaling (Itoi et al., 1994) and is anxiolytic (Skelly and Weiner, 2014). We hypothesized that prazosin—a well-characterized, safe, well-tolerated and FDA-approved drug may be useful in reducing responses to stress during repetitive alcohol deprivations that lead to increased anxiety.
We previously demonstrated that prazosin treatment acutely and chronically decreases voluntary alcohol drinking in P rats (Rasmussen et al., 2009; Froehlich et al., 2013), alcohol drinking in a rat model of relapse to alcohol drinking (Froehlich et al., 2015), alcohol seeking and drinking in operant paradigms (Verplaetse et al., 2011), and drinking during acute withdrawal in alcohol-dependent outbred rats (Walker et al., 2008). Prazosin treatment also facilitates abstinence in treatment-seeking alcohol-dependent men, as demonstrated in a study in which the subjects were unaware of their treatment condition (prazosin vs placebo) and there were no differences in side effects reported (Simpson et al., 2009). In addition, prazosin decreases stress- and cue-induced alcohol craving in alcohol-dependent men and women (Fox et al., 2012). The present study investigated whether prazosin, administered only at the time of stress during alcohol deprivation, would block increases in anxiety behavior after alcohol reinstatement and an additional deprivation. Such a finding would suggest that treatment with prazosin could decrease alcohol drinking not only when administered at the time of alcohol intake, as previously demonstrated, but also potentially by suppressing stress-induced responses that facilitate subsequent alcohol deprivation-induced anxiety.
MATERIALS AND METHODS
Animals
Alcohol-naïve male P rats (n= 40) from generation 71 of selective breeding for alcohol preference were provided by the Alcohol Research Resource Center of the Indiana Alcohol Research Center. The rats were received at 30 days of age and were group housed (three/cage in clear plastic cages) in an isolated room with controlled temperature (21 ± 1°C) and reversed 12-h light/dark cycle (lights off at 10:00 h; procedures during dark periods were performed under dim red illumination). Standard rodent chow (Laboratory Rodent Diet 5001; PMI Nutrition International, Brentwood, MO) was available ad libitum at all times; water was available ad libitum at all times except as noted in the Alcohol Treatment section. Starting at 60–70 days, the rats were individually housed in clear plastic cages. Body weight (BW, 372 ± 8 g at start of study) was determined weekly. All experiments were approved by the VA Puget Sound Health Care System IACUC and conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Alcohol treatment
Alcohol solutions were prepared by diluting 95% alcohol (ethanol) with deionized water. Alcohol and water were presented in two glass drinking tubes (100 ml; BioServ, Frenchtown, NJ) with open-well side-arms extending through adjacent holes on the front of the cage. The rats initially received 3 days access to 10% (v/v) alcohol as the only source of fluid (i.e. alcohol in both drinking tubes), except for a Control group that never received alcohol. During subsequent two-bottle choice between water and 10% alcohol, positions of water and alcohol drinking tubes were alternated daily. Intake of alcohol or water was determined by weighing the drinking tubes to the nearest 0.1 g, with alcohol and water tubes on two empty cages providing estimated losses due to spillage and/or evaporation, which were subtracted from intakes. Net alcohol intake was converted to g alcohol/kg BW.
Experimental design
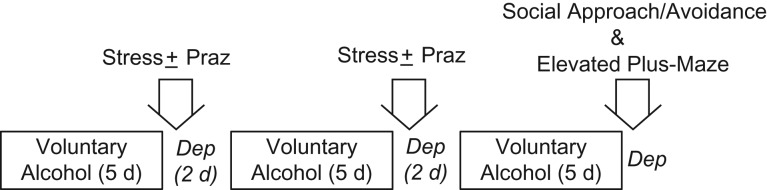
We modified a model originally used by Overstreet et al. (2005) to test drug effects on stress-induced facilitation of anxiety-like behavior during subsequent alcohol deprivation in P rats. As illustrated in Fig. 1 and reviewed in detail by Breese et al. (2005a), this model is based on a protocol in which three cycles of 5 days of voluntary alcohol drinking are each followed by 2 days of alcohol deprivation, with or without restraint stress applied during the first and second alcohol deprivations; anxiety-like behavior is evaluated during a third alcohol deprivation. Drugs are administered before restraint stress during the first and second alcohol deprivations, but no drugs are administered in the third cycle of deprivation when anxiety-like behavior is tested. Consequently, anxiety-like behavior is tested 7 days after the most recent drug treatment.
