Abstract
Purpose
The purpose of this study was to monitor anti-vascular endothelial growth factor (anti-VEGF) treatment regimens for wet age-related macular degeneration (wAMD) in clinical practice and to determine how they impact the physician, patient, and caregiver treatment experience.
Materials and methods
This was a qualitative analysis based on semistructured interviews with 20 ophthalmologists who had practiced both pro re nata (PRN) and treat-and-extend (T&E) anti-VEGF regimens for wAMD. Interview questions were constructed to assess how the different regimens affected patient and caregiver experiences (in the opinion of the ophthalmologist) in addition to the ophthalmologist’s own experience. The interview included questions relating to 1) issues and benefits of PRN and T&E; 2) logistical and operational issues of introducing proactive therapy, especially T&E, to PRN practice; and 3) actions taken to handle the issues raised in 2).
Results
A total of 18 interview results were eligible for analysis. The study demonstrated that the benefits of T&E compared with PRN included decreased burden of patient consultations, decreased patient and caregiver emotional burden, and a sustained period of macular dryness. The issues associated with T&E were increased number of injections and financial burden from prolonged treatment duration. The ophthalmologists also experienced difficulty explaining the significance of proactive injections to patients. Countermeasures to operational issues experienced by ophthalmologists varied by practice.
Conclusion
Patients, caregivers, and the practicing ophthalmologists experienced benefits associated with a T&E regimen. However, in order to encourage better understanding of the T&E regimen, including its smooth implementation and significance for patients, a formal T&E treatment guideline providing standard practice should be considered.
Keywords: wet age-related macular degeneration, anti-vascular growth factor, anti-VEGF as-needed, anti-VEGF treat-and-extend, wAMD patient experience
Introduction
Wet age-related macular degeneration (wAMD) is a chronic disease and a leading cause of blindness in older patients.1–3 Following the recent introduction of anti-vascular endothelial growth factor (anti-VEGF) therapy, wAMD treatment has changed dramatically, with a switch to improving rather than maintaining visual acuity.4
In Japan, after the launch of ranibizumab (Lucentis®; Novartis International AG) in 2009, the pro re nata (PRN) method of administration became the standard approach for anti-VEGF therapy in wAMD. The PRN regimen consists of as-needed injections (depending on patient symptoms) after an initial loading phase of three consecutive monthly injections, with monthly visits to monitor symptoms.5,6
The proactive regimen was introduced with the launch of intravitreal aflibercept (Eylea®; Bayer) in 2012. There are two approaches to proactive therapy: fixed-dosing and treat-and-extend (T&E). Fixed-dosing consists of injections at set intervals (eg, every 8 weeks) after an initial loading phase, regardless of patient symptoms.7 By contrast, T&E does not have a defined injection interval. In general, after a loading phase, monthly injections are continued until no fluid is detected, followed by 2-week extension intervals until the next injection, which is done regardless of symptoms. When fluid is detected, the extension intervals are decreased depending on the severity. Unlike PRN, T&E does not require patients to make monthly visits for monitoring, but rather at intervals determined by the ophthalmologist.5,8
The effectiveness of proactive therapy has been widely reported, and improvements in visual acuity have been achieved with a fewer visits.9–12 Long-term data on anti-VEGF therapy and the various regimens are also becoming available.13,14 While the majority of research focuses on the clinical outcomes of anti-VEGF therapy regimens, several assessments have addressed the logistical difficulties of using anti-VEGF therapy in actual practice.15–18 However, none of these previous assessments address specific differences between the PRN and T&E regimens. With varying visit schedules and approaches to injection criteria, it can be assumed that differences between the two regimens would impact both ophthalmologist and patient treatment experience, ultimately influencing patient adherence to long-term treatment.
The aim of our research was to qualitatively assess the current issues and benefits of the PRN and T&E anti-VEGF therapy regimens and to focus on any issues associated with introducing T&E. Here, we report how proactive therapy is used to treat wAMD in clinical practices in Japan and how the different regimens impact patient, caregiver, and ophthalmologist experience.
Materials and methods
Qualitative assessment was performed using semistructured interviews conducted by IMS Consulting Group™, IMS Japan KK. Interviewees were ophthalmologists selected from a third-party panel by the IMS Consulting Group according to the selection criteria and based on their experience in treating wAMD. Ophthalmologists were required to have introduced proactive therapy for wAMD into their practice and to have current or previous experience with the PRN approach; this stipulation was intended to ensure that the interviewees had working experience with both types of anti-VEGF therapy regimens.
