Abstract
Background
The purpose of this study was to measure how the duration of nonoperative intervention for intestinal obstruction impacted patient outcomes and whether hospital characteristics influenced the timing of operative intervention.
Methods
The State Inpatient Database (Florida) of the Health Care Utilization Project and the Annual Survey database of the American Hospital Association were linked from 2006 to 2011. Included were patients ≥18 years of age with a primary diagnosis of intestinal obstruction. Patient factors included age, sex, socioeconomic factors, and comorbid conditions.
Results
A total of 116,195 patients met our inclusion criteria, and 43,079 underwent operative intervention (37.1%). Patients who required operative correction of the intestinal obstruction after the fifth day of hospitalization, compared with patients who underwent an operation on the day of admission, had increases in mortality (6.1% vs 1.8%, P < .001), complication rates (15.4% vs 4.0%, P < .001), and postoperative hospital stay (9 vs 5 days, P < .001). Patients cared for at a large teaching facility (with surgery residents) had increased odds of early operative intervention by 23% (odds ratio 1.23, [1.20–1.28]), whereas patients at low-volume hospitals had decreased odds of early intervention (odds ratio 0.88, [0.73–0.91]).
Conclusion
Initial nonoperative treatment in patients with uncomplicated intestinal obstruction is an important strategy, but the odds of having an adverse event increase as intestinal obstruction is delayed. Importantly, the presence of surgery residents and increasing bed size are hospital characteristics associated with earlier operative intervention, suggesting a quality benefit for care at large teaching hospitals.
Intestinal obstruction accounts for 20% of all acute operative admissions and can account for as many as 3.1% of emergency surgical admissions.1,2 The optimal outcome for patients presenting with intestinal obstruction is influenced by several factors, such as whether the obstruction is partial or complete, the presence of ischemic or gangrenous bowel, perforation, duration of symptoms, development in the early postoperative period, the admitting service, and etiology.3,4 In patients without evidence of ischemia (uncomplicated intestinal obstruction) and with a clinically stable examination, there is a role for a trial of nonoperative management as an alternative to prompt operative intervention.
The adage, “don’t let the sun rise and set on a bowel obstruction” is evolving into a phrase with mostly historic relevance. In 1981, Bizer et al5 demonstrated the safety of initial nonoperative management for patients with intestinal obstruction. Since that time with appropriate patient selection, several groups have performed both prospective and retrospective studies with outcomes that support those findings.2,6,7 Most experts agree that the majority of patients managed initially with bowel rest, gastric decompression, and fluid resuscitation should be expected to have resolution of the intestinal obstruction.8
The appropriate duration of time to continue with nonoperative management, however, remains controversial. Seror et al9 found that non-operative management for up to 5 days duration resulted in a 73% rate of resolution of obstruction with no significant increase in mortality rate or incidence of strangulated bowel, including those presenting with a complete obstruction. Bickell et al10 found the risk for bowel resection increases substantially if operative intervention is postponed beyond 24 hours and remains increased through 96 hours of unresolved symptoms.
Taken together, current literature suggests that nonoperative management can be delayed safely for as long as 5 days. Importantly, this period of time does not come without risk to the patient for increased mortality and morbidity, especially for patients eventually requiring operative intervention for an intestinal obstruction.11 The purpose of our study was to characterize the magnitude of risk associated with delay in operative treatment of intestinal obstruction by comparing early vs late operative intervention. Our group was specifically interested in evaluating the association between hospital-specific factors and timing of operative treatment of intestinal obstruction.
MATERIALS AND METHODS
This was a cross-sectional, retrospective review in which we used data from 2006 to 2011 from the Health Care Utilization Project State Inpatient Database (HCUP SID) for the state of Florida. The development of HCUP SID was sponsored by the Agency for Healthcare Research and Quality to inform health-related decisions. HCUP SID includes all patient discharge records for all payers for 47 states that participate in the project. Each SID is unique to its individual state. Data are deidentified, protected, and include more than 100 clinical and nonclinical variables.12 The study was deemed exempt from institutional review board approval based on the use of de-identified records.
Patient selection
Included were patients 18 years of age or older who presented with a primary diagnosis of intestinal obstruction. We defined intestinal obstruction as an acute bowel obstruction related to etiologies resulting in a mechanical intestinal obstruction including post-operative adhesions, incarcerated ventral/incisional hernia, inflammatory bowel disease, malignancy, volvulus, and intussusception. Patients were identified by use of the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. ICD-9-CM codes used to define the study population are listed in Table I.
Table I.
Inclusion ICD-9-CM diagnosis and procedure codes with corresponding CCS codes
| Diagnosis | ICD-9 CM code | CCS code |
|---|---|---|
| Adhesions | 560.81, 560.9 | |
| Obstructed Ventral/ Incisional Hernias |
551.2, 551.20, 551.21, 551.29, 552.2, 552.20, 552.21, 552.29 | |
| Intussusception | 560.0 | |
| Volvulus | 560.2 | |
| Other specified | 560.89 | |
| Inflammatory Bowel Disease |
560.89 AND 555.0 or 555.1 or 555.2 or 555.9 or 556.0 or 556.1 or 556.2 or 556.3 or 556.4 or 556.6 or 556.8 or 556.9 |
|
| Malignant | 560.89 AND AHRQ Comorbidity Indicator (Metastatic Disease) | |
| Surgical procedure | ||
| Exploratory laparotomy | 54.11 | 09.21 |
| Exploratory laparoscopy | 54.21 | 09.19 |
| Lysis of adhesions | 54.5, 545.1, 545.9 | 09.22 |
| Small bowel resection | 45.61, 45.62, 45.6.3 | 09.07 |
| Colon resection | 17.33, 45.75, 17.35, 45.75, 17.36, 45.76, 17.31, 17.32, 17.34, 17.39, 45.71, 45.74, 45.79, 45.8, 458.1, 458.2, 458.3 |
ICD-9-CM code used |
| Repair of obstructed or gangrenous ventral hernia |
53.59, 53.63, 53.69 | ICD-9-CM code used |
AHRQ, Agency for Healthcare Research and Quality; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification.