Fig. 1.
Experimental protocol. Praz, prazosin; Dep, alcohol deprivation; d, days.
Adult male P rats were divided into six treatment groups (n = 6–7/group), counterbalanced on the basis of BW. Each group received access to two drinking tubes daily. The Control group received water in both drinking tubes throughout the study; a Constant Access group was provided with a continuous choice between water and 10% (v/v) alcohol in the two drinking tubes (this group served as a control for length of exposure to alcohol); and a Deprivations group received three cycles of 5 days of choice between water and 10% alcohol, interrupted by 2-day alcohol deprivations (i.e. water in both tubes during alcohol deprivation). The remaining three treatment groups received three cycles of 5 days of access to water vs 10% alcohol, interrupted by 2-day alcohol deprivations after cycles 1 and 2, with the addition of 1 h restraint stress starting 4 h after initiation of alcohol deprivations 1 and 2. These rats received 0 (vehicle alone), 1.0 or 1.5 mg/kg BW prazosin administered IP 30 min before restraint stress in cycles 1 and 2 as follows: Deprivation + Stress + Vehicle, Deprivation + Stress + 1.0 mg/kg Prazosin, or Deprivation + Stress + 1.5 mg/kg Prazosin. After the third cycle of 5 days of choice between water and 10% alcohol, a final alcohol deprivation was introduced and each rat was tested 4–5 h later for social approach/avoidance behavior, immediately followed by testing in an elevated plus-maze. All rats were acclimated to the testing room for 3 h with ambient 60 db white noise before any testing began.
Drug treatments
Prazosin HCl (Sigma-Aldrich Corp., St. Louis, MO) was dissolved in water and then diluted 1:1 with 90 mM lactate buffer (pH 5.2) before IP administration in a volume of 4 ml/kg at 30 min prior to restraint stress. Vehicle was 45 mM lactate buffer (pH 5.2). The IP doses of prazosin in this study were the same as the IP doses of prazosin that we have previously demonstrated to decrease alcohol drinking without producing sedation, motor effects, or malaise in male P rats (Rasmussen et al., 2009, 2011; Froehlich et al., 2015).
Restraint stress
Each rat was restrained for 1 h in a flexible clear plastic cone (Decapicone; Braintree Scientific, Inc., Braintree, MA) with the small open end of the cone positioned by the rat's nose and the wide end of the cone closed with two binder clips so the rat could not turn around or escape. Each restrained rat was placed in a clean cage in a dark room (with dim red illumination) separate from the colony room. Rats that did not receive restraint stress remained in their home cages.
The Social Approach/Avoidance Test
The Social Approach/Avoidance Test has been well characterized and extensively used as a test of rat anxiety-like behavior (Toth and Neumann, 2013). We conducted a three-chambered social approach test (Toth and Neumann, 2013) using a rectangular three-chambered arena (40 × 110 cm overall, with 30 cm high opaque plastic walls); divided into a 40 × 20 cm central chamber separating two 40 × 45 cm end chambers by two clear plastic walls with guillotine doors. At the start of the trial, the arena is empty and the test rat is placed in the center chamber with both doors open so the rat can explore all three chambers for 5 min. The doors are then closed with the test rat confined in the center chamber while a 15 × 15 × 15 cm aluminum mesh cage, enclosing an unfamiliar male Wistar ‘target’ rat of the same age, is placed along the distal wall of one end chamber and an identical, but empty, mesh cage is placed along the distal wall of the other end chamber. The mesh cage allows visual, olfactory, auditory and some tactile contact with the unfamiliar target rat. A weighted canister is placed on top of each cage so the target rat cannot move the cage and the test rat cannot climb on top of either cage. The guillotine doors are then opened and the test rat is allowed to explore all three chambers for 5 min, choosing between spending time with the caged target rat, with the empty cage, or in the empty central chamber. The trial is conducted in dim (7–8 lux) light and is video-recorded with an overhead DynaView SSC-590 surveillance camera (Sony USA, New York, NY). Time that the test rat spends in close investigation of the target rat (nose within 2 cm of the cage containing the target rat, oriented toward and sniffing and/or actively examining the cage and enclosed rat) is considered social approach or interaction; time in similar close proximity to the empty cage in the opposite end chamber represents non-social investigation of a novel item.