A total of 20 ophthalmologists were recruited from university hospitals, nonacademic hospitals, and ophthalmologist clinics; the ophthalmologists were recruited from the greater Tokyo area (50%), other regional cities (28%), and rural areas (22%). All interviewees were anonymous to the researchers. Interviews were conducted during the period of November 2014 through February 2015. Written informed consent was obtained from all interviewees, and with their consent, the interviews were recorded for transcripts to be used for analysis. All procedures performed in this study were approved and in accordance with the ethical standards of the institutional review board of Tokyo Women’s University Hospital and with the 1964 Declaration of Helsinki and its later amendments.
The interview questions consisted of three parts: 1) issues and benefits of PRN and T&E; 2) logistical and operational issues of introducing proactive therapy, especially T&E, to PRN practice; and 3) actions taken to handle the issues raised in 2).
Results
Interviewee background
During the interview process, it became clear that two interviewees had a different understanding of either the PRN or T&E treatment regimens, and their interviews were removed from the dataset. Background information on the remaining 18 interviewees is shown in Tables 1 and 2. The distribution of interviewees based on their institution types were as follows: university hospital: n=7, 39%; nonacademic hospital: n=7, 39%; and clinics: n=4, 22%.
Table 1.
Background information of interviewees’ institutions
| All ophthalmologists | University hospital ophthalmologists | Nonacademic hospital ophthalmologists | Clinic ophthalmologists | |
|---|---|---|---|---|
| Number of ophthalmologists in the institution | ||||
| ≥11 | 6 | 6 | 0 | 0 |
| 6–10 | 3 | 0 | 2 | 1 |
| 1–5 | 9 | 1 | 5 | 3 |
| Number of ophthalmologists trained for intravitreal injections | ||||
| ≥11 | 2 | 2 | 0 | 0 |
| 5–10 | 2 | 2 | 0 | 0 |
| 2–4 | 10 | 3 | 6 | 1 |
| 1 | 4 | 0 | 1 | 3 |
| Institution size (bed count) | ||||
| ≥1,000 | 3 | 3 | 0 | 0 |
| 500–999 | 5 | 3 | 2 | 0 |
| 20–499 | 5 | 0 | 5 | 0 |
| Unknown or ≤19 | 5 | 1 | 0 | 4 |
| Payment method | ||||
| DPCa | 14 | 7 | 7 | 0 |
| Non-DPC | 4 | 0 | 0 | 4 |
| Designated treatment location for anti-VEGF injections | ||||
| Outpatient treatment rooms | 7 | 5 | 1 | 1 |
| Operating rooms | 9 | 1 | 6 | 2 |
| Both | 2 | 1 | 0 | 1 |
| Need to reserve space to perform anti-VEGF injections | ||||
| Necessary | 10 | 2 | 7 | 1 |
| Unnecessary | 8 | 5 | 0 | 3 |
Note:
An inpatient bundle payment system similar to the diagnosis-related groups used in Medicare in the US.
Abbreviations: anti-VEGF, anti-vascular endothelial growth factor; DPC, diagnosis procedure combination.
Table 2.
Number of patients seen by the interviewees
| All ophthalmologists | University hospital ophthalmologists | Nonacademic hospital ophthalmologists | Clinic ophthalmologists | |
|---|---|---|---|---|
| Weekly number of patients with ophthalmic conditions | ||||
| ≥201 | 4 | 1 | 1 | 2 |
| 101–200 | 8 | 3 | 4 | 1 |
| ≤100 | 6 | 3 | 2 | 1 |
| Weekly number of wAMD patients (%) | ||||
| ≥70% | 4 | 4 | 0 | 0 |
| 30%–69% | 4 | 3 | 1 | 0 |
| ≤29% | 10 | 0 | 6 | 4 |
| Experience with proactive treatment (number of patients)a | ||||
| ≥101 | 10 | 7 | 2 | 1 |
| 11–100 | 3 | 0 | 2 | 1 |
| ≤10 | 5 | 0 | 3 | 2 |
Note:
Proactive treatment includes both fixed-dosing and T&E.
Abbreviations: T&E, treat-and-extend; wAMD, wet age-related macular degeneration.