Patients who underwent operative treatment were identified using both Clinical Classification Software (CCS) codes and individual ICD-9-CM procedure codes. CCS is a uniform, standardized, and validated coding system used to collapse multiple ICD-9-CM diagnostic and procedure codes into meaningful categories.13 Operative procedures and their respective CCS categories and/or ICD-9-CM procedure codes included are listed in Table I. Patients who responded to nonoperative management were not included in the data analysis.
Patient characteristics
Patient demographic variables based on availability in HCUP SID included sex and race (Caucasian, African-American, Hispanic, Other). Races included under the subgroup “Other” were Asian, Native American, and not reported. Socioeconomic factors included median annual income by zip code ($0–$38,999; $39,000–$47,999; $48,000–$63,999; $64,000+) and primary insurance type (Medicare, Medicaid, Private, Self-Pay, Other).
Clinical characteristics included comorbidities assigned based on Agency for Healthcare Research and Quality software addressing comorbidity. This software uses ICD-9-CM and Diagnosis-Related Group to identify conditions not related to the principle diagnosis and groups them into usable categories to describe patient comorbidity.14
Assessment of hospital factors
Hospital-level variables were assessed by linking HCUP SID to the Annual Survey Database of the American Hospital Association. This is a census of 6,200 hospitals containing 1,000 fields of information categorizing an institution’s organizational structure, facility and service lines, inpatient and outpatient utilization, operation expenses, and staffing. It contains a field for Medicare Provider number, allowing discrete linking to HCUP SID data fields.19
Hospitals with accredited general surgery residencies were identified by the use of data published by the Accreditation Council for Graduate Medical Education and American Osteopathic Association. Hospitals where residents rotated were identified using publicly available information provided by individual residency programs.
Outcomes
Postoperative outcomes of interest included inpatient mortality, hospital complications, and postoperative duration of stay. Complications were categorized as either: (1) major or (2) minor. Major complications were myocardial infarction, pulmonary embolism, and postoperative septicemia. Minor complications were urinary tract infection, deep-vein thrombosis, pneumonia, and wound complications. These classifications were based on previously reported methodologies.15 Complications were obtained by use of the ICD-9-CM codes and excluded if the diagnosis was present on admission.
We defined early operative intervention as occurring at any time before hospital day 5 and late operative intervention as occurring ≥5 days, on the basis of existing literature defining the optimal thresholds for pursuing nonoperative management of intestinal obstruction.16,17,20
Propensity score matching
To control for differences in preoperative patient characteristics, propensity score matching was used to create 2 balanced subgroups. Matching was conducted using an add-on STATA module with the primary treatment defined as timing of operative intervention (early vs late).18 Groups were matched using patient age, sex, diagnosis, congestive heart failure, disorders of coagulation, valvular disease, peripheral vascular disease, liver disease, anemia, malnutrition, diabetes mellitus, chronic hypertension, chronic renal failure, depression, neurologic disorder, and disorder of electrolytes. Propensity scores were matched one to one with no replacement at a caliper of 0.2.
Statistical analysis
Data are summarized using standard descriptive statistics with arithmetic means (standard deviation) or medians (interquartile range) for continuous variables and frequencies (proportions) for categorical variables. Univariate testing on the entire study population was conducted using the Student t test and χ2 tests for continuous and categorical variables, respectively. For comparisons of multiple groups, omnibus testing of continuous variables was done using Kruskal–Wallis tests and categorical variables with Cochran–Mantel–Haenszel tests. Tests of significance in matched groups were performed using paired t tests for continuous variables and McNemar’s test for categorical variables. All multivariable analyses were conducted using mixed-effects, multilevel logistic regression to control for potential hospital effects. All statistical analyses were conducted in STATA, version 12 (StataCorp LP, College Station TX).
RESULTS
Baseline characteristics of study population
The final study population was comprised of 116,195 patients who presented with a primary diagnosis of intestinal obstruction. Baseline demographic, socioeconomic, and clinical characteristics are shown in Table II. The mean age of the population was 65.0 (SD 16.5) years. The primary payer for the majority of patients was Medicare (58.5%), with private insurers being the second most common (26.4%). More than half of the patients lived in a zip code with a median income between $39,000 and $63,999.
Table II.