Elevated plus-maze
This well-characterized and widely used test of rat anxiety-like behavior was conducted as we have previously reported (Rasmussen et al., 2001). The Kinder Scientific (Poway, CA) elevated plus-maze has two closed arms (10 cm wide × 50 cm long) intersected by two perpendicular open arms (10 cm wide × 50 cm long), with a 10 × 10 cm central area. The closed arms have opaque black walls 50 cm high; the open arms were modified to include 1 cm clear plastic edges so the rat can easily turn around without slipping off the arm. This plus-shaped maze is elevated 80 cm above the floor on a center pedestal. The test is conducted in dim (7–8 lux) light immediately after the social interaction test (in the same room but separated by a curtain) and video-recorded with an overhead DynaView SSC-DC590 surveillance camera. The test is initiated by placing the rat in the center of the elevated plus-maze with its head directed toward a closed arm; decreased time on open arms and decreased entries to open arms during the 5-min test are considered to reflect increased anxiety-like behavior. Total arm entries into either open or closed arms reflect overall activity. Entrance to an open or closed arm is determined by all four paws leaving the central area.
Statistics
This study was designed to test the hypothesis that administration of prazosin would block the stress-induced increase in anxiety that occurs during alcohol deprivation, so all possible combinations of alcohol access, stress and prazosin treatments were not tested—i.e. the design is not fully factorial. Results of each test of anxiety-like behavior were compared among the six treatment groups by one-way analysis of variance (ANOVA) followed—when a significant overall F ratio was obtained—by Student–Newman–Keuls (SNK) pairwise comparisons. Analyses were performed with Sigmaplot 11 software (Systat Software, Inc., Chicago, IL) with significance accepted at P ≤ 0.05. Data are presented as mean ± SEM.
RESULTS
Daily alcohol intake during days 11–15 of continuous alcohol access (Constant Access group) averaged 5.8 ± 0.5 g/kg BW/24 h. Alcohol intake during the third 5-day period of alcohol access after the 2nd alcohol deprivation (alcohol access days 11–15) averaged 7.1 ± 0.3, 7.9 ± 0.3, 7.5 ± 0.4 and 7.3 ± 0.6 g/kg BW/24 h, respectively, in the Deprivation group, the Deprivation + Stress + Vehicle group, the Deprivation + Stress + 1.0 mg/kg Prazosin group and the Deprivation + Stress + 1.5 mg/kg Prazosin group. The alcohol intakes were not significantly different among the five treatment groups (P= 0.11). The daily 24-h alcohol intakes during days 11–15 were not significantly correlated with either anxiety-like behavior or the Composite Anxiety Index score within any individual treatment group of 6–7 rats.
Rats treated with vehicle tended to struggle frequently throughout the 1-h restraint stress. In contrast, the prazosin-treated rats tended to lie quietly within ~15 min after onset of restraint and for the remainder of the 1-h trial, without struggling but with their eyes open and with regular breathing. Although times of struggling were not quantitated, the subjectively observed differences between vehicle vs prazosin treatments were striking and consistent. Differences in struggling were not obvious between rats receiving 1.0 vs 1.5 mg/kg doses of prazosin. Rats in all groups were active immediately after release from the restraint tubes.