Benefits and issues of T&E and PRN
The benefits and issues of PRN and T&E were evaluated from two perspectives: the patient or caregiver perspective as understood by the treating ophthalmologist and the treating ophthalmologist’s own perspective.
There were three main areas where the patient or caregiver experienced benefits with T&E, according to the ophthalmologists. These included the following: “Able to tangibly experience treatment efficacy through the sustained period of macular dryness”, “Decrease in emotional burden associated with receiving intraocular injections”, and “Decrease in patient/caregiver time burden” (Table 3). From the treating ophthalmologists’ perspective, the following three areas were identified: “Shorter consultation time per patient”, “Decrease in burden of developing patient specific treatment plans”, and “Decrease in overall psychological stress” (Table 4).
Table 3.
Benefits and issues of T&E compared with PRN from the patient/caregiver perspective as understood by the treating ophthalmologist
| Benefits | |
| Able to tangibly experience treatment efficacy through the sustained period of macular dryness | • Patients know that their treatment is working when their injection intervals increase • Treatment intervals can be adjusted to meet patient needs while maximizing the period of dryness |
| Decrease in emotional burden associated with receiving intraocular injections | • Since their injection schedule is determined months ahead, patients are emotionally prepared on the day of injection, decreasing their emotional burden toward treatment • Patients become proactive toward treatment as their symptoms stabilize with the preventive injections |
| Decrease in patient/caregiver time burden | • Scheduling visits with patients’ family members is easier as the majority of patients are elderly and require accompaniment by their family members • The number of treatments that had to be postponed because family members were unable to accompany patients to the clinic has decreased • Visits only have to be once every few months |
| Issues | |
| Increased financial burden for the patients | • The financial burden may increase since the number of injections increases with T&E • Explaining the administration method is difficult without any aides, which makes it more difficult for patients to accept the financial burden • It is difficult to explain to patients how long they need to continue their injections with the lack of long-term evidence |
| Worry of complications | • The risk of infections may increase with the increased number of injections |
Abbreviations: PRN, pro re nata; T&E, treat-and-extend.
Table 4.
Benefits and issues of T&E compared with PRN from the treating ophthalmologist perspective
| Benefits | |
| Shorter consultation time per patient | • With PRN, at every injection, the patients need to be informed that their symptoms have worsened, but with T&E, such explanations can be omitted since injections are administered regardless of symptoms • If the administration method of T&E and its meaning are explained thoroughly at the first consultation, subsequent consultations are shorter since patients already understand why they are receiving injections • Scheduling does not take as long as with PRN since the treatment goal is clear with T&E |
| Decrease in burden of developing patient-specific treatment plans Decrease in overall psychological stress |
• With a predetermined injection schedule, there is no need to accommodate the patients’ and ophthalmologists’ schedules on a per-injection basis • Since the patients’ visit and injection schedules are based on the previous injection interval, scheduling is much easier • There is no need to explain why the patients require an injection. Moreover, worsening of symptoms does not occur frequently with T&E, so the treatment process is easier to handle • The patients’ disappointed expressions are not seen when they come in for an injection that is predetermined and is not administered because the symptoms have worsened • There is less burden of explaining the treatment process since injection intervals are constant or are even extended in certain cases |
| Easier to set injection schedules | • Injections can be provided months in advance, which makes it easier to set the injection schedules for patients and foresee capacity |
| Issues | |
| Explaining the necessity of proactive injections as prevention | • The significance of proactive injections is not easily accepted by patients who have stabilized and entered maintenance phase since the effectiveness is less tangible than at the beginning of treatment. Patients with wAMD in only one eye or who have not experienced worsening of symptoms require more time in accepting continued preventive injections • Thorough explanation of the significance of proactive injections at the initial consultation is essential in getting patients to continue with long-term treatment • Without cost-effectiveness data, explaining the necessity of proactive injections as a preventive measure is difficult |
| Increased financial burden | • There have been cases where patients refuse T&E due to financial reasons • Thorough explanation of the financial burden is important in order for patients to be willing to undergo long-term treatment. However, this is especially difficult for first-time patients or for patients who have not experienced recurrence or worsening of symptoms |
| Insufficient area designated for injections | • Even though the number of patients has increased, the clinic space to provide injections remains unchanged |
| Insufficient human resources | • While patient numbers have increased with better treatment results and fewer dropouts, it has been difficult to increase the staff to match the increased demand |
| Increased number of appointments | • The time to process scheduling appointments has increased with the increase in patients receiving injections, which has cut into consultation times |
Abbreviations: PRN, pro re nata; T&E, treat-and-extend; wAMD, wet age-related macular degeneration.