Baseline characteristics of study population, including comparison between surgery and nonsurgery groups
| Baseline characteristics | All patients (n = 116,195) | No surgery (n = 73,116) | Surgery (n = 43,079) | P value |
|---|---|---|---|---|
| Mean age, y (SD) | 65.0 (16.5) | 65.8 (16.6) | 65.6 (16.2) | <.0001 |
| Sex, % female | 59.3% | 56.8% | 63.6% | <.0001 |
| Race | ||||
| White | 73.4% | 73.0% | 74.0% | <.0001 |
| Black | 12.1% | 11.4% | 13.3% | |
| Hispanic | 11.2% | 12.1% | 9.5% | |
| Other | 3.4% | 3.5% | 3.2% | |
| Insurance type, % | ||||
| Medicare | 58.5% | 60.9% | 54.5% | <.0001 |
| Medicaid | 6.1% | 5.9% | 6.3% | |
| Private | 26.4% | 24.4% | 29.6% | |
| Other | 9.1% | 8.8% | 9.6% | |
| Income (% in quartile, by zip code) | ||||
| 1 ($0–$38,999) | 28.9% | 28.6% | 29.4% | <.0001 |
| 2 ($39,000–$47,999) | 31.8% | 31.6% | 32.2% | |
| 3 ($48,000–$63,999) | 26.6% | 26.9% | 26.3% | |
| 4 ($64,000+) | 12.7% | 13.0% | 12.2% | |
| Comorbidities | ||||
| Chronic hypertension | 56.9% | 56.1% | 58.2% | <.0001 |
| Disorder of electrolytes | 34.3% | 32.4% | 37.6% | <.0001 |
| Anemia | 18.8% | 16.5% | 22.7% | <.0001 |
| Chronic lung disease | 18.3% | 16.5% | 21.5% | <.0001 |
| Diabetes mellitus | 18.3% | 17.9% | 19.0% | <.0001 |
| Depression | 9.4% | 9.3% | 9.6% | .1376 |
| Chronic renal failure | 7.6% | 7.5% | 7.8% | .0269 |
| Congestive heart failure | 6.7% | 5.8% | 8.3% | <.0001 |
| Peripheral vascular disease | 6.3% | 5.7% | 7.2% | <.0001 |
| Neurologic disorder | 5.9% | 6.0% | 5.7% | .0133 |
| Malnutrition | 5.6% | 3.8% | 8.5% | <.0001 |
| Valvular disease | 5.1% | 4.7% | 5.7% | <.0001 |
| Disorder of coagulation | 3.2% | 2.5% | 4.4% | <.0001 |
| Liver disease | 2.7% | 2.7% | 2.8% | .2051 |
| Age-adjusted CCI | 4.1 (SD = 2.5) | 4.2 (SD = 2.5) | 3.9 (SD = 2.5) | <.0001 |
| Type of obstruction | ||||
| Adhesive | 66.1% | 81.7% | 55.6% | <.0001 |
| Ventral/incisional hernia | 15.2% | 3.9% | 40.5% | <.0001 |
| Volvulus | 3.5% | 1.7% | 7.0% | <.0001 |
| Inflammatory bowel disease | 2.6% | 3.8% | 2.1% | <.0001 |
| Malignancy | 2.1% | 4.7% | 1.0% | <.0001 |
| Intussusception | 1.0% | 1.0% | 1.4% | <.0001 |
| Other | 9.5% | 12.6% | 4.2% | <.0001 |
| Underwent operation | ||||
| Yes | 37.1% | — | — | |
| No | 62.9% | — | — | |
Of the total study population, 43,079 of 116,195 (37.1%) patients underwent operative intervention. The majority of patients (62.9%) responded to nonoperative management. Descriptive statistics of these two groups are shown in Table II. Adhesions and ventral/incisional hernias represented the most common admission diagnoses of the study population. Adhesive disease was the most common diagnosis in both the operative intervention group (55.6%) and nonoperative group (81.7%).
Outcomes for operative intervention compared with duration of nonoperative management
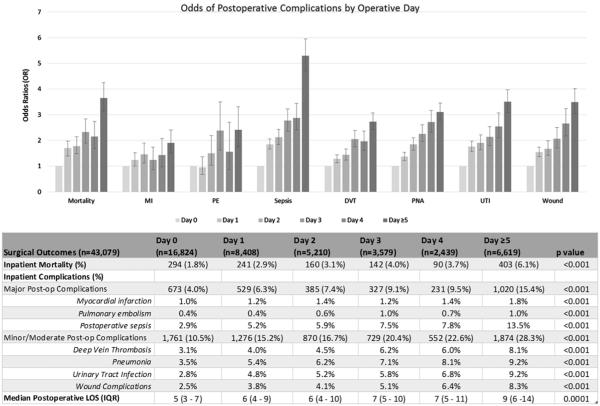
To examine the duration of nonoperative management on mortality and complications, outcomes were assessed based on the hospital day a patient underwent surgery for intestinal obstruction (see Fig 1). Day 0 refers to patients undergoing operation on the day of admission. Inpatient mortality increased each successive day after admission, with a mortality rate of 1.8% for those who had operation within 24 hours of admission compared to 6.1% for patients with operation delayed until day 5 or later (P < .001).
Fig 1.
Comparing hospital day of operation and postoperative outcomes. Error bars = 95% confidence interval of odds ratio. LOS, Length of hospital stay.
The same trend was observed for major and moderate/minor complications. Patients undergoing operation on day 0 had a major postoperative complication rate of 4.0%, whereas those undergoing operative correction more than 4 days after admission had a rate of 15.4% (P < .001). For moderate/minor complications, the complication rate was 10.5% for the group receiving operative correction within 24 hours of admission compared with 28.3% for the group delayed until the fifth day or later (P < .001). When operative intervention did not occur until the fifth day or later, there was nearly a 2-fold increase in the median postoperative hospital duration of stay (5 days vs 9 days, P = .0001).
Patient baseline characteristics of early versus late operative intervention groups
To further characterize the difference observed in postoperative outcomes, patients were grouped into 2 categories: early operative intervention and late operative intervention. Characteristics of the 2 groups are shown in Table III. A total of 36,460 patients were in the early group compared with 6,619 patients in the late group.
Table III.