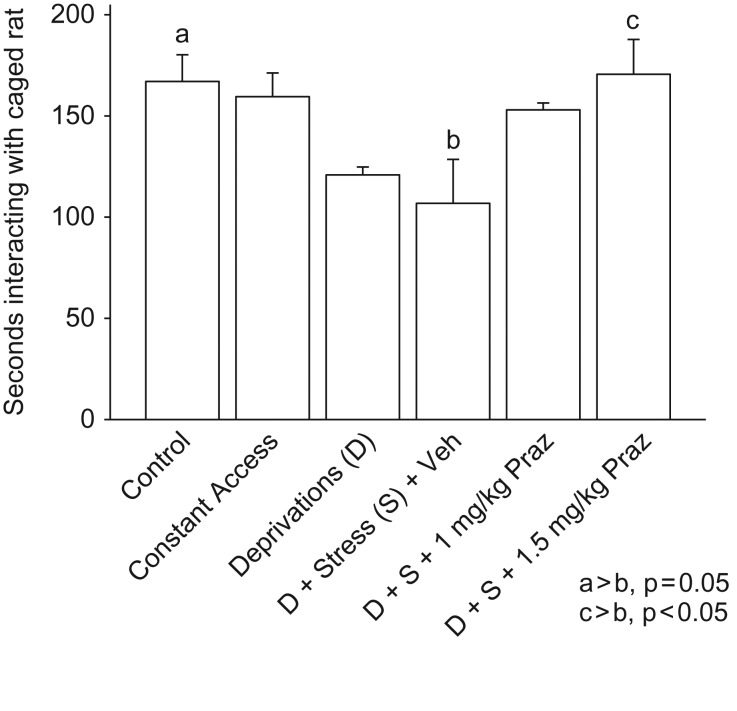
Results of approach/avoidance testing during the final alcohol deprivation are presented in Fig. 2. Rats that had been stressed at the start of the previous two alcohol deprivations and did not receive prazosin treatment [Deprivation + Stress + Vehicle] exhibited decreased social approach behavior during the third (final) deprivation, compared with Control rats (P= 0.05) and compared with rats with continuous alcohol access (constant access; trend at P< 0.06). Prazosin (1.5 mg/kg) treatment before both episodes of stress blocked this stress response (P < 0.05). There was a trend (P = 0.09) for the lower dose of prazosin to also decrease this stress response. There was not a significant difference among the groups with respect to time investigating the novel empty cage.
Fig. 2.
Social Approach/Avoidance Test. Rats that had been stressed at the start of the first two alcohol deprivations (D + Stress + Veh) exhibited increased anxiety-like behavior (i.e. decreased social approach) in the social approach/avoidance test during the third (final) deprivation, compared with Control rats (P= 0.05). Prazosin (1.5 mg/kg) treatment before each of the two stresses blocked this stress effect (P< 0.05). Lower dose prazosin (1 mg/kg) treatment before each of the two stresses tended (P = 0.09) to decrease the stress effect. Each bar represents the mean + SEM of 6–7 rats/group.
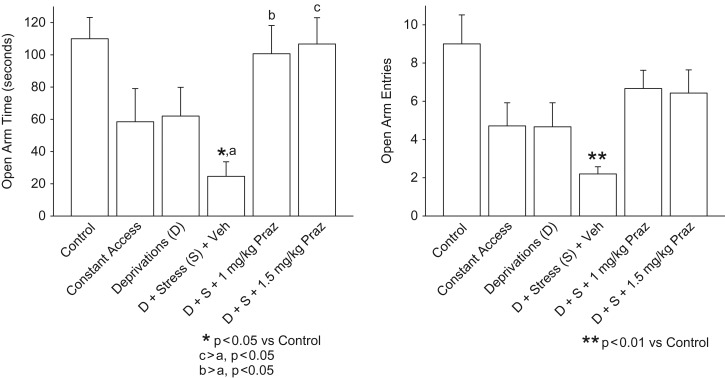
Results of elevated plus-maze testing are presented in Fig. 3. Rats that had been stressed at the start of the first two alcohol deprivations and that did not receive prazosin treatment [Deprivation + Stress + Vehicle] exhibited decreased open arm time (left panel, P< 0.05) and decreased open arm entries (right panel, P< 0.01) during the third deprivation, compared with Control rats. Treatment with either dose of prazosin (1.0 or 1.5 mg/kg) before each of the stresses in the first two deprivations blocked this stress response. Total arm entries were not significantly different among treatment groups.
Fig. 3.