The issues surrounding T&E were those related to the increase in intravitreal injections and concerns arising from the long-term treatment. Concerns over financial burden associated with an increase in intravitreal injections were highlighted from both the patient/caregiver and treating ophthalmologist perspectives. For patients who achieved dryness of the macula and stabilization of symptoms, there appeared to be difficulty in getting them to understand the significance of continuing with proactive injections, which in effect prevent their symptoms from worsening.
Issues arising from the introduction of T&E and countermeasures
Figure 1 summarizes the different issues ophthalmologists faced when introducing T&E to their practice, according to their institution type. These issues can be possibly identified in two stages: the “patient explanation phase” and the “operational phase”. While university hospital ophthalmologists did not consider “Handling concerns of financial burden from long-term treatment” as an issue they faced during consultations, ophthalmologists based in clinics all raised this issue as a concern. Only university hospital ophthalmologists raised “Increased burden of managing appointments” as a concern.
Figure 1.
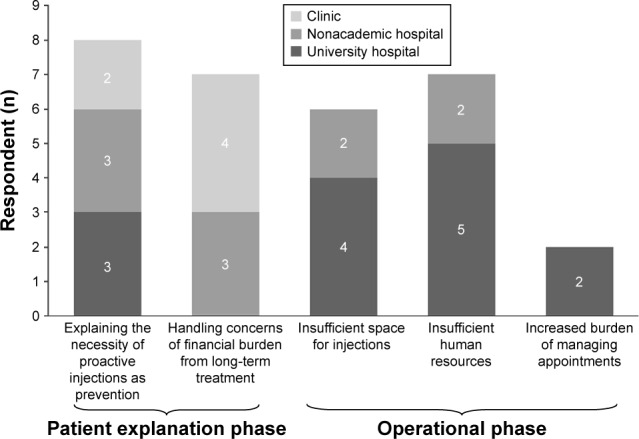
Issues arising from the introduction of T&E categorized by institution type.
Notes: Total number of respondents were 18 (university hospital: seven, nonacademic hospital: seven, and clinic: four). Respondents were asked to identify issues they faced when introducing T&E into their practice after already practicing PRN. Respondents were allowed to answer multiple issues.
Abbreviations: PRN, pro re nata; T&E, treat-and-extend.
Specific examples of the issues raised in Figure 1 are shown in Tables 5–7, organized by institution type. University hospital ophthalmologists mainly raised four issues as difficulties of practicing T&E: “Explaining the necessity of proactive injections as prevention”, “Insufficient space for injections”, “Insufficient human resources”, and “Increased burden of managing appointments”. The following six countermeasures were provided as remedies implemented: “Explained the efficacy/effectiveness in a more tangible way to the patient”, “Strengthened cooperation with other local facilities and referred patients”, “Employed part-time ophthalmologists”, “Delegated tasks of the injection process and defined the roles of each ophthalmologist”, “Shortened the turn-around time of patients receiving injections”, and “Implemented a scheduling system to manage appointments”.
Table 5.