Baseline patient characteristics comparing those who underwent early versus late operative intervention
| Baseline characteristics | Early (<5 d; n = 36,460) | Late (≥5 d; n = 6,619) | P value |
|---|---|---|---|
| Age, y (mean) | 63.1 (SD = 16.2) | 66.4 (SD = 16.1) | <.0001 |
| Sex, n (% female) | 23,219 (63.7%) | 4,180 (63.2%) | .6848 |
| Comorbidities | |||
| Chronic hypertension | 20,925 (57.4%) | 4,163 (62.5%) | <.001 |
| Disorder of electrolytes | 12,536 (34.4%) | 3,681 (55.6%) | <.001 |
| Anemia | 7,530 (20.7%) | 2,270 (34.3%) | <.001 |
| Chronic lung disease | 7,636 (20.9%) | 1,610 (24.3%) | <.001 |
| Diabetes mellitus | 6,909 (19.0%) | 1,288 (19.5%) | .331 |
| Depression | 3,410 (9.4%) | 714 (10.8%) | <.001 |
| Congestive heart failure | 2,782 (7.6%) | 810 (12.2%) | <.001 |
| Chronic renal failure | 2,588 (7.1%) | 780 (11.8%) | <.001 |
| Peripheral vascular disease | 2,512 (6.9%) | 591 (8.9%) | <.001 |
| Neurologic disorder | 1,907 (5.2%) | 537 (8.1%) | <.001 |
| Malnutrition | 2,360 (6.5%) | 1,304 (19.7%) | <.001 |
| Valvular disease | 1,998 (5.5%) | 470 (7.1%) | <.001 |
| Disorder of Coagulation | 1,430 (3.9%) | 460 (7.0%) | <.001 |
| Liver disease | 980 (2.7%) | 227 (3.4%) | .001 |
| Age-adjusted CCI | 3.8 (SD = 2.4) | 4.6 (SD = 2.7) | <.0001 |
| Type of obstruction | |||
| Adhesive | 19,085 (52.4%) | 4,858 (73.4%) | <.001 |
| Ventral hernia | 15,981 (43.8%) | 1,465 (22.1%) | <.001 |
| Volvulus | 2,618 (7.2%) | 394 (6.0%) | <.001 |
| Inflammatory disease | 714 (1.2%) | 212 (3.2%) | <.001 |
| Malignancy | 259 (0.7%) | 146 (2.2%) | <.001 |
| Intussusception | 545 (1.5%) | 44 (0.7%) | <.001 |
| Other | 1,387 (3.8%) | 420 (6.4%) | <.001 |
Patient comorbidity appeared to be correlated with the timing of operative intervention. The prevalence of several comorbid conditions, including electrolyte disorders (55.6% vs 34.4%, P < .001), anemia (34.3% vs 20.7%, P < .001), chronic lung disease (24.3% vs 20.9%, P < .001), congestive heart disease (12.2% vs 7.6%, P < .001), and malnutrition (19.7% vs 6.5%, P < .1 were greater in the late group when compared with the early group. Additionally, comorbid disease severity was greater in the late group compared with the early group (4.6 vs 3.8, P < .0001).
Do postoperative outcomes differ between early and late after propensity score matching?
We next sought to determine whether the difference in operative outcomes between the early and late groups was explained by preoperative comorbid disease. A total of 11,388 patients were matched using propensity scores with minimal bias across covariates with 5,694 patients in each group. Baseline characteristics of matched patients are shown in Table IV.
Table IV.
Baseline characteristics of propensity matched groups, early operative intervention versus late operative intervention
| Characteristic |
Early
(n = 5,694) |
Late
(n = 5,694) |
P value |
|---|---|---|---|
| Age, y | 65.7 | 65.1 | <.001 |
| Sex, female, % | 61.8% | 62.3% | .226 |
| Comorbidities | |||
| Chronic hypertension |
57.7% | 57.4% | .552 |
| Disorder of electrolytes |
40.2% | 39.9% | .512 |
| Chronic lung disease |
19.7% | 20.5% | .022 |
| Anemia | 22.7% | 22.4% | .379 |
| Malnutrition | 8.4% | 7.8% | .006 |
| Diabetes mellitus | 18.9% | 17.4% | <.001 |
| Depression | 10.2% | 10.2% | .843 |
| Chronic renal failure |
8.4% | 7.7% | .004 |
| Congestive heart failure |
8.0% | 7.7% | .245 |
| Peripheral vascular disease |
7.5% | 7.5% | .944 |
| Neurologic disorder |
6.0% | 5.9% | .393 |
| Valvular disease | 6.0% | 6.0% | .923 |
| Disorder of coagulation |
4.2% | 3.8% | .050 |
| Liver disease | 2.9% | 2.5% | .006 |
| Type of obstruction | |||
| Adhesive | 72.3% | 73.0% | .090 |
| Ventral hernia | 15.0% | 18.2% | <.001 |
| Volvulus | 9.7% | 6.3% | <.001 |
| Inflammatory disease |
2.8% | 2.4% | .009 |
| Malignancy | 1.1% | 1.1% | .895 |
| Intussusception | 2.2% | 2.1% | .313 |
| Other | 5.7% | 4.8% | <.001 |
Patient outcomes of the propensity score– matched groups are shown in Table V. Inpatient mortality in the early group was 2.8% compared with 5.2% in the late group (P < .001). Patients undergoing operative intervention after the fourth hospital day had an almost 2-fold increase in incidence of major postoperative complications (13.4% vs 7.2%, P < .001) and a 1.7-fold increase in incidence of minor/moderate postoperative complications (25.9% vs 15.2%, P < .001). Postoperative duration of stay was 3 days longer in the late group compared with the early group (9 vs 6, P < .0005).