Elevated plus-maze test. Rats that had been stressed at the start of the first two alcohol deprivations (D + Stress + Veh) exhibited increased anxiety-like behavior (i.e. decreased open arm time [left panel] and decreased open arm entries [right panel]) in the elevated plus-maze test during the third (final) deprivation, compared with Control rats. Treatment with either dose of prazosin (1 or 1.5 mg/kg) before each of the two stresses in the first two deprivations blocked this stress effect. Each bar represents the mean + SEM of 6–7 rats/group.
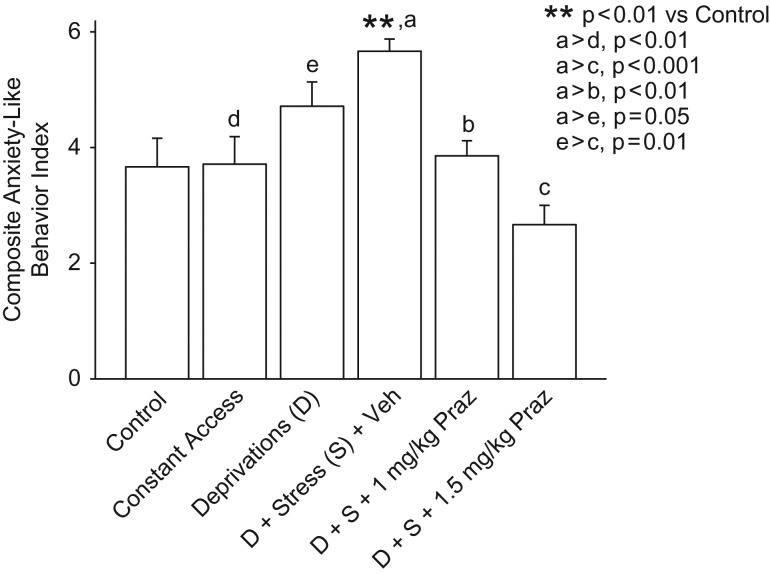
We also expressed the results of the sequential social approach/avoidance and the elevated plus-maze tests as a single composite score. Scores in the social approach/avoidance test (one score for each rat: number of seconds in social approach/interaction) were ranked for all rats and a tertile split was used to assign an anxiety score (range 1–3 in whole increments) to each rat: the third that spent the most time in social approach/interaction (considered to reflect least anxiety) received a score of 1; the middle third received a score of 2; and those that spent the least time in social approach/interaction (considered to reflect the greatest anxiety) received a score of 3. Elevated plus-maze behavior was similarly ranked and scored, with the third that spent the most time on the open arms (considered to reflect the least anxiety) receiving a score of 1 and the third that spent the least time on the open arms receiving a score of 3. These scores on the two behavioral tests were summed for each rat, yielding a single composite index ranging from 2 to 6, with increasing values considered to reflect increasing expression of anxiety. As illustrated in Fig. 4, rats that had been stressed at the start of each of the first two alcohol deprivations, without prazosin treatments, exhibited a robust increase in this composite score of anxiety in the subsequent (third) deprivation period when compared with Control rats (P< 0.01) or rats that had received constant access to alcohol (P< 0.01), as well as a more modest increase (P ≤ 0.05) when compared with rats that had received alcohol deprivations without administrations of stress. The stress-induced increase in the composite index was eliminated by treatment with prazosin in a dose of 1.0 mg/kg (P < 0.01) or 1.5 mg/kg (P < 0.001) before each of the two stresses.
Fig. 4.
Composite anxiety-like behavior score. When anxiety-like behavior was expressed as a composite of the results of social interaction and elevated plus-maze tests, with increasing values reflecting increasing anxiety-like behavior (as described in Results), rats that had been stressed at the start of the first two alcohol deprivations exhibited robust increases (P< 0.01) in anxiety-like behavior in the subsequent (final) deprivation when compared with Control rats and with rats that had received constant access to alcohol, as well as more modest increases (P= 0.05) when compared with rats that had received alcohol deprivations without added stresses. This [deprivation + stress] induced increase in the composite index of anxiety-like behavior was suppressed by treatment with prazosin 1 mg/kg and prazosin 1.5 mg/kg (P < 0.01 and P < 0.001, respectively) before each of the stresses in the first two deprivations, compared with the [deprivation + stress + vehicle] rats. Each bar represents the mean + SEM of 6–7 rats/group.