Issues arising from introducing T&E and their countermeasures as answered by university hospital ophthalmologists
| Phase | Issues | Specific examples of issues | Countermeasures |
|---|---|---|---|
| Patient explanation phase | Explaining the necessity of proactive injections as prevention | • Since the impression of T&E is “more injections” than PRN, it is difficult to get the patient to accept the possible increased burden that comes with more injections | • Explained the efficacy/effectiveness in a more tangible way to the patient – Utilized OCT images so the patients could understand that they were undergoing the best treatment option – Clarified that without continuous injections, their symptoms would worsen |
| Operational phase | Insufficient space for injections | • Even though the number of patients has increased, the clinic space to provide injections remains unchanged | • Strengthened cooperation with other local institutions and referred patients – Suggested switching to local facilities by highlighting better transportation access and more attentive care than at a busy university hospital. Also, at local facilities, anti-VEGF therapy was considered an outpatient procedure, unlike our facility, which means less financial burden – Explained to patients that they will have to return to the university hospitals if they relapse, which resulted in greater acceptance of switching facilities |
| Insufficient human resources | • It is difficult to increase the staff to match the increased number of patients • It takes time to manage both consultations and injections |
• Strengthened cooperation with local facilities and referred appropriate patients • Employed part-time ophthalmologists – With part-time ophthalmologists from temporary agencies, it was possible to focus on injections on the designated injection days and have the part-time staff take on other consultations • Delegated tasks of the injection process and defined the roles of each ophthalmologist – Proactively included the younger staff into the rotations for their experience and to balance the burden of experienced members – Delegated specific tasks to each staff member: consultations, injections, processing appointments, OCT and fundoscopy, explanation of injections, and consultations prior to injections • Shortened the turnaround time of patients receiving injections – Changed the order of each room so that patients move efficiently in one direction: waiting room → examination room → OCT/fundoscopy → consultation room → treatment/injection room – Placed nurses both inside and outside of consultation rooms to efficiently guide patients to the next step in the treatment process – Changed the rules so that patients can step onto the injection table with their shoes on to shorten the time per patient for injections |
|
| Increased burden of managing appointments | • The time to process scheduling appointments has increased with the increase in patients receiving injections, which has affected consultation times | • Strengthened cooperation with local facilities and referred appropriate patients • Implemented a scheduling system to manage appointments – Decreased the processing burden by implementing an original scheduling system |
Abbreviations: anti-VEGF, anti-vascular endothelial growth factor; OCT, optical coherence tomography; PRN, pro re nata; T&E, treat-and-extend.
Table 6.
Issues arising from introducing T&E and their countermeasures as answered by nonacademic hospital ophthalmologists
| Phase | Issues | Specific examples of issues | Countermeasures |
|---|---|---|---|
| Patient explanation phase | Explaining the necessity of proactive injections as prevention | • Since the impression of T&E is “more injections” than PRN, it is difficult to get the patient to accept the possible increased burden that comes with more injections • Cannot explain the cost-effectiveness of the injections during the maintenance phase |
• Explained the efficacy/effectiveness in a more tangible way to the patient – The importance of reducing risk before symptoms worsen was explained to the patient using their OCT images – The patient was also advised that restarting treatment after symptoms have worsened has reduced effects – Although no reports have been presented at academic congresses for high-risk patients on the verge of dropping out, extending their injection interval for >3 months was considered |
| Operational phase | Insufficient space for injections | • Even though the number of patients has increased, the clinic space to provide injections remains unchanged | • Provided injections in outpatient treatment rooms instead of operating rooms |
| Insufficient human resources | • It is difficult to increase the staff to match the increased number of patients • It takes time to manage both consultations and injections |
• Designated roles within the staff – Roles were separated for consultations, which require more experienced staff, and injections, which can be administered uniformly by any of the ophthalmologists, in order to make the process more efficient – The nurses took the patient’s history prior to consultations |
Abbreviations: OCT, optical coherence tomography; PRN, pro re nata; T&E, treat-and-extend.
Table 7.
Issues arising from introducing T&E and their countermeasures as answered by clinic ophthalmologists
| Phase | Issues | Specific examples of issues | Countermeasures |
|---|---|---|---|
| Patient explanation phase | Explaining the necessity of proactive injections as prevention | Since the impression of T&E is “more injections” than PRN, it is difficult to get the patient to accept the possible increased burden that comes with more injections | • Explained the efficacy/effectiveness in a more tangible way to the patient – To patients who were concerned with the financial burden, OCT images of before and after treatment were shown during the initial consultation, so that they could understand the effectiveness of the injections – While the actual number of injections may not differ compared with PRN, it was explained to the patients that the number of visits are fewer with T&E, which can decrease their time and financial burden – Tried to keep patients motivated by discussing the possibility of a cure from IPS research in the future and telling them that they may not have to continue the injections forever |
| Handling concerns of financial burden from long-term treatment | • It is difficult to get patients who have never experienced relapse or worsening of symptoms to understand • Without cost-effectiveness data, explaining the necessity of the financial burden is difficult |
• Prioritized patients who were referred from other hospitals and understood T&E – Patients who have been treated at university hospitals have already received a thorough explanation of T&E and have a better understanding of the treatment as the ophthalmologists tend to be AMD specialists at these hospitals. These patients were prioritized because they tended to be more accepting of the treatment regimen |
Abbreviations: AMD, age-related macular degeneration; OCT, optical coherence tomography; PRN, pro re nata; T&E, treat-and-extend.