Table V.
Outcomes comparing propensity-matched groups, early versus late operative intervention
| Operative outcomes | Early OR (<5 days; n = 5,694) | Late OR (≥5 days; n = 5,694) | P value |
|---|---|---|---|
| Inpatient mortality (%) | 133 (2.8%) | 244 (5.2%) | <.001 |
| Inpatient complications (%) | |||
| Major postoperative complications | 339 (7.2%) | 634 (13.4%) | <.001 |
| Myocardial infarction | 55 (1.2%) | 66 (1.4%) | .314 |
| Pulmonary embolism | 22 (0.5%) | 49 (1.0%) | .001 |
| Postoperative sepsis | 284 (6.0%) | 549 (11.6%) | <.001 |
| Minor/moderate Postoperative complications | 720 (15.2%) | 1,227 (25.9%) | <.001 |
| Deep-vein thrombosis | 172 (3.6%) | 373 (7.9%) | <.001 |
| Pneumonia | 265 (5.6%) | 371 (7.8%) | <.001 |
| Urinary tract infection | 223 (4.7%) | 377 (8.0%) | <.001 |
| Wound complications | 178 (3.8%) | 375 (7.9%) | <.001 |
| Postoperative median duration of stay, d | 6 (IQR = 3–9) | 9 (IQR = 6–14) | .0005 |
IQR, Interquartile range.
Do hospital factors influence early operative intervention
We next attempted to determine whether hospital characteristics played a role in the timing of operative intervention. Hospital teaching status (presence of surgery residents/no surgery residents), number of operations for intestinal obstruction surgeries performed annually (divided into quartiles), and hospital bed size (divided into quartiles) were compared across the early and late groups (see Table VI).
Table VI.
Hospital characteristics in patients with early versus late intervention
| Hospital characteristics | Early (<5 d) | Late (≥5 d) | P value |
|---|---|---|---|
| Teaching status | |||
| Surgery residents | 83.2% (6,936/8,339) | 16.8% (1,403/8,339) | <.001 |
| No surgery residents | 82.1% (28,524/34,740) | 17.9% (6,216/34,740) | |
| Surgical volume (intestinal obstruction cases/per year) | |||
| Very high (245–915) | 85.8% (8,909/10,384) | 14.2% (1,475/10,384) | <.001 |
| High (169–244) | 83.7% (9,079/10,845) | 16.3% (1,766/10,845) | |
| Medium (112–168) | 85.7% (9,642/11,250) | 14.3% (1,608/11,250) | |
| Low (8–111) | 83.3% (8,830/10,600) | 16.7% (1,770/10,600) | |
| Bed size | |||
| Very large hospital (525–2,170) | 86.4% (9,066/10,496) | 13.6% (1,430/10,496) | <.001 |
| Large hospital (319–524) | 85.5% (8,735/10,213) | 14.5% (1,478/10,213) | |
| Medium hospital (199–317) | 83.9% (9,391/11,198) | 16.1% (1,807/11,198) | |
| Small hospital (25–198) | 83.0% (9,268/11,172) | 17.0% (1,904/11,172) | |
Patients at hospitals with surgery residents were more likely to undergo early intervention (83.2%, 6,936/8,339) than those without surgery residents (82.1%, 28,524/34,740, P <.001). Early intervention also occurred most often at very high-volume centers (85.8%, 8,909/10,384) and at those with the greatest number of beds (86.4%, 9,066/10,496).
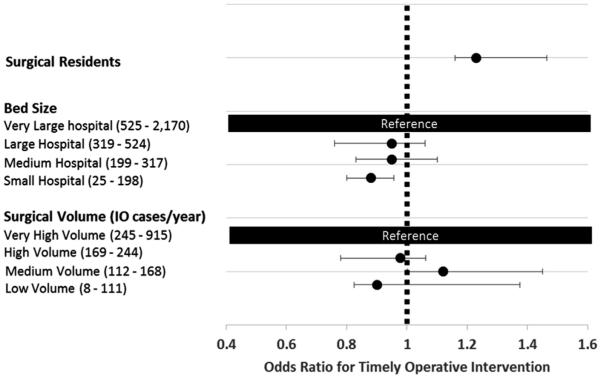
These relationships were assessed further by the use of mixed-level, multivariable analysis in which we controlled for age, sex, comorbidity, and admission diagnosis (see Fig 2). The presence of surgery residents increased the odds of early intervention by 23% (odds ratio 1.23, [1.20–1.28]). When compared with the highest quartile, the smallest hospitals decreased the odds of having an early operative intervention by 12% (odds ratio 0.88, [0.73–0.91]).
Fig 2.
Forest plot comparing hospital characteristics and odds of early vs late operative intervention controlling for patient age, sex, socioeconomic status, and presenting diagnosis.
DISCUSSION
Using a large, population-based study, we demonstrated that each additional day of nonoperative management for intestinal obstruction resulted in increased mortality and morbidity for patients who eventually needed operative intervention. This finding was independent of age, sex, concurrent comorbid disease, and presenting diagnosis. We also identified hospital characteristics that promoted early operative intervention in this patient population.