DISCUSSION
Restraint stress administered during two consecutive alcohol deprivations in male P rats increased anxiety-like behavior when tested during a third deprivation. This finding is consistent with previous reports that repeated alcohol withdrawals increase subsequent deprivation-induced anxiety behavior in rats, and that this response is facilitated by exposures to stress (reviewed by Breese et al., 2005a). Breese et al. (2005a) suggested that repeated withdrawals from alcohol, together with exposure to stressors, sensitize the anxiety response to alcohol withdrawal (similar to multiple withdrawal-induced ‘kindling’ of seizure activity) and that this increase in anxiety reflects a persisting neuroadaptive change. Facilitation of anxiety by repeated alcohol deprivations, enhanced by stress, is consistent with characteristics of alcoholism, which include increased intensity of withdrawal symptoms and negative affect during abstinence after repeated withdrawal episodes, and is thought to increase the likelihood of alcohol relapse (Koob and LeMoal, 1997). Prazosin administration before stress during two alcohol deprivations reduced anxiety-like behavior during a third deprivation in the current study, suggesting that decreasing α1-adrenergic receptor-mediated signaling when encountering stress during alcohol deprivation may prevent the persistent neuroadaptation that Breese et al. (2005a) suggested to be responsible for increased anxiety during alcohol abstinence.
Previous studies using the repeated alcohol deprivation-induced anxiety-like behavioral model in unselected (outbred) rats have demonstrated that drugs which alter benzodiazepine receptor-, serotonin receptor-, CRF type 1 receptor (CRF1)- or less specific mixed receptor-mediated mechanisms can each prevent the increase in abstinence-induced anxiety-like behavior during subsequent alcohol deprivation, suggesting that multiple systems contribute to the facilitation of deprivation-induced anxiety (Breese et al., 2005a,b). However, in P rats the specific blockade of CRF1 during alcohol deprivation was the most effective treatment for blocking this facilitation of deprivation-induced anxiety behavior (Overstreet et al., 2005). In the current study with P rats, specific blockade of α1-adrenergic receptors by prazosin prior to stress during two alcohol deprivations was likewise highly effective in decreasing anxiety-like behavior during a third deprivation. This suggests contributions of two pathways (noradrenergic- and CRF1-mediated), or an integrated pathway, in mediating the facilitation of deprivation-induced anxiety in P rats. Stress increases both noradrenergic and CRF-mediated responses (Koob, 1999); forebrain noradrenergic neurons provide stimulatory innervation of CRF neurons (Yang et al., 1990; Itoi et al., 1994); CRF neurons conversely provide stimulatory innervation of forebrain noradrenergic neurons (Koob, 1999; Jedema and Grace, 2004); and prazosin appears to disrupt a feed-forward loop of extra-hypothalamic CRF-noradrenergic interactions (Yang et al., 1990; Feldman and Weidenfeld, 1998; Koob, 1999; Ryabinin et al., 2002). Consequently, blocking signaling by either CRF1- or α1-adrenergic-mediated mechanisms could potentially block the effects of stress on a noradrenergic-CRF feed-forward loop that has been suggested to mediate increased anxiety during alcohol withdrawal and abstinence (Koob, 1999; Koob and LeMoal, 1997).
Although sympathetic activation is increased during alcohol deprivation in rats and humans (Ehrenreich et al., 1997; Patkar et al., 2004; Rasmussen et al., 2006) and excessive brain and peripheral noradrenergic activation are associated with increased anxiety (Sullivan et al., 1999), it remains to be determined how acutely blocking noradrenergic signaling (including potential interactions between noradrenergic and CRF signaling) at the time of stress suppresses anxiety-like behavior during a subsequent alcohol deprivation 7 days later. It has been demonstrated that administrations of corticosterone instead of stress do not decrease subsequent alcohol deprivation-induced anxiety in this model (Breese et al., 2004), so it is unlikely that prazosin effects on corticosterone secretion play an important mediating role. However, stimulation of noradrenergic activation enhances, and blockade reduces, memory for material with emotional impact (O'Carroll et al., 1999), so prazosin treatment could potentially decrease stress-enhanced increases of alcohol deprivation-induced anxiety by decreasing memory of the stress. Another possibility is that anxiolytic effects of prazosin (Skelly and Weiner, 2014) may have decreased the perceived stressfulness of the stressor, consistent with subjective evidence in the current study suggesting that rats treated with prazosin struggled less and appeared to relax more quickly during the 1-h restraint. A potentially related mechanism is suggested by evidence that pretreatment with prazosin, at the same dose and time of administration as those used in the current study, reduces 1 h restraint stress-induced immediate early gene expression in several telencephalic, diencephalic and brainstem areas of the rodent brain, without altering motor coordination (Stone and Zhang, 1995).