Specific issues raised by nonacademic hospital ophthalmologists were the same as those raised by university hospital ophthalmologists, except for “Increased burden of managing appointments”. A different countermeasure provided was “Provided injections in outpatient treatment rooms instead of operating rooms”, since operating rooms require reservations making scheduling complicated.
Clinic-based ophthalmologists raised two issues relating to “Explaining the necessity of proactive injections as prevention” and “Handling concerns of financial burden from long-term treatment”. The countermeasures implemented for these issues were “Explained the efficacy/effectiveness in a more tangible way to the patient” and “Prioritized patients who were referred from other hospitals and understood T&E”.
Discussion
Interviews with ophthalmologists revealed that compared with PRN, T&E decreased emotional burden for patients/caregivers and time burden for patients/caregivers and ophthalmologists. An additional patient benefit was the sustained period of macular dryness. The main issues raised were the increased number of injections and the associated financial burden from prolonged treatment duration. Issues arising from the introduction of T&E at the “patient explanation phase” or “operational phase” depended on practice type.
The analysis highlighted that predetermined scheduled injections (T&E) provided relief by ensuring that visits did not result in “bad news” and unforeseen injections compared with PRN; this decreased the emotional burden for patients and caregivers. Droege et al19 revealed that patients were more afraid of receiving negative examination results than the actual intravitreal injections, indicating that worsening of symptoms impacts patient experience more than frequency of injections.
These interview results also revealed that T&E reduced time burden for patients and caregivers in clinical practice by decreasing the amount of necessary visits. This is important because monthly monitoring of PRN has been considered burdensome for ophthalmologists, patients, and caregivers in previous studies.5,9,16 For example, Gohil et al20 looked at caregiver burden of PRN in the UK and on average, 70% of caregivers spent at least half a day assisting their patients with clinic visits every 4–6 weeks, and about 25% took time off from work. According to Droege et al,19 patients indicated that travel to and from visits was a major barrier to their wAMD treatment adherence. Combined with our results, it can be inferred that a fewer visits for T&E may result in better adherence by patients to long-term treatment.
The ophthalmologists also spoke of the reality of organizing PRN injections: once the patient was indicated for injection, the facility may not always be able to provide it on the same day, causing the patient to schedule another visit. If the caregiver was unable to accompany the patient for the rescheduled visit, the patient was subjected to even further delay in receiving timely treatment. This is an important issue for treating a time-sensitive condition such as wAMD. A study analyzing a hospital claims database in Japan showed that an average of 11.6 days elapsed between injections and the previous outpatient monitoring visits under PRN.21 Likewise, Takahashi et al22 analyzed two hospitals in Japan and found average injection delays under PRN of between 0 and 104 days. These studies further indicate that injection delays are not rare with PRN approaches in real-life settings. Takahashi et al22 also revealed that visual acuity prognosis worsened with injection delays. Similarly, Rayess et al12 found that patients receiving consecutive maintenance injections with T&E were less susceptible to recurrence of disease activity than PRN, where duration of consecutive injections tends to be prolonged. Previous studies reporting visual acuity outcomes with PRN dosing were conducted with patients receiving injections on the same day,6,14 which, compared with treatment pattern studies, may not reflect real-life clinical practice.21,22 As reported in our analysis, T&E is able to avoid such delays and missed injection opportunities by following a predetermined injection schedule, which leads to prolonged dryness of the macula and prevention of worsening of symptoms. While previous studies have focused on ophthalmologists’ experience of such clinical outcomes, our analysis reveals for the first time that patients (based on ophthalmologist feedback) are also able to tangibly experience the effectiveness of anti-VEGF treatment with T&E, which can be linked to increased treatment satisfaction and likely treatment adherence.