Our observation that delay in the operative management of intestinal obstruction resulted in greater morbidity, mortality, and prolonged post-operative duration of stay is consistent with existing literature. Schraufnagel et al8 also noted an increased risk of mortality and prolonged postoperative duration of stay for those patients operated on after the fourth hospital day. Keenan et al11 reported nonoperative management for uncomplicated adhesive small bowel obstruction exceeding 3 days to be associated with increased morbidity and postoperative duration of hospitalization. Unlike those studies, we did not limit our inclusion criteria only to adhesive small bowl obstructions. To date, our results represent the largest analysis including multiple causes of bowel obstruction. Therefore, our conclusion that earlier operative intervention decreases the risk for mortality and morbidity can be applied to patients presenting with intestinal obstruction of multiple etiologies.
A major assumption of our study was the delay to operation measured the duration of nonoperative treatment. If a patient underwent operative intervention on day 5, we assumed they were managed with a trial of nonoperative therapy from the day of admission until day 4 without resolution. Unfortunately, the exact reasons for progression to operative intervention cannot be abstracted from our data source. As discussed by Schraufnagel et al, who used the National Inpatient Sample to study the impact of delay to operation on outcomes in adhesive small bowl obstructions, we had similar limitations in our ability to assess imaging, laboratory values, and clinical examination findings.8
These limitations are particularly important in framing our assessment of the merits of continued nonoperative intervention. From our clinical experience, there are patients who benefit from a trial of nonoperative management. In our study population, the hospital duration of stay and inpatient mortality of patients who did not undergo operation was 3 days (interquartile range 2–5) and 1.7%, respectively, both significantly less than their operative counterparts. This finding illustrates that in patients with early resolution of intestinal obstruction, nonoperative management can produce acceptable inpatient outcomes. Because we did not directly compare nonoperative with operative intervention, the data presented in this study should not be interpreted as a critique of nonoperative management. Instead, we highlight that failure of intestinal obstruction to respond to conservative care is associated with a significant increase in mortality and morbidity with each additional day that operative intervention of the obstruction is delayed for patients eventually requiring operative intervention.
Furthermore, we found that patients in the late group carried a more substantial burden of comorbid disease. We hypothesize this was, in part, related to an attempt to medically optimize a subpopulation of patients in the late group before operative intervention. Our results suggest that this approach may inadvertently lead to subjecting high-risk patients to a “double hit”---they have substantial baseline comorbid disease states and, in an attempt to optimize these conditions or avoid operative intervention, practitioners may actually be decreasing likelihood of a successful outcome. This observation deserves further study.
Unique to our approach was the ability to assess how hospital factors influenced patient outcomes. Other groups have demonstrated academic status, hospital size, and operative numbers of patients are associated with earlier operative intervention for other surgical diseases including diverticulitis and appendicitis.21,22 Notably, an admitting service can have particular importance in the prompt and appropriate management of intestinal obstruction. Compared with patients with intestinal obstruction admitted to a medical service, those admitted to a surgical service have lesser time to operation, overall duration of stay, and fewer complications.4
We observed during exploratory data analysis that patients at large, quaternary care centers commonly underwent operation earlier in their hospital course. This difference persisted after controlling for comorbidity, socioeconomic status, and admission diagnosis. Leveraging data from the Annual Survey of the America Hospital Association, we were able to identify surgery residents and hospital size as independent predictors for early intervention. A possible explanation for this association is the “round-the-clock” presence of physicians and perioperative resources (operating room staff, advanced diagnostics, etc) at large, academic centers that may play important roles in influencing prompt operative management.23,24
There are several elements of our study that require additional discussion. As with any study using a large sample size, there will be several statistically significant differences which do not hold clinical importance, such as our analysis of socioeconomic factors. Our data suggest that patients of African-American race, with private insurance, and lower-income groups underwent operative management more commonly for intestinal obstruction. The actual difference between groups, however, was 1.9%, 2.7%, and 2.0%. These are unlikely to manifest as clinically important differences, and further analysis would be needed to assess the role, if any, of socioeconomic status on outcomes of patients with intestinal obstruction.
The drawbacks of using an administrative database are well-documented.25 Administrative datasets utilize retrospective data, limiting the ability to draw conclusions regarding causal relationships. Also, there are few incentives for coding minor procedures and diagnoses accurately in administrative datasets unless they impact cost, often leading to under or overreporting in clinical studies. Another important limitation of our study is not being able to assess readmissions adequately due to constraints of the data. The ability to study readmissions, both for patients who underwent operative intervention and those managed nonoperatively, would greatly improve our understanding of the implications associated with each of these treatment strategies.
Despite these limitations, conclusions drawn from these datasets are derived from population-based analyses instead of small, representative samples. Ultimately, our findings are meant to guide efforts toward a prospective study attempting to better understand the implications of nonoperative treatment on operative outcomes and factors responsible for early intervention.
Although our data cannot define an exact threshold for operative intervention or predict which patients are best suited for early intervention, our study does highlight that each additional day during a trial of nonoperative intervention confers an increased risk for a poorer outcome in patients in whom the intestinal obstruction does not resolve. Therefore, it is imperative for surgeons to consider the duration of nonoperative care when deciding to operate or “wait one more day” in this patient population.
Biographies
Dr Brian Harbrecht (Louisville, KY): Anytime that you use a large administrative data set, you exchange the statistical power, as you mentioned, for some of the granularity and specificity of some of the cases. That kind of a dilemma really is the basis for most of my questions.
You didn’t really go into it in your presentation as much as you did in your manuscript, but in your group with intestinal obstruction, you had all patients with all causes of intestinal obstruction, not only adhesions but patients with hernias as well as patients with what you considered to be “other” diagnoses.