Alcohol intakes during the final 5 days before determination of anxiety-like behavior were not significantly different among the treatment groups, although there was a modest trend (P = 0.011)—with least intake by rats receiving continuous alcohol access and most intake by rats receiving Deprivation + Stress + Vehicle treatment. This trend is consistent with changes in alcohol intake previously reported for P rats in this model (Overstreet et al., 2005), although the small sample size in the current study may have been insufficient to demonstrate these anticipated changes. The final 5 days of alcohol intake within each treatment group was also not significantly correlated with behavioral testing results during the subsequent alcohol deprivation, although correlational analyses based on n= 5–6 do not allow meaningful interpretation.
The current results demonstrate that treatment with prazosin, when confined to only the time that stress is encountered during alcohol deprivations, blocks the increase in anxiety-like behavior that is normally seen during subsequent alcohol deprivation. This effect was seen during alcohol deprivation 7 days after the most recent stressor and prazosin treatment. We speculate that prazosin might decrease alcohol drinking through two distinct mechanisms, i.e. an acute effect that is seen when prazosin is administered at the time of alcohol intake, as we have previously reported, and also by suppressing stress-induced increased anxiety during subsequent alcohol deprivation. Acute prazosin-induced suppression of alcohol drinking, together with prazosin's suppression of anxiety during subsequent alcohol deprivation (abstinence), could potentially both contribute to a reduction in alcohol consumption during the repeated cycles of alcohol drinking and relapse that are experienced by many individuals with alcoholism. These results also suggest that using prazosin on an ‘as needed’ basis when stressful events are anticipated during abstinence could be of potential benefit to alcoholic individuals who are trying to avoid relapse. Further studies that incorporate direct measures of alcohol drinking are warranted to test these potential mechanisms and treatments.
FUNDING
Supported by resources from VA Puget Sound Health Care System Mental Health Service, VISN 20 MIRECC and National Institutes of Health Grants AA018604 and AA017839 (Rasmussen); AA018604and AA007611(Froehlich).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- Ballenger JC, Post RM (1978) Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry 133:1–14. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz G (1999) What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res 23:1125–1135. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH (2004) Stress sensitization of the ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A receptor agonist. Neuropsychopharmacology 29:470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ (2005. a) Conceptual framework for the etiology of alcoholism: a ‘kindling’/stress hypothesis. Psychopharmacology 178:367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ (2005. b) Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology 30:1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Chandler J, Hensman C, et al. (1972) Drinking in a London suburb. II. Correlates of trouble with drinking among men. Q J Stud Alcohol 6:94–119. [PubMed] [Google Scholar]
- Ehrenreich H, Schuck J, Stender N, et al. (1997) Endocrine and hemodynamic effects of stress versus systemic CRF in alcoholics during early and medium term abstinence. Alcohol Clin Exp Res 21:1285–1293. [PubMed] [Google Scholar]
- Feldman S, Weidenfeld J (1998) The excitatory effects of the amygdala on the hypothalamo-pituitary-adrenocortical responses are mediated by hypothalamic norepinephrine, serotonin, and CRF-41. Brain Res Bull 45:389–393. [DOI] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, et al. (2012) Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res 36:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer B, Federoff DL, et al. (2013) Prazosin reduces alcohol drinking throughout prolonged treatment and blocks the initiation of drinking in rats selectively bred for high alcohol intake. Alcohol Clin Exp Res 37:1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer B, Fischer S, et al. (2015) Prazosin reduces alcohol intake in an animal model of alcohol relapse. Alcohol Clin Exp Res 39:1538–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoi K, Suda T, Tozawa F, et al. (1994) Microinjection of norepinephrine into the paraventricular nucleus of the hypothalamus stimulates corticotropin-releasing factor gene expression in conscious rats. Endocrinology 135:2177–2182. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA (2004) Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci 24:9703–9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. (1999) Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry 46:1167–1180. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278:52–58. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C (2000) The relationship between anxiety disorders and alcohol use disorders: A review of major perspectives and findings. Clin Psychol Rev 20:149–171. [DOI] [PubMed] [Google Scholar]