As mentioned earlier, issues of introducing T&E into the clinical practice can be identified in two stages: the patient explanation phase and the operational phase. There were benefits associated with decreased emotional burden from simplified consultations with patients who already understood the necessity of receiving injections at their visits. However, ophthalmologists also had difficulties in explaining the preventive effect of T&E, which was identified in all institution types. They had difficulty convincing patients who had entered the maintenance phase of their treatment with stable disease activity to continue with proactive injections before any disease activity resumes. While this difficulty was common to ophthalmologists across all institution types, the management of this issue appeared to differ. This phenomenon may be attributed to the lack of a guideline for T&E articulating how to manage injections during the maintenance phase of a patient’s disease state. A literature review with consensus recommendations on T&E was published in 2015, but there remains a lack of evidence-based resource that can support Japanese practice.8
Issues in the operational phase were raised mostly from university hospital ophthalmologists. University hospitals appear to be overburdened. They have a relatively greater number of wAMD patients compared with hospitals and clinics; T&E also results in increased injections per patient and a fewer dropouts, resulting in an increased number of total patients continuing anti-VEGF treatment. Rearranging the patient flow and dividing staff roles to improve efficiency were provided as effective actions. However, increased staff and space for administering injections seemed to be ultimately desired even after such measures. It should also be noted that issues with staff and spatial capacity have been reported in previous studies with PRN, and this could be an ongoing issue that is not unique to the practice of T&E.15,16,18,22
Strengthening local clinic and university hospital relations in order to increase referrals to clinics was also raised as a potential solution to the operational phase issues. It appears that this is currently practiced only in limited areas. Implementing integrated care pathways is still in its infancy in Japan, particularly in ophthalmology. At present, there have only been pilot trials with glaucoma and ophthalmologic care for patients with dementia.23,24 Disparity in care between practices is also an issue.25 This was also observed by Takahashi et al.22 This variation in care further reiterates the need for an evidence-based standardized method of practicing T&E that can be followed by ophthalmologists across practices.
There are several limitations to this qualitative analysis. First, interviewees were limited and lacked representativeness of a larger population. Second, interviewees were located primarily within the greater Tokyo metropolitan area or other regional cities, and their responses may not reflect the realities of other regional situations. We performed subgroup analyses by region and facility types (hospital vs clinic ophthalmologists) to see if there were any differences. It was revealed that there were no interesting/meaningful results by region (data not shown), but we did find a difference between hospital and clinic ophthalmologists. This is why we only reported the facility type differences in the manuscript.
Conclusion
This qualitative analysis demonstrated that the benefits of T&E compared with PRN as reported by ophthalmologists included decreased burden of patient consultations, decreased patient and caregiver emotional burden, and a sustained period of macular dryness. The analysis revealed that patient treatment satisfaction can be increased via T&E, and this may increase the potential for better adherence to long-term treatment. However, increased number of injections and financial burden from prolonged treatment duration remain issues associated with T&E. The ophthalmologists also experienced difficulty explaining the significance of proactive injections for prevention to patients. Countermeasures to operational issues experienced by ophthalmologists varied by practice. In order to encourage better understanding of the T&E regimen, including its smooth implementation and significance for patients, a formal T&E treatment guideline providing standard practice should be considered.
Acknowledgments
We are most grateful to the ophthalmologists who participated in the interviews. We thank Mr Satoru Ono of IMS Consulting Group™, IMS Japan KK, who was involved in the design of the interviews and analysis of the data. The manuscript was developed and authorized by the authors. The manuscript has also been authorized by Mr Ono as a member who partook in the analysis. This study was funded by Bayer Yakuhin, Ltd.
Footnotes
Disclosure
Tomohiro Iida has received research grants from Bayer Yakuhin, Ltd., Santen Pharmaceutical Co., Ltd., Novartis Pharmaceutical KK, and Alcon Japan Ltd. Keirei Ishii is an employee of Bayer Yakuhin, Ltd. The authors report no other conflicts of interest in this work.