Is that the same group that formed the basis for the analysis in your presentation? When patients present with an obstruction from an incarcerated hernia, those patients aren’t likely to get a trial of nonoperative therapy in my hospital or likely in many hospitals.
Looking at some of the tables in your manuscript, the vast majority of the patients with the diagnosis of “other,” almost 90%, were treated nonoperatively, which would be appropriate if those patients had Crohn disease or some other conditions.
Do you really think it’s appropriate to lump all those groups together, or would your analysis be a little bit more informative if you just isolated that group to the ones with adhesions, because those are the ones in whom much of the dilemma exists.
You did highlight the issues of medical comorbidities. I think that’s an important part. Your data do suggest that the patients who are operated on late are the ones who have the most comorbidities. Are they operated on late because they have those comorbidities and people are trying to avoid operative management, and that’s what drives the complications, or is it truly an inappropriate delay for other reasons? Can the severity of complications be assessed in your database? I think I know the answer to that, but I would like to hear your answer.
Finally, I’m not a statistician but in your propensity analysis, many of the factors that you controlled for were still statistically different between your 2 groups. Do you have an explanation for that?
Dr Jordan Liles: We found that a large number of patients who presented with adhesions may not have been coded for adhesions with ICD-9. There is another classification that includes “other.” For this data set, I believe it was relevant to include the other category because they contained approximately 50% of our patient database. When we lumped “other” with adhesions, they made up between 90 and 95% of the patient database, which seems to be in line with what the literature recommends for those patients that present with intestinal obstruction.
I believe the second question was why was surgical operation or intervention was delayed. Unfortunately, one of the drawbacks of retrospective analysis is we can’t determine what the physicians were thinking when they decided to delay operative intervention, but one thing that we can do is control for those comorbid conditions.
When we went through these data, we saw that even controlling for nearly all those comorbid conditions as best we could, we found that delayed operative intervention, regardless of comorbid conditions, was associated with an increased complication risk. I believe that’s the main point we wanted to drive home. It was less to do with the presence of the comorbid conditions, but just that the delayed operative intervention increased the likelihood of complications.
I am going to defer the third question to Dr Luchette or Dr Kothari.
Dr Frederick Luchette: The third question dealt with the propensity analysis. We’ve consulted with our statisticians, and these are valid tests. I don’t know that we can get much granular, as Jordan just mentioned.
I was trained that the sun never rises or sets on a bowel obstruction. My take-home message is the sicker the patient, you have to lower your threshold for surgical intervention rather than delaying it. I hope that resonates with everybody in the audience.
Dr Christopher R. McHenry (Cleveland, OH): I was interested to hear that you identified some disparities in care. It seemed to me that African-American patients were more likely to undergo delayed operation whereas patients of a greater socioeconomic status were more likely to undergo earlier operation.
I recognize that these are administrative data, but can you surmise what may be the explanation for those disparities? When we identify disparities like this, there should be some plan to deal with those disparities. I would be interested in your comments.
Dr Jordan Liles: First, examining cultural differences wasn’t one of the purposes of this study, so we didn’t do too much analysis. I think that is an appropriate follow-up question that we will definitely look into in the future.
Additionally, you mentioned that a greater socioeconomic status played a role in earlier operative intervention. I think there may have been some confusion, because actually the socio-economic status was very similar between the early versus delayed group.
I think what you may have seen was that patients with private insurance were more likely to undergo early operative intervention. It’s not something that we really delved into for this study, but I think in the future we’ll probably look into that as well.
Dr Kenric Murayama (Abington, PA): Your cut-off between early or timely and delayed intervention was 5 days. As you went through the data, was there some period in that 5 days where you hit a tipping point that you saw a sudden increase in the mortality and complication rate? That would be the day that we all need to know when we are going to suddenly see a significant increase in mortality and complication.
Dr Jordan Liles: That’s a good question. The reason we picked 5 days as the cutoff was because literature is recommending that between 3 and 5 is when you operate for best outcomes. We wanted to be conservative with our cutoff dates. We picked day 5 just for this study. Unfortunately, we did not find a specific day.
What we did find was that every day of delayed operative intervention increased the likelihood for morbidity, mortality, postoperative length of stay, and major and minor complications.
Dr Margo Shoup (Maywood, IL): As a surgeon in a community practice where we don’t have any residents, I have to tell you I’m not really sure what to do with this information.
You have to realize that when you are in an academic setting, the residents are always eager to operate on everything they see. In reality, it’s not always the right thing to do. The easiest thing is to take someone to surgery with a bowel obstruction. That’s simple. You take them to surgery, you see it, and you fix it if you can. The hardest thing is to sit there and wait on them. Sometimes that’s a judgment call from people that have been in practice for a long time.
Figuring out why they had the obstruction is so important. For example, operating on everybody with obstruction from carcinomatosis is not going to be the right thing to do. They are still going to die in the next 30–60 days. So just keep that in mind.
Dr Scott Gruber (Detroit, MI): You sort of didn’t give us the good side of holding off after 5 days. In other words, how many patients by day were actually able to avoid surgery? There is a positive side of that coin. I think you have to give us the whole picture.