- Langen B, Fink H (2004) Anxiety as a predictor of alcohol preference in rats. Prog Neuropsychopharmacol Biol Psychiatry 28:961–968. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stevens DE, Fenton B, et al. (1998) Co-morbidity and familial aggregation of alcoholism and anxiety disorders. Psychol Med 28:773–788. [DOI] [PubMed] [Google Scholar]
- O'Carroll RE, Drysdale E, Cahill L, et al. (1999) Stimulation of the noradrenergic system enhances and blockade reduces memory for emotional material in man. Psychol Med 29:1083–1088. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR (2005) Pharmacological modulation of repeated ethanol withdrawal-induced anxiety-like behavior differs in alcohol-preferring P and Sprague-Dawley rats. Pharmacol Biochem Behav 81:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar AA, Marsden CA, Naik PC, et al. (2004) Differences in peripheral noradrenergic function among actively drinking and abstinent alcohol-dependent individuals. Am J Addict 13:225–235. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, et al. (2009) The α1-noradrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res 33:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Beckwith LE, Kincaid CL, et al. (2011) Combining the α1-adrenergic receptor antagonist, prazosin, with the β-adrenergic receptor antagonist, propranolol, reduces alcohol drinking more effectively than either drug alone. Alcohol Clin Exp Res 38:1532–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Mitton DR, Green J, et al. (2001) Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res 25:999–1005. [PubMed] [Google Scholar]
- Rasmussen DD, Wilkinson CW, Raskind MA (2006) Chronic daily ethanol and withdrawal: 6. effects on rat sympathoadrenal activity during ‘abstinence’. Alcohol 38:173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Bachtell RK, Heinrichs SC, et al. (2002) The corticotropin-releasing factor/urocortin system and alcohol. Alcohol Clin Exp Res 26:714–722. [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, et al. (2009) A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res 33:255–263. [DOI] [PubMed] [Google Scholar]
- Sinha R, Robinson J, O'Malley S (1998) Stress response dampening: Effects of gender and family history of alcoholism and anxiety disorders. Psychopharmacology 137:311–320. [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Weiner JL (2014) Chronic treatment with prazosin or duloxetine lessens concurrent anxiety-like behavior and alcohol intake: evidence of disrupted noradrenergic signaling in anxiety-related alcohol use. Brain Behav 4:468–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, et al. (1995) Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology 122:369–373. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, et al. (1993) Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol 10:1–10. [DOI] [PubMed] [Google Scholar]
- Stone EA, Zhang Y (1995) Adrenoceptor antagonists block c-fos response to stress in the mouse brain. Brain Res 694:279–286. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Copland JD, Kent JM, et al. (1999) The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biol Psychiatry 46:1205–1218. [DOI] [PubMed] [Google Scholar]
- Toth I, Neumann ID (2013) Animal models of social avoidance and social fear. Cell Tissue Res 354:107–118. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, Rasmussen DD, Froehlich JC, et al. (2011) Effects of prazosin, an alpha-1-adrenergic receptor antagonist, on the seeking and intake of alcohol and sucrose in alcohol-preferring (P) rats. Alcohol Clin Exp Res 36:881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, et al. (2008) The effects of α1-noradrenergic receptor antagonism on dependence-induced increases in responding for ethanol. Alcohol 42:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ (1990) The involvement of the central noradrenergic systems and corticotropin-releasing factor in defensive-withdrawal behavior in rats. J Pharmacol Exp Ther 255:1064–1070. [PubMed] [Google Scholar]