References
- 1.Friedman DS, O’Colmain BJ, Munoz B, et al. Eye Diseases Prevalence Research Group Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 3.Kawasaki R, Yasuda M, Song SJ, et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. 2010;117(5):921–927. doi: 10.1016/j.ophtha.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Sivaprasad S, Hykin P. What is new in the management of wet age-related macular degeneration? Br Med Bull. 2013;105:201–211. doi: 10.1093/bmb/ldt004. [DOI] [PubMed] [Google Scholar]
- 5.Hatz K, Prunte C. Treat and extend versus pro re nata regimens of ranibizumab in neovascular age-related macular degeneration: a comparative 12 month study. Acta Ophthalmol. 2016 Mar 24; doi: 10.1111/aos.13031. Epub. [DOI] [PubMed] [Google Scholar]
- 6.Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heier JS, Brown DM, Chong V, et al. VIEW 1 and VIEW 2 Study Groups Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Freund KB, Korobelnik JF, Devenyi R, et al. Treat-and-extend regimens with anti-VEGF agents in retinal diseases: a literature review and consensus recommendations. Retina. 2015;35(8):1489–1506. doi: 10.1097/IAE.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 9.Gupta OP, Shienbaum G, Patel AH, Fecarotta C, Kaiser RS, Regillo CD. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology. 2010;117(11):2134–2140. doi: 10.1016/j.ophtha.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 10.Arnold JJ, Campain A, Barthelmes D, et al. Two-year outcomes of “treat and extend” intravitreal therapy for neovascular age-related macular degeneration. Ophthalmology. 2015;122(6):1212–1219. doi: 10.1016/j.ophtha.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Homer N, Grewal DS, Mirza RG, Lyon AT, Gill MK. Transitioning to intravitreal aflibercept following a previous treat-and-extend dosing regimen in neovascular age-related macular degeneration: 24-month results. Eye (Lond) 2015;29(9):1152–1155. doi: 10.1038/eye.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rayess N, Houston SK, 3rd, Gupta OP, Ho AC, Regillo CD. Treatment outcomes after 3 years in neovascular age-related macular degeneration using a treat-and-extend regimen. Am J Ophthalmol. 2015;159(1):3–8. doi: 10.1016/j.ajo.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120(11):2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 14.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Maguire MG, Martin DF, Ying GS, et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(8):1751–1761. doi: 10.1016/j.ophtha.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amoaku W, Blakeney S, Freeman M, et al. Action on AMD Group Action on AMD. Optimising patient management: act now to ensure current and continual delivery of best possible patient care. Eye (Lond) 2012;26(suppl 1):S2–S21. doi: 10.1038/eye.2011.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casaroli-Marano R, Roura M, Grupo de Estudio Optimal Disponibilidad de recursos para pacientes con degeneración macular asociada a la edad de tipo húmedo. Estudio Optimal [Availability of resources for patients with wet age-related macular degeneration. Optimal study] Arch Soc Esp Oftalmol. 2013;88(8):307–312. doi: 10.1016/j.oftal.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Michelotti MM, Abugreen S, Kelly SP, et al. Transformational change: nurses substituting for ophthalmologists for intravitreal injections – a quality-improvement report. Clin Ophthalmol. 2014;8:755–761. doi: 10.2147/OPTH.S59982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prenner JL, Halperin LS, Rycroft C, Hogue S, Williams LZ, Seibert R. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol. 2015;160(4):725–731. doi: 10.1016/j.ajo.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Droege KM, Muether PS, Hermann MM, et al. Adherence to ranibizumab treatment for neovascular age-related macular degeneration in real life. Graefes Arch Clin Exp Ophthalmol. 2013;251(5):1281–1284. doi: 10.1007/s00417-012-2177-3. [DOI] [PubMed] [Google Scholar]
- 20.Gohil R, Crosby-Nwaobi R, Forbes A, Burton B, Hykin P, Sivaprasad S. Caregiver burden in patients receiving ranibizumab therapy for neovascular age related macular degeneration. PLoS One. 2015;10(6):e0129361. doi: 10.1371/journal.pone.0129361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iida T, Narimatsu A, Adachi K, Wang ECY. Anti-vascular endothelial growth factor outpatient treatment patterns in patients with exudative age-related macular degeneration from a Japanese hospital claims database. JHEOR. 2014;2(1):41–52. doi: 10.36469/9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi H, Ohkubo Y, Sato A, et al. Relationship between visual prognosis and delay of intravitreal injection of ranibizumab when treating age-related macular degeneration. Retina. 2015;35(7):1331–1338. doi: 10.1097/IAE.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 23.Kashiwagi K. より質の高い緑内障診療を目指して [Toward a high quality glaucoma care] Nippon Ganka Gakkai Zasshi. 2012;116(3):269–296. [PubMed] [Google Scholar]
- 24.Miyamura Y, Sotozono C, Higashihara H, Hoshi S, Kinoshita S. 認知症を伴う高齢者の重症眼感染症 [Severe ocular infection in elderly patients with dementia: a case study] Nippon Ganka Gakkai Zasshi. 2015;119(12):863–867. [PubMed] [Google Scholar]
- 25.Suto S, Hiraoka T, Okamoto Y, Okamoto F, Oshika T. スマートフ ォンによる前眼部および眼底撮影 [Photography of anterior eye segment and fundus with smartphone] Nippon Ganka Gakkai Zasshi. 2014;118(1):7–14. [PubMed] [Google Scholar]