REFERENCES
- 1.Miller G, Boman J, Shrier I, Gordon PH. Etiology of small bowel obstruction. Am J Surg. 2000;180:33–6. doi: 10.1016/s0002-9610(00)00407-4. [DOI] [PubMed] [Google Scholar]
- 2.Hayanga AJ, Bass-Wilkins K, Bulkley GB. Current management of small-bowel obstruction. Adv Surg. 2005;39:1–33. doi: 10.1016/j.yasu.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Teixeira PG, Karamanos E, Talving P, Inaba K, Lam L, Demetriades D. Early operation is associated with a survival benefit for patients with adhesive bowel obstruction. Ann Surg. 2013;258:459–65. doi: 10.1097/SLA.0b013e3182a1b100. [DOI] [PubMed] [Google Scholar]
- 4.Malangoni MA, Times ML, Kozik D, Merlino JI. Admitting service influences the outcomes of patients with small bowel obstruction. Surgery. 2001;130:706–13. doi: 10.1067/msy.2001.116918. [DOI] [PubMed] [Google Scholar]
- 5.Bizer LS, Liebling RW, Delany HM, Gliedman ML. Small bowel obstruction: the role of non-operative treatment in simple intestinal obstruction and predictive criteria for strangulation obstruction. Surgery. 1981;89:407–13. [PubMed] [Google Scholar]
- 6.Scott FI, Osterman MT, Mahmoud NN, Lewis JD. Secular trends in small-bowel obstruction and adhesiolysis in the United States: 1988–2007. Am J Surg. 2012;204:315–20. doi: 10.1016/j.amjsurg.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zielinski MD, Bannon MP. Current management of small bowel obstruction. Adv Surg. 2011;45:1–29. doi: 10.1016/j.yasu.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Schraufnagel D, Rajaee S, Millham FH. How many sunsets? timing of surgery in adhesive small bowel obstruction: a study of the nationwide inpatient sample. J Trauma Acute Care Surg. 2013;74:181–7. doi: 10.1097/TA.0b013e31827891a1. discussion 187–189. [DOI] [PubMed] [Google Scholar]
- 9.Seror D, Feigin E, Szold A, Allweis TM, Carmon M, Nissan S, et al. How conservatively can postoperative small bowel obstruction be treated? Am J Surg. 1993;165:121–6. doi: 10.1016/s0002-9610(05)80414-3. [DOI] [PubMed] [Google Scholar]
- 10.Bickell NA, Federman AD, Aufses AH. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201:847–54. doi: 10.1016/j.jamcollsurg.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Keenan JE, Turley RS, McCoy CC, Migaly J, Shapiro ML, Scarborough JE. Trials of non-operative management exceeding 3 days are associated with increased morbidity in patients undergoing surgery for uncomplicated adhesive small bowel obstruction. J Trauma Acute Care Surg. 2014;76:1367–72. doi: 10.1097/TA.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 12.HCUP databases . healthcare cost and utilization project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: 2014. [PubMed] [Google Scholar]
- 13.Elixhauser A, Steiner C, Palmer L. Clinical classifications software (CCS) Agency for Healthcare Research and Quality; Rockville, MD: 2008. [Google Scholar]
- 14.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 15.Worni M, Schudel IM, Østbye T, Shah A, Khare A, Pietrobon R, et al. Worse outcomes in patients undergoing urgent surgery for left-sided diverticulitis admitted on week- ends vs weekdays: A population-based study of 31 832 patients. Arch Surg. 2012;147:649–55. doi: 10.1001/archsurg.2012.825. [DOI] [PubMed] [Google Scholar]
- 16.Maung AA, Johnson DC, Piper GL, Barbosa RR, Rowell SE, Bokhari F, et al. Evaluation and management of small-bowel obstruction: An eastern association for the surgery of trauma practice management guideline. J Trauma Acute Care Surg. 2012;73:S362–9. doi: 10.1097/TA.0b013e31827019de. [DOI] [PubMed] [Google Scholar]
- 17.Catena F, Di Saverio S, Kelly MD, Biffl WL, Ansaloni L, Mandal'a V, et al. Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2010 evidence-based guidelines of the world society of emergency surgery. World J Emerg Surg. 2011;6:21. doi: 10.1186/1749-7922-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leuven E, Sianesi B. PSMATCH2: Stata module to perform full mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical Software Components. 2014 Available from: https://ideas.repec.org/c/boc/bocode/s432001.html. Accessed July 26, 2015.
- 19.American Hospital Association . The AHA annual survey database. American Hospital Association; Washington, DC: 2005. Available from: http://www.ahadataviewer.com/book-cd-products/AHA-Survey/. Accessed July 26, 2015. [Google Scholar]
- 20.Williams SB, Greenspon J, Young HA, Orkin BA. Small bowel obstruction: conservative vs surgical management. Dis Colon Rectum. 2005;48:1140–6. doi: 10.1007/s10350-004-0882-7. [DOI] [PubMed] [Google Scholar]
- 21.Poh BR, Cashin P, Dubrava Z, Blamey S, Yong WW, Croagh DG. Impact of an acute care surgery model on appendicectomy outcomes. ANZ J Surg. 2013;83:735–8. doi: 10.1111/ans.12351. [DOI] [PubMed] [Google Scholar]
- 22.Bosio RM, Smith BM, Aybar PS, Senagore AJ. Implementation of laparoscopic colectomy with fast-track care in an academic medical center: benefits of a fully ascended learning curve and specialty expertise. Am J Surg. 2007;193:413–6. doi: 10.1016/j.amjsurg.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–20. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 24.Harmon JW, Tang DG, Gordon TA, Bowman HM, Choti MA, Kaufman HS, et al. Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg. 1999;230:404–11. doi: 10.1097/00000658-199909000-00013. discussion 411–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haut ER, Pronovost PJ, Schneider EB. Limitations of administrative databases. JAMA. 2012;307:2589–90. doi: 10.1001/jama.2012.6626. [DOI] [PubMed] [Google Scholar]